Abstract
Background/Aims:
Altered extracellular matrix (ECM) remodeling and podocyte apoptosis are characteristic features of diabetic nephropathy (DN). Aliskiren (ALI) inhibits the renin-catalyzed conversion of angiotensinogen to angiotensin I. This study tested ALI's effect on podocyte ECM accretion and survival in a high-glucose environment in vitro.
Methods:
Conditionally immortalized mouse podocytes were incubated in normal glucose (NG; 5.5 mM) or high glucose (HG; 40 mM) for 24–48 h with and without ALI (20 nM). Real-time RT-PCR was performed for fibronectin (FN), collagen α5(type IV) (Cola5IV), matrix metalloproteinases 2 and 9 (MMP2 and MMP9), and tissue inhibitor of metalloproteinases 1 and 2 (TIMP1 and TIMP2). Western blots were performed for FN, Cola5IV, MMP2, MMP9, TIMP1 and cleaved (activated) caspase-3.
Results:
ALI significantly reduced the mRNA and protein levels of FN, Cola5IV and TIMP1, and the mRNA of TIMP2 and cleaved caspase-3. ALI had no effect on MMP2 mRNA or protein or MMP9 mRNA tested under HG conditions. Under NG conditions, ALI had no effect on FN, Cola5IV, MMP2, MMP9 and activated caspase-3 proteins. ALI decreased the activated caspase-3 protein and evidence of apoptosis by TUNEL staining observed in podocytes cultured under HG conditions.
Conclusion:
These results show for the first time that renin inhibition with ALI mitigates the profibrotic and apoptotic effects of HG in cultured podocytes. These data strengthen the therapeutic rationale for renin inhibition with ALI beyond its hemodynamic effects.
Key Words: Aliskiren, Diabetic nephropathy, Extracellular matrix, High glucose, Podocytes, Renin inhibition
Introduction
Proteinuria is a cardinal manifestation of diabetic nephropathy (DN) and reflects pathological filtration across the glomerular capillary wall. One defining characteristic of DN is thickening of the glomerular basement membrane (GBM). The podocyte has a major role in synthesizing the GBM. The regulated maintenance of the GBM represents a balance between extracellular matrix (ECM) degradation and synthesis. Matrix metalloproteinases (MMPs) degrade ECM and tissue inhibitors of matrix metalloproteinases (TIMPs) inhibit MMPs. The podocyte is a polarized epithelial cell that produces the components of the ECM and the MMPs and TIMPs responsible for ECM remodeling. While the role of GBM pathology in proteinuria has been eclipsed in recent years by the abundance of data that implicate a predominant role for structural and signaling pathology in podocytes as the cause of decreased permselectivity [1,2], alterations in the GBM may nevertheless also contribute.
Podocyte pathology has emerged as key in the evolution of albuminuria in most albuminuric states [3,4], including DN [5,6]. Experimentally, the demonstration of podocyte apoptosis in models of DN [7,8,9,10] supports assertions that podocyte apoptosis diminishes GBM coverage. In patients with DN, podocytes are effaced [11], the podocyte number is diminished [3,6], podocytes are excreted in the urine [4,12] and there is a clear relationship between podocyte loss and proteinuria [1].
Apoptosis is induced in cultured podocytes by numerous injurious stimuli that simulate conditions of diabetes, such as elevated levels of TGF-β [13,14,15,16,17], angiotensin II [18] and glucose [7,15]. Cell death progresses via activation of the apoptotic effector molecule caspase-3 [14]. Because podocytes are terminally differentiated and usually do not undergo replication, injuries that lead to apoptosis diminish the overall podocyte number and lead to patches of denuded GBM.
Podocytes express all of the components of the renin-angiotensin system (RAS) required to synthesize angiotensin II [18,19,20]. Angiotensin II is synthesized as the result of the 2 enzymatically catalyzed steps of the RAS. First, renin catalyzes the upstream rate-limiting conversion of angiotensinogen to angiotensin I. Angiotensin-converting enzyme (ACE) then catalyzes the conversion of angiotensin I to angiotensin II. Angiotensin II is believed to function in an autocrine manner in podocytes [18]. Thus, the intrarenal RAS is regulated independently of the systemic RAS. Apart from high glucose (HG) itself, angiotensin II is implicated as a primary mediator of injury in DN. One aim of the current study is to assess whether inhibiting angiotensin II via upstream renin antagonism prevents podocyte apoptosis induced by incubation in high-glucose medium.
The current studies also address whether RAS-blockade with aliskiren (ALI) preserves podocyte ECM regulation and podocyte survival during HG exposure. Glucose-stimulated glomerular cells are common in vitro models for angiotensin II-induced ECM accumulation [21,22,23] and for DN. This study is the first to test whether the direct renin inhibitor ALI ameliorates HG-induced ECM accumulation and podocyte apoptosis in cultured mouse podocytes via measurements of the synthesis of the following proteins: fibronectin (FN), the podocyte-specific collagen α5(type IV) (Cola5IV), MMP2 (gelatinase A), MMP9 (gelatinase B), TIMP1 (MMP9 inhibitor), TIMP2 (MMP2 inhibitor) and cleaved caspase-3.
Materials and Methods
Cell Culture
Conditionally immortalized mouse podocytes, carrying a thermosensitive SV40 transgene, proliferated at 33°C in RPMI 1640 with 5.5 mM glucose, 10% fetal bovine serum (FBS), γ-IFN (tapered from 50 units/µl to 10 units/µl), 100 U/ml penicillin, 100 U/ml streptomycin and 100 U/ml amphotericin B. Podocytes were then thermoshifted to 37°C and allowed to differentiate for 14 days without γ-IFN. Following a 14-day differentiation period, podocytes were serum-starved for 24 h with 0.4% FBS prior to treatment. The starved cells were exposed to normal glucose (NG, 5.5 mM) or HG (40 mM), and 20 nM ALI for 24 (matrix-related) or 48 h (activated caspase-3). All ALI-treated cells underwent a 1-hour pretreatment period with 5.5 mM glucose and 20 nM ALI before HG/ALI cotreatment began. Mannitol served as an osmotic control.
