Abstract
The dual burden of enteric infection and childhood malnutrition continues to be a global health concern and a leading cause of morbidity and death among children. Campylobacter infection, in particular, is highly prevalent in low- and middle-income countries, including Bangladesh. We examined longitudinal data to evaluate the trajectories of change in child growth, and to identify associations with Campylobacter infection and household factors. The study analyzed data from 265 children participating in the MAL-ED Study in Mirpur, Bangladesh. We applied latent growth curve modelling to evaluate the trajectories of change in children’s height, as measured by length-for-age z-score (LAZ), from age 0–24 months. Asymptomatic and symptomatic Campylobacter infections were included as 3- and 6-month lagged time-varying covariates, while household risk factors were included as time-invariant covariates. Maternal height and birth order were positively associated with LAZ at birth. An inverse association was found between increasing age and LAZ. Campylobacter infection prevalence increased with age, with over 70% of children 18–24 months of age testing positive for infection. In the final model, Campylobacter infection in the preceding 3-month interval was negatively associated with LAZ at 12, 15, and 18 months of age; similarly, infection in the preceding 6-month interval was negatively associated with LAZ at 15, 18, and 21 months of age. Duration of antibiotic use and access to treated drinking water were negatively associated with Campylobacter infection, with the strength of the latter effect increasing with children’s age. Campylobacter infection had a significant negative effect on child’s growth and this effect was most powerful between 12 and 21 months. The treatment of drinking water and increased antibiotic use have a positive indirect effect on linear child growth trajectory, acting via their association with Campylobacter infection.
Author summary
Campylobacter infection is a gastrointestinal bacterial infection that is widespread, particularly in low- and middle-income countries. Children in poor living conditions are particularly at risk for infection and are often also suffering from poor linear growth. We conducted an analysis of data collected from children in urban Bangladesh who participated in the MAL-ED study, a project that examined the effect of gastrointestinal infection on growth. We used innovative analytic methods to examine the linear growth of the children from 0 to 24 months of age and whether Campylobacter infection as well as household factors were associated with growth. We found that the height of the mother and the birth order of the child had a positive relationship with child’s length at birth. Campylobacter infection was found to have a negative impact on linear growth in children between 12–21 months of age. A longer duration of antibiotic use in the children, as well as the treatment of drinking water in the household had a negative relationship with Campylobacter infection, thus indirectly had a positive effect on the children’s growth. The negative relationship between Campylobacter and growth highlights the importance of reducing infection and addressing associated factors, such as water quality.
Introduction
Growth impairment in children continues to be a major global health concern, particularly in low- and middle-income countries (LMICs). Linear growth in early childhood is an important indicator of nutritional status and is associated with both short- and long-term negative consequences, such as mortality, chronic disease, neurodevelopmental outcomes, and poor economic performance in adulthood [1–4]. Linear growth impairment, expressed as low height/length for age, is partly a consequence of fetal growth restriction, preterm birth, intergenerational effects. It also is a result of low household socioeconomic status which leads to inadequate dietary intake and inadequate sanitation, water quality and poor hygienic conditions; all of which contribute to greater exposure of children to infections and clinical disease [2,5–7]. Enteric infection has been recognized as an important cause of child growth impairment since enteric infection rates are negatively associated with child growth, particularly in LMICs [8]. This may be due to decreased nutrient absorption, inflammatory responses, reduced appetite, changing feeding practices, and the high prevalence of frequent episodes of infection in these settings [5,9–11].
The ‘Etiology, Risk Factors, Interactions of Enteric Infections and Malnutrition, and the Consequences for Child Health and Development Study’ (MAL-ED) was a multi-centre longitudinal research project designed to examine the effect of enteric infection and risk factors on child growth. The study screened stools collected from both diarrheal episodes and non-diarrheal stools for a wide variety of pathogens to estimate the pathogen-specific burden of diarrhea in children 0–24 months of age. Campylobacter was identified as one of the most prevalent enteric pathogens among children who participated in the study and was negatively associated with growth attainment at 24 months of age [2,12]. Campylobacter species are bacterial pathogens transmitted to humans through food, contact with animals, water sources and through person-to-person transmission via fecal oral route or fomites [13]. Asymptomatic infections in MAL-ED study participants were negatively associated with linear growth, a finding consistent with other studies that suggest that asymptomatic enteric infections may play a larger role in growth faltering and malnutrition than has previously been appreciated [2,14,15]. A cohort study in Peru also identified a marginal association between both symptomatic and asymptomatic Campylobacter infection and linear growth impairment in the 9 months following infection [16,17]. Asymptomatic Campylobacter infection is associated with prolonged excretion in many cases and is said to be an opportunistic infection as it is more common among children who are malnourished [18]. In a recent MAL-ED analysis, Rouhani et al reported that Campylobacter infection was associated with disruptions in the gut microbiota, which may explain the effects of asymptomatic infection on linear growth[19].
Bangladesh has one of the highest burden of growth impairment in the world despite important declines in recent decades [20]. Dhaka, Bangladesh’s capital city, has become one of the world’s most densely populated urban areas, and urbanization continues to increase (12). Urban settlements disproportionately attract economically disadvantaged rural population, resulting in crowded housing provision with poor environmental conditions [20]. This presents a public health challenge, as the households and their children are more vulnerable to disease. Studying the factors associated with enteric infection and childhood linear growth in such an urban context is crucial in addressing this public health problem.
