Key Points
Real-world experience endorses higher response rate with blinatumomab compared with conventional chemotherapy in RR ALL.
Blinatumomab and tyrosine kinase inhibitor combination is safe and effective in improving long-term outcome of RR Ph+ B-cell ALL patients.
Abstract
The availability and use of blinatumomab symbolizes a paradigm shift in the management of B-cell acute lymphoblastic leukemia (ALL). We conducted a retrospective multicenter cohort analysis of 239 ALL patients (227 relapsed refractory [RR], n = 227; minimal residual disease [MRD], n = 12) who received blinatumomab outside of clinical trials to evaluate safety and efficacy in the “real-world” setting. The median age of patients at blinatumomab initiation was 48 years (range, 18-85). Sixty-one (26%) patients had ≥3 prior therapies and 46 (19%) had allogeneic hematopoietic cell transplantation before blinatumomab. The response rate (complete remission/complete remission with incomplete count recovery) in patients with RR disease was 65% (47% MRD−). Among 12 patients who received blinatumomab for MRD, 9 (75%) patients achieved MRD negativity. In patients with RR disease, median relapse-free survival and overall survival (OS) after blinatumomab was 32 months and 12.7 months, respectively. Among patients who received blinatumomab for MRD, median relapse-free survival was not reached (54% MRD− at 2 years) and OS was 34.7 months. Grade ≥3 cytokine release syndrome, neurotoxicity, and hepatotoxicity were observed in 3%, 7%, and 10% of patients, respectively. Among patients who achieved complete remission/complete remission with incomplete count recovery, consolidation therapy with allogeneic hematopoietic cell transplantation retained favorable prognostic significance for OS (hazard ratio, 0.54; 95% confidence interval, 0.30-0.97; P = .04). In this largest “real-world” experience published to date, blinatumomab demonstrated responses comparable to those reported in clinical trials. The optimal sequencing of newer therapies in ALL requires further study.
Visual Abstract
Introduction
Treatment of relapsed refractory (RR) B-cell acute lymphoblastic leukemia (ALL) is challenging because of chemo-resistance and toxicity of cytotoxic therapies. Response rates to conventional salvage chemotherapy regimens are in the range of 20% to 40% and the duration of remissions are short-lived.1-4 The aim in this subset of patients is to achieve remission with minimal toxicity and to attain response for sufficient duration to successfully bridge to allogeneic hematopoietic cell transplantation (allo-HCT). In this context, blinatumomab has emerged as a novel therapy and promises to achieve desired results. Blinatumomab is a bispecific T-cell antibody construct that binds and allows CD3+ cytotoxic T cells to recognize and eradicate CD19+ ALL blasts.5 In a phase 3, randomized controlled trial (Blinatumomab Versus Standard of Care Chemotherapy in Patients With Relapsed or Refractory Acute Lymphoblastic Leukemia), when compared with standard of care conventional chemotherapy, blinatumomab was superior in inducing complete remission (CR) (34% vs 16%, P < .001) and improving overall survival (OS; 7.7 vs 4.0 months, P = .01) in patients with RR B-cell ALL.5 Cytokine release syndrome (CRS) and neurological adverse events were more common in the blinatumomab arm compared with conventional chemotherapy. More recently, Stein et al investigated exposure-adjusted adverse events in patients from phase 3 Blinatumomab Versus Standard of Care Chemotherapy in Patients With Relapsed or Refractory Acute Lymphoblastic Leukemia study and demonstrated more frequent neurological adverse events in standard of care arm compared with blinatumomab.6
Although information regarding the efficacy and toxicity of blinatumomab is mainly available through the experience reported in clinical trials, numerous real-world experiences in other hematological malignancies suggest that clinical outcomes may differ outside of these controlled settings with generally more fit patients.5,7-11 In this study, we evaluated survival outcome and toxicities of blinatumomab in B-cell ALL patients in the real-world setting. Furthermore, we explored the feasibility of allo-HCT following blinatumomab treatment. To our knowledge we report the largest series of B-cell ALL patients treated with blinatumomab outside of clinical trials.
Methods
We conducted a retrospective multicenter study in collaboration with 11 academic institutions in the United States. This study was approved by the institutional review board from each participating institution. B-cell ALL patients who were age 18 years or older at the time of blinatumomab administration and who received drug outside of clinical trials were enrolled in this study. Patients with Philadelphia chromosome [t9,22] (Ph+) B-cell ALL or those who received blinatumomab for minimal residual disease (MRD) were also included. Medical records were reviewed to collect demographic, patient-related, disease-related and clinical outcome data. These patients were evaluated for response, relapse-free survival (RFS), OS from the time of blinatumomab initiation, and toxicities. Responses and survival outcome of patients who received blinatumomab for MRD were analyzed separately.
CR was defined as 5% or less bone marrow blasts, no evidence of disease in the bone marrow, and recovery of peripheral blood count (platelet count of >100 000 per microliter and absolute neutrophil count of >1000 per microliter). Complete remission with incomplete count recovery (CRi) was defined as 5% or less bone marrow blast and no evidence of disease in the bone marrow but with incomplete recovery of peripheral blood count. MRD was assessed at each participating institution with the use of multicolor flow cytometry. Extramedullary disease (EMD) was determined by treating physician’s assessment, supported by radiological (computed tomography scan or positron emission tomography scan) and/or histological confirmation.
Statistical analysis
Demographic and disease characteristics were summarized using descriptive statistics and compared between the study group using Wilcoxon rank-sum test for continuous and χ2 tests for categorical outcomes. Survival curves were estimated using the Kaplan-Meier method and compared between groups via the log-rank test. The cumulative incidence of allo-HCT was estimated using the Nelson-Aalen estimate with death without allo-HCT as a competing risk. RFS was estimated among patients achieving a CR/CRi after blinatumomab from the time of CR/CRi to death or progression. Cox regression was used for the multivariate analysis with time of allo-HCT as a time-dependent covariate.