Western Blotting
The cell lysate was harvested using a cell scraper after the addition of RIPA buffer with proteinase inhibitor (1:100; Sigma, St. Louis, Mo., USA) and phosphatase inhibitor cocktails 1 and 2 (1:100; Sigma). Cells were sonicated for 1 min, centrifuged for 10 min at 10,000 g and the lysates stored at −20°C. Protein concentration was quantified using Protein Assay Dye Reagent (Bio-Rad Laboratories, Hercules, Calif., USA) and known bovine serum albumin (BSA) concentrations. Western blots were performed under reducing conditions. 50–100 µg of protein was loaded onto SDS-PAGE gels (6–14% gradient). Protein was transferred overnight to nitrocellulose membranes (GE Osmonics Labstore, Minnetonka, Minn., USA). Membranes were blocked with 5% milk in tris-buffered saline solution with 0.2% Tween 20 (TBST), incubated with primary antibody, washed (3 times for 10 min with TBST), incubated with secondary antibody and rewashed. The film was developed using enhanced chemiluminescence solutions (Pierce Biotechnology Inc., Rockford, Ill., USA). Arbitrary densitometric units were obtained during analysis with AlphaDigiDoc 1000 (Alpha Innotech Corporation, San Leandro, Calif., USA).
Antibodies
Primary antibodies were mouse anti-FN IgM (F6140; Sigma), mouse anti-α5 type(IV) collagen (MAB5; Wieslab AB, Lund, Sweden), rabbit anti-mouse MMP2 (sc-10736; Santa Cruz Biotechnology, Santa Cruz, Calif., USA), rabbit anti-MMP9 (2270; Cell Signaling Technology Inc., Danvers, Mass., USA), rabbit anti-TIMP1 (sc-5538; Santa Cruz Biotechnology), rabbit anti-cleaved caspase-3 (9661; Cell Signaling Technology Inc.) and mouse anti-GAPDH (RDI-TRK5G4-6C5; Fitzgerald Industries International Inc., Concord, Mass., USA). HRP-linked secondary antibodies were goat anti-mouse IgG (sc-2005; Santa Cruz Biotechnology), goat anti-mouse IgM (sc-2064; Santa Cruz Biotechnology) and goat anti-rabbit (7074; Cell Signaling Technology Inc.).
Real-Time Reverse Transcription-Polymerase Chain Reaction
Total RNA from cultured podocytes was extracted according to the RNA STAT-60 protocol (Tel-Test Inc., Friendswood, Tex., USA). Genomic DNA digestion (Qiagen Inc., Valencia, Calif., USA) was followed by RNA Cleanup according to the RNeasy Mini Protocol (Qiagen). Reverse transcription was performed on 2 µg of total RNA with random primer (Roche Diagnostics Corp., Indianapolis, Ind., USA) for 1 h at 37°C using the Omniscript Reverse Transcriptase Kit (Qiagen). Real-time RT-PCR was performed with cDNA using a Quantitect SYBR Green PCR Kit (Qiagen). Quantitative gene expression patterns, reflected by calculated Ct values of FN, Cola5IV, MMP2, TIMP2, MMP9, TIMP1 and β-actin normalizer cDNA were measured with the Applied Biosystems 7000 Real-Time PCR System (Applied Biosystems, Foster City, Calif., USA). Primer sequences are provided in table 1.
Table 1.
Primers used in real-time RT-PCR
| Gene | Forward primer | Reverse primer | Base pairs |
|---|---|---|---|
| Fibronectin | 5′-catgcggttttggttcagact-3′ | 5′-cggtgtaattccggttgttgtac-3′ | 88 |
| Cola5IV | 5′-aaaggagagcctggtggaat-3′ | 5′-gccacaccttgtatgccttt-3′ | 153 |
| MMP2 | 5′-accagaacaccatcgagacc-3′ | 5′-tacttttaaggcccgagcaa-3′ | 182 |
| TIMP2 | 5′-acacggccccctcttca-3′ | 5′-tctgcctttcctgcaattagatact-3′ | 82 |
| MMP9 | 5′-ttccccaaagacctgaaaacc-3′ | 5′-gcccgggtgtaaccatagc-3′ | 83 |
| TIMP1 | 5′-aaatcaacgagaccaccttatacca-3′ | 5′-atccacagaggctttccatga-3′ | 132 |
| β-Actin | 5′-ctttctacaatgagctgcgtg-3′ | 5′-tcatgaggtagtctgtcagg-3′ | 304 |
TUNEL Staining
Podocytes were harvested after 48 h of treatment, fixed with ice-cold 4% PFA for 15 min on ice and permeabilized in 0.5% Tween-20, 0.2% BSA in phosphate-buffered saline (PBS) for 15 min. The TUNEL reaction mixture was prepared by adding terminal deoxynucleotidyl transferase to the nucleotide mixture, as per the manufacturer (Millipore, Temecula, Calif., USA). Each slide was incubated with 50 µl of TUNEL reaction mixture at room temperature for 60 min, and cells were labeled by avidin-FITC solution in the dark for 30 min. After rinsing with PBS, the nuclei on the slides were counterstained with DAPI, mounted with Vectashield fluorescence mounting medium (Vector Laboratories, Burlingame, Calif., USA) and visualized under a fluorescence microscope. Quantification of positive cells by TUNEL staining was performed by taking images in 5 regions of sections at a magnification of ×400. Cell counts were obtained by counting nuclei for healthy and apoptotic cells. Counts of positive cells were given in the results as percentages of total number of cells.
Statistical Analysis
Analysis of variance followed by the Mann-Whitney test for nonparametric data was utilized for analysis of all results. Statistical calculations were performed using StatMost (Data Most Corp., Salt Lake City, Utah, USA). All data are expressed as means ± SEM; p < 0.05 was considered statistically significant.
