Abstract
Catheter ablation for ventricular tachycardia (VT) has been increasingly used over the past two decades in patients with structural heart disease (SHD). In these individuals, a substrate mapping strategy is being more commonly applied to identify targets for VT ablation, which has been shown to be more effective versus targeting mappable VTs alone. There are a number of substrate mapping methods in existence that aim to explore potential VT isthmuses, although their success rates vary. Most of the reported electrogram-based mapping studies have been performed with ablation catheters; meanwhile, the use of multipolar mapping catheters with smaller electrodes and closer interelectrode spacing has emerged, which allows for an assessment of detailed near-field abnormal electrograms at a higher resolution. Another recent advancement has occurred in the use of imaging techniques in VT ablation, particularly in refining the substrate. The goal of this paper is to review the key developments and limitations of current mapping strategies of substrate-based VT ablation and their outcomes. In addition, we briefly summarize the role of cardiac imaging in delineating VT substrate.
Keywords: Ablation, cardiac imaging, substrate, three-dimensional mapping system, ventricular tachycardia
Introduction
Over the last decade, catheter ablation for ventricular tachycardia (VT) has been increasingly performed as an adjunctive therapy to antiarrhythmic drugs.1,2 With this came an increased understanding of the mechanisms of VT; notably, it commonly results due to scar-related reentry in patients with structural heart disease (SHD). Activation mapping and entrainment mapping are reasonable approaches to identify and target critical sites of the reentrant VT circuit for ablation in patients with tolerated reentrant VT.3–6 However, the majority of patients presenting for catheter ablation have unstable VT that hampers the accurate definition of the critical areas of the reentrant circuit with activation or entrainment mapping.7,8
Thus, substrate ablation is increasingly favored as a VT ablation strategy, with or without entrainment/activation mapping methods. Substrate-based approaches involve the identification of local abnormal ventricular electrograms that represent diseased areas consistent with potential isthmuses capable of supporting reentrant VT and may be followed even when VTs are not inducible or not hemodynamically tolerated.9,10 Although a combination of several approaches is commonly employed during VT ablation, a number of studies have examined with variable results whether a substrate-based ablation strategy may be superior or comparable to one guided predominantly by the activation/entrainment mapping of inducible and hemodynamically tolerated VTs.11–18 Critical in achieving more successful results with a substrate-based approach is an accurate representation of the pathologic substrate; various strategies to delineate this have been proposed to date.4,10,13–22
With the advent of three-dimensional (3D) electroanatomical mapping (EAM) systems in the 1990s,23 there has been a significant improvement in our ability to represent both anatomical and functional electrical information in a real-time model of the ventricle. Furthermore, the development of multipolar catheters facilitates precise scar detection,24–26 with potentially more successful results.27 In addition, cardiac imaging in the form of cardiac magnetic resonance (CMR) imaging or multidetector computed tomography (MDCT) may play a potentially important role in the preprocedural assessment of cardiac anatomy and myocardial substrate and the intraprocedural integration of the structural and electrophysiological VT substrate.28–31
In this paper, we review substrate mapping and ablation strategies and clinical outcomes for VTs in the setting of SHD and also discuss the importance of modalities for substrate detection in addition to those based on electrograms.
Substrate mapping in patients with structural heart disease
Electrogram-based substrate detection
The identification and modification of the arrhythmogenic substrate in the endocardium and/or epicardium are increasingly considered as composing a primary ablation strategy in patients with SHD. This technique was originally developed in the absence of 3D mapping9,32–34; however, the development of 3D mapping systems in the late 1990s23 accelerated the use of electrogram-based techniques in detecting and localizing substrate reproducibly and feasibly. Furthermore, the recent use of ultra-high-density mapping with catheters with multiple small electrodes and closer interelectrode spacing has enhanced the speed, density, resolution, and detailed near-field signal assessment of mapping acquisition, reducing interpolation and possibly improving clinical outcomes25–27,35–38 (Figure 1).
Figure 1:
Left: Comparison of bipolar voltage maps (endocardial: scar < 0.5 mV, border zone 0.5–1.5 mV, and healthy tissue > 1.5 mV; epicardial: border zone 0.5–1 mV and healthy tissue ; 1 mV) using Navistar® (Biosense Webster, Diamond Bar, CA, USA) (NAV) mapping versus PentaRay® (Biosense Webster, Diamond Bar, CA, USA) (PR) mapping in the endocardium (A) and epicardium (B) of a sheep model with an iatrogenic-created anteroseptal scar and in humans (C) using the CARTO® 3 system (Biosense Webster, Diamond Bar, CA, USA). All images are shown in an anteroposterior view. A: LAVA is represented in pink, the proximal conduction (left-sided His) system is in blue, and the Purkinje system is in yellow. Border zone areas and LAVA channels are visible within the scar with PR mapping, but none are visible with NAV mapping. B: Three LAVA channels are visible with PR mapping but none with NAV mapping. The border zone is smaller and demonstrates increased detail using PR mapping. C: Larger scar area and improved border zone definition using PR versus NAV mapping. Right: Point pair analysis (≤ 3 mm of distance between a PR and NAV point) from two examples by manual signal analysis in two different patients. Substrate maps are shown at 100% transparence. LAVA using PR (in purple) and NAV (in white) are tagged. The distance between tags is measured using the distance measurement tool in the mapping system. A red arrow indicates a clear LAVA visible with PR mapping but one that is barely or not visible with NAV mapping. Reproduced with permission from: Berte B, Relan J, Sacher F, et al. Impact of electrode type on mapping of scar-related VT. J Cardiovasc Electrophysiol. 2015;26(11):1213–1223. LAVA: local abnormal ventricular activity; ENDO: endocardial; EPI: epicardial.
Bipolar voltage mapping to evaluate the electrogram peak-to-peak voltage is a widely accepted and frequently used technique to characterize substrate. Endocardially, a bipolar voltage amplitude of 1.5 mV or more normally identifies healthy tissue. Additionally, normal ventricular myocardial bipolar electrograms are defined as sharp, biphasic or triphasic signals with a duration of 70 ms or less and/or with an amplitude-to-duration ratio of more than 0.046.9,39 Areas with voltages of 0.5 mV to 1.5 mV are often considered as border zones in the setting of a 3.5-mm to 4-mm-tip, 1-mm ring electrode, and 2-mm interelectrode spacing mapping catheter (Table 1),10,32,34 even in the right ventricle.40 The definitions above have been validated by human pathologic data and radiologic studies.41–43 Areas with voltages of less than 0.5 mV are generally considered as “dense scar”; however, low-amplitude abnormal electrograms are frequently observed in these areas.44 Therefore, in order to define unexcitable scar, the area should contain no visible electrograms (ideally using mapping catheters with smaller and narrower-spaced bipolar electrodes) and have no local pacing capture.45 In the epicardium, a bipolar voltage cutoff of 1 mV or more46 or 1.5 mV47 is considered normal. With regard to the right ventricle, epicardial 1.5 mV can also be a reasonable bipolar voltage cutoff.40 The majority of VTs have critical circuits located in the scar border zone,48 which harbors abnormal electrograms9,32,33 [ie, fractionated potentials,49 double potentials, and late potentials (LPs), discrete and separated from the QRS by 40 ms50], which can be targeted by catheter ablation. However, it has been reported that a certain proportion of abnormal potentials are also located in regions with bipolar voltages of more than 1.5 mV,19,21 with abnormal electrograms occasionally unmasked by extrastimuli.51,52 We have recorded at least 3% of substrate defined as local abnormal ventricular activity (LAVA) in voltage zones of more than 1.5 mV (because of far-field signal annotation)53 (Figures 2A–2D). Moreover, Tung et al.54 found that 18% of critical VT isthmuses were within low-voltage areas during pacing from the site but within normal amplitude (> 1.5 mV) areas with pacing from another site, indicating that voltage is affected by the activation wavefront.54–56 Therefore, a voltage map based on standard (0.5–1.5 mV) voltage criteria is not necessarily capable of delineating the entire possible VT substrate.
