Abstract
Rationale: Because encouraging rates for hospital and long-term survival of immunocompromised patients in ICUs have been described, these patients are more likely to receive invasive therapies, like extracorporeal membrane oxygenation (ECMO).
Objectives: To report outcomes of immunocompromised patients treated with ECMO for severe acute respiratory distress syndrome (ARDS) and to identify their pre-ECMO predictors of 6-month mortality and main ECMO-related complications.
Methods: Retrospective multicenter study in 10 international ICUs with high volumes of ECMO cases. Immunocompromised patients, defined as having hematological malignancies, active solid tumor, solid-organ transplant, acquired immunodeficiency syndrome, or long-term or high-dose corticosteroid or immunosuppressant use, and severe ECMO-treated ARDS, from 2008 to 2015 were included.
Measurements and Main Results: We collected demographics, clinical data, ECMO-related complications, and ICU- and 6 month–outcome data for 203 patients (median Acute Physiology and Chronic Health Evaluation II score, 28 [25th–75th percentile, 20–33]; age, 51 [38–59] yr; PaO2/FiO2, 60 [50–82] mm Hg before ECMO) who fulfilled our inclusion criteria. Six-month survival was only 30%, with a respective median ECMO duration and ICU stay of 8 (5–14) and 25 (16–50) days. Patients with hematological malignancies had significantly poorer outcomes than others (log-rank P = 0.02). ECMO-related major bleeding, cannula infection, and ventilator-associated pneumonia were frequent (36%, 10%, and 50%, respectively). Multivariate analyses retained fewer than 30 days between immunodeficiency diagnosis and ECMO cannulation as being associated with lower 6-month mortality (odds ratio, 0.32 [95% confidence interval, 0.16–0.66]; P = 0.002), and lower platelet count, higher Pco2, age, and driving pressure as independent pre-ECMO predictors of 6-month mortality.
Conclusions: Recently diagnosed immunodeficiency is associated with a much better prognosis in ECMO-treated severe ARDS. However, low 6-month survival of our large cohort of immunocompromised patients supports restricting ECMO to patients with realistic oncological/therapeutic prognoses, acceptable functional status, and few pre-ECMO mortality-risk factors.
Keywords: extracorporeal membrane oxygenation, acute respiratory distress syndrome, immunodeficiency, outcome, hematological malignancies
At a Glance Commentary
Scientific Knowledge on the Subject
Because encouraging hospital and long-term survival rates have been reported for immunocompromised patients admitted to ICUs, those patients are more likely to receive invasive therapies, such as extracorporeal membrane oxygenation (ECMO). Although the negative impact of immunodeficiency on the survival of patients with acute respiratory distress syndrome, ECMO-treated or not, has been constantly stressed, patients’ prognoses and ECMO-related complications have not been thoroughly investigated so far.
What This Study Adds to the Field
On the basis of a cohort of 203 severely ill, ECMO-treated, immunocompromised patients, 6-month overall survival was only 30%, with significantly worse outcomes for patients with hematological malignancies. Notably, a time span of fewer than 30 days between immunodeficiency diagnosis and ECMO cannulation was independently associated with a better 6-month survival rate, whereas higher PaCO2, age, and driving pressure, and lower pre-ECMO platelet count were associated with 6-month mortality.
Over the past decade, ICU and hospital survival rates for immunocompromised patients have gradually improved (1–5). Although mortality remains high compared with the general population of critically ill patients, especially when associated with mechanical ventilation (4), those patients are increasingly admitted to ICUs. Concurrently, successful extracorporeal membrane oxygenation (ECMO) support of the most severe acute respiratory distress syndrome (ARDS) cases, as shown for the recent influenza A(H1N1) pandemic or, in the randomized CESAR (Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure) trial (6–9), led to a steep increase of the number of venovenous (VV)-ECMO procedures performed over the past decade (10, 11). Thus, the reported encouraging rates for hospital and long-term survival of immunocompromised patients in ICUs (4) mean that those patients are more likely to receive invasive therapies, like ECMO. Indeed, 19% to 31% of ECMO-treated Patients with ARDS in recently published cohorts (6, 12, 13) were immunocompromised. Although the negative impact of immunosuppression on survival of patients who have ARDS—with or without ECMO (12–14)—has been constantly emphasized, their prognoses and ECMO-related complications have not been thoroughly examined yet. To date, data that are available on this specific population were mainly described in a single-center cohort study that usually included small numbers of patients (15).
The objectives of this international, multicenter, retrospective study were 1) to report outcomes of ECMO-treated moderate or severe ARDS in immunocompromised patients; 2) to identify pre-ECMO predictors of 6-month mortality; and 3) to describe ECMO-related complications and their main risk factors within this population.
Methods
Study Design and Patients
This study included immunocompromised patients hospitalized in 10 ICUs in seven countries between 2008 and 2015. Fourteen ICUs with large volumes of ECMO cases (>20 ECMOs/yr) (16) were invited to participate. All participating ICUs obtained Institutional Review Board approval in accordance with their local legislation. All consecutive immunodeficient patients older than 15 years old, who received extracorporeal lung support (i.e., venoarterial-ECMO, VV-ECMO, or extracorporeal CO2 removal) for acute respiratory failure were screened. Patients undergoing extracorporeal CO2 removal or with end-stage chronic respiratory failure were excluded from the final analysis. Thus, the final analysis focused on adult immunocompromised patients who received ECMO for moderate or severe ARDS. Immunodeficiency was defined as 1) hematological malignancies, 2) active solid tumor or having received specific antitumor treatment within the previous year, 3) solid-organ transplant, 4) AIDS, or 5) long-term or high-dose corticosteroids (CS) or immunosuppressants (IS). Long-term CS was defined as >7.5 mg of prednisone per day for more than 3 months, with a high dose defined as >1 mg/kg for more than 1 week within the last 3 months. IS had to have been received during the 6 months before ECMO or within the first 48 hours after ECMO cannulation. A recent immunodeficiency diagnosis means that the diagnosis was confirmed fewer than 30 days before ICU admission (4).
Data Collection
Baseline information was recorded for the time immediately preceding ECMO implantation. It included age, sex, the Acute Physiology and Chronic Health Evaluation (APACHE) II score (17), the Sequential Organ Failure Assessment (SOFA) score at cannulation (18), the Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score (13), the Predicting Death for Severe ARDS on VV-ECMO (PRESERVE) score (12), the Murray score (19), dates of hospital and ICU admissions, origin of immunodeficiency and its date of diagnosis, risk factors for ARDS, concomitant therapies before starting ECMO (neuromuscular blocking agents, nitric oxide, prone positioning), date that mechanical ventilation started, ventilator settings (positive end-expiratory pressure [PEEP], FiO2, plateau pressure, tidal volume), arterial blood-gas parameters, and standard laboratory parameters. Driving pressure was calculated as described elsewhere (20). The Berlin definition and grading of ARDS were applied (21).
Follow-up variables that were recorded were the use of antimicrobials, vasopressors, and renal replacement therapy; bleeding complications; other ECMO-related complications (hemolysis, ischemic stroke); ECMO and mechanical-ventilation durations; length of ICU and hospital stays; and ICU and 6-month post–ICU admission survival rates.
Major bleeding was defined as requiring ≥2 units of packed red blood cells due to an obvious hemorrhagic event, leading to a surgical or interventional procedure, an intracerebral hemorrhage, or a fatal outcome. Neutropenia was defined as an absolute neutrophil count <500/μl, whereas massive hemolysis was defined as plasma-free hemoglobin >500 mg/L associated with clinical signs of hemolysis. Nosocomial infection definitions agreed with those of the Centers for Disease Control and the Prevention/National Nosocomial Infections Surveillance System (22). Quantitative cultures of distal BAL fluids or protected specimen-brush samples growing, respectively, ≥104 or ≥103 cfu/ml, diagnosed ventilator-associated pneumonia before antibiotics were started. Cannula infection diagnosis required local signs of infection at the access site with a positive culture of subcutaneous needle aspirate from that site. When the same organism(s) was (were) recovered from both blood and the cannula site, bloodstream infections were considered cannula-related.