Previous studies have explored the drivers of growth impairment in children; including household-level factors such as water, sanitation, and hygiene (WASH), child characteristics, and maternal factors. However; few have linked these factors to both specific enteric pathogen data and longitudinal growth trajectories. Latent growth curve modelling, a less commonly used approach outside of the social sciences, allows the application of many predictors and determines which exert important effects on the rate of change in growth, including both time-varying and time-invariant predictors or covariates. In addition, this method allows the opportunity to also explore the indirect effect of household factors on LAZ via infection. The application of such a method could contribute additional detail to the growing body of information on Campylobacter and growth.
Therefore, using the latent growth curve modelling approach, this study aims to investigate, for children in the Dhaka, Bangladesh MAL-ED study site: 1) whether these household-level factors and Campylobacter infection are associated with changes in LAZ across the first 24 months of life; 2) whether the effect of Campylobacter infection on changes in LAZ is consistent across the first 24 months of a child’ age or whether children are more susceptible to this effect at certain ages; and, 3) whether the effects of household-level factors on LAZ operated directly, or operated indirectly via their association with Campylobacter infection.
Methods
Study sample
A total of 265 children were enrolled within 17 days of birth between 2010 and 2012 from the Bauniabadh area of Mirpur, one of the 21 administrative units of Dhaka. Inclusion criteria included a birthweight or enrolment weight of greater than 1500g, a mother aged at least 16 years, singletons, and no other siblings being enrolled. Children with congenital or severe disease were excluded from the study [2,12,21].
Study data collection
Length and weight measures of children were taken every month following their enrollment, using standard scales (Seca GmbH & Co. KG., Hamburg, Germany). LAZ scores (length-for-age z-score), were then calculated by mapping the individual child length, standardized by age and size, onto the WHO reference population distribution [22]. Non-diarrheal stool samples were collected from children every month for the first 12 months of age, and every 3 months from children between 12 and 24 months of age. Diarrheal stool samples were collected during a diarrheal episode or up to one day after. Diarrhea was defined as the maternal report of three or more loose stools in 24 hours [12]. Our analysis included the enteropathogen data collected from the non-diarrheal stool samples. Laboratory analyses of all stool samples were undertaken at a laboratory at icddr,b in Dhaka, Bangladesh, in accordance with a standardized microbiology protocol. Campylobacter was identified using Enzyme Linked Immunosorbent Assay (ELISA) ProSpecT kits, in accordance with manufacturers procedures [12,23]. [12,23]. The ProSpecT Campylobacter microplate assay (Alexon-Trend) detects Campylobacter Specific Antigen (SA), a surface antigen. The ELISA method was the preferred analysis method as it was found to have higher sensitivity than culture [24]. Campylobacter species was the most prevalent enteropathogen in the MAL-ED Bangladesh site, with prevalence increasing with age, from 18% children having positive stool samples at 3 months of age to 69% children at 24 months of age (S1 Table).
Household sanitation, and hygiene (WASH) characteristics were collected from caregiver interviews at study baseline. These included water source, treatment of drinking water, toilet facility type, whether toilets were shared, handwashing behaviours, and the presence of a refrigerator. Caregiver data, such as years of education, age, and anthropometric measures, including height, were also collected at study baseline.
Statistical analysis
Latent growth curve modelling (LGCM) was used to assess the overall shape of growth in LAZ, variation in this pattern between the children studied, and antecedents of that variation. LGCM, a form of longitudinal analysis that utilizes a structural equation modelling (SEM) framework, models change in an outcome through estimating latent variables for the intercept (starting point) and slope (trajectory) of change, using the observed values of the variable across time as indicators of these underlying ‘true’ latent variables. Potential predictors of the intercept and trajectory of change can then be included in the model, both as subject-level predictors (i.e., time invariant constructs) and time-specific effects. [25]
Given the exploratory nature of part of our analyses—we did not have a clear hypothesis regarding the shape of the change in LAZ and the temporal pattern of the relationship between Campylobacter infection and LAZ—we split the data into random halves, with one half of the data used to build our model, and the other used for testing the fit of the resulting model and the statistical significance of its parameters. This was necessary to ensure robustness when evaluating our final model: a model built and then tested on the same subjects would naturally be likely to achieve a higher level of fit than when tested on any other random sample from the population to which we wish to generalize.
Model building was initiated with the development of an ‘unconditional’ growth curve model, which identified the trajectory of LAZ without the inclusion of any covariates. In this model LAZ measurements at 3-month intervals (0, 3, 6, 9, 12, 15, 18, 21, 24 months) were used as indicators of the intercept and slope latent variables, with the intercept mean describing the population-average baseline LAZ, the (linear) slope mean representing the population average rate of change of LAZ over time (per 3 month period), and intercept and slope variances respectively describing how starting level and change in LAZ varied amongst our subjects. To examine the improvement offered by a curvilinear model, an additional latent variable representing quadratic change was added to the model. We used the model chi-square statistics, Comparative Fit Index (CFI), Tucker-Lewis Index (TLI), Root Mean Square Error Approximation (RMSEA) and the Standardized Root Mean Square Residual (SRMR) Root to assess model fit [26]. A CFI value of above 0.95, a TLI value above 0.95, an SRMR value of below 0.08, and an RMSEA below 0.06 are all indicative of a good model fit [27]. Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) were also used to compare between models when assessing relative model fit.