Results
ALI Attenuates FN and Cola5IV mRNA and Protein
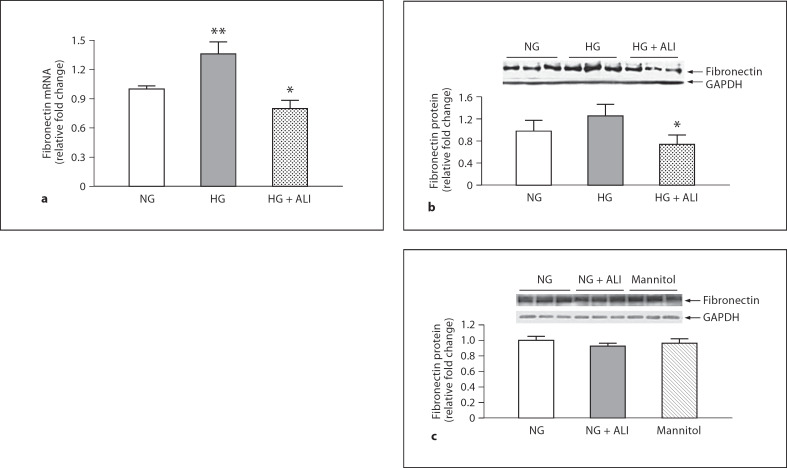
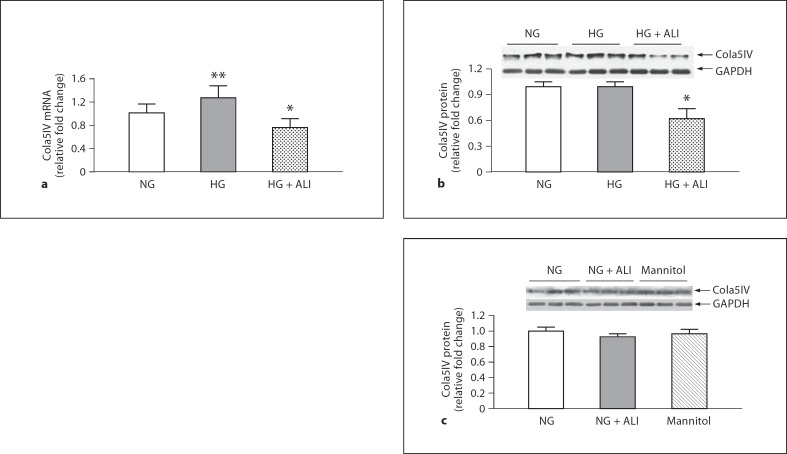
Similar trends for 2 components of the GBM, FN and Cola5IV were observed after 24 h of incubation. The 1.4-fold (p < 0.05) increase in podocyte FN mRNA with exposure to HG was reduced 1.7-fold by ALI (p < 0.05; fig. 1a). Similar results were obtained at the protein level where FN was numerically increased 1.3-fold after HG exposure and was reduced 1.7-fold by ALI (p = 0.05; fig. 1b). In podocytes cultured in NG, ALI did not reduce FN protein, nor was FN protein changed in NG with mannitol added to isotonicity with HG (5.5 mM glucose + 34.5 mM mannitol; fig 1c). In a similar manner, the statistically significant 1.3-fold increase of Cola5IV mRNA upon exposure to HG was significantly reduced 1.2-fold by ALI administration (p < 0.05; fig. 2a). Although an increase in Cola5IV protein with HG exposure was not observed, presumably due to the short exposure time of 24 h, ALI nevertheless reduced Cola5IV 1.7-fold in podocytes cultured in HG (p < 0.05; fig. 2b). In podocytes cultured in NG, ALI did not reduce Cola5(IV) protein, nor was Cola5(IV) protein changed in NG with mannitol added (5.5 mM glucose + 34.5 mM mannitol; fig 2c).
Fig. 1.
FN mRNA and protein under NG and HG conditions with and without ALI in podocytes. a FN mRNA in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. b FN protein in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 6. c In NG, ALI did not increase FN protein, nor was FN protein changed by isotonic mannitol (5.5 mM glucose + 34.5 mM mannitol, n = 3 in each group). * p ≤ 0.05 vs. HG; ** p < 0.05 vs. NG.
Fig. 2.
Cola5IV in podocytes. a Cola5IV mRNA in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. b Cola5IV protein in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 6. c In NG, ALI did not increase Cola5IV protein, nor was Cola5IV protein changed by isotonic mannitol (5.5 mM glucose + 34.5 mM mannitol, n = 3 in each group). ** p < 0.05 vs. NG, * p < 0.05 vs. HG.