Table 1:
Studies Investigating Different Substrate Ablation Strategies for VT
| Ablation Method | Study | Patients | Age | LVEF | Endpoint(s) [% of Patients Who Met the Endpoint(s)] | Epicardial and Endocardial Ablation/Mapping | 3D Mapping System |
|---|---|---|---|---|---|---|---|
| Linear ablation | Marchlinski et al. 200010 | 9 ICM and 7 NICM | 58 ± 16 years | 32% ± 15% | Noninducibility (47%) | 0/0 | CARTO® in 13 patients; none in 3 patients |
| Soejima et al. 200172 | 40 ICM | 67 ± 11 years | 28.9% | Noninducibility (58%) | 0/0 | CARTO® in 40 patients | |
| LP ablation | Volkmer et al. 200616 | 25 ICM | 65 ± 8 years | 30% ± 8% | Elimination of LPs and noninducibility (81%) | 0/0 | CARTO® in 25 patients |
| Arenal et al. 200373 | 21 ICM, 2 NICM, and 1 TOF | 66 ± 9 years | 30% ± 9% | Elimination of LPs and noninducibility (88%) | 0/0 | CARTO in 22 patients; none in 2 patients | |
| Nogami et al. 200874 | 18 ARVC | 48 ± 11 years | N/A | Change of isolated delayed component and noninducibility (33%) | 0/0 | CARTO® in 18 patients | |
| Garcia et al. 200975 | 13 ARVC | 43 ± 15 years | N/A | Elimination of LPs and noninducibility (85%) | 13/13 (1 underwent both types of mapping and epicardial ablation but not endocardial ablation) | CARTO® in 13 patients | |
| Bai et al. 201176 | 26 ARVC | 37 ± 11 years | 53% ± 10% | Elimination of LPs and noninducibility (100%) | 26/26 | CARTO® in 26 patients | |
| Vergara et al. 201277 | 36 ICM and 14 NICM | 66 ± 10 years | 32% ± 9% for ICM; 36% ± 10% for NICM | Elimination of LPs (84%) | 21/21 (3 underwent both types of mapping and epicardial ablation but not endocardial ablation) | CARTO®/NavX™ (number unknown) | |
| Arenal et al. 201378 | 59 ICM | 67 ± 9 years | 30% ± 11% | Elimination of LPs (78%) | 0/0 | CARTO® in 59 patients | |
| LAVA | Jaïs et al. 201219 | 56 ICM 14 NICM | 67 ± 11 years | 35% ± 10% | Elimination of LAVA (70%) and noninducibility | 17/21 | CARTO®/NavX™ (number unknown) |
| Wolf et al. 201820 | 159 ICM | 65 ± 11 years | 34% ± 11% | Elimination of LAVA (64%) and noninducibility | 27/46 | CARTO®/NavX™/Rhythmia™ in a total of 119 patients; none in 40 patients | |
| Scar homogenization | Di Biase et al. 201511 | 58 ICM | 67 ± 9 years | 32% ± 10% | Elimination of any abnormal potential ± loss of pacing capture (NA). | NA | CARTO® in 58 patients |
| Di Biase et al. 201217 | 43 ICM | 62 ± 8 years | 24% ± 8% | Elimination of any abnormal potential ± loss of pacing capture (NA). | 14/43 | CARTO® in 43 patients | |
| Scar dechanneling | Berruezo et al. 201238 | 11 ARVC | 42 ± 13 years | 55% ± 7% | Elimination of LP channels (NA) | 11/11 | CARTO® in 11 patients |
| Tung et al. 201381 | 15 ICM, 2 NICM, 2 ARVC, 1 sarcoid, 1 noncompaction, and 1 Chagas | 63 (52–70) years | 25% (25%–30%) | Change or elimination of LPs, failure to capture ± impedance drop of ; 10 Ω plus noninducibility (84%) | 9/9 | CARTO® in 11 patients patients/NavX™ in 10 patients | |
| Berruezo et al. 201582 | 75 ICM and 26 NICM | 65 ± 12 years | 36% ± 13% | Elimination of LP channels (84%) | N/A/27 | CARTO® in 96 patients/NavX™ in 5 patients | |
| Fernández-Armenta et al. 2016102 | 19 ICM and 5 NICM | 66 ± 11 years | 36% ± 14% | Elimination of delayed components (87.5%) | 5/5 (1 underwent epicardial ablation and mapping but not endocardial ablation or mapping) | CARTO/NavX (n = N/A) | |
| Core isolation | Tzou et al. 201579 | 32 ICM and 12 NICM | 63 ± 14 years | 31% ± 13% | Isolation with exit block (84%) | 5/6 | CARTO® in 44 patients |
| MRI-based scar dechanneling | Andreu et al. 201783 | 37 ICM and 17 NICM | 64 ± 11 years | 38% ± 12% | Elimination of LP channels detected on EAM guided by CMR (84%) | 18/18 | CARTO® in 54 patients |
ARVC: arrhythmogenic right ventricular cardiomyopathy; CMR: cardiac magnetic resonance imaging; EAM: electroanatomical mapping; Epi: epicardial; ICM: ischemic cardiomyopathy; LAVA: local abnormal ventricular activity; LP: late potential; LVEF: left ventricular ejection fraction; LP: late potential; MRI: magnetic resonance imaging; N/A: not available; NICM: nonischemic cardiomyopathy; noncompaction: noncompaction cardiomyopathy; RF: radiofrequency; sarcoid: cardiac sarcoidosis; TOF: tetralogy of Fallot; VT: ventricular tachycardia. CARTO® is the property of Biosense Webster, Diamond Bar, CA, USA. NavX™ is the property of Abbott Laboratories, Chicago, IL, USA. Rhythmia™ is the property of Boston Scientific, Natick, MA, USA.
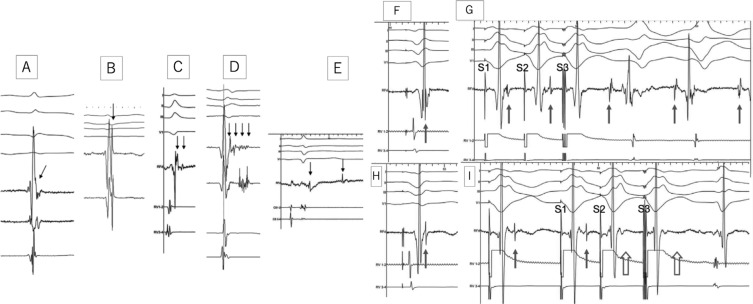
Figure 2:
Left: Electrogram recordings from different patients showing characteristics of LAVAs (arrows). A: The potential representing LAVA is fused with the terminal portion of the far-field ventricular signal, making it difficult to identify the LAVA as a separate signal. B: The LAVA potential occurs just after and with a slightly higher frequency than the far-field ventricular potential. LAVAs in A and B occur within the QRS complex. C: The LAVA is a double-component potential that closely follows the far-field ventricular signal. The early component is a high-frequency potential that is almost fused with the preceding far-field ventricular potential. It occurs within the terminal portion of the QRS complex. Another low-amplitude signal follows an isoelectric interval and represents the late component of the LAVA, which occurs after the QRS complex. D: LAVAs are represented by pluricomponent signals without isoelectric intervals. These signals can be visualized distinctly from the preceding far-field ventricular signal. E: A double-component LAVA signal. Although the early component is recorded just after the QRS complex, the late component is recorded after the inscription of the T-wave on the surface electrocardiogram. Right: Role of LAVAs in the induction of VT and the influence of radiofrequency (RF) energy on LAVAs. F: The local ventricular electrogram during the baseline paced rhythm at first sight looks simple. However, in the terminal portion, a very high-frequency component (LAVA) may be identified. G: Programmed electric stimulation from the right ventricle (RV) unmasks the LAVA potential by increasing the delay from the far-field signal. The delay observed during RV pacing suggests poor coupling of the muscle bundle generating the LAVA signal. The delay is maximal with S3, which is associated not only with a change in the polarity of the LAVA but also with the induction of VT. H: After delivery of RF energy, there is a remarkable delay (see A) between the far-field ventricular signal and LAVAs during baseline paced rhythm. I: Repeat programmed electric stimulation from the RV results in the absence of LAVA signals after the far-field ventricular potential during S2 and S3 (open arrows). The absence of LAVAs is associated with an inability to induce VT. Although ablation has rendered the VT noninducible, further RF energy application is indicated to completely eliminate the LAVAs. Reproduced with permission from Jaïs P, Maury P, Khairy P, et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125(18):2184–2196.
Furthermore, with the use of high-density mapping with small electrodes and narrower interelectrode spacing (Figure 1), traditional definitions of substrate voltage need to be adjusted depending on the mapping catheter.24,36 The electric field recorded by a pair of electrodes on a novel mapping catheter is relatively small, recording precise local signals located just underneath the electrodes.57,58 Meanwhile, endocardial unipolar voltage mapping has a large field of view and is useful to identify septal, intramural, and/or epicardial substrate,58–69 with different amplitude thresholds present depending on the etiology of the disease. The use of bipolar mapping with small electrodes and closer interelectrode spacing in combination with unipolar mapping may constitute an optimal strategy.
In summary, there are several limitations55 of conventional voltage mapping for substrate detection, with variations occurring due to the wavefront of activation,54–56 catheter interelectrode spacing,24,25 interpolation,63 far-field peak annotation of multicomponent electrograms, catheter orientation64 and contact,65,66 and surrounding insulating tissue (eg, fat, edema). Conversely, nonelectrogram techniques of substrate detection such as cardiac imaging29,30,67–69 are unaffected by directions of wavefront activation or techniques based on specific electrogram characterizations (see later). Lastly, omnipolar mapping is a new development that may resolve some of these limitations by providing instantaneous catheter-tip wavefront direction and speed.70,71 With this mapping technology, local electrical field signals are determined and used to assess the traveling wavefront on a multielectrode catheter, rather than activation-based data acquisition, which may allow for a beat-to-beat determination of wave propagation information that is independent of electrode orientation or activation time. Further clinical validation of this technology is ongoing.