Campylobacter infection, defined as a positive stool sample collected from children regardless of clinical status, was then included as a time-varying covariate of LAZ. As such, our 3-monthly observations of LAZ (as opposed to the latent variables representing intercept and slope for change in LAZ) were regressed upon our dichotomous measurement of Campylobacter presence/absence. Given that the effect of infection on linear growth is delayed by several months, we lagged the effect of Campylobacter infection on LAZ by 3 and 6 month periods (e.g. LAZ at 9 months was regressed upon Campylobacter infection at both 3 and 6 months; LAZ at 12 months was regressed upon Campylobacter infection at 6 and 9 months, etc) [28–30]. We explored temporal variation in the effect of Campylobacter infection on LAZ by first fitting a model in which the 3-month lag and 6-month lag effects were both free to vary across all time points; having examined the pattern of coefficients from this model, we then fitted a series of simpler models based on these patterns, with fixings in place to model the effect of Campylobacter infection on LAZ being stronger/weaker at particular periods of the child’s development.
Finally, the subject-level demographic, family, and household factors collected at study baseline were considered as potential predictors of the latent variables for intercept, slope and quadratic change (i.e., having a direct effect upon LAZ), and of Campylobacter infection at each time point (therefore enabling an indirect effect on LAZ via Campylobacter infection). Specific household factors considered were exposure to treated drinking water, type of toilet facility, shared toilet facilities, and presence of a refrigerator; demographic factors were sex of the child, birth order, and maternal education. Duration of antibiotic use and presence of exclusive breastfeeding were also included as time-varying covariates in the model given their importance in infection and growth [8]. We examined whether the effects of these demographic and household variables on Campylobacter infection were consistent across time or showed variation by first fitting a model in which they were free to vary, and then checking whether there was loss in model fit when applying temporal fixings to these relationships: specifically, whether they were each constant across time, or whether they would have an increasingly (or decreasingly) important effect on Campylobacter infection throughout the 24 months. Again, models were compared using the fit indices listed above. The model emerging from this building process was then tested on the other half of the data, to obtain a robust evaluation of model fit and the statistical significance of model parameters. Mplus version 8.0 software was used for all LGCM modelling [31]. Models were estimated using Monte Carlo integration.
Ethics statement
The MAL-ED study was approved in Bangladesh by the icddr,b review committee. Informed consent was obtained from the parent or guardian of every participating child in the study. The project was granted an Exemption to Ethics Review at the University of Queensland, as a secondary data analysis. An ethics application was also submitted to the regional ethics board in Switzerland (Ethikkommission Nordwest- und Zentralschweiz” (EKNZ)), on behalf of the Swiss Tropical and Public Health Institute, which granted a Declaration of No Objection.
Results
Characteristics of study participants included in the final model are show in Table 1.
Table 1. Characteristics of study participants in the final model.
| Child Characteristics (N = 265) | N | % |
| Sex (female) | 136 | 51.3 |
| Birth order | ||
| 1 | 108 | 40.8 |
| 2 | 94 | 35.5 |
| 3+ | 63 | 23.77 |
| Mean | SD | |
| Duration of antibiotic use (mean days) | 109.6 | 56.7 |
| Duration of exclusive breastfeeding (mean days) | 98.64 | 57.4 |
| Household Characteristic (N = 242) | N | % |
| Drinking water source | ||
| Piped into dwelling | 197 | 81.4 |
| Piped to yard/plot, public tap/stand pipe | 45 | 18.6 |
| Households treating water | 146 | 60.3 |
| Improved toilet facility | 184 | 76.0 |
| Shared toilet facility | 201 | 83.1 |
| Presence of refrigerator | 68 | 28.1 |
| Maternal Characteristics (N = 265) | Mean | SD |
| Maternal education (years) | 4.54 | 3.2 |
| Maternal height (cm) | 148.9 | 5.2 |
Our exploratory (model building) analyses on one half of the data involved the development of an unconditional model, which estimated the starting point and change in the outcome variable LAZ without the inclusion of any covariates. The initial model included only the intercept and slope as latent variables, with their means and variances freely estimated (AIC = 1292.534, BIC = 1335.78, CFI = 0.918, TLI = 0.924, SRMR = 0.092, RMSEA = 0.162). To investigate whether a curvilinear trajectory of LAZ offered a better fit to the data, a quadratic term was added to the model, resulting in a substantial improvement in all model fit indices (AIC = 1237.20, BIC = 1291.98, CFI = 0.95, TLI = 0.95, SRMR = 0.078, RMSEA = 0.128).
We then explored temporal variation in the relationship between Campylobacter infection and LAZ. A model in which these regression coefficients were free to differ across time suggested that the effect was strongest for children over 12 months old but diminished as the children approached 24 months of age. Therefore, we compared the free model to one in which the effects on LAZ at 12, 15, 18, and 21 months were fixed to be equal. This was done separately for the 3- and 6-month lag Campylobacter infection to LAZ paths to account for potential difference in time length effect. We then separately fixed the effects on LAZ at 3, 6, 9 and 24 months to be equal. This latter model (i.e. the one with two sets of fixed equal paths (12–21 month and 3–9, 24 month) provided the best fit.
Finally, we explored the direct and indirect effect of household factors. The latent variables for the intercept, slope and quadratic term, and Campylobacter infection at each time point were regressed on child, maternal and household characteristics. Maternal age was not found to improve the model and therefore completely removed. Paths from toilet facility, antibiotic use, and exclusive breastfeeding to LAZ intercept, slope and quadratic terms, were likewise removed. The relationships with Campylobacter infection were fixed across all intervals as per exploratory results, instead of being allowed to vary freely. LAZ at each time point was regressed on the time-lagged Campylobacter infection variables, again with the equality restrictions across time suggested by our exploratory analysis in place.