ALI Remodels Matrix Turnover through Regulation of TIMP2 but Not MMP2
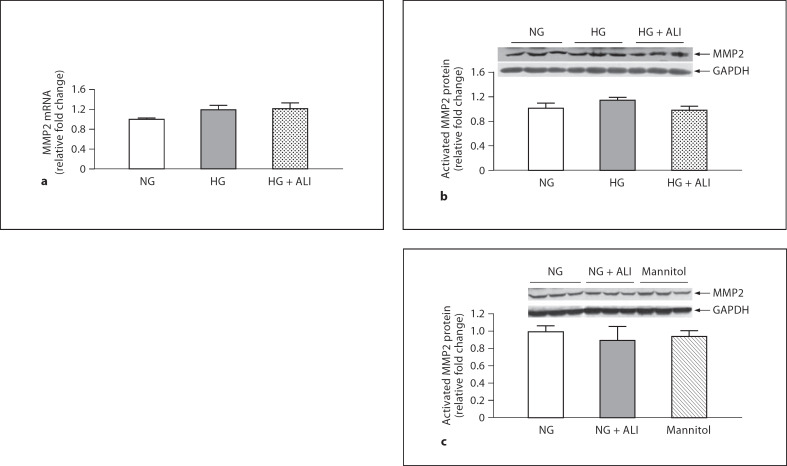
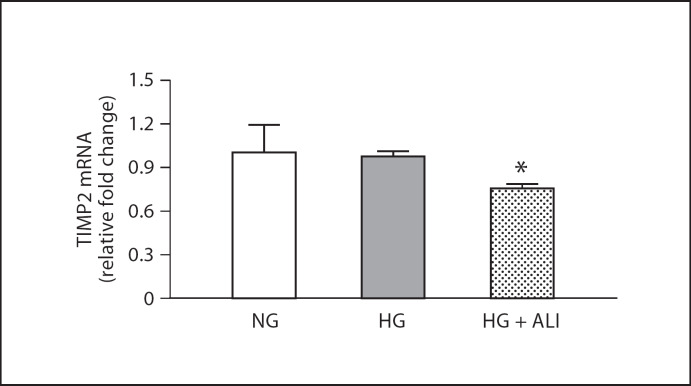
GBM thickening in DN reflects an imbalance between matrix synthesis and degradation. Matrix degradation reflects the balance between MMPs and TIMPs. HG induced a statistically insignificant 1.2-fold increase in MMP2 mRNA and ALI had no effect on this increment (fig. 3a). Western blotting revealed 2 bands for MMP2: inactive and activated MMP2. Only the activated band at approximately 63 kDa was considered for analysis. At the protein level, HG increased MMP2 protein by 1.1-fold and ALI reduced MMP2 protein by 1.2-fold, but these small changes were not statistically significant (fig. 3b). HG did not induce a change in TIMP2 mRNA, but ALI decreased TIMP2 mRNA 1.3-fold versus HG (p < 0.05; fig. 4; the protein concentration of TIMP2 in the cell lysate of mouse podocytes was too low at both 24 and 48 h to obtain results via Western blotting). Moreover, ALI significantly increased the MMP2/TIMP2 podocyte mRNA ratio 1.3-fold (p < 0.05). In podocytes cultured in NG, ALI did not reduce MMP2 protein, nor was MMP2 protein changed in NG with mannitol added (5.5 mM glucose + 34.5 mM mannitol; fig 3c). TIMP2 was not tested with a mannitol control. Taken together, ALI induces changes in the balance between MMP2 and TIMP2 in cultured podocytes that favor matrix turnover.
Fig. 3.
24-hour MMP2 in podocytes. a MMP2 mRNA in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. b Activated MMP2 protein in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. c In NG, ALI did not reduce MMP2 protein, nor was MMP2 protein changed by isotonic mannitol (5.5 mM glucose + 34.5 mM mannitol, n = 3 in each group).
Fig. 4.
24-hour TIMP2 in podocytes. TIMP2 mRNA in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. * p < 0.05 vs. HG.
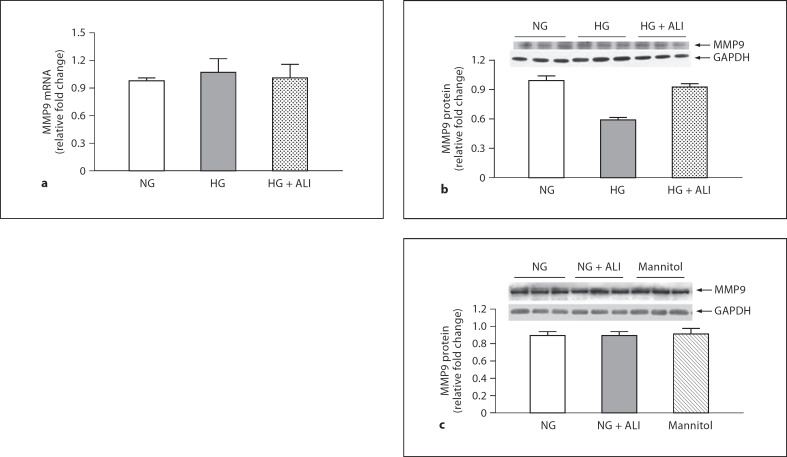
The Impact of ALI on MMP9 and TIMP1 Favors Matrix Degradation
GBM thickening in DN may also result from decreased degradation by MMP9 and increased MMP9 inhibition by TIMP1. A statistically insignificant 1.1-fold increase of podocyte MMP9 mRNA was observed in a HG environment (fig. 5a). Moreover, ALI did not significantly increase MMP9 mRNA versus HG (fig. 5a). Both the inactive and active bands for MMP9 protein were observed on Western blotting, but only the activated band at approximately 88–92 kDa was considered for analysis. At the protein level, HG tended to decrease MMP9 1.7-fold (p = 0.10). In HG, ALI tended to increase and restore podocyte MMP9 protein to basal levels by a 1.6-fold change when compared to HG alone (p = 0.18; fig. 5b). In podocytes cultured in NG, ALI did not reduce MMP9 protein, nor was MMP9 protein changed in NG with mannitol added (5.5 mM glucose + 34.5 mM mannitol; fig. 5c).
Fig. 5.
24-hour MMP9 in podocytes. a MMP9 mRNA in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. b MMP9 protein in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. c In NG, ALI did not reduce MMP9 protein, nor was MMP9 protein changed by isotonic mannitol (5.5 mM glucose + 34.5 mM mannitol, n = 3 in each group).
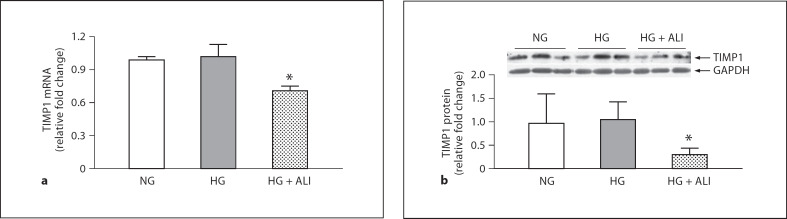
The trends for the mRNA and protein of TIMP1, which endogenously inhibits MMP9, were similar. HG had no effect on TIMP1, but statistically significant 1.4-fold and 3.2-fold decreases in TIMP1 were observed in mRNA and protein, respectively, with ALI (p < 0.05; fig. 6a). HG induced no significant change in TIMP1 protein, but ALI significantly lowered TIMP1 protein under HG culture conditions (fig 6b). TIMP1 was not tested with a mannitol control. Thus, ALI restored the trend toward a decline in MMP9 protein that was induced by podocyte incubation in HG, while decreasing TIMP1 protein levels. The numerical 1.3-fold increase in the MMP9/TIMP1 mRNA ratio was not significant, but the 4.5-fold increase in the MMP9/TIMP1 protein ratio did reach statistical significance (p < 0.05). Taken together, in cultured podocytes ALI induced changes in the balance between MMP9 and TIMP1 that favor matrix turnover.