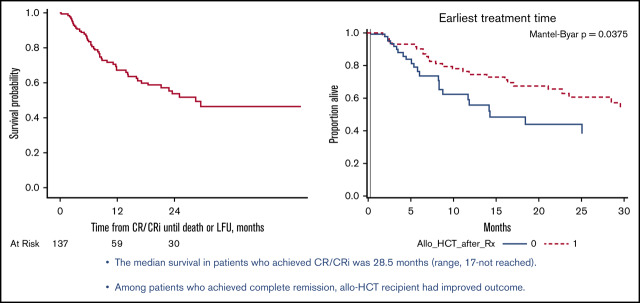
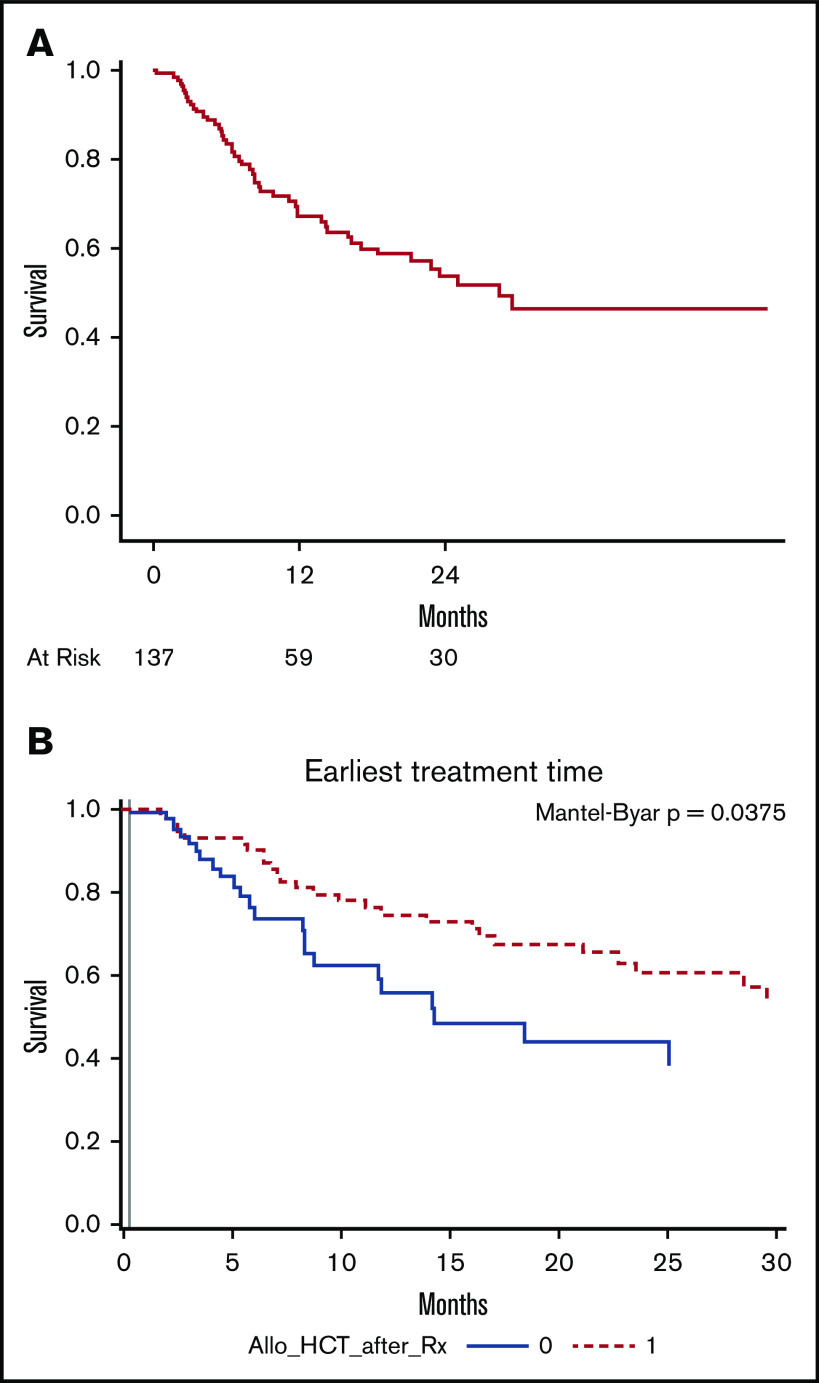
The utilization and effect of allo-HCT was examined among patients who achieved CR/CRi, with follow-up starting at the first documented response. The effect of allo-HCT on survival was visualized using a Simon-Makuch plot, which estimates the survival experience of 2 hypothetic patients, 1 of whom never gets the intervention, whereas the other gets it at the time of the earliest treatment start. The P values were based on Mantel-Byar test. The incidence of acute graft-versus-host disease (aGVHD) and chronic graft-versus-host disease (cGVHD) was determined based on timing of GVHD after allo-HCT. aGVHD within first 100 days of allo-HCT and cGVHD after 100 days after allo-HCT, as documented in medical records.