Statistical Analyses
This study followed the Consolidated Standards of Reporting Trials (CONSORT) recommendations for reporting cohort studies (STROBE statement) (23). Continuous variables (expressed as median [25th–75th percentile]) were compared with the student’s t test or the Mann-Whitney U-test, as appropriate. Categorical variables were compared with χ2 tests. Patients’ demographic, clinical, and pre-ECMO ventilation characteristics and laboratory results were tested in univariable analyses for association with death 6 months after ICU admission or major ECMO-related bleeding. Factors achieving P ≤ 0.10 in our univariable analyses were entered into the multivariate model. Thereafter, multiple backward-stepwise logistic-regression analyses eliminated variables with an exit threshold set at P > 0.05. All potential explanatory variables included in the multivariable analyses were subjected to collinearity analysis with a correlation matrix. Variables associated with one another were not included in the multivariable model. The main analyses were on the basis of data from patients with complete information available for all variables. Sensitivity analyses were computed using multiple imputations (24) (additional details are provided in the online supplement).
Patients for whom pre-ECMO data permitted calculation of the PRESERVE score (12) and nonimmunocompromised ECMO-supported patients with ARDS extracted from the PRESERVE cohort (12) were matched (1:1 without replacement) on an identical PRESERVE score. Of note, the parameter “immunocompromised status” was not integrated in the PRESERVE score calculation. Multiple backward-stepwise logistic-regression analyses were repeated on this matched population to determine risk factors of death 6 months after ECMO initiation.
The area under the receiver operating characteristic curve was used to compare the discrimination and calibration of the RESP, PRESERVE, SOFA at cannulation, and APACHE II scores to predict 6-month mortality. Pairwise comparisons of the area under the curves of all scores used the Delong test (25). Results are reported as odds ratios (ORs) (95% confidence interval [CI]). Lastly, Kaplan-Meier survival curves were computed for immunodeficiency etiologies and the timing of immunosuppressant use associated with 6-month mortality, and compared with the Mantel-Cox log-rank tests with P < 0.05 defined significance. Analyses were computed with StatView v5.0 (SAS Institute Inc.) and SPSS 22 (SPSS Inc.) software.
Results
Study Population
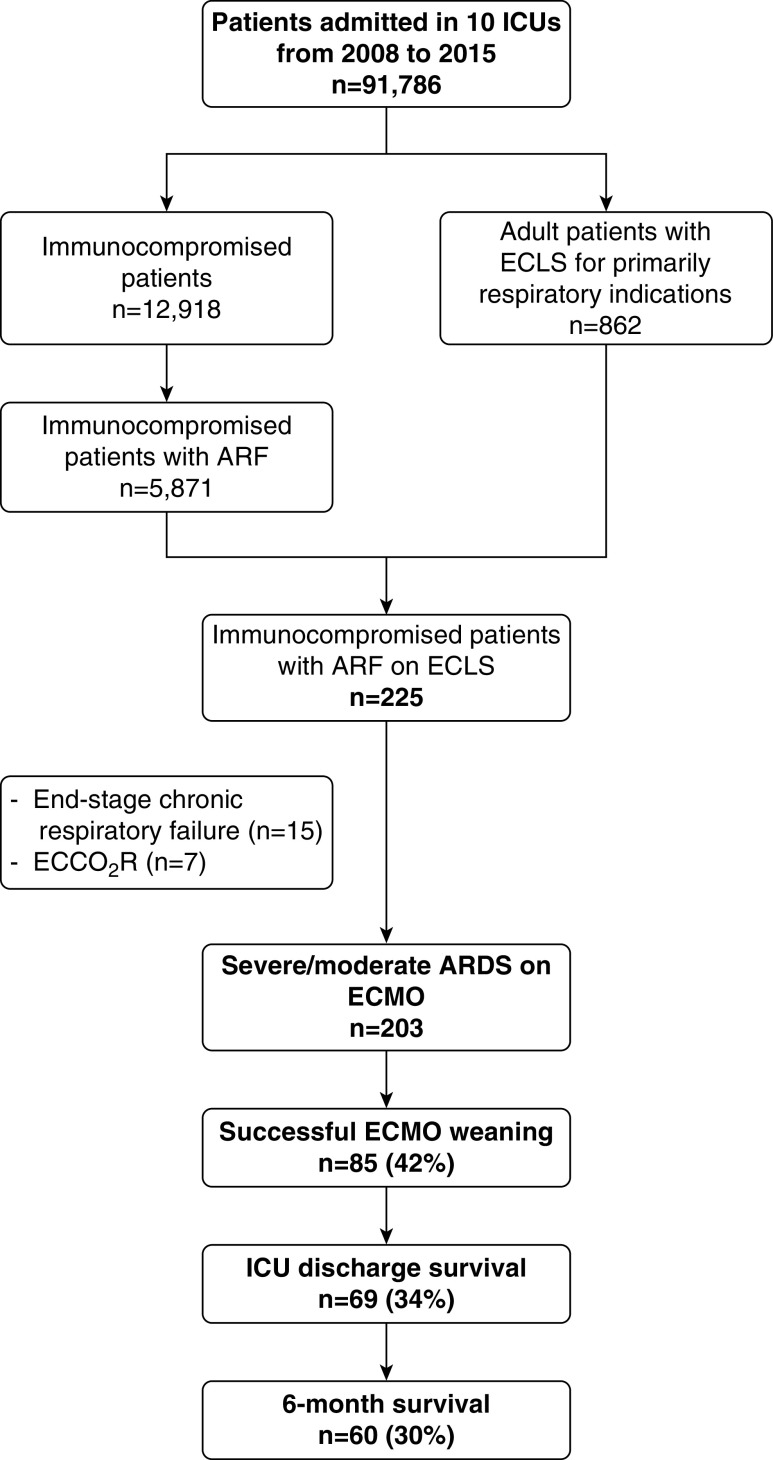
During the 8-year study period, 203 ECMO-treated immunocompromised patients (Figure 1) (age, 51 [38–59] yr; 62% male; APACHE II score, 28 [20–33]) with ARDS (197 severe and 6 moderate) were enrolled in 10 international centers. The main center characteristics and their local management of patients on VV-ECMO are detailed in Table E1 in the online supplement. Patient characteristics at ICU admission are reported in Tables 1, 2, and E2. Briefly, long-term CS/IS use and hematological malignancies were the most frequent causes of immunodeficiency (28% and 30%, respectively) with 51 (25%) patients recently diagnosed as immunocompromised.
Figure 1.
Flow chart of the study. ARDS = acute respiratory distress syndrome; ARF = acute respiratory failure; ECCO2R = extracorporeal CO2 removal; ECLS = extracorporeal life support; ECMO = extracorporeal membrane oxygenation.
Table 1.