Specific electrogram-based substrate ablation strategies
Substrate-based ablation approaches may differ between VT ablation centers. A number of methods are implemented during substrate modification, with a large variation in the amount of ablation energy delivered according to the preset mapping and endpoints of the procedure. The major strategies are summarized in Table 1. These studies must be cautiously interpreted because the mapping details or endpoints are often heterogeneous, even for the same strategy. In addition, although the advantage of multipolar mapping catheters has now been recognized, many previous studies have used ablation catheters for mapping.
Local abnormal ventricular activity–guided ablation
In a seminal study by our group, we reported a mapping and ablation strategy to homogenize substrate defined as LAVAs (Figure 2).19–21 Elimination of all LAVAs is associated with improved midterm and long-term arrhythmia-free survival.19–21 LAVAs are defined as sharp, high-frequency ventricular potentials, possibly of low amplitude, that are distinct from the far-field ventricular electrogram that occurs at any time during or after the far-field ventricular electrogram in sinus rhythm or before the far-field ventricular electrogram during VT, which sometimes display fractionation or double or multiple components separated by very-low-amplitude signals or an isoelectric interval and which are poorly coupled to the rest of the myocardium.19,21 Importantly, this strategy also targets abnormal substrate in so-called normal-voltage areas, although most LAVAs are generally observed in low-voltage areas.53 Clinical outcomes have been reported as including a 55% (88/159) VT freedom rate during a median follow-up of 47 months (range: 33–82 months) without antiarrhythmic drug therapy except for β-blockers.20
Linear ablation with cross-section of the scar and border-zone
Marchlinski et al.10 first described the use of linear ablation lesions to target multiple unmappable VTs. The technique involved the creation of contiguous lesions from the dense infarct area through the infarct border-zone and anchored to anatomic barriers or healthy myocardium. In their study,10 the ablation strategy resulted in a 75% (4/16) freedom from VT recurrence rate at the median follow-up point of eight months (range: 3–36 months). Additionally, Soejima et al.72 reported a VT freedom rate of 62.5% (25/40) at a mean follow-up point of 12 months ± six months in their study. In addition, this linear ablation approach was the main approach used in the Substrate Mapping and Ablation in Sinus Rhythm to Halt VT (SMASH VT) trial,22 a randomized study showing promising results.
Late-potential ablation
Definitions of LPs differ among studies.16,73–78 The initial description was of any electrogram with a duration extending beyond the end of the surface QRS.32 Modified definitions have subsequently been reported, often with an isoelectric line among multiple components in the bipolar signals.73,74 Regarding the clinical result, Arenal et al.73 first reported that after a mean follow-up of nine months ± four months, no VT recurrence was observed in 19 (79%) of 24 patients. Volkmer et al.16 additionally demonstrated a 71% VT freedom rate (7/25) in a follow-up period of 26 months ± 14 months. Nogami et al.74 reported a 67% (6/18) VT freedom rate over a relatively long follow-up period of 61 months ± 38 months in patients with ARVC. Garcia et al.75 and Bai et al.76 also demonstrated results of the elimination of delayed potentials or LPs in patients with ARVC with follow-up [VT freedom in 77% (10/13) patients during 18 months ± 13 months of follow-up and ventricular arrhythmia or implantable cardioverter-defibrillator (ICD) appropriate therapy freedom in 84.6% (22/26) during 39 months ± four months of follow-up, respectively]. More recently, Vergara et al.77 reported that, after a mean follow-up of 13 months ± four months, VT recurred in 10 patients (20%).
Scar homogenization
Di Biase et al.17 reported on a scar homogenization approach targeting all abnormal electrograms within low-voltage areas defined with conventional bipolar voltage criteria when mapping in sinus or paced rhythm. With this approach, abnormal electrograms are defined as any electrograms that have more than three deflections, an amplitude of less than 1.5 mV, and a duration of more than 70 ms. The acute ablation endpoint was either the elimination of abnormal electrograms or the loss of local capture at high-output pacing (20 mA output at a 10-ms pulse width). This approach can potentially eliminate more possible critical sites than more focused mapping approaches, but has limitations in patients with massive substrate, particularly under unstable conditions. During a mean follow-up of 25 months ± 10 months, the freedom from VT recurrence rate was 81% (35/43) in patients with ICM who showed scar homogenization.17 More recently, the results of a multicenter randomized study comparing scar homogenization with standard limited substrate ablation in patients with ICM were reported.11 At one year of follow-up, freedom from VT recurrence was achieved in 52% (31/60) of patients who underwent clinical VT ablation only versus in 85% (49/58) of patients who underwent scar homogenization.
Border-zone ablation/core isolation
The core isolation approach was recently developed by Tzou et al.79 in an effort to limit the number of lesions required to eliminate all of the areas critical for VT maintenance within the dense scar. This is a stepwise approach that starts with the identification of the potential critical isthmus within the dense scar that is related to the patient’s clinical and/or induced VTs based on conventional criteria including voltage channels; sites with LPs; sites with good pacemaps; and the existence of long stimulus to QRS intervals, isthmus sites defined by entrainment mapping, and sites of VT termination with ablation. Therefore, this approach acts as a combined approach between conventional and substrate-based approaches. These areas are typically within areas of dense scar (< 0.5 mV). Once identified, the critical area is targeted with contiguous ablation lesions either completely surrounding the region of interest or by using anatomic anchors to minimize the amount of ablation necessary. The authors demonstrated that, after a mean follow-up of 18 months ± nine months, no VT recurrence was observed in 38 (86%) of 44 patients.79
Scar dechanneling
This substrate ablation approach, which targets channels within the abnormal substrate, was originally described by Soejima et al.45 and Arenal et al.80 Although, in all studies, the concept of scar dechanneling encompasses targeting the VT channels, the identification of the channels differs in terms of technique. In the study by Soejima et al.,45 channels were identified within the low-voltage area using high-output pacing (10 mA, with pulse width of 2 ms). Electrically unexcitable scar was defined as a loss of capture at high-output pacing and marked on the voltage maps. Conversely, Arenal et al.80 were able to visualize channels after adjusting voltage cutoffs on EAM. More recently, Tung et al.81 and Berruezo et al.82 described an approach that targets interconnected activation channels within the abnormal substrate, adopting clear endpoints with clinical follow-up. The method involved high-density mapping of the channels of activation of LPs. Once a specific sequence of LP activation has been identified, focal ablation of the earliest LP is delivered with the end goal of eliminating a consecutive series of LPs. Tung et al.81 demonstrated a rate of freedom from VT recurrence of 86% (18/21) during a median follow-up of 11 months (range: 6–18 months), while Berruezo et al.82 noted that, during a median follow-up of 21 months (range: 11–29 months), the rate of freedom of VT recurrence was 80% (80/101). In addition, Andreu et al.83 recently demonstrated scar dechanneling by using CMR imaging in conjunction with EAM, which showed corridors formed by conducting channel points in the scar tissue. In that study, the rate of VT freedom during a mean follow-up of 20 months ± 19 months was 81.5% (44/54).83
Frequency analysis mapping
High-frequency electrogram components are more often associated with critical sites of reentry as compared with low-frequency, large-amplitude components. Several studies have demonstrated that the frequency analysis of electrograms may aid with substrate identification84–86; however, this analysis is still only available as an offline tool and the feasibility of an automated real-time tool needs to be further investigated.
Use of imaging to identify substrate
Cardiac imaging may play an important role in the preprocedural assessment of cardiac anatomy and myocardial scar as well as in the intraprocedural integration of the structural VT substrate.28 Cardiac imaging has been mainly used as an adjunct either offline or online (real-time image integration)27,83,87,88 to support the localization of scar in 3D mapping systems during substrate mapping and ablation (Figure 3). Cardiac imaging has several advantages, as follows: (1) it may provide precise anatomical information including endocardial/intramural/epicardial scar location, while EAM systems can only provide derived 3D reconstructions from catheter–electrode contact at the myocardial surface; (2) there is no possibility of inaccuracy due to extrapolation, lack of catheter contact, confounding effects of far-field electrograms, or the influence of wavefront activations; (3) it provides information about adjacent anatomical structures, which may affect mapping and ablation, such as papillary muscles, coronary arteries, phrenic nerves, and epicardial fat. However, there are also limitations in imaging techniques in terms of feasibility (eg, magnetic resonance imaging in some patients with old ICDs, MDCT in patients with severe chronic kidney failure), the availability of images with 3D mapping systems, and registration issues.