Results
Baseline characteristics
From December 2014 to May 2019, 239 patients with B-cell ALL were identified. Two hundred and twenty-seven (95%) patients received blinatumomab for RR B-cell ALL and 12 (5%) patients received blinatumomab for MRD. Baseline characteristics are summarized in Table 1. The median age of patients at blinatumomab initiation was 48 years (range, 18-85), and 101 (42%) patients were females. Sixty-one (26%) patients had ≥3 prior therapies and 46 (19%) patients had allo-HCT before blinatumomab (data missing on 1 patient). Fifteen (6.5%) patients had central nervous system (CNS) disease at the time of diagnosis and 39 (17%) patients had CNS disease at the time of relapse/progression. In the RR group, 221 (97%) patients had bone marrow disease at blinatumomab initiation, among them 9 (4%) patients had concurrent active CNS disease. Overall, 10 (4%) patients had EMD, among them 8 patients had EMD relapse without bone marrow infiltration at blinatumomab initiation. The median number of blinatumomab cycles given was 2 (range, 1-5). Nine patients with active CNS disease received intrathecal chemotherapy concurrently with blinatumomab. Three patients received chemotherapy with blinatumomab and 1 patient received rituximab with blinatumomab. Overall, 61 (25%; n = 55 [RR], n = 6 [MRD]) patients with Ph+ B-cell ALL received blinatumomab; among them, 29 (48%) patients received blinatumomab with a tyrosine kinase inhibitor (TKI). Among these 29 patients, 16 (55%) patients received ponatinib and 13 (45%) patients received dasatinib with blinatumomab concomitantly. The dosing of TKI was based on anticipated tolerance as per treating physician’s assessment. Overall, 22 (9%) patients were previously treated with inotuzumab ozogamicin.
Table 1.
Baseline characteristics (N = 239)
| Value | |
|---|---|
| Age, median (range),* y | 48 (18-85) |
| Female | 138 (58) |
| KPS* | |
| 80%-100% | 131 (84) |
| <80% | 25 (16) |
| Missing, n | 83 |
| WBC,* median (range) | 4.2 (0.01-205) |
| Missing, n | 1 |
| Peripheral blood blast %,* median (range) | 0.2 (0.2-97) |
| Missing, n | 10 |
| Ph+ | 61 (25.5) |
| t(4,11) | 28 (12) |
| Any cytogenetic abnormality | 164 (69) |
| CNS disease | |
| At diagnosis | 15 (6.5) |
| At relapse/progression | 39 (17) |
| At blinatumomab initiation | 9 (4) |
| Extramedullary disease | 10 (4) |
| No. of therapies before blinatumomab, median (range) | 1 (1-7) |
| Missing, n | 1 |
| Blinatumomab as first salvage | 126 (53) |
| Blinatumomab as second salvage | 51 (21) |
| Blinatumomab as third salvage or beyond | 62 (26) |
| No. of patients with ≥3 therapies | 61 (26) |
| Primary refractory | 55 (24) |
| Time to progression after induction, y | |
| <1 | 93 (40) |
| ≥1-<2 | 45 (19) |
| ≥2-<3 | 16 (7) |
| ≥3 | 18 (7.5) |
| Allo-HCT before blinatumomab | 46 (19) |
| Previous treatment with inotuzumab ozogamicin | 22 (9) |
| Blinatumomab with TKI | 29 (12) |
| Blinatumomab with ponatinib | 16 (7) |
| Blinatumomab with dasatinib | 13 (5) |
| Blinatumomab given for MRD | 12 (5) |
| Standard blinatumomab dosing† | 218 (91) |
| MRD dosing8 | 12 (5) |
| Reduced dose due to anticipated tolerance | 9 (4) |
Values are n (%) unless otherwise noted. MRD dosing: cycle 1: 28 mcg/day continuous IV infusion on days 1-28 of a 42-day cycle. Consolidation, cycle 2-4: 28 mcg/day continuous IV infusion on days 1-28 of a 42-day cycle.
KPS, Karnofsky performance status.
At blinatumomab initiation.
Continuous infusion of 9 μg/kg for 1 week followed by 28 μg/kg infusion for 3 weeks in 6 weekly cycles.
Response
The response rate (CR/CRi) in patients with RR disease was 67% with 49% of patients achieving CR with MRD negativity (Table 2). Four (2%) patients were nonevaluable. Among the 12 patients who received blinatumomab for MRD, 9 (75%) achieved MRD−. In patients who received blinatumomab for MRD, 1 patient had prior therapy with inotuzumab ozogamicin. This patient did not achieve MRD− with blinatumomab. In patients with RR Ph+ B-cell ALL, the CR/CRi rate was 74% (n = 28 [68%] MRD−). In a subgroup of patients with RR Ph+ B-cell ALL, 23/55 (42%) received blinatumomab with TKI with CR/CRi rate of 83%. In 32/55 (58%) patients with Ph+ RR ALL who received blinatumomab without TKI, the CR/CRi rate was 69%. In MRD+ Ph+ B-cell ALL, 4/6 (66%) achieved MRD−. Among the patients who were primary refractory to induction chemotherapy, 35/55 (64%) achieved CR/CRi. One hundred and twenty-six patients received blinatumomab as first salvage with CR/CRi rate of 70% and 51 patients received blinatumomab as second salvage with CR/CRi rate of 65%. Among patients who had early relapse (<1 year after CR1; n = 78), CR/CRi rate was 65%. Patients who had late relapse (>2 years after CR1; n = 31), CR/CRi rate was 51%. Among 10 patients with EMD besides CNS, only 2 had a response with blinatumomab.
Table 2.