Baseline Characteristics of Patients according to 6-Month Survival Status
| Characteristics | All Patients (N = 203) | Status 6 Months after ICU
Admission |
P Value | |
|---|---|---|---|---|
| Survivors (n = 60) | Nonsurvivors (n = 143) | |||
| Male sex | 127 (63) | 33 (55) | 94 (66) | 0.15 |
| Age, yr | 51 (38–59) | 49 (33–59) | 52 (40–59) | 0.10 |
| APACHE II score | 28 (20–33) | 28 (22–33) | 28 (19–33) | 0.54 |
| SOFA score at ICU admission | 12 (8–15) | 12 (8–16) | 11 (7–15) | 0.41 |
| Body mass index, kg/m2 | 24.7 (21.7–28.2) | 24.9 (21.5–28.1) | 24.7 (22.0–28.1) | 0.64 |
| Charlson comorbidity score | 3 (2–4) | 3 (1–4) | 3 (2–4) | 0.54 |
| Recently diagnosed immunodeficiency* | 51 (25) | 26 (43) | 25 (17) | 0.0002 |
| Hematological malignancies | 62 (30) | 15 (25) | 47 (33) | 0.27 |
| AML/ALL/MDS | 15 (7) | 2 (3) | 13 (9) | 0.26 |
| NHL/Hodgkin’s/myeloma | 38 (19) | 10 (17) | 28 (20) | 0.62 |
| CML/others | 9 (4) | 3 (5) | 6 (4) | 0.48 |
| Recently diagnosed <30 d | 14 (7) | 7 (12) | 7 (5) | 0.08 |
| Allogeneic HSCT† | 14 (7) | 1 (2) | 13 (9) | 0.05 |
| Solid tumor | 39 (19) | 8 (13) | 31 (22) | 0.17 |
| Lung cancer | 18 (9) | 3 (5) | 15 (10) | 0.21 |
| Recently diagnosed (<30 d) | 14 (7) | 6 (10) | 8 (6) | 0.26 |
| Solid-organ transplant | 27 (13) | 11 (18) | 16 (11) | 0.17 |
| Lung | 13 (6) | 6 (10) | 7 (5) | 0.17 |
| Kidney | 7 (3) | 3 (5) | 4 (3) | 0.24 |
| Heart/liver | 7 (3) | 2 (3) | 5 (3) | 0.32 |
| <30 d | 6 (3) | 4 (7) | 2 (1) | 0.19 |
| AIDS | 19 (9) | 5 (8) | 14 (10) | 0.74 |
| Diagnosed at ICU admission | 4 (2) | 0 (0) | 4 (3) | 0.19 |
| AIDS opportunistic infection | 6 (3) | 2 (3) | 4 (3) | 0.57 |
| CD4 lymphocyte count, cells/mm3 | 130 (29–362) | 130 (83–226) | 109 (22–400) | 0.83 |
| Long-term CS/IS | 56 (28) | 21 (35) | 35 (24) | 0.12 |
| Recently diagnosed <30 d | 13 (6) | 9 (15) | 4 (3) | 0.001 |
| Connective tissue disease | 27 (13) | 11 (18) | 16 (11) | 0.22 |
| NSIP | 8 (4) | 0 (0) | 8 (6) | 0.09 |
| Vasculitis | 7 (3) | 4 (7) | 3 (2) | 0.21 |
| Crohn’s/ulcerative colitis | 5 (2) | 3 (5) | 2 (1) | 0.37 |
| Others | 9 (4) | 3 (5) | 6 (4) | 0.62 |
| Severe ARDS (Berlin definition) | 197 (97) | 59 (98) | 138 (97) | 0.48 |
| ARF etiologies | ||||
| Bacterial pneumonia | 63 (31) | 14 (23) | 49 (34) | 0.12 |
| Viral pneumonia‡ | 38 (19) | 14 (23) | 24 (17) | 0.27 |
| Pneumocystis jirovecii pneumonia | 9 (4) | 2 (3) | 7 (5) | 0.62 |
| Specific lung involvement§ | 26 (13) | 13 (22) | 13 (9) | 0.01 |
| Post–lung transplantation | 6 (3) | 3 (5) | 3 (2) | 0.26 |
| Aspiration pneumonia | 9 (4) | 4 (7) | 5 (3) | 0.31 |
| No definite diagnosis | 21 (10) | 6 (10) | 15 (10) | 0.91 |
| Miscellaneous | 41 (20) | 9 (15) | 32 (22) | 0.23 |
| Surgery <7 d before cannulation | 27 (13) | 11 (18) | 16 (11) | 0.17 |
Definition of abbreviations: ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; ARF = acute respiratory failure; CML = chronic myeloid leukemia; CS = corticosteroids; HSCT = hematopoietic stem-cell transplant; IS = immunosuppressant use; MDS = myelodysplastic syndrome; NHL = non-Hodgkin’s lymphoma; NSIP = nonspecific interstitial pneumonia; SOFA = Sequential Organ Failure Assessment.
Results are expressed as n (%) or median (25th–75th percentile).
Recently diagnosed immunocompromised status was defined as confirmed fewer than 30 days before ICU admission.
Two patients received both allogeneic and autogeneic HSCTs.
Eleven patients had viral and bacterial coinfections.
Tumoral lung infiltrates or pulmonary vasculitis.
Table 2.
Clinical and Biological Findings at the Time of ECMO Initiation according to 6-Month Survival Status
| Characteristics | All Patients (N = 203) | Status 6 Months after ICU
Admission |
P Value | |
|---|---|---|---|---|
| Alive (n = 60) | Dead (n = 143) | |||
| Disease severity | ||||
| RESP score* | −1 (−2 to 1) | 1 (−1 to 2) | −1 (−3 to 1) | 0.002 |
| PRESERVE score† | 7.0 (5.0 to 8.0) | 6.0 (4.5 to 8.0) | 8.0 (6.0 to 8.5) | 0.001 |
| SOFA at cannulation | 13 (9 to 16) | 13 (9 to 16) | 13 (9 to 16) | 0.83 |
| Murray score | 3.5 (3.25 to 3.75) | 3.5 (3.25 to 3.75) | 3.5 (3.25 to 3.75) | 0.90 |
| Ventilation parameters‡ | ||||
| No endotracheal MV | 8 (4) | 1 (2) | 7 (5) | 0.28 |
| FiO2, % | 100 (100 to 100) | 100 (100 to 100) | 100 (100 to 100) | 0.86 |
| Tidal volume, ml/kg IBW | 5.8 (4.8 to 6.4) | 5.9 (5.0 to 6.6) | 5.5 (4.8 to 6.3) | 0.29 |
| Respiratory rate, breaths/min | 30 (25 to 34) | 30 (24 to 32) | 30 (25 to 35) | 0.37 |
| Plateau pressure, cm H2O | 32 (30 to 35) | 31 (28 to 34) | 32 (30 to 35) | 0.11 |
| PEEP, cm H2O | 10 (8 to 13) | 10 (10 to 14) | 10 (8 to 12) | 0.33 |
| Driving pressure, cm H2O | 21.0 (17.5 to 25.0) | 20.0 (15.0 to 22.0) | 22.0 (19.0 to 25.5) | 0.01 |