We then tested our proposed final LGCM on the other half of the data. This model explained 69% of the variance in LAZ observed scores, through both the growth curve structure and the time-varying covariates of Campylobacter infection and breast-feeding; likewise our subject level predictors explained 17% of the variance in the intercept factor (i.e. initial level) of LAZ, 15% of the variance in the linear slope factor for change in LAZ, and 13% of the variance in the quadratic slope factor for LAZ. The percentage of within-subjects variance in LAZ uniquely explained by Campylobacter was 7.1%. Model results are presented in Table 2 and Fig 1.
Table 2. Final LGCM results for LAZ change over time and association with Campylobacter infection and household-level factors.
| Dependent variable | Independent variable | Unstandardized Path Estimate | Standard error | P-value |
|---|---|---|---|---|
| Growth factors associations with household level risk factors | ||||
| LAZ intercept | Sex of child (1 = male, 2 = female) | 0.144 | 0.195 | 0.459 |
| Birth order (1,2,3+) | 0.274 | 0.122 | 0.025 | |
| Maternal education (years) | 0.011 | 0.037 | 0.772 | |
| Maternal height (cm) | 0.041 | 0.01 | 0.037 | |
| Water treated (y/n) | -0.007 | 0.203 | 0.973 | |
| Shared toilet (y/n) | 0.044 | 0.275 | 0.872 | |
| Presence of refrigerator (y/n) | 0.294 | 0.231 | 0.203 | |
| LAZ slope | Sex of child (1 = male, 2 = female) | 0.114 | 0.067 | 0.091 |
| Birth order (1,2,3+) | -0.052 | 0.042 | 0.215 | |
| Maternal education (years) | 0.016 | 0.013 | 0.204 | |
| Maternal height (cm) | 0.001 | 0.007 | 0.998 | |
| Water treated (y/n) | 0.109 | 0.070 | 0.116 | |
| Shared toilet (y/n) | -0.003 | 0.094 | 0.974 | |
| Presence of refrigerator (y/n) | 0.009 | 0.079 | 0.905 | |
| Effect of Campylobacter infection and exclusive breastfeeding on LAZ intervals | ||||
| LAZ 6 months | Campylobacter—3 months | 0.006 | 0.033 | 0.853 |
| Exclusive breastfeeding—3 months (y/n) | 0.080 | 0.043 | 0.065 | |
| Exclusive breastfeeding—6 months (y/n) | 0.079 | 0.135 | 0.558 | |
| LAZ 9 months | Campylobacter—3 months | -0.018 | 0.036 | 0.614 |
| Campylobacter—6 months | 0.006 | 0.033 | 0.853 | |
| Exclusive breastfeeding—6 months (y/n) | 0.000 | 0.115 | 0.999 | |
| LAZ 12 months | Campylobacter—6 months | -0.018 | 0.036 | 0.592 |
| Campylobacter—9 months | -0.115 | 0.030 | <0.0005 | |
| LAZ 15 months | Campylobacter—9 months | -0.098 | 0.030 | 0.001 |
| Campylobacter—12 months | -0.115 | 0.030 | <0.0005 | |
| LAZ 18 months | Campylobacter—12 months | -0.098 | 0.030 | 0.001 |
| Campylobacter—15 months | -0.115 | 0.030 | <0.0005 | |
| LAZ 21 months | Campylobacter—15 months | -0.098 | 0.030 | 0.001 |
| Campylobacter—18 months | 0.006 | 0.033 | 0.872 | |
| LAZ 24 months | Campylobacter—18 months | -0.018 | 0.036 | 0.592 |
| Campylobacter—21 months | 0.006 | 0.033 | 0.872 | |
| Effect of household factors on Campylobacter Infection | ||||
| Time-fixed covariates | ||||
| Campylobacter 1-24mos | Sex of child (1 = male, 2 = female) | 0.055 | 0.189 | 0.770 |
| Maternal education (years) | -0.011 | 0.030 | 0.722 | |
| Water treated (y/n) | -0.430 | 0.193 | 0.026 | |
| Improved toilet facility (y/n) | 0.141 | 0.208 | 0.499 | |
| Shared toilet (y/n) | 0.135 | 0.240 | 0.574 | |
| Antibiotic duration (days) | -0.048 | 0.009 | 0.000 | |
| Time-Varying covariates | ||||
| Campylobacter 3mos | Exclusive breastfeeding—3 months (days) | -0.647 | 0.602 | 0.283 |
| Campylobacter 6mos | Exclusive breastfeeding—3 months (y/n) | -0.294 | 0.500 | 0.557 |
| Exclusive breastfeeding—6 months (y/n) | 1.607 | 0.860 | 0.062 | |
| Campylobacter 9mos | Exclusive breastfeeding—6 months (y/n) | -0.402 | 0.843 | 0.634 |
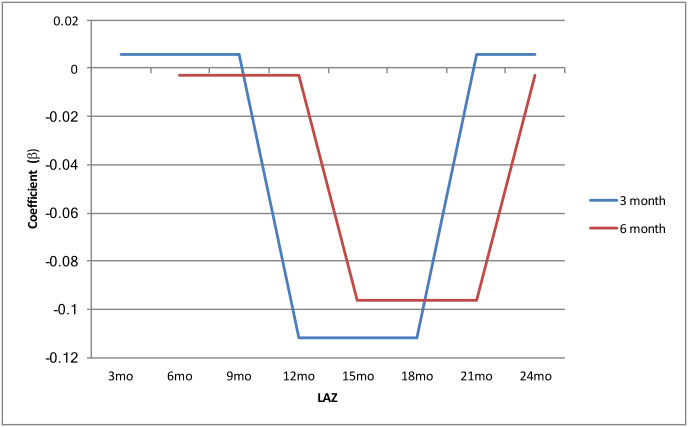
Campylobacter infection in the previous 3-month interval was negatively associated with LAZ at 12, 15, and 18 months (b = -0.115, p = <0.001) (Figs 1 and 2). Infection in the previous 6-month interval was negatively associated with LAZ at 15, 18, and 21 months, (b = -0.098, p = 0.001) (Figs 1 and 2).