Response to blinatumomab
| Value, n (%) | |
|---|---|
| Response with blinatumomab in R/R B-cell ALL (N = 227) | |
| CR + CRi | 149 (67) |
| CR with MRD− | 109 (49) |
| CR with MRD+ | 37 (17) |
| CRi | 3 (1) |
| Partial remission | 8 (4) |
| Nonevaluable | 4 (2) |
| CR + CRi in Ph+ (n = 55) | 41 (74.5) |
| CR + CRi in primary refractory (n = 55) | 35 (64) |
| CR + CRi after salvage 1 (n = 126) | 90 (71) |
| CR + CRi after salvage 2 (n = 51) | 33 (65) |
| CR + CRi after early relapse (<18 months of first remission) (n = 121) | 78 (64) |
| CR + CRi after late relapse (≥36 months of first remission) (n = 17) | 12 (71) |
| Response to EMD (n = 10) | 2 (20) |
| Response with blinatumomab in B-cell ALL with MRD (n = 12) | |
| No. of patients who achieved MRD− | 9 (75) |
Eighty-five (35.5%) patients who achieved remission were successfully bridged to allo-HCT. Among 142 RR ALL patients who did not receive allo-HCT after blinatumomab, 41 (29%) patients had prior allo-HCT. The median age of the patients was 51 (range, 18-85), 35 (25%) patients were Ph+ and 22 (15%) patients had MLL gene rearrangement. Twenty-five (18%) patients had primary refractory disease and the median number of therapies before blinatumomab was 2 (range, 1-8). Thirty-four (24%) patients had a history of CNS disease. Among these nontransplant patients, 4 patients died from toxicity, 1 each from CRS, liver failure, cerebrovascular accident, and septic shock. Sixty-four (45%) patients achieved CR/CRi (4 patients were not evaluable), among them 29 patients who continued therapy until last follow-up. Three patients received CAR-T cells and 2 patients received donor lymphocyte infusion with prior history of allo-HCT after responding to blinatumomab. The remaining 30 patients progressed after responding initially. The median event-free survival was 5.2 months (95% confidence interval [CI], 3.4-6.9), RFS was 6.9 months (95% CI, 3.5-10.2), and OS was 5.5 months (95% CI, 4.0-10.2).
We performed a logistic regression analysis to evaluate predictors for response (CR/CRi). Patients who received blinatumomab in combination with TKI and/or chemotherapy had an odds ratio of 4.97 (95% CI, 1.51-16.43; P = .008), predicting high likelihood of achieving CR/CRi. The number of prior therapies >3 vs 1 had an odds ratio of 0.33 (95% CI, 0.15-0.71; P = .004), suggesting less likelihood of achieving CR/CRi. Ph+ (P = .57), MLL gene rearranged (P = .38), and CNS+ ALL (P = .8) did not demonstrate significant impact on CR/CRi. Similarly, number of prior therapies (2 vs 1; P = .8), time to progression after CR1 <1 year vs primary refractory (P = .53) or 1 to <2 years vs primary refractory (P = .92) did not show significance for achievement of CR/CRi (supplemental Table 1).
Adverse events of interest
Adverse events of interest are outlined in Table 3. Among the 236 evaluable patients, CRS of any grade (G) was reported in 94 (40%) patients (G1-G2, 36%; G3-G4, 3%). Forty-four (44%) patients were treated with corticosteroids and 8 (8%) patients required tocilizumab with steroids for CRS. Almost all patients with CRS had complete resolution of symptoms apart from 1 patient who died from severe CRS. In patients who had G3 CRS and received tocilizumab, blinatumomab was discontinued transiently. Once CRS was resolved, blinatumomab was rechallenged with 9 mcg/day for 7 days followed by 28 mcg/day after 7 days and continued without recurrence of CRS. In 1 patient with G4 CRS, blinatumomab was discontinued permanently. This patient died, despite receiving high-dose steroids and tocilizumab.
Table 3.
Adverse events of interest (N = 239)
| Adverse events | Any grade | Grade 1-2 | Grade ≥3 |
|---|---|---|---|
| Cytokine release syndrome (evaluable; n = 236) | 94 (40) | 86 (36) | 8 (3) |
| Neurological adverse events (evaluable; n = 236) | 55 (23) | 36 (15) | 17 (7) |
| Encephalopathy | 22 (9) | 10 (4) | 12 (5) |
| Headache | 27 (11) | 24 (10) | 3 (1) |
| Seizure | 3 (1) | 2 (1) | 1 (0.4) |
| Stroke | 3 (1) | 0 | 1 (0.4) |
| Hepatotoxicity (evaluable; n = 238) | 83 (35) | 58 (24) | 26 (10) |
| Transaminases | 62 (26) | 40 (17) | 22 (9) |
| Hyperbilirubinemia | 20 (8) | 17 (7) | 3 (1) |
| Veno-occlusive disease | 2 (1) | 1 (0.4) | 1 (0.4) |
| Dose interruptions | |||
| Total no. of patients with dose interruptions | 50 (21) | ||
| 1 | 36 (15) | ||
| 2 | 9 (4) | ||
| 3 | 5 (2) | ||
| Discontinuation of blinatumomab because of AE | 17 (7) | ||
| Neurotoxicity | 8 (3) | ||
| Hepatoxicity | 4 (2) | ||
| Cytokine release syndrome | 3 (1) | ||
| Infections | 3 (1) | ||
| Pain | 1 (0.4) | ||
Among the 236 evaluable patients, neurotoxicity of any grade was observed in 55 (23%) patients, with 17 (7%) patients experiencing G3-G4 toxicity. Among the 238 evaluable patients, hepatotoxicity of any grade was observed in 84 (35%) patients; 62 (26%), 20 (8%), and 2 (1%) patients experienced transaminase elevation, hyperbilirubinemia, and veno-occlusive disease (VOD), respectively. Of the 2 patients who had VOD, none had prior therapy with inotuzumab ozogamicin. Four (2%) patients died from blinatumomab-related toxicities (n = 1 septic shock, n = 1 liver failure, n = 1 stroke, n = 1 CRS). Sixteen (7%) patients had to discontinue blinatumomab because of adverse events. Eight (3%) and 4 (2%) patients discontinued blinatumomab as a result of neurotoxicity and hepatoxicity, respectively. Three (1%) patients each had to discontinue blinatumomab because of CRS and infections. One (0.4%) patient had uncontrollable pain that led to blinatumomab discontinuation. Fifty (21%) patients had dose interruptions resulting from adverse events. Most of the patients (n = 36) had 1-time dose interruption with resumption of therapy upon resolution of symptoms.