| Static compliance, ml/cm H2O | 17.3 (12.5 to 22.3) | 20.3 (13.3 to 26.4) | 16.7 (12.4 to 21.0) | 0.11 |
| Pre-ECMO blood gases | ||||
| pH | 7.26 (7.18 to 7.36) | 7.28 (7.20 to 7.33) | 7.25 (7.17 to 7.37) | 0.98 |
| PaCO2, mm Hg | 57 (48 to 70) | 53 (45 to 64) | 60 (48 to 73) | 0.06 |
| HCO3−, mmol/L | 26 (22 to 31) | 24 (20 to 28) | 26 (23 to 32) | 0.007 |
| SaO2, % | 90 (82 to 94) | 89 (82 to 95) | 90 (81 to 94) | 0.96 |
| Arterial lactate, mmol/L | 2.2 (1.6 to 4.2) | 2.8 (1.5 to 5.1) | 2.2 (1.6 to 3.5) | 0.51 |
| PaO2/FiO2, mm Hg | 60 (50 to 81) | 59 (49 to 76) | 60 (51 to 83) | 0.97 |
| Adjuvant therapies | ||||
| Neuromuscular blockers | 152 (75) | 40 (67) | 112 (78) | 0.14 |
| Recruitment maneuvers | 45 (22) | 15 (25) | 30 (21) | 0.45 |
| Prone positioning | 97 (48) | 24 (40) | 73 (51) | 0.21 |
| Nitric oxide | 93 (46) | 24 (40) | 69 (48) | 0.37 |
| High-dose corticosteroids | 65 (32) | 17 (28) | 48 (34) | 0.54 |
| Vasopressors | 157 (77) | 44 (73) | 113 (79) | 0.38 |
| Renal replacement therapy | 47 (23) | 17 (29/8) | 30 (21) | 0.21 |
| Antibiotics at cannulation | 196 (97) | 58 (97) | 138 (97) | 0.95 |
| Pre-ECMO cardiac arrest | 19 (9) | 6 (10) | 13 (9) | 0.83 |
| Pre-ECMO pneumothorax | 18 (9) | 4 (7) | 14 (10) | 0.46 |
| Pre-ECMO neutropenia | 28 (14) | 5 (8) | 23 (16) | 0.14 |
| Hemoglobin, g/dl | 8.8 (7.9 to 10.0) | 9.3 (8.1 to 10.6) | 8.7 (7.6 to 10.0) | 0.04 |
| Platelet count, ×103/μl | 126 (64 to 215) | 158 (95 to 247) | 101 (56 to 211) | 0.01 |
| Fluid balance 24 h before cannulation, ml | 830 (−38 to 2,192) | 1,150 (−244 to 2,850) | 800 (70 to 1,987) | 0.45 |
| Interval, d | ||||
| Hospital admission–ECMO | 9 (4 to 16) | 5 (2 to 11) | 11 (5 to 18) | 0.003 |
| ICU admission–ECMO | 4 (1 to 10) | 2 (1 to 5) | 5 (2 to 12) | <0.0001 |
| MV–ECMO | 3 (1 to 8) | 2 (0 to 5) | 3 (1 to 10) | 0.004 |
| Main indication for ECMO | ||||
| Refractory hypoxemia | 157 (77) | 46 (77) | 111 (78) | 0.88 |
| Uncontrolled plateau pressure | 21 (10) | 8 (13) | 13 (9) | 0.36 |
| Respiratory and cardiac failure | 11 (5) | 2 (3) | 9 (6) | 0.39 |
| Uncompensated hypercapnia | 14 (7) | 4 (7) | 10 (7) | 0.93 |
| Mobile ECMO team | 89 (44) | 26 (43) | 63 (44) | 0.92 |
| Venovenous-ECMO | 191 (94) | 55 (92) | 137 (96) | 0.23 |
| Femoral–jugular | 148 (73) | 46 (77) | 102 (71) | 0.43 |
| Femoral–femoral | 33 (16) | 10 (17) | 23 (16) | 0.91 |
| Dual lumen cannula | 15 (7) | 2 (3) | 13 (9) | 0.15 |
| Venoarterial-ECMO | 9 (4) | 4 (7) | 5 (3) | 0.31 |
| Venoarteriovenous-ECMO | 2 (1) | 1 (2) | 1 (1) | 0.52 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; IBW = ideal body weight; MV = mechanical ventilation; PEEP = positive end-expiratory pressure; PRESERVE = Predicting Death for Severe ARDS on VV-ECMO; RESP = Respiratory Extracorporeal Membrane Oxygenation Survival Prediction; SOFA = Sequential Organ Failure Assessment; VV = venovenous.
Results are expressed as n (%) or median (25th–75th percentile).
Complete for 150 patients.
Complete for 135 patients.
Invasive MV dates were complete for 143 patients.
Only VV-ECMO was used for 94% of the patients (median PaO2/FiO2 ratio, 60 [50–82)]) and was started after a median of 3 (1–8) days on mechanical ventilation, with a median PEEP level of 10 (8–13) cm H2O, neuromuscular blocking agents (77%), prone positioning (48%), and inhaled nitric oxide (46%). Notably, bacterial and viral pneumonias were the two main risk factors for ARDS.
Patient Outcomes and Predictors of 6-Month Mortality
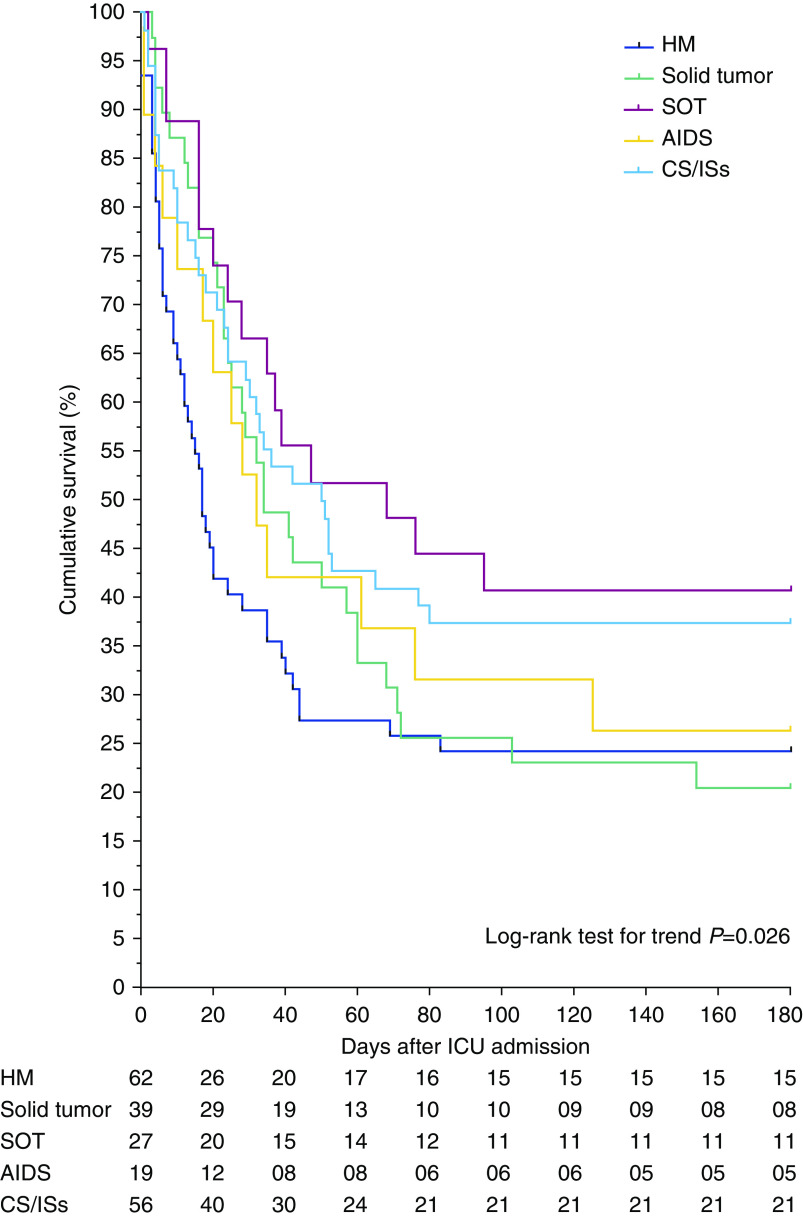
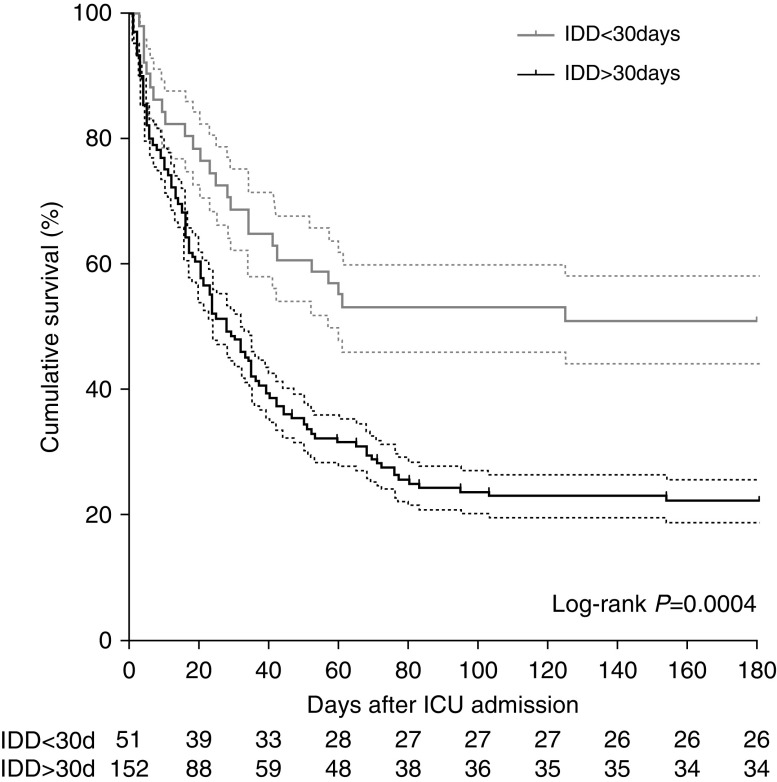
Eighty-five (42%) patients were successfully weaned off ECMO, 69 (34%) survived to ICU discharge, and 60 (30%) were alive at 6 months after discharge. The latter rate was stable throughout the study period (Figure E1). Notably, four (2%) patients were bridged to lung transplantation. Respective median ECMO-support, mechanical-ventilation, and hospital-stay durations for 6-month survivors were 8 (5–14), 17 (12–39), and 53 (41–82) days (Table 3). As shown in Figure 2, cumulative survival at 6 months after ICU admission depended on the underlying immunodeficiency etiology. Six-month survivals were 40%, 37%, 26%, 24%, and 20% in the solid-organ transplant, long-term or high-dose CS or IS, AIDS, hematological malignancies, and solid tumor groups, respectively. Interestingly, when each immunosuppression category was compared with other immunocompromised patients, patients with hematological malignancies had a significantly worse outcome (log-rank P = 0.02) (Figure E2). In addition, 6-month survival was also significantly lower for patients diagnosed as immunocompromised more than 30 days before ICU admission (Figure 3 and Table E3) (P = 