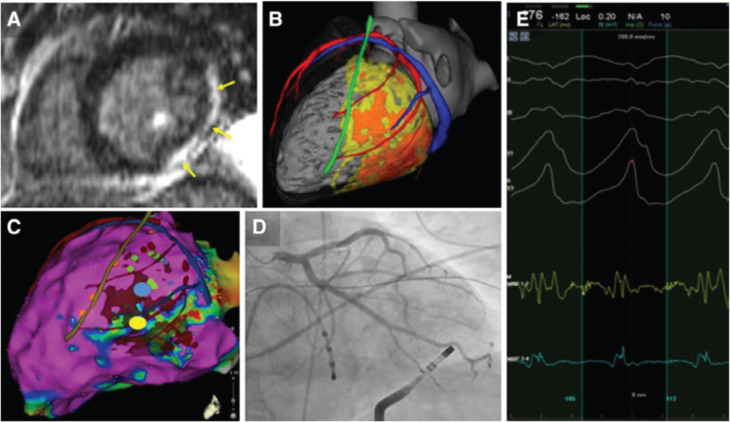
Figure 3:
A: Lateral and inferior LV scar on CMR (arrows). B: Patient-specific 3D model built from merged computed tomography (CT) (anatomy) and magnetic resonance imaging (scar) data. Cardiac chambers (gray), coronary arteries, veins (in red and blue, respectively), and left phrenic nerve (green) as segmented from CT and dense scar and gray zone (in orange and yellow, respectively) as segmented from magnetic resonance imaging. C: Epicardial bipolar voltage map with merged imaging model. Voltage mapping (color-coded from purple to red) underestimates the substrate extent as compared with imaging. Fractionated and LPs (green dots) are identified in normal voltage areas. Middiastolic potentials (yellow and blue signals in E) are recorded during VT on an epicardial lateral LV site (yellow dot in C). This potential target for epicardial ablation is far enough from the left phrenic nerve path derived from imaging (green line in C), which accurately matches sites of phrenic capture (orange dots in C). However, CT demonstrates the proximity of this site to a marginal branch of the circumflex artery on the registered imaging model. D: Confirmatory coronary angiography demonstrates contact between the tip of the ablation catheter and the coronary artery. Ablation was thus performed on a different site of the VT isthmus (blue dot in C), resulting in successful VT termination. Reproduced with permission from Mahida S, Sacher F, Dubois R, et al. Cardiac imaging in patients with ventricular tachycardia. Circulation. 2017;136(25):2491–2507.
Recently, in an attempt to refine targeted VT ablation strategies further, several studies have focused on identifying specific scar regions that harbor critical VT isthmuses.29,67,89 At this time, scar regions with increased transmurality, scar border zones, and regions at the scar-core–border-zone transition point have been identified as potential targets.29,90 CMR has been widely used in this regard, and several studies have shown good correlation with EAM30,31,62,83,88 and a positive clinical impact.27,52,87 Further potential benefits of real-time CMR guidance91–93 could include improved procedural supervision without exposure to radiation/contact EAM as well as improved substrate detection and lesion visualization according to CMR-defined endpoints. However, CMR may be unavailable, contraindicated, or of suboptimal quality because of ICD-related artifacts, and MDCT represents a valuable alternative for imaging integration. MDCT has been used in combination with EAM to accurately identify dense scar and border-zone regions with significantly higher special resolution29,68 as compared with CMR and with high clinical effectiveness.27 Studies have mostly included patients with ICM,29 while the correlation in patients with NICM is less robust.67,68,87,89 A further advantage of MDCT is in the definition of high-resolution cardiac anatomy.47,94 CMR and MDCT may visualize potential isthmuses as VT substrate; however, a certain proportion of circuits are at least partially functional95 and the registration needs to be accurate and reproducible.
Other modalities such as intracardiac echocardiography96,97 and nuclear imaging98,99 may also help to identify substrate. In addition, electrocardiographic imaging incorporated with CMR or MDCT has the potential to identify VT isthmuses noninvasively.100 Furthermore, an entirely noninvasive approach that combines anatomical imaging with electrocardiographic imaging and noninvasive cardiac radiotherapy for ablation has been reported, which represents another intriguing strategy that employs cardiac imaging.101
Clinical outcomes of substrate-based ablation
Overall, the clinical outcome of substrate-based ablation is a VT recurrence-free rate of approximately 54% to 91% in mid- to long-term follow-up (Table 1). As described above, the success rates, however, vary widely with different strategies and across studies. We have also compared clinical outcomes between substrate- and nonsubstrate-based ablation (Table 2). Di Biase et al.11 demonstrated a superior VT-free survival rate at 12 months in conjunction with extensive scar homogenization in patients with ICM in a randomized trial [ie, the Ablation of Clinical VT versus Addition of Substrate Ablation on the Long-term Success Rate of VT Ablation (VISTA) trial], while Fernández-Armenta et al.102 also conducted a randomized study comparing substrate-based ablation to conventional ablation and demonstrated comparable VT-free survival rates between the two strategies. A recent meta-analysis has also revealed similar acute procedural efficacy, complication, VT recurrence, and mortality rates while comparing a predominantly substrate-based ablation strategy to a strategy guided by activation and entrainment mapping of inducible and hemodynamically tolerated VTs.103 A separate meta-analysis104 also showed a significantly lower risk of the composite primary outcome of long-term VA recurrence and all-cause mortality among those undergoing substrate modification in comparison with standard ablation in a cohort of mostly patients with ICM. Furthermore, in this study, long-term success was improved when performing complete substrate modification.104 Hence, substrate ablation may be superior to a conventional strategy in terms of VT recurrence when extensive substrate ablation is performed.11
Table 2:
Summary of Studies Comparing Outcomes between Substrate and Conventional Ablation Strategies
| Study | Design | Patients | Age (Mean or Median) | LVEF (%) | Strategy (Substrate Ablation) | Endpoint(s) | Follow-up (Mean or Median) | No. of Sub | Sub VT | Sub Complications | Sub Cardiac Death | Sub All-cause Death | No. of Controls | Non-sub VT | Non-sub Complications | Non-sub Cardiac Death | Non-sub All Death | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Di Biase et al. 201511 | Multicenter, prospective randomized study | 118 ICM | 67 ± 9 years (sub) and 65 ± 12 years (control) | 32% ± 10% (sub) and 33% ± 14% (control) | Scar homogenization | Elimination of any abnormal potential ± loss of pacing capture (NA) | 12 months | 58 | 9 (16%) | 3 (5%) | 3 (5%) | 5 (9%) | 60 | 29 (48%) | 3 (5%) | 5 (8%) | 9 (15%) | Substrate ablation better in ventricular arrhythmia recurrence during one-year follow-up (log-rank < 0.001); mortality or complication was comparable (p = 0.54 and p = 0.61) |
| Bunch et al. 201213 | Multicenter, retrospective case-control study | 31 (20 ICM and 11 NICM) | 62.5 years (sub) and 59.7 years (control) | 25% (sub) and 20% (control) | LP ablation/linear ablation | Noninducibility | 9 ± 3 months | 18 | 8 (44%) | 3 (17%) | 4 (22%) | 4 (22%) | 13 | 4 (31%) | 4 (31%) | 1 (8%) | 1 (8%) | No statistical difference in VT recurrence, complication, cardiac death, or all-cause death |
| Makimoto et al. 201514 | Single-center, retrospective case-control study | 85 (34 ARVC, 16 ICM, 14 DCM, 1 HCM, 2 D-HCM, 11 sarcoidosis, and 6 congenital) | 53.1 ± 16.2 years | 51.7% ± 16.4% | LP ablation/linear ablation | Elimination of fractionated or isolated delayed potentials, confirmation of a linear lesion blockade PVC elimination, noninducibility | 61 ± 40 months | 50 | 15 (30%) | 0 (0%) | NA | NA | 35 | 15 (43%) | 0 (0%) | 3 (9%) | NA | No statistical difference in VT recurrence, heart failure, or death during five years of follow-up |
| Ventura et al. 200715 | Single-center, prospective cohort study | 30 ICM | 65 ± 7 years | 32% ± 6% | Pacemap-based ablation | Local pacing capture loss and noninducibility | 14 ± 6 months | 14 | 6 (43%) | 0 (0%) | NA | NA | 16 | 4 (25%) | 1 (6%) | NA | NA | No statistical difference in VT recurrence |
| Volkmer et al. 200616 | Single-center, retrospective case-control study | 47 ICM | 65 ± 8 years | 30% ± 7% (sub) and 30% ± 7% (control) | LP ablation | Elimination of LPs related to the VT and noninducibility | 24 ± 12 (sub) months, 26 ± 14 (control) months | 25 | 7 (28%) | 3 (12%) | 3 (12%) | 3 (12%) | 22 | 6 (27%) | 2 (9%) | 3 (14%) | 4 (18%) | No statistical difference in VT recurrence or death |
| Di Biase et al. 201217 | Multicenter, prospective cohort study | 92 ICM | 62 ± 13 years | 27% ± 5% (sub) and 24% ± 8% (control) | Scar homogenization | Elimination of any abnormal potential ± loss of pacing capture (NA) | 25 ± 10 months | 43 | 8 (19%) | 0 (0%) | 0 (0%) | 0 (0%) | 49 | 23 (47%) | 0 (0%) | 0 (0%) | 0 (0%) | Substrate ablation better in ventricular arrhythmia recurrence during follow-up (log-rank = 0.006) |
| Fernández-Armenta et al.102 | Single-center, prospective randomized study | 48 (37 ICM and 11 NICM) | 66 ± 11 years (sub) and 69 ± 8 years (control) | 36% ± 14% (sub) and 36% ± 11% (control) | Scar dechanneling | Elimination of delayed components | 22 ± 14 months | 24 | 10 (42%) | 0 (0%) | 0 (0%) | NA | 24 | 8 (33%) | 4 (17%) | 0 (0%) | NA | No statistical difference in VT recurrence during follow-up |
ARVC: arrhythmogenic right ventricular cardiomyopathy; DCM: dilated cardiomyopathy; D-HCM: hypertrophic cardiomyopathy dilated phase; HCM: hypertrophic cardiomyopathy; ICM: ischemic cardiomyopathy; LP: late potential; LVEF: left ventricular ejection fraction; N/A: not available; NICM: nonischemic cardiomyopathy; No.: number; sub: substrate ablation strategy; VT: ventricular tachycardia recurrence.