Fig. 6.
24-hour TIMP1 in podocytes. a TIMP1 mRNA in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. b HG (40 mM) induced no significant change in TIMP1 protein vs. NG (5.5 mM), but ALI significantly lowered TIMP1 protein under HG culture conditions (40 mM glucose + 20 mM ALI), n = 3. * p < 0.05 vs. HG.
ALI Attenuates HG-Induced Podocyte Apoptosis
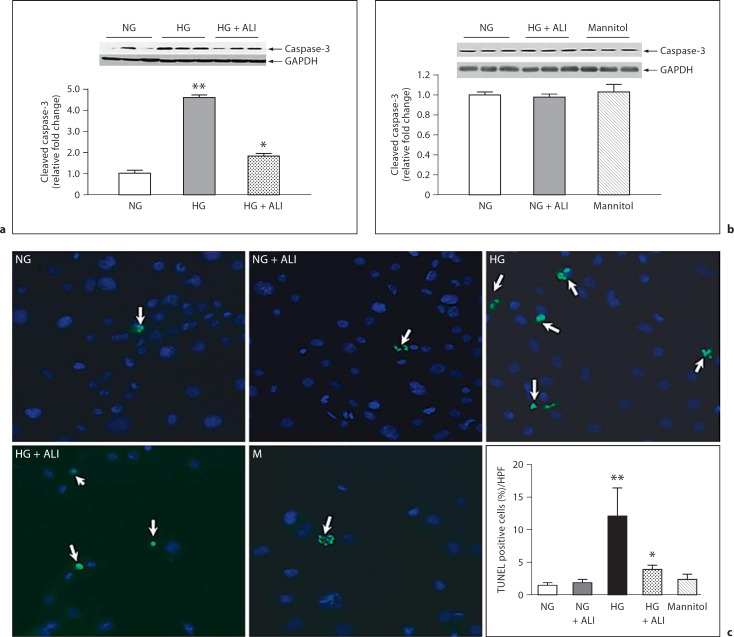
To induce apoptosis, podocytes were incubated in HG for 48 h. HG induced a 4.4-fold increase in cleaved caspase-3 protein versus incubation in NG (p < 0.05). ALI substantially reduced HG-induced cleaved caspase-3 production 2.5-fold (p < 0.05; fig. 7a). In podocytes cultured in NG, ALI did not reduce cleaved caspase-3, nor was cleaved caspase-3 changed in NG with mannitol added (5.5 mM glucose + 34.5 mM mannitol; fig 7b; n = 3 in each group). TUNEL staining showed a marked increment in apoptotic podocytes in HG versus NG, which was significantly attenuated by ALI (fig 7c). Thus, in addition to its effects on ECM, ALI may further preserve the structure of the glomerular capillary wall in a HG environment by attenuating the apoptotic pathway in cultured podocytes.
Fig. 7.
48-hour cleaved caspase-3 in podocytes. a Cleaved caspase-3 protein in NG (5.5 mM glucose), HG (40 mM glucose) and HG + ALI (40 mM glucose + 20 mM ALI), n = 3. b In NG, ALI did not reduce cleaved caspase-3 protein, nor was cleaved caspase-3 protein changed by isotonic mannitol (5.5 mM glucose + 34.5 mM mannitol, n = 3 in each group). c TUNEL staining showed a significant increment in apoptotic podocytes in HG vs. NG, which was significantly attenuated by ALI. * p < 0.05 vs. HG; ** p < 0.05 vs. NG.
Discussion
The glomerular contribution to proteinuria in DN reflects pathology of the glomerular capillary wall. Thickening of the GBM in DN results from the abnormal deposition of ECM proteins as well as an imbalance between ECM synthesis and degradation. Podocytes efface themselves and are lost through apoptosis. The current in vitro study utilized a hyperglycemic environment to mimic the diabetic milieu in cultured mouse podocytes in order to determine the effects of ALI on podocyte ECM synthesis, degradation and survival. These studies showed that in cultured podocytes exposed to HG, ALI induced changes that favored matrix turnover rather than matrix accretion, and attenuated podocyte apoptosis.
In this report, the in vitro increases in FN mRNA observed are consistent with previous findings demonstrating that glomerular FN mRNA is increased in hyperglycemic diabetic rats [24]. Our in vitro studies also showed a HG-induced increase in Cola5IV mRNA, which is consistent with other previous findings in cultured podocytes [25]. ALI decreased HG-induced mRNA increments in both FN and Cola5IV. Due to the relatively short (24 h) HG exposure, neither FN nor Cola5IV protein was increased in our experiments. Other laboratories reported HG-induced FN protein in cultured mouse podocytes with 4-day treatments [26] and HG-induced increases in Cola5IV protein with 14-day treatments [25]. Diabetes-induced increases in type IV collagen were reported in vivo after DN of 1-month duration [23]. ALI nevertheless significantly inhibited FN and Cola5IV protein compared to the basal level and to the level observed in HG. Taken together, our data on FN and podocyte-specific type IV collagen in podocytes cultured in HG with ALI are similar to the in vitro and in vivo data obtained with ACEi (angiotensin converting enzyme inhibitors) and ARBs (angiotensin II receptor blockers) [27,28], and reflect nonhemodynamic direct biochemical effects of ALI on ECM synthesis and degradation.