0.004). Although RESP- and PRESERVE-score performances were significantly better than those of classical ICU disease-severity scores, they showed modest discrimination (c = 0.69 [95% CI, 0.6–0.76] and c = 0.65 [95% CI, 0.56–0.73], respectively; Figure E3). Multivariable analyses retained pre-ECMO recently diagnosed immunodeficiency (OR, 0.32 [95% CI, 0.16–0.66]; P = 0.002), older age, lower platelet count, higher Pco2, and higher driving pressure as being independently associated with 6-month mortality (Table 4). Another logistic-regression model, using multiple imputation methods to ascribe pre-ECMO mechanical-ventilation data with missing observations, showed the same tendencies (Table E4). Lastly, a direct relationship was observed between the 6-month mortality rate and pre-ECMO driving pressure, pre-ECMO Pco2, and platelet count quartiles (Figures E4 and E5).
Table 3.
ECMO Management and ECMO-related Complications according to 6-Month Survival Status
| Parameter | All Patients (N = 203) | Status 6 Months after ICU
Admission |
P Value | |
|---|---|---|---|---|
| Alive (n = 60) | Dead (n = 143) | |||
| Fluid balance: Day 1 on ECMO, ml | 1,552 (200 to 3,454) | 867 (−130 to 2,700) | 1,921 (380 to 3,900) | 0.04 |
| Fluid balance: Day 3 on ECMO, ml | 741 (−279 to 1,673) | 887 (−244 to 1,510) | 636 (−287 to 1,846) | 0.40 |
| SOFA: Day 3 on ECMO | 12 (8 to 15) | 11 (8 to 15) | 12 (8 to 16) | 0.80 |
| ΔSOFA Days 1–3 | 0 (−2 to 2) | −1 (−3 to 2) | 0 (−2 to 1) | 0.13 |
| Surgery on ECMO | 44 (22) | 12 (20) | 32 (22) | 0.70 |
| New cannulation/site change | 18 (9) | 4 (7) | 14 (10) | 0.47 |
| To control bleeding | 13 (6) | 3 (5) | 10 (7) | 0.59 |
| Lung transplantation | 2 (1) | 0 (0) | 2 (1) | 0.35 |
| ECMO-related | 35 (17) | 11 (18) | 24 (17) | 0.79 |
| ECMO-initiation–surgery interval, d | 3 (1 to 21) | 1 (0 to 10) | 3 (1 to 21) | 0.57 |
| Ventilatory adjuvant therapies on ECMO | ||||
| Neuromuscular blockers | 106 (52) | 27 (45) | 79 (55) | 0.18 |
| Prone positioning | 14 (7) | 6 (10) | 8 (6) | 0.26 |
| Nitric oxide/prostacyclin | 30 (15) | 5 (8) | 25 (17) | 0.09 |
| Other therapies on ECMO | ||||
| Renal replacement therapy | 107 (53) | 34 (57) | 73 (51) | 0.46 |
| Chemotherapy on ECMO | 15 (7) | 7 (12) | 8 (6) | 0.13 |
| Plasmapheresis | 10 (5) | 7 (12) | 3 (2) | 0.004 |
| Neutropenia on ECMO | 41 (20) | 13 (22) | 28 (20) | 0.73 |
| ECMO-related major bleeding | 74 (36) | 12 (20) | 62 (43) | 0.001 |
| Oronasal bleeding | 22 (11) | 3 (5) | 19 (13) | 0.08 |
| Hemothorax | 15 (7) | 1 (2) | 14 (10) | 0.04 |
| Cerebral bleeding | 13 (6) | 0 (0) | 13 (9) | 0.01 |
| Gastric ulcer | 14 (7) | 1 (2) | 13 (9) | 0.06 |
| Transfused RBC units | 8 (4 to 16) | 8 (4 to 16) | 8 (4 to 16) | 0.85 |
| Transfused platelet units | 1 (0 to 8) | 0 (0 to 7) | 2 (0 to 8) | 0.49 |
| ECMO-related infections | ||||
| Ventilator-associated pneumonia | 101 (50) | 33 (55) | 68 (48) | 0.33 |
| Cannula infection | 20 (10) | 3 (5) | 17 (12) | 0.13 |
| ECMO–cannula-infection interval, d | 14 (9 to 33) | 37 (26 to 38) | 14 (9 to 27) | 0.12 |
| Other complications on ECMO | ||||
| Major hemolysis | 29 (14) | 6 (10) | 23 (16) | 0.26 |
| Cardiac arrest | 35 (17) | 2 (3) | 33 (23) | 0.0007 |
| Pneumothorax on ECMO | 22 (11) | 3 (5) | 19 (13) | 0.07 |
| Tracheotomy | 79 (41) | 29 (48) | 50 (35) | 0.07 |
| Tracheotomy on ECMO | 49 (24) | 13 (22) | 36 (25) | 0.59 |
| Outcomes | ||||
| Number of days on vasopressor | 6 (3 to 12) | 5 (2 to 8) | 7 (4 to 16) | 0.003 |
| ECMO duration, d | 9 (5 to 25) | 8 (5 to 14) | 11 (5 to 29) | 0.14 |
| Bridge to transplantation | 4 (2) | 3 (5) | 1 (1) | 0.04 |
| Successful weaning | 85 (42) | 57 (95) | 28 (20) | <0.0001 |
| Mechanical ventilation duration, d | 20 (9 to 41) | 17 (12 to 39) | 21 (9 to 41) | 0.99 |
| ICU length of stay, d | 24 (14 to 43) | 25 (16 to 50) | 24 (12 to 42) | 0.14 |
| Hospital length of stay, d | 40 (21 to 65) | 53 (41 to 82) | 33 (16 to 54) | <0.0001 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; RBC = red blood cell; SOFA = Sequential Organ Failure Assessment.
Results are presented as n (%) or median (25th–75th percentile).
Figure 2.
Kaplan-Meier estimates during the 180 days after ICU admission, depending on patients’ underlying immunodeficiency. CS = corticosteroids; HM = hematological malignancies; IS = immunosuppressant use; SOT = solid-organ transplant.
Figure 3.
Kaplan-Meier survival estimates for immunocompromised patients with refractory acute respiratory distress syndrome on extracorporeal membrane oxygenation 180 days after ICU admission, according to the time of immunodeficiency diagnosis (IDD) (<30 or >30 d) (log-rank test; P = 0.0004). The dashed lines represent the 95% confidence interval.