However, despite the substantial progress that has been made in the use of cardiac imaging to guide VT ablation, there is insufficient evidence at present to suggest that the use of imaging can add value to clinical outcomes. Although observational, nonrandomized studies suggest that image integration may have an impact on procedural outcomes,27,83,87,88 well-designed, prospective randomized studies are needed to assess the true impact of image integration as well as to evaluate the potential mechanism(s) of any benefit.
Conclusion
Several substrate-based ablation strategies have been developed, which include extensive or less-extensive ablation lesions according to the preset mapping and endpoints of the procedure. Although multipolar mapping catheters with smaller and more-narrowly-spaced bipolar electrodes are now widely used, most currently available studies use data acquired by way of ablation catheters. Advances in cardiac imaging may be helpful in providing refined anatomical substrate details.
At this time, clinical outcomes of substrate-based ablation are at least comparable with and possibly superior to conventional VT ablation. The further development of mapping technologies, cardiac imaging, and novel modalities and the incorporation of these modalities in delineating VT substrate may additionally improve the clinical outcomes of substrate-based ablation.
Table 1:
Studies Investigating Different Substrate Ablation Strategies for VT (continued)
| Ablation Method | Study | Mapping Catheter | Voltage Map Cutoff | Procedure Time | Ablation Catheter | RF Time or No. of Lesions |
|---|---|---|---|---|---|---|
| Linear ablation | Marchlinski et al. 200010 | Ab cath | 0.5–1.5 mV | 9.4 hours | Nonirrigated | 59 ± 34 lesions |
| Soejima et al. 200172 | Ab cath | 0.5–1.5 mV | 7.4 hours | Nonirrigated/open irrigated/closed irrigated | 21 ± 10 lesions | |
| LP ablation | Volkmer et al. 200616 | Ab cath | 0.5–1.5 mV | 3.9 ± 1.1 hours | Noirrigated | 11 ± 8 lesions |
| Arenal et al. 200373 | Ab cath | 0.5–1.5 mV | 7.9 ± 2.1 hours | Nonirrigated/open irrigated | 14 ± 6 lesions | |
| Nogami et al. 200874 | Ab cath | 0.1–1.5 mV | 3.6 ± 1.1 hours | Nonirrigated | 17 ± 10 lesions | |
| Garcia et al. 200975 | Ab cath | 0.5–1.5 mV (endo)/ 0.5–1.0 mV (epi) | N/A | Nonirrigated/open irrigated | 35 ± 26 lesions (endo)/ 37 ± 21 lesions (epi) | |
| Bai et al. 201176 | Ab cath | 0.5–1.5 mV (endo)/ 0.5–1.5 mV (epi) | 5.3 ± 1.2 hours | Open irrigated | 26 ± 14 minutes | |
| Vergara et al. 201277 | Ab cath/Livewire™/AFocus™ II | 0.5–1.5 mV (endo)/ 0.5–1.0 mV (epi) | NA | Open irrigated | N/A | |
| Arenal et al. 201378 | Ab cath | 0.1–1.5 mV | 2.9 ± 0.8 hours | Open irrigated | 11 ± 5 minutes | |
| LAVA | Jaïs et al. 201219 | Ab cath/PentaRay® | 0.5–1.5 mV (endo)/ 0.5–1.0 mV (epi) | 2.5 ± 1.2 hours | Open irrigated | 23 ± 11 minutes |
| Wolf et al. 201820 | Multielectrode catheter in 89 patients/Ab cath in 70 patients | 0.5–1.5 mV (endo)/ 0.5–1.0 mV (epi); 0.2–0.8 mV was used in 6 patients with Rhythmia™ | 4.1 ± 1.3 hours | Open irrigated | 36 ± 20 minutes | |
| Scar homogenization | Di Biase et al. 201511 | Ab cath | 0.5–1.5 mV (endo)/ 0.5–1.5 mV (epi) | 4.2 ± 1.3 hours | Open irrigated | 68 ± 21 minutes |
| Di Biase et al. 201217 | Ab cath | 0.5–1.5 mV (endo)/ 0.5–1.5 mV (epi) | 4.8 ± 1.5 hours | Open irrigated | 74 ± 21 minutes | |
| Scar dechanneling | Berruezo et al. 201238 | Ab cath | 0.5–1.5 mV (endo)/ 0.5–1.5mV (epi) | 3.0 ± 1.0 hours | Open irrigated | 6.3 (4–8.7) lesions |
| Tung et al. 201381 | DecaNav® in 3 patients/Livewire™ in 16 patients/Constellation™ in 2 patients | 0.5–1.5 mV (endo)/ 0.5–1.5 mV (epi) | N/A | Open irrigated (including Thermocool® SF)/closed irrigated | 7 (4–14) lesions | |
| Berruezo et al. 201582 | NA | 0.5–1.5 mV (endo)/ 0.5–1.5 mV (epi) | 3.8 ± 1.1 hours | Open irrigated | 28 ± 16 minutes | |
| Fernández-Armenta et al. 2016102 | Ab cath | 0.5–1.5 mV (endo)/ 0.5–1.5 mV (epi) | 3.5 ± 1.1 hours | Open irrigated | 23 ± 14 minutes | |
| Core isolation | Tzou et al. 201579 | Ab cath | 0.5–1.5 mV (endo)/ 0.5–1.0 mV (epi) | 5.4 ± 2.0 hours | Open irrigated (including Thermocool® SF) | 111 ± 91 lesions |
| MRI-based scar dechanneling | Andreu et al. 201783 | Ab cath | 0.5–1.5 mV (endo)/ 0.5–1.5 mV (epi) | 3.8 ± 1.1 hours | Open irrigated | 19 ± 12 minutes |
Ab cath: ablation catheter; LAVA: local abnormal ventricular activity; LP: late potential; MRI: magnetic resonance imaging; No.: number; RF: radiofrequency. Livewire™ and AFocus™ II are the property of Abbott Laboratories, Chicago, IL, USA. DecaNav®, PentaRay®, and Thermocool® SF are the property of Biosense Webster, Diamond Bar, CA, USA. Constellation™ is the property of Boston Scientific, Natick, MA, USA.
Table 1:
Studies Investigating Different Substrate Ablation Strategies for VT (continued)
| Ablation Method | Study | Complication(s) | Follow-up (Mean or Median) | VT Recurrence (%) | Mortality (%) |
|---|---|---|---|---|---|
| Linear ablation | Marchlinski et al. 200010 | 1 (stroke) | 8 (3–36) months | 4 (25%) | N/A |
| Soejima et al. 200172 | 4 (1 iliac artery dissection, 1 femoral artery pseudoaneurysm, 1 embolism to lower leg, 1 retroperitoneal hematoma) | 12 ± 6 months | 15 (37.5%) | 9 (22.5%); 5 noncardiac-related and unrelated to the procedure, 3 due to cardiac failure, 1 sudden death | |
| LP ablation | Volkmer et al. 200616 | 0 | 9 ± 4 months | 5 (21%) | 1 (4.2%) noncardiac cause |
| Arenal et al. 200373 | 0 | 26 ± 14 months | 7 (29%) | 2 (8%); 1 tamponade in hospital, 1 nonarrhythmogenic death | |
| Nogami et al. 200874 | N/A | 61 ± 38 months | 6 (33%) | 3 (17%); 2 heart failure, 1 malignancy | |
| Garcia et al. 200975 | 0 | 18 ± 13 months | 3 (23%) | N/A | |
| Bai et al. 201176 | 1 (groin hematoma) | 39 ± 4 months | 4 (15%) | 0 (0%) | |
| Vergara et al. 201277 | N/A | 13 ± 4 months | 10 (20%) | 1 (2%) heart failure | |
| Arenal et al. 201378 | 0 | 39 ± 21 months | 25 (42%) | 13 (22%); 6 heart failure, 3 recurrent incessant VT, 1 sudden cardiac death, 3 noncardiac-related | |
| LAVA | Jaïs et al. 201219 | 2 (1 cardiac tamponade, 1 RV perforation) | 22 (14–27) months | 32 (46%) | 13 (19%); 2 died within 24 hours from ablation, 1 PEA, 2 heart failure, 1 sudden death, 1 VT storm, 6 noncardiac-related |
| Wolf et al. 201820 | 12 (9 epicardial bleeding, 2 complete AV block, 1 acute heart failure) | 47 (33–82) months | 71(45%) | 40 (25%); 3 arrhythmia-related, 20 heart failure, 17 noncardiac-related | |
| Scar homogenization | Di Biase et al. 201511 | 3 (pericardial effusion) | 12 months | 9 (15.5%) | 5 (8.6%); 3 nonarrhythmic cardiac-related 2 noncardiac-related |
| Di Biase et al. 201217 | 1 (groin hematoma) | 21 (19–25) months | 8 (19%) | 1 (2%) noncardiac-related | |
| Scar dechanneling | Berruezo et al. 201238 | 1 (RV puncture) | 11 (6–24) months | 1 (9%) | 0 (0%) |
| Tung et al. 201381 | N/A | 11 (6–18) months | 3 (14%) | N/A | |
| Berruezo et al. 201582 | 7 (2 tamponade, 2 complete AV block, 2 pericardial effusion, 1 TIA, 1 PN palsy) | 21 (11–29) months | 20 (20%) | 9 (8.9%); 4 heart failure, 1 sudden cardiac death, 1 arrhythmic storm, 2 noncardiac-related, 1 unknown | |
| Fernández-Armenta et al. 2016102 | 0 | 22 ± 14 months | 10 (41.7%) | N/A | |
| Core isolation | Tzou et al. 201579 | 2 (1 arterial pseudoaneurysm, 1 transient hypotension) | 18 ± 9 months | 6 (14%) | 0 (0%) |
| MRI-based scar dechanneling | Andreu et al. 201783 | N/A | 20 ± 19 months | 10 (18.5%) | 0 (0%) |
AV: atrioventricular; CHF: congestive heart failure; DVT: deep vein thrombosis; LP: late potential; LAVA: local abnormal ventricular activity; MRI: magnetic resonance imaging; PN: phrenic nerve; RV: right ventricle/ventricular; TIA: transient ischemic attack.