In subset analysis, among 29 patients who received blinatumomab with TKI, CRS of any grade was observed in 10 (34%) patients, but no G3-G4 CRS was observed. The symptoms resolved with temporarily holding therapy and providing supportive care. Transaminitis of any grade was observed in 7 (24%) patients; 1 (3%) patient had G3 toxicity. Neurological adverse events of any grade were observed in 4 (14%) patients, and 1 (3%) patient had G3 encephalopathy.
Allo-HCT
Eighty-five (35.5%) patients who achieved remission were successfully bridged to allo-HCT. The median age of the patients transplanted was 59 (range, 18-72) years and the median number of therapies received before allo-HCT was 2 (range, 2-6). Five (6%) patients had second allo-HCT after blinatumomab. The median number of blinatumomab cycles before allo-HCT was 2 (range, 1-4). The median time from remission to allo-HCT was 1.7 months (range, 0.20-13.2). The proportion of patients who had MRD, matched unrelated donor, haploidentical, and cord blood allo-HCT were 36.5%, 42%, 15%, and 9.5%, respectively. aGVHD, cGVHD, VOD, and infectious complications after allo-HCT were observed in 28 (33%), 14 (16.5%), 2 (2%), and 12 (14%) patients, respectively. There were no reports of B-cell aplasia after allo-HCT. Six (7%) patients had transplant-related mortality within 100 days of allo-HCT. The median progression-free survival and OS among transplant recipients was not reached (NR); 66% were progression free and 62% were alive at 2 years (Table 4).
Table 4.
Baseline characteristics and clinical outcome of patients who received allo-HCT transplantation after blinatumomab (N = 85)
| Value | |
|---|---|
| Age, median (range), y | 59 (18-72) |
| Median number or prior therapies (range) | 2 (2-6) |
| Prior allo-HCT | 5 (6) |
| Time from remission to allo-HCT, median (range), mo | 1.7 (0.2-13.2) |
| Type of allo-HCT | |
| Matched related donor | 31 (37) |
| Matched unrelated donor | 36 (42) |
| Haploidentical | 13 (15) |
| Cord blood | 5 (6) |
| Complication after allo-HCT | |
| aGVHD | 28 (33) |
| cGVHD | 14 (16.5) |
| Veno-occlusive disease | 2 (2) |
| Infectious complication | 12 (14) |
| Transplant-related mortality in 100 d | 6 (7) |
| Progression-free survival | NR (66% progression free at 2 y) |
| Overall survival | NR (62% alive at 2 y) |
Values are n (%) unless otherwise noted.
RFS
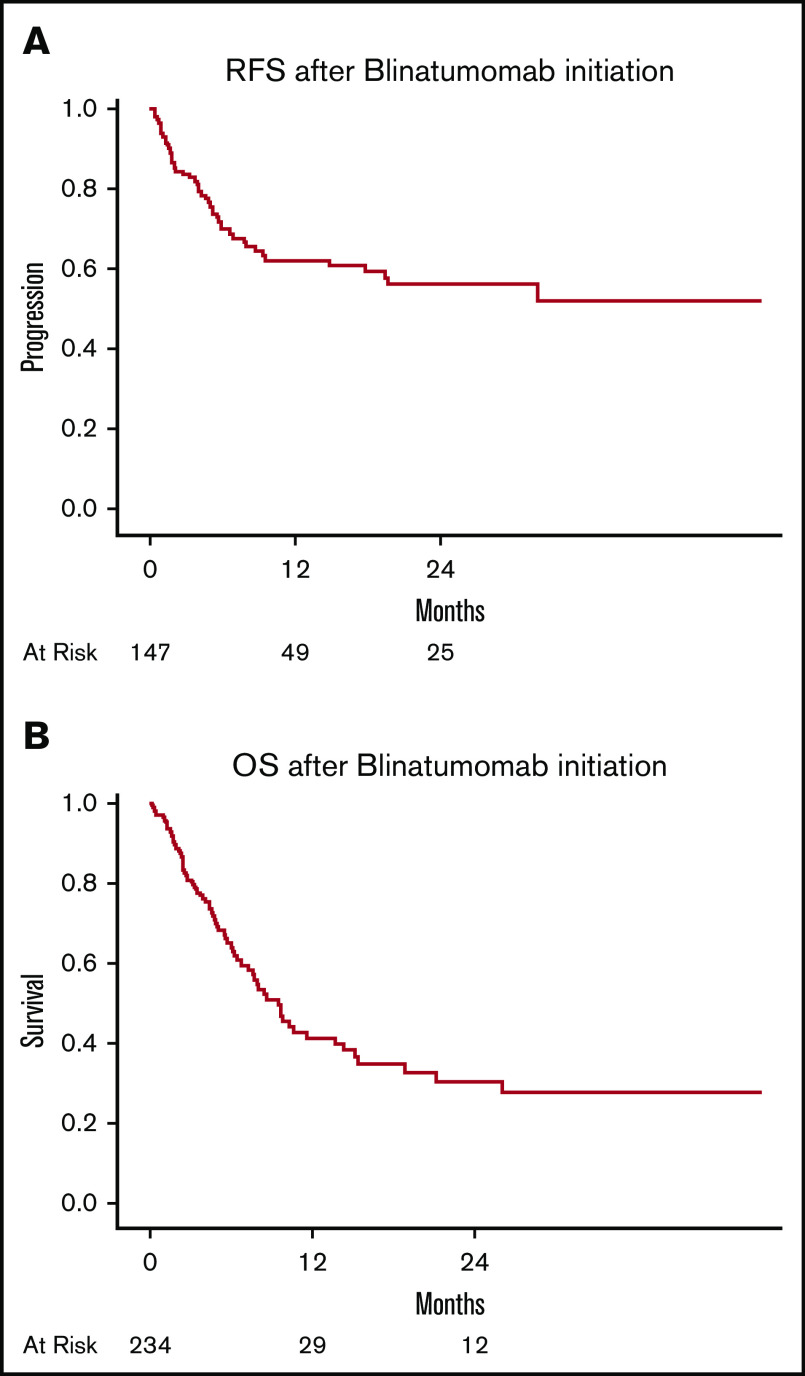
The median RFS in patients with RR disease was 32.1 months (95% CI, 9.5-NR; Figure 1A). The median RFS in patients who received blinatumomab for MRD was NR and 54% of patients were MRD− at the 2-year follow-up. In a subgroup of RR Ph+ B-cell ALL patients who received blinatumomab with TKI, RFS was NR (76% of patients were relapse free at 2 years) compared with 32 months in RR Ph+ B-cell ALL patients who received blinatumomab alone (P = .28).