MMP2, also called gelatinase A, degrades the protein components of the GBM (predominantly FN and laminin) and plays a role in the pathogenesis of GBM structural changes in DN [2,27]. MMP2 undergoes cleavage for activation and is inhibited by TIMP2 [29]. There is abundant literature on the effects of MMP2 on GBM remodeling. Some studies have shown that MMP2 mRNA and protein decrease in human podocytes cultured with HG [30]. Others found that in rats, ACE inhibition reversed a HG-induced decrease in MMP2 activity, though TIMP2 remained unchanged [27]. Still, others found that glycation of the matrix has no effect on MMP2 activity in cultured mesangial cells [31]. The data in the current study not only reproduce the finding that a hyperglycemic environment, at least short-term, has no effect on MMP2 in cultured mouse podocytes [32], but goes further to show that ALI has no effect on MMP2 and that HG has no effect on TIMP2 (MMP2's inhibitor). ALI does, however, decrease TIMP2. The ratio of MMP2 to TIMP2 was significantly increased by ALI, resulting in an environment favoring increased MMP2 activity. Based on these results, the data suggest that ALI's role in attenuating GBM thickening, particularly in the setting of relatively short-term HG exposure, may not occur by increasing degradation of the GBM via direct increases in MMP2, but by indirectly regulating MMP2 activation through a reduction in TIMP2.
MMP9, also known as gelatinase B, also plays a role in GBM remodeling in DN based on its role in collagen degradation. MMP9 is converted to its active form by cleavage and is inhibited by TIMP1 [33]. There is abundant literature on changes in MMP9 in the glomerulus in diabetes. Elevation of MMP9 activity has been reported in podocytes in a HG environment followed by a return to basal levels after 10 days by some investigators [32], while others have reported no HG-induced changes in MMP9 activity [30]. Still, others have reported that ACEi prevent a decrease in MMP9 activity in diabetic rats [27]. In the current study, there were no significant changes in MMP9 mRNA in podocytes in HG medium over the short-term, but ALI prevented the HG-induced 1.7-fold decrease of MMP9 protein. The finding of an ALI-induced decrease in the endogenous MMP9 inhibitor, TIMP1, demonstrates a mechanism whereby ALI may participate in the regulation of ECM turnover. Taken together, these results suggest that GBM pathology in DN may be significantly attenuated by an indirect increase in ECM degradation via decreases in TIMP1 induced by ALI.
Though trends in mRNA generally correlate with trends in protein, HG had no effect on MMP9 mRNA, but it tended to decrease MMP9 protein 1.7-fold. Other in vitro studies utilizing human prostate cancer cells found that TGF-β regulates MMP9, though not MMP2, before secretion by increasing mRNA stability, and that this regulation is independent of RNA synthesis [34]. These latter data raise the possibility that the discrepancy we observed between MMP9 mRNA and protein reflects nontranscriptional regulation of MMP9 protein synthesis.
Our work bears some similarities, but also some differences, from work published by Bai et al. [32]. The general methodologies used are similar. We both used the same cell line, similar ambient glucose concentrations for the normal and HG media, and a similar duration of incubation (e.g. 24–48 h). For MMP2, the results are concordant, e.g. that a HG environment did not appear to affect mRNA, protein (work reported herein) or activity [32]. For Cola5IV protein, we showed no change in the comparison between NG and HG at 24–48 h (although there was a marginal but statistically significant increase for Cola5IV mRNA). Bai et al. [32] also showed no change for Cola5IV protein at 24–48 h, some decrement from 72–96 h, and then no change again at time points >96 h. These results are therefore also concordant. The apparent discordance with regard to MMP9 may relate to timing. Bai et al. [32] show an increase in MMP9 in podocytes cultured in HG at 48 h, but a return to baseline at 5 days. Our measurements were made at 24 h, which may explain the difference. Given that the paper by Bai et al. [32] shows that the effects of HG on podocyte expression may be time-dependent, it is worth noting that both studies show internal consistencies. In the paper by Bai et al. [32], HG induced increased MMP9 activity and an anticipated decrement in Cola5IV protein. Our in vitro results show no statistically significant change in MMP9 or TIMP1 mRNA or protein, as well as an increase in Cola5IV mRNA, but no change in Cola5IV protein. Of interest, as another point of agreement, neither study showed an increase in Cola5IV protein that one might have anticipated in diabetes.
The current results have a direct bearing on recent work using ALI in experimental and clinical settings of DN. In diabetic hypertensive rats with nephropathy, ALI showed consistent anti-albuminuric effects [35,36]. Moreover, ALI protected against glomerulosclerosis in this model of DN [35]. While the antihypertensive action of ALI observed in these studies probably accounted for a significant portion of these renoprotective effects, it is reasonable to postulate that a direct protective effect on podocytes, as suggested by the current work, may have also contributed to these findings. Indeed, the antihypertensive and anti-albuminuric effects of ALI were poorly correlated [35], suggesting a possible intrarenal effect of the drug.
ACEi and ARBs are standard treatments for proteinuria and DN [5,37]. Recently ALI has been shown to confer antiproteinuric protection with losartan [38]. It has been posited that ALI, which exerts its inhibitory effect by binding to the active site of renin [39], may provide superior efficacy due to its more proximal site of action in the RAS pathway compared to ACEi and ARBs. Moreover, the studies reported herein suggest that the relative activity of MMP2 and MMP9 may be increased indirectly in HG+ALI-treated podocytes, owing to ALI-induced suppression of TIMP1 and TIMP2. Overall, an increment in MMP activity would be a desired effect in DN, a disorder in which ECM accretion may contribute to the glomerular pathophysiology. In contrast, in the blood of patients with glomerulonephritis, ACEi actually reduced the activity of MMP2 and MMP9, while the effects of an ARB were not statistically significant. TIMP1 and TIMP2 were also unchanged [40]. Although our data are in cultured podocytes, and the data referred to above are in patient's blood, the data nevertheless suggest that some of the ameliorating cellular actions of ALI may differ from actions of ACEi and ARBs, and further support a rationale for synergistic clinical applications.
The studies reported herein demonstrate robust protection of podocytes from HG-induced apoptosis as evidenced by a decrement in caspase-3 activity. HG stimulates TGF-β [27,41] and TGF-β has been shown to induce apoptosis in cultured mouse podocytes through Bax synthesis and caspase-3 activation [18] and in rat podocytes [42]. RAS blockade prevents TGF-β production in diabetic patients via ACEi [41,43,44] and in vitro with ARBs [45]. Indeed, treatment of diabetic hypertensive rats with ALI reduced renal cortical levels of TGF-β mRNA [36]. The data reported herein are the first to demonstrate that blockade of the RAS with ALI prevents HG-induced apoptosis in cultured podocytes. Therefore, ALI may mitigate proteinuria by maintaining the integrity of the glomerular filtration barrier by facilitating the survival of podocytes.