Table 4.
Pre-ECMO Predictors of 6-Month Mortality of Immunocompromised Patients with ARDS Rescued by ECMO
| Variable* | OR (95% CI) | P Value |
|---|---|---|
| Recently diagnosed immunodeficiency† | 0.364 (0.148–0.899) | 0.028 |
| Platelet count | 0.996 (0.992–0.999) | 0.008 |
| Pco2 | 1.031 (1.005–1.058) | 0.019 |
| Age | 1.032 (1.002–1.062) | 0.035 |
| Driving pressure | 1.079 (1.001–1.164) | 0.047 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; CI = confidence interval; ECMO = extracorporeal membrane oxygenation; OR = odds ratio.
Obtained for 134 patients with complete data.
A recently diagnosed immunocompromised status was defined as confirmed fewer than 30 days before ICU admission.
Matched Cohort Analysis
One hundred and thirty-six immunocompromised patients with severe ARDS were compared with 94 nonimmunocompromised patients who had received ECMO for severe ARDS (PRESERVE cohort) (12). These two groups differed in many aspects (see Table E5, “before matching”). Nonimmunocompromised patients who received ECMO were younger, more frequently overweight, and had signs of more severe pulmonary failure before ECMO (lower pH, PaO2/FiO2 ratio, SaO2, and more frequent use of nitric oxide). ARDS etiology was more frequently bacterial or viral infection in nonimmunocompromised patients. Alternatively, immunocompromised patients frequently had more Pneumocystis jirovecii pneumonia and disease-specific lung involvement. The 6-month mortality rate was significantly higher for immunocompromised patients (70% versus 26%; P < 0.0001). In the 80 pairs of patients matched with identical PRESERVE scores (see Table E5, “after matching”), multivariable analysis retained immunodeficiency (OR, 5.72 [95% CI, 2.67–12.22]; P < 0.0001), older age, and higher driving pressure as being independently associated with 6-month mortality (Table E6).
Major ECMO-Related Complications
ECMO-related major hemorrhages, ventilator-associated pneumonias, and cannula infections occurred in 36%, 50%, and 10% of the patients, respectively. More than half of the patients required renal replacement therapy, whereas 7% received chemotherapy on ECMO (Table 3). Multivariable analyses retained AIDS, platelet count (≤75 × 103/μl), and a longer ICU-admission-to-ECMO-cannulation interval as pre-ECMO predictors of major bleeding (Tables E7 and E8). Notably, major ECMO-related hemorrhage was associated with higher in-ICU and 6-month mortality (84% vs. 63% without major bleeding, P = 0.002).
Discussion
To our knowledge, this report describes the largest cohort of immunocompromised patients treated with ECMO for moderate or severe ARDS (i.e., 203 patients from 7 countries over 8 yr). In this cohort, 6-month survival rates were 40%, 37%, 26%, 24%, and 20% in the solid-organ transplant, long-term or high-dose CS or IS, AIDS, hematological malignancy, and solid tumor groups, respectively. Notably, a short immunodeficiency-diagnosis-to-ICU-admission interval (<30 d) was independently associated with a better prognosis. As expected for this at-risk population, ECMO-related bleeding and nosocomial infections were frequent.
Promising hospital and 1-year survival rates, compared with the poor outcomes reported 15–20 years ago (4, 26, 27), have encouraged ICU admissions for immunocompromised patients with acute respiratory failure. Indeed, immunocompromised patients with ARDS represented 15% of the patients included in the PROSEVA (Proning Severe ARDS Patients) study (28) and 12% of the patients included in the LUNG SAFE (Large Observational Study to Understand the Global Impact of Severe ARDS) (29) study. Nonetheless, compared with the general population, their mortality remained high, especially when mechanical ventilation was necessary (4). The results of a recent subanalysis of the LUNG SAFE study highlighted the independent association of active cancer, hematological malignancy, or immunodeficiency with increased hospital mortality of patients with ARDS (14). For such patients, the relevance of treatment intensification is of major importance, because it involves ethical and medical-economic stakes. For instance, Extracorporeal Life Support Organization guidelines still consider “major pharmacological immunosuppression (absolute neutrophil count <400/mm3)” a relative contraindication, although recent VV-ECMO cohorts enrolled 19% to 31% immunocompromised patients (12, 13).
Some subgroups of critically ill immunodeficient patients on ECMO still have a despairingly low survival rate, associated with the characteristics of underlying malignancies. Herein, 6-month survival was lower for patients with hematological malignancies, AIDS, or solid tumors. Allogeneic hematopoietic stem-cell transplant (HSCT) recipients had a 20% greater risk of ICU admission than other immunocompromised patients and they had a higher fatality rate, especially when invasive ventilation was required (30). Thus, HSCT recipients should continue to be viewed as a high-risk subgroup for ICU admission that seemingly will not benefit from ECMO. As recently highlighted by Wohlfarth and colleagues, the ICU-survival and hospital-survival rate for these fragile patients on ECMO was only 19% (31), which is consistent with our findings. In addition, the poor prognoses of critically ill patients with solid cancers were previously emphasized, with probabilities of attaining 12 and 18 months of quality-adjusted survival after an ICU stay reported for only 30% and 19%, respectively (32). Moreover, maintenance of optimal antitumor treatment might often be jeopardized in these patients with severely impaired functional status or residual organ dysfunctions after ECMO weaning. On the other hand, ECMO may serve as a bridge to response in ARDS cases related to a newly diagnosed malignancy if effective antitumor therapies are available. However, the poor outcome of our ECMO patients should be interpreted in light of 1) the cause of the immunodepression and 2) the outcome of immunocompromised patients with ARDS not supported with ECMO. Indeed, in a recent study of 1,004 cancer patients with ARDS who met the Berlin definition, overall hospital survival was only 36% (31% in the severe ARDS group) (5). More recently, hospital survival for a large population of immunocompromised patients on invasive mechanical ventilation mixing hematological malignancy, solid tumor, solid-organ transplant, systemic diseases, and drug-related immunosuppression was only 44% (33). Lastly, hospital survival was 55% in 2,584 AIDS patients on mechanical ventilation for various reasons, with mechanical ventilation during an ICU stay being strongly associated with mortality according to multivariate analysis (OR, 3.5 [95% CI, 2.9–4.2]) (3).
Prediction models have been developed recently to help clinicians identify patients most likely to survive once ECMO has been initiated (34), with immunodeficiency consistently associated with poorer outcomes (12, 13, 35, 36). The external validations of the RESP and PRESERVE scores with our population showed acceptable performances (i.e., areas under the receiver operating characteristic curve of 0.70 and 0.68, respectively), which were considerably better than the “classical” ICU disease severity. However, those scores were not calculated at the same time during the ICU course (i.e., ICU admission versus the day of ECMO cannulation). Integration of the pre-ECMO platelet count and the immunodeficiency-diagnosis-to-cannulation interval might improve the RESP score’s performance for this population. Similarly, because higher driving pressure and higher Pco2 were the two pre-ECMO ventilatory parameters that were independently associated with 6-month mortality in this cohort, these variables may further improve ECMO prediction scoring systems. Indeed, the driving pressure was recently recognized as one of the most important prognosis factors for patients with ARDS either with ECMO (37) or without (12, 13, 20).
Bleeding complications and ECMO-related infections are two major threats for immunodeficient patients on ECMO (38). A low pre-ECMO platelet count was an independent predictor of mortality, with AIDS or a longer ICU stay before cannulation significantly associated with a higher risk of major bleeding. The potential platelet-reducing effect of ECMO is clinically important for this population, because ECMO patients who become thrombocytopenic may have an increased risk of bleeding (39). However, results of a recent study suggested that the severity of the critical illness and platelet counts at the time of cannulation, rather than ECMO duration, were both associated with platelet-count declines on ECMO (40). As reported herein, this point is crucial for immunocompromised patients on ECMO, because it seems to impact the risks of bleeding and 6-month mortality (Tables 4 and E6). Moreover, ECMO-related infections were frequent, with 10% and 50% of the patients, respectively, developing cannula infections and ventilator-associated pneumonia. Antibiotic prophylaxis or continuous antibiotics, which are frequently used in ECMO centers despite no evidence of their benefit (41), might be of interest for this population to limit the burden of such complications.