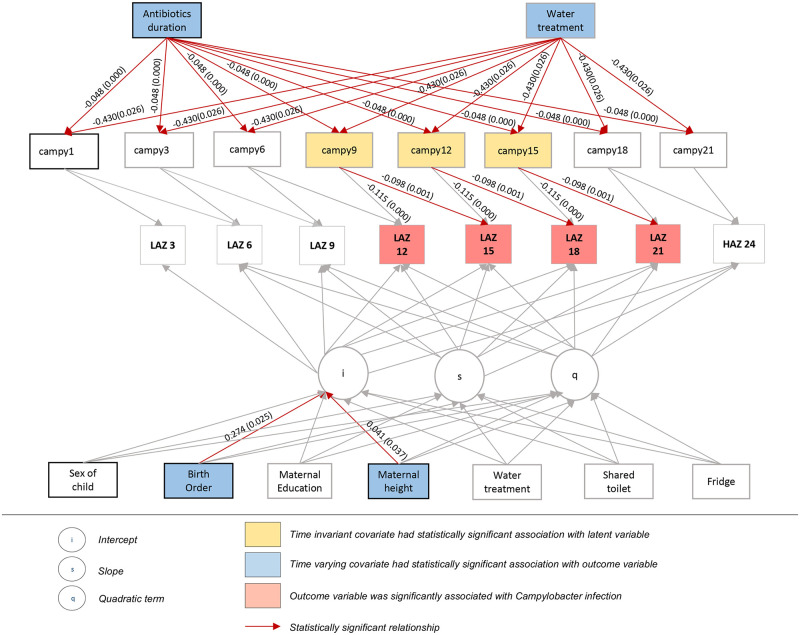
Fig 1. Final latent growth curve model: Final model results are presented in this figure.
Campylobacter infection in the previous 3- and 6-month interval was negatively associated with LAZ at 12 to 21 months. Birth order and maternal height were significantly associated with baseline LAZ. Antibiotic use and treatment of water was negatively associated with Campylobacter infection.
Of the demographic and household predictors, maternal height (b = 0.042, p = 0.033) was unsurprisingly positively associated with the intercept factor (i.e. the initial level of LAZ). Birth order was also significantly associated with initial LAZ (b = 0.274, p = 0.025). Increased duration of antibiotic use was negatively associated with Campylobacter infection (b = -0.048, p = <0.001). Water treatment was negatively associated with Campylobacter infection, with this effect equally fixed across time (b = -0.430, p = 0.026). Type of toilet facility, having a shared facility, and the presence of a refrigerator were not associated with Campylobacter infection at any time point.
Significant indirect effects, both operating via Campylobacter infection between the 12th and 21st months of the child’s life, were found between water treatment and LAZ (total indirect effect for the 3 and 6 month Campylobacter infection-LAZ lags, indirect effect = 0.092, SE = 0.046, p = 0.047) and between antibiotic use and LAZ (total indirect effect for the 3 and 6 month Campylobacter infection-LAZ lags, indirect effect = 0.010, SE = 0.003, p = 0.001). Indirect effects of water treatment and antibiotic use outside of the 12 to 21 month age range, and of improved toilet facility and toilet sharing at any age, were not statistically significant.
Discussion
To our knowledge, this study describes the first application of LGCM to household-level risk factors associated with enteric infection and childhood linear growth. The study population is representative of an urban slum population in Bangladesh, where growth impairment is a particular challenge [20,32].
The decrease of LAZ across the 24 months is widely described in the literature [4,20]. A significant portion of growth impairment takes place between 6 and 24 months of age, when children are more mobile and may experience potential nutritional challenges and exposure to poor environmental conditions as breastfeeding is reduced and complementary foods are introduced [20,33].
As in previous studies, we have found a high prevalence of Campylobacter infection in this community. increasing throughout the first 24 months to reach a peak prevalence at 18 months of age (S1 Table). Again, supporting prior research, we found that Campylobacter infection has a negative association with linear growth; however, this study extends these observations by identifying the age intervals in the first 24 months of life where infection is most strongly associated with growth. Our analysis specifically identified that children between 12 and 21 months old are most likely to show a negative relationship between having had a Campylobacter episode 3 to 6 months previously and their age adjusted length (Fig 2).
Fig 2. Relationship between Campylobacter infection across time intervals: Campylobacter infection in the previous 3-month interval (blue) was negatively associated with LAZ at 12, 15, and 18 months, while Infection in the previous 6-month interval (red) was negatively associated with LAZ at 15, 18, and 21 months.