A randomized, double-blind clinical study concluded that ALI may be beneficial as a renoprotective agent [38]. The current results show for the first time that renin inhibition with ALI mitigates profibrotic and apoptotic effects of HG in cultured podocytes, and suggests potential differences versus ACEi and ARB in biological actions of ALI that would support a role for clinical additivity or synergism with the latter 2 agents. These data strengthen the therapeutic rationale for renin inhibition with ALI beyond its hemodynamic effects.
Disclosure Statement
Novartis Institutes for Biomedical Research provided the ALI and paid for the supplies utilized in these experiments. Novartis did not pay the salaries of authors L.M.P., Y.W., T.D., J.L. or S.G.A. D.L.F. is an employee of Novartis. This work was supported in part by a grant from Novartis Pharmaceuticals.
References
- 1.White KE, Bilous RW. Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant. 2004;19:1437–1440. doi: 10.1093/ndt/gfh129. [DOI] [PubMed] [Google Scholar]
- 2.Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, Striker LJ. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells. Diabetes. 1999;48:1638–1644. doi: 10.2337/diabetes.48.8.1638. [DOI] [PubMed] [Google Scholar]
- 3.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15:1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 5.Mifsud SA, Allen TJ, Bertram JF, Hulthen UL, Kelly DJ, Cooper ME, Wilkinson-Berka JL, Gilbert RE. Podocyte foot process broadening in experimental DN: amelioration with renin-angiotensin blockade. Diabetologia. 2001;44:878–882. doi: 10.1007/s001250100561. [DOI] [PubMed] [Google Scholar]
- 6.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Investigat. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MAF, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 8.Menini S, Iacobini C, Oddi G, Ricci G, Simonelli P, Fallucca S, Grattarola M, Pugliese F, Pesce C, Pugliese G. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007;50:2591–2599. doi: 10.1007/s00125-007-0821-y. [DOI] [PubMed] [Google Scholar]
- 9.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 10.Chuang PY, Yu Q, Fang W, Uribarri J, He JC. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007;72:965–976. doi: 10.1038/sj.ki.5002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjorn SF, Bangstad HJ, Hanssen KF, Nyberg G, Walker JD, Viberti GC, Osterby R. Glomerular epithelial foot processes and filtration slits in IDDM patients. Diabetologia. 1995;38:1197–1204. doi: 10.1007/BF00422369. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Ushiyama C, Shimada N, Sekizika K, Ebihara I, Hara M, Kiode H. Effect of the antiplatelet drug dilazep dihydrochloride on urinary podocytes in patients in the early stage of diabetic nephropathy. Diabetes Care. 2000;23:1168–1171. doi: 10.2337/diacare.23.8.1168. [DOI] [PubMed] [Google Scholar]
- 13.Asanuma K, Campbel KN, Kim K, Faul C, Mundel P. Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes. Proc Natl Acad Sci USA. 2007;104:10134–10139. doi: 10.1073/pnas.0700917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffer M, Bitzer M, Roberts ISD, Kopp JB, Dijke P, Mundel P, Bottinger EP. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitu GM, Wang S, Hirschberg R. BMP7 is a podocyte survival factor and rescues podocytes from diabetic injury. Am J Physiol Renal. 2007;293:F1641–F1648. doi: 10.1152/ajprenal.00179.2007. [DOI] [PubMed] [Google Scholar]
- 16.Logan CM, Brinkkoetter PT, Krofft RD, Poppin JW, Shankland SJ. Darbepoetin alfa protects podocytes from apoptosis in vitro and in vivo. Kidney Int. 2007;72:489–498. doi: 10.1038/sj.ki.5002362. [DOI] [PubMed] [Google Scholar]
- 17.Wu DT, Bitzer M, Ju W, Mundel P, Bottinger EP. TGF-beta concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol. 2005;16:3211–3221. doi: 10.1681/ASN.2004121055. [DOI] [PubMed] [Google Scholar]
- 18.Liebau MC, Lang D, Bohm J, Endlich N, Bek MJ, Witherden I, Mathieson PW, Saleem MA, Pavenstadt H, Fischer KG. Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol. 2006;290:F710–F719. doi: 10.1152/ajprenal.00475.2004. [DOI] [PubMed] [Google Scholar]
- 19.Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, Choi HY, Kim SJ, Kim HJ, Han SH, Lee JE, Han DS, Kang SW. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int. 2007;71:1019–1027. doi: 10.1038/sj.ki.5002195. [DOI] [PubMed] [Google Scholar]
- 20.Velez JCQ, Bland AM, Arthur JM, Raymond JR, Janech MG. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol. 2007;293:F398–F407. doi: 10.1152/ajprenal.00050.2007. [DOI] [PubMed] [Google Scholar]
- 21.Bolick DT, Hatley ME, Srinivasan S, Hedrick CC, Nadler JL. Lisofylline, a novel antiinflammatory compound, protects mesangial cells from hyperglycemia and angiotensin II-mediated extracellular matrix deposition. Endocrinology. 2003;144:5227–5231. doi: 10.1210/en.2003-0739. [DOI] [PubMed] [Google Scholar]
- 22.Ikehara K, Hisaya T, Kuboki K, Inokuchi T. Role of kinase C-angiotensin II pathway for extracellular matrix production in cultured human mesangial cells exposed to high glucose levels. Diabetes Res Clin Pract. 2003;59:25–30. doi: 10.1016/s0168-8227(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 23.Kang SW, Natarajan R, Shahed A, Nast CC, LaPage J, Mundel P, Kashtan C, Adler SG. Role of 12-lipoxygenase in the stimulation of p38 mitogen-activated protein kinase and collagen α5(IV) in experimental diabetic nephropathy and glucose-stimulated podocytes. J Am Soc Nephrol. 2003;14:3178–3187. doi: 10.1097/01.asn.0000099702.16315.de. [DOI] [PubMed] [Google Scholar]