The significant numbers of ECMO-related complications, the human and financial costs, and the high mortality in this at-risk immunocompromised population plead for restricting ECMO use to high-volume and expert referral centers with appropriate and accurate selection of the patients who are most likely to obtain benefits over the standard therapies (42). On the basis of our results, a realistic oncological/therapeutic prognosis and adequate functional status are the first, essential features. Specifically, ECMO initiation in patients with severe ARDS who also have hematological malignancies, including HSCT, and solid tumor should be thoughtfully discussed. In addition, our finding that a shorter immunodeficiency-diagnosis-to-ECMO interval was independently associated with lower mortality might improve the selection of good candidates among this high-risk population and offer them some hope. Moreover, the duration of pre-ECMO mechanical ventilation was significantly longer for patients who died within 6 months after ICU admission (Table 2), which is consistent with previous observations of large cohorts (12, 13, 43).
Our study’s strengths include the large cohort investigated and characterized in detail, and its multicenter and longitudinal design, with a 6-month post–ICU admission follow-up. However, it also has limitations. First, data were collected in international centers with extensive experience with ECMO and caring for immunocompromised patients, which may limit the generalizability of our results. Second, data collection spanned 8 years. Over the last decade, new generations of ECMO devices have been developed (44), and a landmark randomized trial was published (9). Therefore, global ECMO management of ARDS may have changed during the study period. Third, although all participating centers applied ultraprotective ventilation in ECMO patients, we did not report daily detailed mechanical-ventilation settings, which has been highlighted as an important determinant of the outcome of patients on ECMO for respiratory failure (37, 45). Fourth, due to our study’s retrospective design, our 6-month evaluation was limited to vitals status and did not explore survivors’ health-related quality of life. Quality-adjusted life-year assessment would have been useful for this population (32), because it provides a patient-centered outcome measure that adequately captures the consequences of a disease and the effects of therapies and interventions (46). Further prospective evaluation of ECMO for immunocompromised patients with ARDS is warranted. Lastly, not having taken into account residual confounding factors associated with the origin of the immunodeficiency might have biased our results.
In conclusion, on the basis of 203 ECMO-treated immunocompromised patients with severe ARDS, analyzed retrospectively, the overall 6-month survival was only 30%. The best results were obtained with patients on long-term CS/IS or with solid-organ transplants, with respective 38% and 41% 6-month survival rates. Alternatively, ECMO initiation may be discouraged in most patients with ARDS who have hematological malignancies, including HSCT, and solid tumor. Considering the growing number of immunocompromised patients admitted to ICUs, further research should focus on decreasing the burden of infectious and bleeding complications. ECMO treatments with no invasive mechanical ventilation (47), low anticoagulation (48), and antibiotic prophylaxis warrant further investigations for this specific population. In the interim, it is worth considering that recently diagnosed immunodeficiency is associated with a much better prognosis for patients with severe ARDS who receive ECMO. However, the low 6-month survival of our large cohort of immunocompromised patients supports restricting ECMO to patients with realistic oncological/therapeutic prognoses, acceptable functional status, and few pre-ECMO mortality-risk factors.
Acknowledgments
Acknowledgment
The authors thank all the medical and nursing staff of participating centers.
The IDEA Study Group collaborators are as follows: Service de Reanimation Medicale, Centre Hospitalier Universitaire d’Angers, Angers, France: Marc Pierrot and Alain Mercat; Assistance Publique–Hôpitaux de Paris (AP-HP), Bichat Hospital, Medical and Infectious Diseases Intensive Care Unit, Paris, France: Jean Reuter, Romain Sonneville, Lila Bouadma, and Jean-Francois Timsit; Department of Intensive Care, Hôpital Erasme–Université Libre de Bruxelles, Brussels, Belgium: Alexandre Brasseur, Olivier Lheureux, Jacques Creteur, Philippe Lemaitre, Xavier Bechtold, Gerdy Debeuckelaere, and Franky Partipilo; Department of Intensive Care, Erasmus MC–University Medical Center, Rotterdam, the Netherlands: Dinis Reis Miranda, Robert van Thiel, and Corstiaan den Uil; Department of Anaesthesia and Intensive Care, General University Hospital, Prague, Czech Republic: Martin Balik, Jan Rulisek, Petr Kopecky, and Jan Kunstyr; Department of Emergency and Intensive Care, San Gerardo Hospital, Monza, Italy: Michela Bombino, Jonata Pizzagalli, Alessandra Ponti, Antonio Pesenti, and Nicolò Patroniti; Department of Pulmonary, Allergy, and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea: Hyoung Soo Kim and Sunghoon Park; Medical Intensive Care Unit, Hôpital de la Pitié–Salpêtrière, AP-HP, Paris, France: Charles-Edouard Luyt, Nicolas Brechot, Guillaume Hekimian, Sebastien Besset, Maxime Coutrot, Ania Nieszkowska, Simon Bourcier, Guillaume Lebreton, Pascal Leprince, Alain Combes, Guillaume Franchineau, and Matthieu Schmidt; AP-HP, Groupe Hospitalier Pitié–Salpêtrière–Charles Foix, Service de Pneumologie et Reanimation Medicale (Departement “R3S”), Paris, France: Julien Mayaux, Alexandre Demoule, and Helene Prodanovic; Department of Medicine I, Intensive Care Unit 13i2, Medical University of Vienna, Vienna, Austria: Andja Bojic, Nina Buchtele, Alexander Hermann, Peter Jaksch, Oliver Robak, Peter Schellongowski, Wolfgang R. Sperr, Thomas Staudinger, and Philipp Wohlfarth.
Footnotes
A complete list of members for the IDEA Study Group may be found before the beginning of the References.