References
- 1.Sapp JL, Wells GA, Parkash R, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375(2):111–121. doi: 10.1056/NEJMoa1513614. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Santangeli P, Muser D, Maeda S, et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13(7):1552–1559. doi: 10.1016/j.hrthm.2016.03.004. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.El-Shalakany A, Hadjis T, Papageorgiou P, Monahan K, Epstein L, Josephson ME. Entrainment/mapping criteria for the prediction of termination of ventricular tachycardia by single radiofrequency lesion in patients with coronary artery disease. Circulation. 1999;99(17):2283–2289. doi: 10.1161/01.cir.99.17.2283. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Stevenson WG, Friedman PL, Sager PT, et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997;29(6):1180–1189. doi: 10.1016/s0735-1097(97)00065-x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.de Chillou C, Lacroix D, Klug D, et al. Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation. 2002;105(6):726–731. doi: 10.1161/hc0602.103675. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009;6(6):886–933. doi: 10.1016/j.hrthm.2009.04.030. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Stevenson WG, Friedman PL, Kocovic D, Sager PT, Saxon LA, Pavri B. Radiofrequency catheter ablation of ventricular tachycardia after myocardial infarction. Circulation. 1998;98(4):308–314. doi: 10.1161/01.cir.98.4.308. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Callans DJ, Zado E, Sarter BH, Schwartzman D, Gottlieb CD, Marchlinski FE. Efficacy of radiofrequency catheter ablation for ventricular tachycardia in healed myocardial infarction. Am J Cardiol. 1998;82(4):429–432. doi: 10.1016/s0002-9149(98)00353-1. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Cassidy DM, Vassallo JA, Marchlinski FE, Buxton AE, Untereker WJ, Josephson ME. Endocardial mapping in humans in sinus rhythm with normal left ventricles: Activation patterns and characteristics of electrograms. Circulation. 1984;70(1):37–42. doi: 10.1161/01.cir.70.1.37. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Marchlinski FE, Callans DJ, Gottlieb CD, Zado ES. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101(11):1288–1296. doi: 10.1161/01.cir.101.11.1288. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Di Biase L, Burkhardt JD, Lakkireddy D, et al. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol. 2015;66(25):2872–2882. doi: 10.1016/j.jacc.2015.10.026. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Dello Russo A, Casella M, Pieroni M, et al. Drug-refractory ventricular tachycardias after myocarditis: Endocardial and epicardial radiofrequency catheter ablation. Circ Arrhythmia Electrophysiol. 2012;5(3):492–498. doi: 10.1161/CIRCEP.111.965012. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Bunch TJ, Darby A, May HT, et al. Efficacy and safety of ventricular tachycardia ablation with mechanical circulatory support compared with substrate-based ablation techniques. Europace. 2012;14(5):709–714. doi: 10.1093/europace/eur347. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makimoto H, Nakajima I, Miyamoto K, et al. Clinical impact of mapping strategies for treatment of ventricular tachycardias in patients with structural heart disease. Pacing Clin Electrophysiol. 2015;38(5):630–640. doi: 10.1111/pace.12601. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Ventura R, Klemm HU, Rostock T, et al. Stable and unstable ventricular tachycardias in patients with previous myocardial infarction: A clinically oriented strategy for catheter ablation. Cardiology. 2007;109(1):52–61. doi: 10.1159/000105326. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Volkmer M, Ouyang F, Deger F, et al. Substrate mapping vs. tachycardia mapping using CARTO in patients with coronary artery disease and ventricular tachycardia: Impact on outcome of catheter ablation. Europace. 2006;8(11):968–976. doi: 10.1093/europace/eul109. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Di Biase L, Santangeli P, Burkhardt DJ, et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60(2):132–141. doi: 10.1016/j.jacc.2012.03.044. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Gökoğlan Y, Mohanty S, Gianni C, et al. Scar homogenization versus limited-substrate ablation in patients with nonischemic cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol. 2016;68(18):1990–1998. doi: 10.1016/j.jacc.2016.08.033. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Jaïs P, Maury P, Khairy P, et al. Elimination of local abnormal ventricular activities: A new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125(18):2184–2196. doi: 10.1161/CIRCULATIONAHA.111.043216. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Wolf M, Sacher F, Cochet H, et al. Long-term outcome of substrate modification in ablation of post-myocardial infarction ventricular tachycardia. Circ Arrhythmia Electrophysiol. 2018;11(2):e005635. doi: 10.1161/CIRCEP.117.005635. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 21.Sacher F, Lim HS, Derval N, et al. Substrate mapping and ablation for ventricular tachycardia: the LAVA approach. J Cardiovasc Electrophysiol. 2015;26(4):464–471. doi: 10.1111/jce.12565. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357(26):2657–2665. doi: 10.1056/NEJMoa065457. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gepstein L, Hayam G, Ben-haim SA. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation. 1997;95(6):1611–1622. doi: 10.1161/01.cir.95.6.1611. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Berte B, Relan J, Sacher F, et al. Impact of electrode type on mapping of scar-related VT. J Cardiovasc Electrophysiol. 2015;26(11):1213–1223. doi: 10.1111/jce.12761. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Anter E, Tschabrunn CM, Josephson ME. High-resolution mapping of scar-related atrial arrhythmias using smaller electrodes with closer interelectrode spacing. Circ Arrhythmia Electrophysiol. 2015;8(3):537–545. doi: 10.1161/CIRCEP.114.002737. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Tschabrunn CM, Roujol S, Dorman NC, Nezafat R, Josephson ME, Anter E. High-resolution mapping of ventricular scar: comparison between single and multielectrode catheters. Circ Arrhythmia Electrophysiol. 2016;9(6):pii: e003841. doi: 10.1161/CIRCEP.115.003841. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita S, Cochet H, Sacher F, et al. Impact of new technologies and approaches for post-myocardial infarction ventricular tachycardia ablation during long-term follow-up. Circ Arrhythmia Electrophysiol. 2016;9(7):1–12. doi: 10.1161/CIRCEP.116.003901. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 28.Mahida S, Sacher F, Dubois R, et al. Cardiac imaging in patients with ventricular tachycardia. Circulation. 2017;136(25):2491–2507. doi: 10.1161/CIRCULATIONAHA.117.029349. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Yamashita S, Sacher F, Hooks DA, et al. Myocardial wall thinning predicts transmural substrate in patients with scar-related ventricular tachycardia. Heart Rhythm. 2017;14(2):155–163. doi: 10.1016/j.hrthm.2016.11.012. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 30.Wijnmaalen AP, Van Der Geest RJ, Taxis CFB, et al. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011;32(1):104–114. doi: 10.1093/eurheartj/ehq345. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Armenta J, Berruezo A, Andreu D, et al. Three-dimensional architecture of scar and conducting channels based on high resolution ce-CMR: Insights for ventricular tachycardia ablation. Circ Arrhythmia Electrophysiol. 2013;6(3):528–537. doi: 10.1161/CIRCEP.113.000264. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 32.Cassidy DM, Vassallo JA, Miller JM, et al. Endocardial catheter mapping in patients in sinus rhythm: Relationship to underlying heart disease and ventricular arrhythmias. Circulation. 1986;73(4):645–652. doi: 10.1161/01.cir.73.4.645. [PubMed] [DOI] [PubMed] [Google Scholar]
- 33.Cassidy DM, Vassallo JA, Buxton AE, Doherty JU, Marchlinski FE, Josephson ME. The value of catheter mapping during sinus rhythm to localize site of origin of ventricular tachycardia. Circulation. 1984;69(6):1103–1110. doi: 10.1161/01.cir.69.6.1103. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Kienzle MG, Miller J, Falcone RA, Harken A, Josephson ME. Intraoperative endocardial mapping during sinus rhythm: Relationship to site of origin of ventricular tachycardia. Circulation. 1984;70(6):957–965. doi: 10.1161/01.cir.70.6.957. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Acosta J, Penela D, Andreu D, et al. Multielectrode vs. point-by-point mapping for ventricular tachycardia substrate ablation: a randomized study. Europace. 2018;20(3):512–519. doi: 10.1093/europace/euw406. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Sacher F, Duchateau J, Capellino S, et al. Voltage threshold for high density mapping catheter with short interspersed small electrodes. Heart Rhythm. 2016;13:S32. [Google Scholar]