We explored the impact of Campylobacter infection on age intervals instead of total attained height at the end of the study. This approach was applied to specifically examine the effect of infection on short term growth faltering and to identify the period most critical for the association between infection and growth impairment. A previous MAL-ED analysis across the 8 study sites described the second year of life as being the most critical period for Campylobacter-associated growth impairment. [8] Our findings provide further support that effect of exposure to potential Campylobacter pathogen reservoirs and resulting infection on growth may have been limited in the first year of child’s life, given that we did not find an association between infection and growth during this period. Interestingly, our analysis did not find an association between preceding infection and LAZ in the latest time interval, specifically at 24 months of age. This may suggest that the increasing and initial exposure to Campylobacter has the most significant effect on the growth trajectory of children. Most children may have already suffered an incident of Campylobacter infection by 18 to 21 months of age, with few new incidents of infection occurring after this period; however, further analyses would need to confirm this. In addition, children may develop protective immunity following early infection, which may explain the reduction in infection rates in addition to the high number of asymptomatic cases [34]. As pathogen data used for this analysis was collected from asymptomatic stool samples, this analysis further highlights the importance of asymptomatic Campylobacter infection on child growth. Additional analyses should also compare the effect of symptomatic stool samples.
Treatment of drinking water and increased duration of antibiotic use were important negative predictors for Campylobacter infection. Treatment of drinking water, as an important predictor for Campylobacter infection, may therefore be indirectly impacting positively upon growth. All participating households received their water from an improved piped water source as defined by World Health Organization Joint Monitoring Programme [35]; however, treatment of drinking water was positively associated with LAZ. Interestingly, the WASH Benefits Bangladesh study did not find a benefit in water treatment to the reduction of reported diarrhea. In addition, the integration of WASH interventions to nutrition did not have a benefit to child LAZ. [36,37]. This was in contrast to a previous intervention conducted in another community, which identified a 36% reduction in diarrhea in children from the water chlorination plus safe storage arm when compared to controls [37]. Another study reported that children from households with better conditions had higher LAZ scores compared with household with poorer hygienic conditions [38]. Our findings suggest that water treatment is having an effect on Campylobacter infection and indirectly on LAZ. Previous studies may not have found an effect of water treatment on enteric disease due to inadequately interrupting the pathogen-specific transmission pathways. The relationship between water treatment and Campylobacter infection identified in this analysis needs to be explored further.
Antibiotic use was prevalent in this study population. A previous study reported that the Bangladesh site had one of the highest rates of antibiotic use, second to the Pakistan site. They also found that antibiotic use in the prior month was associated with a reduced risk of Campylobacter detection in the stool [2]. While it was negatively associated with Campylobacter infection, there needs to be special consideration about its long-term effects beyond the first 24 months of age, given the current global health challenge of the increasing rate of antibiotic resistance and potential negative effects on the gut microbiota [39].
Previous research has suggested the exposure to chickens in the home as a risk factor for childhood impaired growth with LAZ; however, our analysis did not identify an association between chicken ownership and LAZ [8]. Interestingly, the Bangladesh site had one of the lowest rates of reported chicken ownership of all participating MAL-ED sites, with few households reporting the presence of chickens in the home (1.3%). However, reported chicken and fowl ownership may not accurately reflect children’s exposure to such zoonosis as Campylobacter since chickens from other households wander freely throughout the community and so may be a source of children’s exposure.
Increased maternal education in previous research has also as being associated with improved linear growth in young children [18,40,41]. In our preliminary models, maternal education was significantly associated with the rate of change of growth; however, after adjusting for other covariates in our final model, the relationship was no longer present. An analysis of the MAL-ED cohort did not find an association with breastfeeding practices and growth in the first 24 months, and commented that duration of exclusive breastfeeding was low among the participants [15]. Similarly, we did not identify an association between LAZ and Campylobacter among the Bangladesh participants.
Research on the implication of birth order on child growth is inconsistent. Several studies have reported that firstborns have lower birth weight but that they become significantly taller in later childhood and in adulthood, while others reported that they remained shorter [42,43]. In this analysis, increased birth order was positively associated with baseline LAZ of the children; however, it was not associated with the rate of change in LAZ. Maternal height was also associated with the intercept, or the birth length, of the participating children. However, they were not associated with the slope or rate of change of LAZ in the first 24 months of age.
Limitations
Our small sample size limited our ability to include additional variables in this pathogen-specific analysis, given our focus on exploring the various household predictors for growth and Campylobacter infection. Dietary intake has widely been identified as having an effect on linear child growth and so future analysis of the extensive dietary intake data collected for the MAL-ED analysis should aim to identify key variables to incorporate in latent grow curve models. Our team is currently developing such models. Also, our analysis uses pathogen data collected from regularly-collected non-diarrheal samples; however, future analyses should also consider the pathogen information collected from diarrheal samples. Finally, the urban slum population may result in a generalizability limitation, with findings being specific to a densely populated urban settlement. However, given rapid rate of urbanization on the global scale, particularly in low- and middle-income countries, child health in urban settings is an increasingly important global health concern.
Conclusion
Linear growth impairment in children is a complex public health challenge resulting from a variety of factors associated with poor socioeconomic conditions. At the same time, Campylobacter infection continues to be widely prevalent as part of these conditions, particularly in the Bangladesh MAL-ED population. To our knowledge, this is the first application of the latent growth curve modelling to explore Campylobacter infection, childhood linear growth, and the association with household factors. he negative association between Campylobacter infection and LAZ at 12, 15, 18 and 21 months of age reinforces the importance of addressing and controlling Campylobacter infection. Our household-level model also highlights the need to reduce the prevalence of infection by addressing the factors associated with it. While water sources in the households of participating children at the Bangladesh site were considered to be “improved” piped sources, treatment of drinking water was identified as being negatively associated with Campylobacter infection. Further examination using this modelling framework is needed to understand the relationship between water treatment and enteric infection.