Author Contributions: M.S. and A.C. contributed to the conception of the study, data collection, and data analysis and interpretation, and they drafted the manuscript; P.S., N.P., F.S.T., D.R.M., J.R., H.P., M.P., A.D., S.P., M.B., A.D., I.A.C, A.M., P.W., and R.S. contributed to data collection and interpretation and revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201708-1761OC on January 3, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the International ECMO Network (ECMONet), the REVA Research Network, and the IDEA Study Group, Marc Pierrot, Alain Mercat, Jean Reuter, Romain Sonneville, Lila Bouadma, Jean-Francois Timsit, Alexandre Brasseur, Olivier Lheureux, Jacques Creteur, Philippe Lemaitre, Xavier Bechtold, Gerdy Debeuckelaere, Franky Partipilo, Dinis Reis Miranda, Robert van Thiel, Corstiaan den Uil, Martin Balik, Jan Rulisek, Petr Kopecky, Jan Kunstyr, Michela Bombino, Jonata Pizzagalli, Alessandra Ponti, Antonio Pesenti, Nicolò Patroniti, Hyoung Soo Kim, Sunghoon Park, Charles-Edouard Luyt, Nicolas Bréchot, Guillaume Hékimian, Sébastien Besset, Maxime Coutrot, Ania Nieszkowska, Simon Bourcier, Guillaume Lebreton, Pascal Leprince, Alain Combes, Matthieu Schmidt, Julien Mayaux, Alexandre Demoule, Hélène Prodanovic, Andja Bojic, Nina Buchtele, Alexander Hermann, Peter Jaksch, Oliver Robak, Peter Schellongowski, Wolfgang R. Sperr, Thomas Staudinger, and Philipp Wohlfarth
References
- 1. Zuber B, Tran TC, Aegerter P, Grimaldi D, Charpentier J, Guidet B, et al. CUB-Réa Network. Impact of case volume on survival of septic shock in patients with malignancies. Crit Care Med. 2012;40:55–62. doi: 10.1097/CCM.0b013e31822d74ba. [DOI] [PubMed] [Google Scholar]
- 2. Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A, et al. Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med. 2012;40:43–49. doi: 10.1097/CCM.0b013e31822b50c2. [DOI] [PubMed] [Google Scholar]
- 3. Barbier F, Roux A, Canet E, Martel-Samb P, Aegerter P, Wolff M, et al. Temporal trends in critical events complicating HIV infection: 1999-2010 multicentre cohort study in France. Intensive Care Med. 2014;40:1906–1915. doi: 10.1007/s00134-014-3481-7. [DOI] [PubMed] [Google Scholar]
- 4. Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium--a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol. 2013;31:2810–2818. doi: 10.1200/JCO.2012.47.2365. [DOI] [PubMed] [Google Scholar]
- 5. Azoulay E, Lemiale V, Mokart D, Pène F, Kouatchet A, Perez P, et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. 2014;40:1106–1114. doi: 10.1007/s00134-014-3354-0. [DOI] [PubMed] [Google Scholar]
- 6. Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 7. Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 8. Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, et al. REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 9. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt M, Hodgson C, Combes A. Extracorporeal gas exchange for acute respiratory failure in adult patients: a systematic review. Crit Care. 2015;19:99. doi: 10.1186/s13054-015-0806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889–896. doi: 10.1007/s00134-016-4273-z. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 14. Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, et al. LUNG SAFE Investigators and the ESICM Trials Group. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42:1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 15. Wohlfarth P, Ullrich R, Staudinger T, Bojic A, Robak O, Hermann A, et al. Arbeitsgruppe für hämato-onkologische Intensivmedizin der Österreichischen Gesellschaft für Internistische und Allgemeine Intensivmedizin und Notfallmedizin (ÖGIAIN) Extracorporeal membrane oxygenation in adult patients with hematologic malignancies and severe acute respiratory failure. Crit Care. 2014;18:R20. doi: 10.1186/cc13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, et al. International ECMO Network (ECMONet) Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190:488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 17. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 18. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 19. Ferguson ND, Frutos-Vivar F, Esteban A, Fernández-Segoviano P, Aramburu JA, Nájera L, et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33:2228–2234. doi: 10.1097/01.ccm.0000181529.08630.49. [DOI] [PubMed] [Google Scholar]
- 20. Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 21. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 23. CONSORT Group. CONSORT statement [accessed 2017 Jan]. Available from: http://www.consort-statement.org/
- 24. Bartlett JW, Seaman SR, White IR, Carpenter JR. Alzheimer’s Disease Neuroimaging Initiative. Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat Methods Med Res. 2015;24:462–487. doi: 10.1177/0962280214521348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 26. Lloyd-Thomas AR, Dhaliwal HS, Lister TA, Hinds CJ. Intensive therapy for life-threatening medical complications of haematological malignancy. Intensive Care Med. 1986;12:317–324. doi: 10.1007/BF00261745. [DOI] [PubMed] [Google Scholar]
- 27. Tremblay LN, Hyland RH, Schouten BD, Hanly PJ. Survival of acute myelogenous leukemia patients requiring intubation/ventilatory support. Clin Invest Med. 1995;18:19–24. [PubMed] [Google Scholar]
- 28. Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 29. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 30. Lengliné E, Chevret S, Moreau AS, Pène F, Blot F, Bourhis JH, et al. Changes in intensive care for allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2015;50:840–845. doi: 10.1038/bmt.2015.55. [DOI] [PubMed] [Google Scholar]
- 31. Wohlfarth P, Beutel G, Lebiedz P, Stemmler HJ, Staudinger T, Schmidt M, et al. Intensive Care in Hematologic and Oncologic Patients (iCHOP); Caring for Critically Ill Immunocompromised Patients Multinational Network (NINE-I) Characteristics and outcome of patients after allogeneic hematopoietic stem cell transplantation treated with extracorporeal membrane oxygenation for acute respiratory distress syndrome. Crit Care Med. 2017;45:e500–e507. doi: 10.1097/CCM.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 32. Normilio-Silva K, de Figueiredo AC, Pedroso-de-Lima AC, Tunes-da-Silva G, Nunes da Silva A, Delgado Dias Levites A, et al. Long-term survival, quality of life, and quality-adjusted survival in critically ill patients with cancer. Crit Care Med. 2016;44:1327–1337. doi: 10.1097/CCM.0000000000001648. [DOI] [PubMed] [Google Scholar]
- 33. Azoulay E, Pickkers P, Soares M, Perner A, Rello J, Bauer PR, et al. Efraim investigators and the Nine-I study group. Acute hypoxemic respiratory failure in immunocompromised patients: the Efraim multinational prospective cohort study. Intensive Care Med. 2017;43:1808–1819. doi: 10.1007/s00134-017-4947-1. [DOI] [PubMed] [Google Scholar]
- 34. Rozencwajg S, Pilcher D, Combes A, Schmidt M. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care. 2016;20:392. doi: 10.1186/s13054-016-1568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Enger T, Philipp A, Videm V, Lubnow M, Wahba A, Fischer M, et al. Prediction of mortality in adult patients with severe acute lung failure receiving veno-venous extracorporeal membrane oxygenation: a prospective observational study. Crit Care. 2014;18:R67. doi: 10.1186/cc13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheng Y-TWM-Y, Wu MY, Chang YS, Huang CC, Lin PJ. Developing a simple preinterventional score to predict hospital mortality in adult venovenous extracorporeal membrane oxygenation: A pilot study. Medicine (Baltimore) 2016;95:e4380. doi: 10.1097/MD.0000000000004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Serpa Neto A, Schmidt M, Azevedo LC, Bein T, Brochard L, Beutel G, et al. ReVA Research Network and the PROVE Network Investigators. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis : Mechanical ventilation during ECMO. Intensive Care Med. 2016;42:1672–1684. doi: 10.1007/s00134-016-4507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmidt M, Brodie D, Combes A. Patients with hematologic malignancies have many reasons to die during extracorporeal membrane oxygenation. Crit Care. 2014;18:522. doi: 10.1186/s13054-014-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paden ML, Rycus PT, Thiagarajan RR. ELSO Registry. Update and outcomes in extracorporeal life support. Semin Perinatol. 2014;38:65–70. doi: 10.1053/j.semperi.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 40. Abrams D, Baldwin MR, Champion M, Agerstrand C, Eisenberger A, Bacchetta M, et al. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: a cohort study. Intensive Care Med. 2016;42:844–852. doi: 10.1007/s00134-016-4312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kao LS, Fleming GM, Escamilla RJ, Lew DF, Lally KP. Antimicrobial prophylaxis and infection surveillance in extracorporeal membrane oxygenation patients: a multi-institutional survey of practice patterns. ASAIO J. 2011;57:231–238. doi: 10.1097/MAT.0b013e31820d19ab. [DOI] [PubMed] [Google Scholar]
- 42. Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Selewski DT, Cornell TT, Lombel RM, Blatt NB, Han YY, Mottes T, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37:1166–1173. doi: 10.1007/s00134-011-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012;38:210–220. doi: 10.1007/s00134-011-2439-2. [DOI] [PubMed] [Google Scholar]
- 45. Schmidt M, Stewart C, Bailey M, Nieszkowska A, Kelly J, Murphy L, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med. 2015;43:654–664. doi: 10.1097/CCM.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 46. Prieto L, Sacristán JA. Problems and solutions in calculating quality-adjusted life years (QALYs) Health Qual Life Outcomes. 2003;1:80. doi: 10.1186/1477-7525-1-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schmidt M, Spieth PM, Zanella A. Will all ARDS patients be receiving mechanical ventilation in 2035? No. Intensive Care Med. 2017;43:570–572. doi: 10.1007/s00134-016-4487-0. [DOI] [PubMed] [Google Scholar]
- 48. Agerstrand CL, Burkart KM, Abrams DC, Bacchetta MD, Brodie D. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg. 2015;99:590–595. doi: 10.1016/j.athoracsur.2014.08.039. [DOI] [PubMed] [Google Scholar]