- 37.Maagh P, Christoph A, Dopp H, Mueller MS, Plehn G, Meissner A. High-density mapping in ventricular tachycardia ablation: a PentaRay® study. Cardiol Res. 2017;8(6):293–303. doi: 10.14740/cr636w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berruezo A, Fernández-Armenta J, Mont L, et al. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythmia Electrophysiol. 2012;5(1):111–121. doi: 10.1161/CIRCEP.110.960740. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 39.Haqqani HM, Kalman JM, Roberts-Thomson KC, et al. Fundamental differences in electrophysiologic and electroanatomic substrate between ischemic cardiomyopathy patients with and without clinical ventricular tachycardia. J Am Coll Cardiol. 2009;54(2):166–173. doi: 10.1016/j.jacc.2009.04.024. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Baldinger SH, Kapur S, et al. Right ventricular scar-related ventricular tachycardia in nonischemic cardiomyopathy: electrophysiological characteristics, mapping, and ablation of underlying heart disease. J Cardiovasc Electrophysiol. 2018;29(1):79–89. doi: 10.1111/jce.13346. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Deneke T, Müller KM, Lemke B, et al. Human histopathology of electroanatomic mapping after cooled-tip radiofrequency ablation to treat ventricular tachycardia in remote myocardial infarction. J Cardiovasc Electrophysiol. 2005;16(11):1246–1251. doi: 10.1111/j.1540-8167.2005.40826.x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Koa-Wing M, Ho SY, Kojodjojo P, Peters NS, Davies DW, Kanagaratnam P. Radiofrequency ablation of infarct scar-related ventricular tachycardia: correlation of electroanatomical data with post-mortem histology. J Cardiovasc Electrophysiol. 2007;18(12):1330–1333. doi: 10.1111/j.1540-8167.2007.00886.x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Codreanu A, Odille F, Aliot E, et al. Electroanatomic characterization of post-infarct scars comparison with 3-dimensional myocardial scar reconstruction based on magnetic resonance imaging. J Am Coll Cardiol. 2008;52(10):839–842. doi: 10.1016/j.jacc.2008.05.038. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Anter E, Tschabrunn C, Rajoul S. In vivo high-resolution mapping of post-infarction reentrant ventricular tachycardias. Heart Rhythm. 2015;12:S203. [Google Scholar]
- 45.Soejima K, Stevenson WG, Maisel WH, Sapp JL, Epstein LM. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation. Circulation. 2002;106(13):1678–1683. doi: 10.1161/01.cir.0000030187.39852.a7. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 46.Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54(9):799–808. doi: 10.1016/j.jacc.2009.05.032. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 47.Desjardins B, Morady F, Bogun F. Effect of epicardial fat on electroanatomical mapping and epicardial catheter ablation. J Am Coll Cardiol. 2010;56(16):1320–1327. doi: 10.1016/j.jacc.2010.04.054. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Verma A, Marrouche NF, Schweikert RA, et al. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J Cardiovasc Electrophysiol. 2005;16(5):465–471. doi: 10.1046/j.1540-8167.2005.40443.x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 49.De Bakker JMT, Van Capelle FJL, Janse MJ, et al. Slow conduction in the infarcted human heart: ‘zigzag’ course of activation. Circulation. 1993;88(3):915–926. doi: 10.1161/01.cir.88.3.915. [PubMed] [DOI] [PubMed] [Google Scholar]
- 50.Kettering K, Weig HJ, Reimold M, et al. Catheter ablation of ventricular tachycardias in patients with ischemic cardiomyopathy: Validation of voltage mapping criteria for substrate modification by myocardial viability assessment using FDG PET. Clin Res Cardiol. 2010;99(11):753–760. doi: 10.1007/s00392-010-0182-2. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.de Riva M, Naruse Y, Ebert M, et al. Targeting the hidden substrate unmasked by right ventricular extrastimulation improves ventricular tachycardia ablation outcome after myocardial infarction. JACC Clin Electrophysiol. 2018;4(3):316–327. doi: 10.1016/j.jacep.2018.01.013. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 52.Porta-Sánchez A, Jackson N, Lukac P, et al. Multicenter study of ischemic ventricular tachycardia ablation with decrement evoked potential (DEEP) mapping with extra stimulus. JACC Clin Electrophysiol. 2018;4(3):307–315. doi: 10.1016/j.jacep.2017.12.005. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.Komatsu Y, Daly M, Sacher F, et al. Electrophysiologic characterization of local abnormal ventricular activities in postinfarction ventricular tachycardia with respect to their anatomic location. Heart Rhythm. 2013;10(11):1630–1637. doi: 10.1016/j.hrthm.2013.08.031. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 54.Tung R, Josephson ME, Bradfield JS, Shivkumar K. Directional influences of ventricular activation on myocardial scar characterization: voltage mapping with multiple wavefronts during ventricular tachycardia ablation. Circ Arrhythmia Electrophysiol. 2016;9(8):pii: e004155. doi: 10.1161/CIRCEP.116.004155. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 55.Josephson ME, Anter E. Substrate mapping for ventricular tachycardia assumptions and misconceptions. JACC Clin Electrophysiol. 2015;1(5):341–352. doi: 10.1016/j.jacep.2015.09.001. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 56.Roberts DE, Hersh LT, Scher AM. Influence of cardiac fiber orientation on wavefront voltage, conduction velocity, and tissue resistivity in the dog. Circ Res. 1979;44(5):701–712. doi: 10.1161/01.res.44.5.701. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 57.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108(6):704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Haqqani HM, Tschabrunn CM, Tzou WS, et al. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: Incidence, characterization, and implications. Heart Rhythm. 2011;8(8):1169–1176. doi: 10.1016/j.hrthm.2011.03.008. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 59.Polin GM, Haqqani H, Tzou W, et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8(1):76–83. doi: 10.1016/j.hrthm.2010.09.088. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 60.Hutchinson MD, Gerstenfeld EP, Desjardins B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythmia Electrophysiol. 2011;4(1):49–55. doi: 10.1161/CIRCEP.110.959957. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campos B, Jauregui ME, Park KM, et al. New unipolar electrogram criteria to identify irreversibility of nonischemic left ventricular cardiomyopathy. J Am Coll Cardiol. 2012;60(21):2194–2204. doi: 10.1016/j.jacc.2012.08.977. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 62.Desjardins B, Crawford T, Good E, et al. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009;6(5):644–651. doi: 10.1016/j.hrthm.2009.02.018. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duchateau J, Dubois R. High-density mapping of atrial tachycardias: importance of interpolation. J Cardiovasc Electrophysiol. 2018;29(5):E7–E8. doi: 10.1111/jce.13475. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 64.Blauer JJE, Swenson D, Higuchi K, et al. Sensitivity and specificity of substrate mapping: an in silico framework for the evaluation of electroanatomical substrate mapping strategies. J Cardiovasc Electrophysiol. 2014;25(7):774–780. doi: 10.1111/jce.12444. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teijeira-Fernandez E, Cochet H, Bourier F, et al. Influence of contact force on voltage mapping: a combined magnetic resonance imaging and electroanatomic mapping study in patients with tetralogy of Fallot. Heart Rhythm. 2018;15(8):1198–1205. doi: 10.1016/j.hrthm.2018.03.022. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.Mizuno H, Vergara P, MacCabelli G, et al. Contact force monitoring for cardiac mapping in patients with ventricular tachycardia. J Cardiovasc Electrophysiol. 2013;24(5):519–524. doi: 10.1111/jce.12080. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 67.Esposito A, Palmisano A, Antunes S, et al. Cardiac CT with delayed enhancement in the characterization of ventricular tachycardia structural substrate: relationship between CT-segmented scar and electro-anatomic mapping. JACC Cardiovasc Imaging. 2016;9(7):822–832. doi: 10.1016/j.jcmg.2015.10.024. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Tian J, Jeudy J, Smith MF, et al. Three-dimensional contrast-enhanced multidetector CT for anatomic, dynamic, and perfusion characterization of abnormal myocardium to guide ventricular tachycardia ablations. Circ Arrhythmia Electrophysiol. 2010;3(5):496–504. doi: 10.1161/CIRCEP.109.889311. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 69.Berruezo A, Paetsch I. Inception: implanting the idea of magnetic resonance imaging-guided ventricular tachycardia substrate ablation. Europace. 2018;20(FI2):f143–f145. doi: 10.1093/europace/eux367. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 70.Massé S, Magtibay K, Jackson N, et al. Resolving myocardial activation with novel omnipolar electrograms. Circ Arrhythmia Electrophysiol. 2016;9(7):e004107. doi: 10.1161/CIRCEP.116.004107. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deno DC, Balachandran R, Morgan D, Ahmad F, Masse S, Nanthakumar K. Orientation-independent catheter-based characterization of myocardial activation. IEEE Trans Biomed Eng. 2017;64(5):1067–1077. doi: 10.1109/TBME.2016.2589158. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 72.Soejima K, Suzuki M, Maisel WH, et al. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001;104(6):664–669. doi: 10.1161/hc3101.093764. [DOI] [PubMed] [Google Scholar]