Supporting information
(PDF)
Acknowledgments
The authors would like to thank the staff and participants of the MAL-ED network for their important contributions. They acknowledge the Governments of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support to icddr,b.
Data Availability
The MAL-ED dataset is publicly available as of early 2019, and can be accessed in the following URL: https://clinepidb.org/ce/app/record/dataset/DS_3dbf92dc05.
Funding Statement
This research protocol (MAL-ED birth cohort study) was funded by University of Virginia (UVA) with support from MAL-ED Network Investigators in the Foundation of National Institute of Health (FNIH) (https://fnih.org/), Fogarty International Centre (FIC) (https://www.fic.nih.gov) with overall support from the Bill & Melinda Gates Foundation (BMGF) (https://www.gatesfoundation.org/), grant number GR-681. This analysis of the Bangladesh MAL-ED data did not receive additional funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mosites E, Dawson-hahn E, Walson J, Rowhani-rahbar A, Neuhouser ML, Mosites E, et al. Paediatrics and International Child Health Piecing together the stunting puzzle : a framework for attributable factors of child stunting Piecing together the stunting puzzle : a framework for attributable factors of child stunting. Paediatr Int Child Health [Internet]. 2016;9047(October):1–8. Available from: 10.1080/20469047.2016.1230952 [DOI] [Google Scholar]
- 2.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Heal. 2018;6(12):e1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473–80. 10.1542/peds.2009-1519 [DOI] [PubMed] [Google Scholar]
- 4.Rieger M, Trommlerová SK. Age-Specific Correlates of Child Growth. Demography. 2016;53(1):241–67. 10.1007/s13524-015-0449-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budge S, Parker AH, Hutchings PT, Garbutt C. Environmental enteric dysfunction and child stunting. Nutr Rev. 2019;77(4):240–53. 10.1093/nutrit/nuy068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danaei G, Andrews KG, Sudfeld CR, Fink G, McCoy DC, Peet E, et al. Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels. PLoS Med. 2016;13(11):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwinger C, Fadnes LT, Shrestha SK, Shrestha PS, Chandyo RK, Shrestha B, et al. Predicting Undernutrition at Age 2 Years with Early Attained Weight and Length Compared with Weight and Length Velocity. J Pediatr [Internet]. 2016;1–7. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022347616312410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, Mcgrath M, et al. Epidemiology and Impact of Campylobacter Infection in Children in 8 Low-Resource Settings : Results From the MAL-ED Study. 2016;63:1171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard SA, Barrett LJ, Guerrant RL, Checkley W, Miller MA. Disease surveillance methods used in the 8-site MAL-ED cohort study. Clin Infect Dis. 2014;59(Suppl 4):S220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrant RL, Leite AM, Pinkerton R, Medeiros PHQS, Cavalcante PA, DeBoer M, et al. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in northeast Brazil. PLoS One. 2016;11(9):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosek MN, Ahmed T, Bhutta Z, Caulfield L, Guerrant R, Houpt E, et al. Causal Pathways from Enteropathogens to Environmental Enteropathy: Findings from the MAL-ED Birth Cohort Study. EBioMedicine. 2017;18:109–17. 10.1016/j.ebiom.2017.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob Heal. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of campylobacter infection. Clin Microbiol Rev. 2015;28(3):687–720. 10.1128/CMR.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richard SA, McCormick BJJ, Miller MA, Caulfield LE, Checkley W. Modeling environmental influences on child growth in the MAL-ED cohort study: Opportunities and challenges. Clin Infect Dis. 2014;59(Suppl 4):S255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caulfield LE. Relationship between growth and illness, enteropathogens and dietary intakes in the first 2 years of life: findings from the MAL-ED birth cohort study. BMJ Glob Heal [Internet]. 2017;2(4):e000370. Available from: http://gh.bmj.com/lookup/doi/10.1136/bmjgh-2017-000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riddle MS, Guerry P. Status of vaccine research and development for Campylobacter jejuni. Vaccine [Internet]. 2016;34(26):2903–6. Available from: 10.1016/j.vaccine.2016.02.080 [DOI] [PubMed] [Google Scholar]
- 17.Lee GO, Richard SA, Kang G, Houpt ER, Seidman JC, Pendergast LL, et al. A comparison of diarrheal severity scores in the MAL-ED multisite community-based cohort study. J Pediatr Gastroenterol Nutr. 2016;63(5):466–73. 10.1097/MPG.0000000000001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee G, Pan W, Peñataro Yori P, Paredes Olortegui M, Tilley D, Gregory M, et al. Symptomatic and Asymptomatic Campylobacter Infections Associated with Reduced Growth in Peruvian Children. PLoS Negl Trop Dis. 2013;7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouhani S, Griffin NW, Yori PP, Olortegui MP, Siguas Salas M, Rengifo Trigoso D, et al. Gut Microbiota Features Associated With Campylobacter Burden and Postnatal Linear Growth Deficits in a Peruvian Birth Cohort. Clin Infect Dis. 2019;(Xx Xxxx):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam MM, Sanin KI, Mahfuz M, Ahmed AMS, Mondal D, Haque R, et al. Risk factors of stunting among children living in an urban slum of Bangladesh: Findings of a prospective cohort study. BMC Public Health. 2018;18(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed T, Mahfuz M, Islam M, Mondal D, Hossain I, Ahmed AMS, et al. The MAL-ED Cohort Study in Mirpur, Bangladesh. 2014;59(Suppl 4):280–6. [DOI] [PubMed] [Google Scholar]