Figure 1.
Kaplan-Meier curves in RR B-cell ALL patients. (A) Relapse-free survival after blinatumomab. (B) OS after blinatumomab.
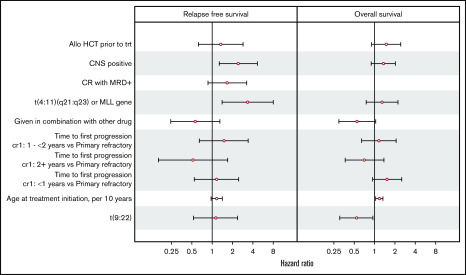
In multivariate analysis for RFS in patients with RR disease, the presence of CNS disease at the time of blinatumomab initiation (hazard ratio [HR], 2.44; 95% CI, 1.28-4.66; P = .006) and MLL gene rearrangement (HR, 3.38; 95% CI, 1.41-8.07; P = .006) retained independent negative prognostic significance. Age at blinatumomab initiation, CR with MRD+ vs MRD−, CR1 of <1 year vs primary refractory disease, CR1 between 1 and < 2 years vs primary refractory disease or CR1 of >2 years vs primary refractory disease did not show significance in multivariate analysis (supplemental Table 2A; Figure 3).
Figure 3.
Forest plot illustrating hazard ratios of variables tested for significance for relapse-free survival and overall survival.
Overall survival
The median follow-up of the entire cohort was 14 months (range, 1-52). In RR ALL group the median follow-up was 14 months (range, 1-52) and in the MRD+ ALL group; 6 months (range, 4-32). The median OS of patients after blinatumomab was 12.7 months (95% CI, 9.2-17.9) (Figure 1B). Among patients who received blinatumomab for MRD, the median OS was 34.7 months (95% CI, 8.8-34.7). In subset analyses for OS in patients with RR disease, the OS after blinatumomab was not significantly different between patients with primary refractory disease vs others (15.1 vs 12.0 months; P = .7; supplemental Figure 1A). Among RR patients with Ph+ disease, the median OS was NR (57% alive at 2 years) vs 9.6 months (95% CI, 7.7-14.3), with P value of .001 (supplemental Figure 1B). In a subgroup of RR Ph+ B-cell ALL patients who received blinatumomab with TKI, OS was NR (69% of patients were alive at 2 years) compared with 13.1 months in RR Ph+ patients who received blinatumomab alone (P = .03). B-cell ALL patients with t(4:11) (MLL gene rearrangement) had inferior survival of 7.5 months vs 14.3 months (P = .008), as illustrated in supplemental Figure 1C. The median survival in patients who achieved CR/CRi was 28.5 months (range, 17-not reached) (Figure 2A). Allo-HCT retained favorable prognostic significance (HR, 0.54; 95% CI, 0.30-0.97; P = .04) for survival in multivariate analysis, among patients who achieved CR/CRi. Similarly, the Simon-Makuch curve showed significant improvement in survival outcome of patients who received allo-HCT (P = .03; Figure 2B).
Figure 2.
Survival in responding patients. (A) Kaplan-Meier curves for OS in patients who achieved CR/CRi. (B) Simon-Makuch curve demonstrating survival outcome in patients who achieved CR and received allo-HCT vs no allo-HCT.
In multivariate analysis for OS, Ph+ B-cell ALL (HR, 0.38; 95% CI, 0.21-0.66; P = .0008) and allo-HCT after blinatumomab (HR, 0.40; 95% CI, 0.24-0.67; P = .0005) retained favorable prognostic significance for OS. Age at blinatumomab initiation (HR, 1.14; 95% CI, 1.01-1.30; P = .02) and CR1 of <1 year vs primary refractory disease (HR, 1.69; 95% CI, 1.02-2.79; P = .04) retained independent negative prognostic significance for OS. MLL gene rearrangement, CNS disease, and allo-HCT before blinatumomab did not retain prognostic significance for OS in multivariate analysis (supplemental Table 2B; Figure 3).
Discussion
Our real-world data with multicenter collaboration demonstrates that blinatumomab was well tolerated and led to significant responses in patients with RR disease. Adverse events secondary to blinatumomab were comparable to the initial studies and were effectively treated with supportive care. Among the patients who achieved remission, more than one-third of the patients were successfully bridged to allo-HCT with improved long-term survival.