- 24.Kang SW, Adler SG, Nast CC, LaPage J, Gu J, Nader JL, Natarajan R. 12-Lipoxygenase is increased in glucose-stimulated mesangial cells and in experimental diabetic nephropathy. Kidney Int. 2001;59:1354–1362. doi: 10.1046/j.1523-1755.2001.0590041354.x. [DOI] [PubMed] [Google Scholar]
- 25.Iglesias-De La Cruz MC, Ziyadeh FN, Isono M, Kouahou M, Han DC, Kalluri R, Mundel P, Chen S. Effects of high glucose and TGF-β1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62:901–913. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Zhou J, Minto AW, Hack BK, Alexander JJ, Haas M, Li TC, Heilig CW, Quigg RJ. Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int. 2006;70:882–891. doi: 10.1038/sj.ki.5001624. [DOI] [PubMed] [Google Scholar]
- 27.McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, Gilbert RE. Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002;45:268–275. doi: 10.1007/s00125-001-0730-4. [DOI] [PubMed] [Google Scholar]
- 28.Morano S, Cipriani R, Santangelo C, Fallarino M, Carnovale A, Mandosi E, Gatti A, Sensi M, Di Mario U. Angiotensin blockade and matrix synthesis by glomerular epithelial cells in high glucose: a further experimental insight into the pathophysiology of diabetic nephropathy. Clin Ter. 2008;159:151–154. [PubMed] [Google Scholar]
- 29.Imai K, Ohuchi E, Aoki T, Nomura H, Fujii Y, Sato H, Seiki M, Okada Y. Membrane-type matrix metalloproteinase 1 is a gelatinolytic enzyme and is secreted in a complex with tissue inhibitor of metalloproteinases 2. Cancer Res. 1996;56:2707–2710. [PubMed] [Google Scholar]
- 30.Kitsiou PV, Tzinia AK, Stetler-Stevenson WG, Michael AF, Fan WW, Zhou B, Tsilibary C. Glucose-induced changes in integrins and matrix-related function in cultured human glomerular epithelial cells. Am J Physiol Renal Physiol. 2003;284:F671–F679. doi: 10.1152/ajprenal.00266.2002. [DOI] [PubMed] [Google Scholar]
- 31.McLennan SV, Martell SKY, Yue DK. Effects of mesangium glycalation on matrix metalloproteinase activities: possible role in diabetic nephropathy. Diabetes. 2002;51:2612–2618. doi: 10.2337/diabetes.51.8.2612. [DOI] [PubMed] [Google Scholar]
- 32.Bai Y, Wang L, Li Y, Liu S, Li J, Wang H, Huang H. High ambient glucose levels modulates the production of MMP9 and alpha5(IV) collagen by culture podocytes. Cell Physiol Biochem. 2005;17:57–68. doi: 10.1159/000091464. [DOI] [PubMed] [Google Scholar]
- 33.Vempati P, Karagiannis ED, Popel AS. A biochemical model of matrix metalloproteinase 9 activation and inhibition. J Biol Chem. 2007;282:37585–37596. doi: 10.1074/jbc.M611500200. [DOI] [PubMed] [Google Scholar]
- 34.Sehgal I, Thompson TC. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and −2) activities by transforming growth factor-B1 in human prostate cancer cell lines. Mol Biol Cell. 1999;10:407–416. doi: 10.1091/mbc.10.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly DJ, Zhang Y, Moe G, Naik G, Gilbert RE. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia. 2007;50:2398–2404. doi: 10.1007/s00125-007-0795-9. [DOI] [PubMed] [Google Scholar]
- 36.Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130–136. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- 37.Rossing K, Jacobsen P, Pietraszek L, Parving HH. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy. Diabetes Care. 2003;28:2268–2274. doi: 10.2337/diacare.26.8.2268. [DOI] [PubMed] [Google Scholar]
- 38.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenburg NK. Aliskiren combined with losartan in type-2 diabetes and nephropathy. N Engl J Med. 2008;353:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 39.Wood JM, Maibaum J, Rahuel J, Grutter MG, Cohen N, Rasetti V, Ruger H, Goschke R, Stutz S, Fuhrer W, Schilling W, Rigollier P, Yamaguchi Y, Cumin F, Baum H, Schnell CR, Herold P, Mah R, Jensen C, O'Brien E, Stanton A, Bedigian MP. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003;308:698–705. doi: 10.1016/s0006-291x(03)01451-7. [DOI] [PubMed] [Google Scholar]
- 40.Lods N, Ferrari P, Frey FJ, Kappeler A, Berthier C, Vogt B, Marti H. Angiotensin-converting enzyme inhibition but not angiotensin II receptor blockade regulates matrix metalloproteinase activity in patients with glomerulonephritis. J Am Soc Nephrol. 2003;14:2861–2872. doi: 10.1097/01.asn.0000092789.67966.5c. [DOI] [PubMed] [Google Scholar]
- 41.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by HG is mediated by autocrine activation of TGF-beta. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol. 2002;283:F173–F180. doi: 10.1152/ajprenal.00240.2001. [DOI] [PubMed] [Google Scholar]
- 43.Leehey DJ, Singh AK, Alavi N, Singh R. Role of angiotensin II in diabetic nephropathy. Kidney Int. 2000;58:S93–S98. doi: 10.1046/j.1523-1755.2000.07715.x. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert RE, Cox A, Wu LL, Allen TJ, Hulthen UL, Jerums G, Cooper ME. Expression of transforming growth factor-beta1 and type IV collagen in the renal tubulointerstitium in experimental diabetes: effects of ACE inhibition. Diabetes. 1998;47:414–422. doi: 10.2337/diabetes.47.3.414. [DOI] [PubMed] [Google Scholar]
- 45.Yao Q, Qian JQ, Lin XH, Lindholm B. Inhibition of the effect of high glucose on the expression of Smad in human peritoneal mesothelial cells. Int J Artif Organs. 2004;27:828–834. doi: 10.1177/039139880402701003. [DOI] [PubMed] [Google Scholar]