- 22.Roth DE, Krishna A, Leung M, Shi J, Bassani DG, Barros AJD. Early childhood linear growth faltering in low-income and middle-income countries as a whole-population condition: analysis of 179 Demographic and Health Surveys from 64 countries (1993–2015). Lancet Glob Heal. 2017;5(12):e1249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahim SM, Das S, Gazi MA, Mahfuz M, Ahmed T. Association of intestinal pathogens with faecal markers of environmental enteric dysfunction among slum-dwelling children in the first 2 years of life in Bangladesh. Trop Med Int Heal. 2018;23(11):1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houpt E, Gratz J, Kosek M, Zaidi AKM, Qureshi S, Kang G, et al. Microbiologic Methods Utilized in the MAL-ED Cohort Study. 2014;59(day 0):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park I, Schutz RW. An Introduction to Latent Growth Model. Res Q Exerc Sport [Internet]. 2005;76(2):176–92. Available from: 10.1080/02701367.2005.10599279 [DOI] [PubMed] [Google Scholar]
- 26.Curran PJ, Obeidat K, Losardo D. Twelve Frequently Asked Questions About Growth Curve Modeling. J Cogn Dev. 2010;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felt JM, Depaoli S, Tiemensma J. Latent growth curve models for biomarkers of the stress response. Front Neurosci. 2017;11(JUN):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157(2):166–75. 10.1093/aje/kwf179 [DOI] [PubMed] [Google Scholar]
- 29.Checkley W, Gilman RH, Epstein LD, Suarez M, Diaz JF, Cabrera L, et al. Asymptomatic and Symptomatic Cryptosporidiosis : Their Acute Effect on Weight Gain in Peruvian Children. 1997;145(2):156–63. [DOI] [PubMed] [Google Scholar]
- 30.Richard SA, Black RE, Gilman RH, Guerrant RL, Kang G, Lanata CF, et al. Catch-Up Growth Occurs after Diarrhea in Early Childhood. J Nutr. 2014;144(6):965–71. 10.3945/jn.113.187161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthen LK, Muthén BO. MPlus User’s Guide. Eighth. Los Angeles, CA: Muthen & Muthen; [Google Scholar]
- 32.Akram R, Sultana M, Ali N, Sheikh N, Sarker AR. Prevalence and Determinants of Stunting Among Preschool Children and Its Urban–Rural Disparities in Bangladesh. Food Nutr Bull [Internet]. 2018;39(4):521–35. Available from: 10.1177/0379572118794770 [DOI] [PubMed] [Google Scholar]
- 33.Onyango AW, Borghi E, De Onis M, Casanovas C, Garza C. Complementary feeding and attained linear growth among 6–23-month-old children. Public Health Nutr. 2013;17(9):1975–83. 10.1017/S1368980013002401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of campylobacter infection. Clin Microbiol Rev. 2015;28(3):687–720. 10.1128/CMR.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Progress on Drinking Water, Sanitation and Hygiene. Geneva, Switzerland; 2017. [Google Scholar]
- 36.Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Heal [Internet]. 2018;6(3):e302–15. Available from: 10.1016/S2214-109X(17)30490-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ercumen A, Naser AM, Unicomb L, Arnold BF, Colford JM, Luby SP. Effects of source-versus household contamination of tubewell water on child diarrhea in Rural Bangladesh: A randomized controlled trial. PLoS One. 2015;10(3):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin A, Arnold BF, Afreen S, Goto R, Huda TMN, Haque R, et al. Household environmental conditions are associated with enteropathy and impaired growth in rural bangladesh. Am J Trop Med Hyg. 2013;89(1):130–7. 10.4269/ajtmh.12-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casals-pascual C, Vergara A, Vila J. Intestinal microbiota and antibiotic resistance : Perspectives and solutions. Hum Microbiome J [Internet]. 2018;9(March):11–5. Available from: 10.1016/j.humic.2018.05.002 [DOI] [Google Scholar]
- 40.Miller LC, Joshi N, Lohani M, Rogers B, Mahato S, Ghosh S, et al. Women’s education level amplifies the effects of a livelihoods-based intervention on household wealth, child diet, and child growth in rural Nepal. Int J Equity Health. 2017;16(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abuya BA, Ciera J, Kimani-Murage E. Effect of mother’s education on child’s nutritional status in the slums of Nairobi. BMC Pediatr [Internet]. 2012;12(1):1. Available from: BMC Pediatrics [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells JCK, Hallal PC, Reichert FF, Dumith SC, Menezes AM, Victora CG. Associations of birth order with early growth and adolescent height, body composition, and blood pressure: Prospective birth cohort from Brazil. Am J Epidemiol. 2011;174(9):1028–35. 10.1093/aje/kwr232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells JCK, Devakumar D, Manandhar DS, Saville N, Chaube SS, Costello A, et al. Associations of stunting at 2 years with body composition and blood pressure at 8 years of age: longitudinal cohort analysis from lowland Nepal. Eur J Clin Nutr [Internet]. 2018; Available from: 10.1038/s41430-018-0291-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The MAL-ED dataset is publicly available as of early 2019, and can be accessed in the following URL: https://clinepidb.org/ce/app/record/dataset/DS_3dbf92dc05.