Over the past few decades, the clinical outcome of B-cell ALL has improved because of better treatment strategies and supportive care; however, the outcome of those patients with RR disease remains poor.1,4,12 In fact, the rate of response to conventional chemotherapy decreases with each successive salvage regimen.12 Blinatumomab has been shown to improve the outcome of these chemo-resistant patients. Kantarjian et al, in a pivotal phase 3 study, reported significantly improve outcome of RR B-cell ALL patients treated with blinatumomab compared with conventional chemotherapy; this served as the data that led to the approval of blinatumomab for patients with RR B-cell ALL. The agent was subsequently approved for MRD based on efficacy data in prospective clinical trials.13,14 Our study reinforces prior reports of blinatumomab efficacy in clinical trials and supports its used as a more effective therapy than conventional salvage chemotherapies.12 Sixty-five percent (n = 149/227) of patients with RR disease achieved CR/CRi, among them 73% achieved MRD−. The response rate observed were higher than reported in the Kantarjian et al study, most likely because of the latter study studying more heavily pretreated patients with ∼60% patients receiving blinatumomab as second salvage/beyond and 30% of patients with prior allo-HCT. Moreover, among the 12 patients who received blinatumomab for MRD, 75% achieved MRD−, which is also consistent with the results observed in clinical trials.8
Although blinatumomab has improved response rates in RR B-cell ALL patients, responses are short lived and allo-HCT is recommended in eligible patients to improve the long-term outcome and offer a possible cure. In our study, 35.5% of patients who achieved remission were successfully bridged to allo-HCT. Patients who received allo-HCT had significantly improved progression-free survival (66%) and OS (62%) at 2 years’ follow-up. The favorable impact of allo-HCT on RFS and OS was retained in multivariate analysis. The rates of transplant-related complications such as aGVHD (33%), VOD (2%), and transplant-related mortality within 100 days (7%) were comparable to those reported in the literature.14 Our results are consistent with previous reports that allo-HCT is safe and effective after blinatumomab in patients with RR B-cell ALL.5,15
Philadelphia chromosome+ B-cell ALL is considered as a high-risk disease with outcome shown to be even worse in patients with RR disease.16 Blinatumomab has shown efficacy in this group of patients and deeper molecular responses were achieved with single-agent blinatumomab as reported in a phase 2, multicenter study.17 In our patient cohort, 55 RR Ph+ B-cell ALL patients received blinatumomab and 25 (45%) patients received TKI in addition to blinatumomab. The CR rate in this group of patients was 74% (MRD− 68%), which was higher than that reported (36%) in the clinical trial setting. The superior response rates observed may be due to the addition of TKI, which were received by nearly one-half of the patients in our study. The response rates observed translated into superior OS compared with rest of the patients. All of our patients either received dasatinib or ponatinib concurrently with TKI. Second-generation TKI such as dasatinib inhibits both Src and LYN in addition to ABL kinase. Moreover, T-cell receptor signaling is also dependent on Src family kinase activity, and Src inhibitors may affect the efficacy of immunotherapies such as blinatumomab that are dependent on native T-cell function. Although in vitro studies suggest that blinatumomab combination with dasatinib (Src/ABL kinase inhibitor) may repeal efficacy of blinatumomab, clinical studies suggest that dasatinib may increase clonal expansion of cytotoxic T cells and improve responses.18,19 Moreover, dasatinib has more profound activity against regulatory T cells compared with cytotoxic T cells, which facilitates blinatumomab activity.20 Our and other small series suggest that dasatinib or ponatinib given concomitantly with blinatumomab does not affect its effectiveness.21 Larger studies are warranted to evaluate efficacy of blinatumomab plus TKI combination.
Extramedullary disease in ALL confers poor prognosis with inferior progression-free survival and OS.22 In our cohort, 10 patients had EMD besides CNS involvement; 5 of these patients had prior allo-HCT and only 2 patients had a response to blinatumomab. Future studies incorporating CAR T-cell therapy may be able to overcome poor outcome associated with extramedullary relapse.22,23
Cytokine release syndrome is 1 of the most significant adverse effects associated with blinatumomab and is a result of immune activation secondary to therapy.24 In the initial studies, the incidence of G1-G2 CRS in RR B-cell ALL patients treated with blinatumomab was reported in the range of 10% to 15%, whereas severe CRS (G ≥ 3) was rare (2%-4%).5,7,24 We observed a higher proportion of patients (36%) having G1-G2 CRS but the incidence of G3-G4 CRS (3%) was comparable to earlier studies. Most of the patients had complete resolution of CRS with supportive care. The higher incidence G1-G2 CRS in our analysis may be due to heterogeneity in patient’s disease burden at the initiation of blinatumomab in our study compared with clinical trials. The rate of grade 3 or higher neurological toxicities (17 [7%]) was comparable to observations in clinical trials. Seventeen (7%) patients had to discontinue blinatumomab because of adverse events and 4 (2%) patients died from severe adverse events. The mortality secondary to blinatumomab observed in our study was significantly lower than what has been reported after conventional salvage chemotherapies.3,4 We have observed relatively lower incidence of CRS in the blinatumomab plus TKI subgroup, with none of the patients developing G3-G4 CRS. This suggests that TKI such as dasatinib and ponatinib may have caused impaired T-cell proliferation and cytokine production resulting in lower incidence of CRS. Future clinical studies in a larger group of patients are warranted to evaluate off target kinase inhibition with TKI and effectiveness of blinatumomab.
We acknowledge the limitations of our retrospective analysis, including the possibilities of selection bias and inability to capture all consecutive B-cell ALL patients treated with blinatumomab outside of clinical trials and therapies patients received after progressing on blinatumomab. Second, it would be preferable to have blood-based biomarker data, analyzing effect of blinatumomab on regulatory and cytotoxic T cells and response to therapy, which this data set is lacking. Future studies that can evaluate effect of blinatumomab on T-cell proliferation/expansion would be highly intriguing. Nevertheless, our real-world data support the effectiveness of blinatumomab in chemo-resistant B-cell ALL patients as well as the feasibility of allo-HCT after blinatumomab to improve long-term outcomes. High response rates in RR ALL compared with traditional salvage chemotherapy suggests that blinatumomab can be more beneficial early in the treatment course during induction/consolidation. Eastern Cooperative Oncology Group trial 1910 has recently closed, so new data will be available soon.