- 73.Arenal A, Glez-Torrecilla E, Ortiz M, et al. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41(1):81–92. doi: 10.1016/s0735-1097(02)02623-2. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 74.Nogami A, Sugiyasu A, Tada H, et al. Changes in the isolated delayed component as an endpoint of catheter ablation in arrhythmogenic right ventricular cardiomyopathy: predictor for long-term success. J Cardiovasc Electrophysiol. 2008;19(7):681–688. doi: 10.1111/j.1540-8167.2008.01104.x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 75.Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120(5):366–375. doi: 10.1161/CIRCULATIONAHA.108.834903. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 76.Bai R, Di Biase L, Shivkumar K, et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythmia Electrophysiol. 2011;4(4):478–485. doi: 10.1161/CIRCEP.111.963066. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Vergara P, Trevisi N, Ricco A, et al. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23(6):621–627. doi: 10.1111/j.1540-8167.2011.02246.x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Arenal Á, Hernández J, Calvo D, et al. Safety, long-term results, and predictors of recurrence after complete endocardial ventricular tachycardia substrate ablation in patients with previous myocardial infarction. Am J Cardiol. 2013;111(4):499–505. doi: 10.1016/j.amjcard.2012.10.031. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 79.Tzou WS, Frankel DS, Hegeman T, et al. Core isolation of critical arrhythmia elements for treatment of multiple scar-based ventricular tachycardias. Circ Arrhythmia Electrophysiol. 2015;8(2):353–361. doi: 10.1161/CIRCEP.114.002310. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 80.Arenal A, Del Castillo S, Gonzalez-Torrecilla E, et al. Tachycardia-related channel in the scar tissue in patients with sustained monomorphic ventricular tachycardias: influence of the voltage scar definition. Circulation. 2004;110(17):2568–2574. doi: 10.1161/01.CIR.0000145544.35565.47. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 81.Tung R, Mathuria NS, Nagel R, et al. Impact of local ablation on interconnected channels within ventricular scar: mechanistic implications for substrate modification. Circ Arrhythmia Electrophysiol. 2013;6(6):1131–1138. doi: 10.1161/CIRCEP.113.000867. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berruezo A, Fernández-Armenta J, Andreu D, et al. Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythmia Electrophysiol. 2015;8(2):326–336. doi: 10.1161/CIRCEP.114.002386. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 83.Andreu D, Penela D, Acosta J, et al. Cardiac magnetic resonance-aided scar dechanneling: Influence on acute and long-term outcomes. Heart Rhythm. 2017;14(8):1121–1128. doi: 10.1016/j.hrthm.2017.05.018. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 84.Kuroki K, Nogami A, Igarashi M, et al. New substrate-guided method of predicting slow conducting isthmuses of ventricular tachycardia: preliminary analysis to the combined use of voltage limit adjustment and fast-fourier transform analysis. Circ Arrhythmia Electrophysiol. 2018;11(4):e005705. doi: 10.1161/CIRCEP.117.005705. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 85.Lin CY, Silberbauer J, Lin YJ, et al. Simultaneous Amplitude Frequency Electrogram Transformation (SAFE-T) mapping to identify ventricular tachycardia arrhythmogenic potentials in sinus rhythm. JACC Clin Electrophysiol. 2016;2(4):459–470. doi: 10.1016/j.jacep.2016.01.013. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 86.Campos B, Jauregui ME, Marchlinski FE, Dixit S, Gerstenfeld EP. Use of a novel fragmentation map to identify the substrate for ventricular tachycardia in postinfarction cardiomyopathy. Heart Rhythm. 2015;12(1):95–103. doi: 10.1016/j.hrthm.2014.10.002. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 87.Yamashita S, Sacher F, Mahida S, et al. Image integration to guide catheter ablation in scar-related ventricular tachycardia. J Cardiovasc Electrophysiol. 2016;27(6):699–708. doi: 10.1111/jce.12963. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 88.Bogun FM, Desjardins B, Good E, et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy. Utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009;53(13):1138–1145. doi: 10.1016/j.jacc.2008.11.052. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Komatsu Y, Cochet H, Jadidi A, et al. Regional myocardial wall thinning at multidetector computed tomography correlates to arrhythmogenic substrate in postinfarction ventricular tachycardia: Assessment of structural and electrical substrate. Circ Arrhythmia Electrophysiol. 2013;6(2):342–350. doi: 10.1161/CIRCEP.112.000191. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Piers SRD, Tao Q, De Riva Silva M, et al. CMR-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. JACC Cardiovasc Imaging. 2014;7(8):774–784. doi: 10.1016/j.jcmg.2014.03.013. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 91.Grothoff M, Gutberlet M, Hindricks G, et al. Magnetic resonance imaging guided transatrial electrophysiological studies in swine using active catheter tracking – experience with 14 cases. Eur Radiol. 2017;27(5):1954–1962. doi: 10.1007/s00330-016-4560-7. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 92.Sommer P, Grothoff M, Eitel C, et al. Feasibility of real-time magnetic resonance imaging-guided electrophysiology studies in humans. Europace. 2013;15(1):101–108. doi: 10.1093/europace/eus230. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 93.Dukkipati SR, Mallozzi R, Schmidt EJ, et al. Electroanatomic mapping of the left ventricle in a porcine model of chronic myocardial infarction with magnetic resonance-based catheter tracking. Circulation. 2008;118(8):853–862. doi: 10.1161/CIRCULATIONAHA.107.738229. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 94.Yamashita S, Sacher F, Mahida S, et al. Role of high-resolution image integration to visualize left phrenic nerve and coronary arteries during epicardial ventricular tachycardia ablation. Circ Arrhythmia Electrophysiol. 2015;8(2):371–380. doi: 10.1161/CIRCEP.114.002420. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Anter E, Tschabrunn CM, Buxton AE, Josephson ME. High-resolution mapping of postinfarction reentrant ventricular tachycardia: electrophysiological characterization of the circuit. Circulation. 2016;134(4):314–327. doi: 10.1161/CIRCULATIONAHA.116.021955. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khaykin Y, Skanes A, Whaley B, et al. Real-time integration of 2D intracardiac echocardiography and 3D electroanatomical mapping to guide ventricular tachycardia ablation. Heart Rhythm. 2008;5(10):1396–1402. doi: 10.1016/j.hrthm.2008.06.025. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 97.Bunch TJ, Weiss JP, Crandall BG, et al. Image integration using intracardiac ultrasound and 3D reconstruction for scar mapping and ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 2010;21(6):678–684. doi: 10.1111/j.1540-8167.2009.01680.x. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 98.Dickfeld T, Lei P, Dilsizian V, et al. Integration of three-dimensional scar maps for ventricular tachycardia ablation with positron emission tomography-computed tomography. JACC Cardiovasc Imaging. 2008;1(1):73–82. doi: 10.1016/j.jcmg.2007.10.001. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 99.Klein T, Abdulghani M, Smith M, et al. Three-dimensional 123I-meta-iodobenzylguanidine cardiac innervation maps to assess substrate and successful ablation sites for ventricular tachycardia: feasibility study for a novel paradigm of innervation imaging. Circ Arrhythmia Electrophysiol. 2015;8(3):583–591. doi: 10.1161/CIRCEP.114.002105. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 100.Cochet H, Dubois R, Sacher F, et al. Cardiac arrythmias: multimodal assessment integrating body surface ECG mapping into cardiac imaging. Radiology. 2014;271(1):239–247. doi: 10.1148/radiol.13131331. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 101.Cuculich PS, Schill MR, Kashani R, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377(24):2325–2336. doi: 10.1056/NEJMoa1613773. [CrossRef] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernández-Armenta J, Penela D, Acosta J, et al. Substrate modification or ventricular tachycardia induction, mapping, and ablation as the first step? A randomized study. Heart Rhythm. 2016;13(8):1589–1595. doi: 10.1016/j.hrthm.2016.05.013. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 103.Kumar S, Baldinger SH, Romero J, et al. Substrate-based ablation versus ablation guided by activation and entrainment mapping for ventricular tachycardia: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2016;27(12):1437–1447. doi: 10.1111/jce.13088. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 104.Briceño DF, Romero J, Villablanca PA, et al. Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: systematic review and meta-analysis. Europace. 2018;20(1):104–115. doi: 10.1093/europace/eux109. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]