Supplementary Material
The full-text version of this article contains a data supplement.
Footnotes
Send data sharing requests via e-mail to the corresponding author, Ehab Atallah (eatallah@mcw.edu).
Authorship
Contribution: T.B. undertook study design, writing the original manuscript draft, and methodology; A.S. undertook methodology and statistical analysis; A.A. and M.R.L. contributed patients and provided manuscript editing; M.W. contributed patients and provided manuscript editing. S.A., M.A.K., C.S., E.S., M.H., I.Y., A.A.P., S.B., and M.B. undertook data curation; I.A., N.P., E.C., J.Y., R.J.M., S.D., and M.L. contributed patients; R.M.S. undertook data curation and manuscript editing; and E.A. undertook study design and methodology, contributed patients, and edited the manuscript.
Conflict-of-interest disclosure: A.A. reports receiving honoraria/serving as a consultant for Glycomimetics, KiTE Pharmaceuticals, Novartis, and Pfizer; and receives research support from Amgen, AbbVie, Macrogenics, Glycomimetics, and Pfizer. R.J.M. has served on an advisory board for Pfizer. N.P. reports receiving honoraria/serving as a consultant for Alexion, Pfizer, Agios Pharmaceuticals, Blueprint Medicines, Incyte, Novartis, Celgene, Bristol-Myers Squibb, and CTI biopharma; receives research funding (all to the institution) from Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Sunesis Pharmaceuticals, Jazz Pharmaceuticals, Pfizer, Astex Pharmaceuticals, CTI biopharma, Celgene, Genentech, AI Therapeutics, Samus Therapeutics, Arog Pharmaceuticals, Kartos Therapeutics; and receives grant funding from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Ehab Atallah, Division of Hematology and Oncology, Medical College of Wisconsin, 9200 W Wisconsin Ave, Milwaukee WI 53226; e-mail: eatallah@mcw.edu.
References
- 1.Kantarjian HM, Thomas D, Ravandi F, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer. 2010;116(24):5568-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oriol A, Vives S, Hernández-Rivas JM, et al. ; Programa Español de Tratamiento en Hematologia Group . Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95(4):589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavernier E, Boiron JM, Huguet F, et al. ; Australasian Leukaemia and Lymphoma Group . Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907-1914. [DOI] [PubMed] [Google Scholar]
- 4.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86(7):1216-1230. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein AS, Larson RA, Schuh AC, et al. Exposure-adjusted adverse events comparing blinatumomab with chemotherapy in advanced acute lymphoblastic leukemia. Blood Adv. 2018;2(13):1522-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134-4140. [DOI] [PubMed] [Google Scholar]
- 8.Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia [published correction appears in Blood. 2018;131(14):1522-1531]. Blood. 2018;131(14):1522-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishnoi R, Shah C, Moreb J, Murthy HS. Single center real-world experience of daratumumab and extra-medullary myeloma. Blood. 2018;132(suppl 1):5668. [Google Scholar]
- 10.Mato AR, Allan JN, Pagel JM, et al. Front-line ibrutinib therapy for chronic lymphocytic leukemia (CLL) in the real world: responses, toxicity, outcomes and subsequent therapies. Blood. 2017;130(suppl 1):3011. [Google Scholar]
- 11.Mato AR, Thompson M, Allan JN, et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica. 2018;103(9):1511-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gökbuget N, Stanze D, Beck J, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia . Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032-2041. [DOI] [PubMed] [Google Scholar]
- 13.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493-2498. [DOI] [PubMed] [Google Scholar]
- 14.Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein A, Topp MS, Goekbuget N, et al. Allogeneic hematopoietic stem cell transplantation following anti-CD19 BiTE® blinatumomab in adult patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL). Blood. 2014;124(21):965. [Google Scholar]
- 16.Moorman AV, Harrison CJ, Buck GA, et al. ; Adult Leukaemia Working Party, Medical Research Council/National Cancer Research Institute . Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109(8):3189-3197. [DOI] [PubMed] [Google Scholar]
- 17.Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795-1802. [DOI] [PubMed] [Google Scholar]
- 18.Leonard J, Kosaka Y, Malla P, et al. T-cell suppression by Src/ABL kinase inhibitors when combined with blinatumomab in Ph+ acute lymphoblastic leukemia. Blood. 2018;132(suppl 1):2694. [Google Scholar]
- 19.Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23(8):1398-1405. [DOI] [PubMed] [Google Scholar]
- 20.Najima Y, Yoshida C, Iriyama N, et al. Regulatory T cell inhibition by dasatinib is associated with natural killer cell differentiation and a favorable molecular response-the final results of the D-first study. Leuk Res. 2018;66:66-72. [DOI] [PubMed] [Google Scholar]
- 21.King AC, Pappacena JJ, Tallman MS, Park JH, Geyer MB. Blinatumomab administered concurrently with oral tyrosine kinase inhibitor therapy is a well-tolerated consolidation strategy and eradicates measurable residual disease in adults with Philadelphia chromosome positive acute lymphoblastic leukemia. Leuk Res. 2019;79:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng J, Lai P, Qin L, et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia [published correction appears in J Heamtol Oncol. 2019;12(1):117]. J Hematol Oncol. 2018;11(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yates B, Shalabi H, Civelek AC, Delbrook C, Fry TJ, Shah NN. Efficacy and kinetics of CAR-T cell therapy in children and young adults with extramedullary acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). Biol Blood Marrow Transplant. 2018;24(3):S75-S76. [Google Scholar]
- 24.Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.