Abstract
Optogenetics has recently gained recognition as a biological technique to control the activity of cells using light stimulation. Many studies have applied optogenetics to cell lines in the central nervous system because it has the potential to elucidate neural circuits, treat neurological diseases and promote nerve regeneration. There have been fewer studies on the application of optogenetics in the peripheral nervous system. This review introduces the basic principles and approaches of optogenetics and summarizes the physiology and mechanism of opsins and how the technology enables bidirectional control of unique cell lines with superior spatial and temporal accuracy. Further, this review explores and discusses the therapeutic potential for the development of optogenetics and its capacity to revolutionize treatment for refractory epilepsy, depression, pain, and other nervous system disorders, with a focus on neural regeneration, especially in the peripheral nervous system. Additionally, this review synthesizes the latest preclinical research on optogenetic stimulation, including studies on non-human primates, summarizes the challenges, and highlights future perspectives. The potential of optogenetic stimulation to optimize therapy for peripheral nerve injuries (PNIs) is also highlighted. Optogenetic technology has already generated exciting, preliminary evidence, supporting its role in applications to several neurological diseases, including PNIs.
Keywords: Optogenetics, opsin, nerve regeneration, central nervous systems, peripheral nervous system
Introduction
Optogenetics is a novel biological technology that has been a useful and popular tool to better understand the nervous system. It has recently been a focus in neuroscience research due to its spatial and temporal accuracy, and it offers a way to study the brain’s behavior, physiology, cognition and action (Deisseroth 2015; Mukerjee and Lazartigues 2019). Optogenetics uses genetic variations to increase the expression of light sensitive ion channels called opsins, allowing control of cells in living tissues (Govorunova et al. 2015; Camporeze et al. 2018; Anderson and Weir 2019).
Previous research demonstrates that optogenetics can play a critical role in uncovering the functions of the central nervous system (CNS), treating epilepsy and depression, relieving pain, and enhancing nerve regeneration (Emiliani et al. 2015; Tyree and de Lecea 2017; Barnett et al. 2018; Kubiak et al. 2018; Ward et al. 2018; Liu et al. 2019; Viana Magno et al. 2019). Because of the high spatial and temporal resolution, optogenetic regulation can bidirectionally stimulate or silence specific cells and neuronal circuits within a millisecond, facilitating analysis of neural circuit operation or induction of cell pathways in different diseases (Mahmoudi et al. 2017; Wiegert et al. 2017). Several studies have shown that the optogenetic technique promotes neuronal repair and peripheral nerve growth by activating neurons, growing axons, and differentiating Schwann cells (Xiao et al. 2015; Kubiak et al. 2018; Ward et al. 2018).
Peripheral nerve injury (PNI) commonly presents with trauma, tumors and medical disorders and affects approximately 1 million people worldwide every year (Jiang, Jones, et al. 2017), including 360,000 patients in the United States alone (Walocko et al. 2016; Song et al. 2018). Unlike the CNS, the peripheral nervous system (PNS) has regenerative capacity. However, peripheral nerve regeneration is often incomplete, especially in cases of neurotmesis with large gaps. Novel engineered nerve scaffolds, growth factors, and electrical stimulation (ES) have also been reported to improve the outcomes after peripheral nerve injures (Johnson et al. 2015; Du, Chen, et al. 2018; Du, Zhen, et al. 2018; Li R et al. 2018). However, patients are often unsatisfied with sensory and functional recovery despite the treatment’s significant economic burden on society: annually, approximately $150 billion is spent on PNI treatments in the United States (Qing et al. 2018).
Optogenetics has been widely researched and has spawned breakthroughs in the fields of behavior, physiology, pathology, cognition, and action. Most of these studies have focused on rodents, and no clinical applications have been reported, but the recent studies in non-human primates (NHP) are promising (Ebina et al. 2019; Williams et al. 2019). This review introduces the basic principles and approaches of optogenetic stimulation and summarizes the existing knowledge of optogenetic stimulation and its translational potential. Although optogenetic stimulation has been applied in neuroscientific studies, information regarding optogenetic stimulation in nerve regeneration is limited. Prior reviews focus on the development of optogenetic stimulation technology and its application to neural circuits and specific neurological diseases (such as depression, epilepsy and Parkinson's disease). This paper synthesizes the latest applications to neural regeneration in the nervous system. It incorporates the latest advances in neuroscience, analyzing current research in optogenetic applications to the CNS as well as the PNS, and focuses specifically on optogenetics’ role in nerve regeneration in the PNS (Figure 1). The recent development of optogenetic stimulation techniques in peripheral nerves hints at revolutionary therapeutic potential. The promotion of axonal growth and secretion of neurotrophic factors are possible approaches that are reviewed to offer novel options for treatment of PNIs.
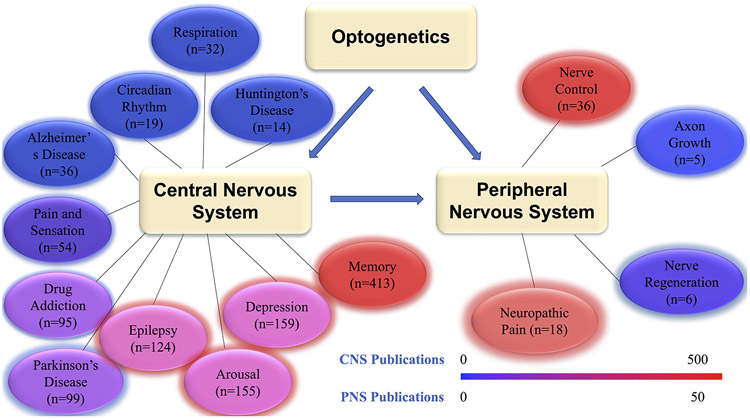
Figure 1:
A visual representation of the distribution of different optogenetic applications in the nervous system. n=number of publications. Filter: Since 2015, search term = optogenetics and “***term***”.
Opsin family
Optogenetics regulates cellular activation using opsins, which were first identified in 1971 by Oesterhelt and Stoeckenius in the form of halorhodopsin (Oesterhelt and Stoeckenius 1971). Since then, various opsins have been discovered, including natural forms and engineered variants. They are widely distributed in algae, fungi, prokaryotes and eukaryotes (Gupta et al. 2019; Kuhne et al. 2019). Opsins are classified into two distinct families: microbial opsins (type I rhodopsin, ion transport proteins) and animal opsins (type II rhodopsin, G protein-coupled receptors and melanopsin) (Deisseroth 2015; Duebel et al. 2015; Govorunova et al. 2015; Mahmoudi et al. 2017; Rost et al. 2017). Microbial opsins are the primary tools to regulate cellular activities, but animal opsins can be used as optogenetic tools to control neuronal excitability (Esquiva et al. 2016; de Assis et al. 2018).
Microbial opsins can be divided into two types depending on their cellular effect: depolarizing microbial opsins (channelrhodopsins) and hyperpolarizing microbial opsins (bacterorhodopsins and halorhodopsins) (Zhang et al. 2014; Deisseroth 2015; Dolgikh et al. 2015; Govorunova et al. 2015; Mukerjee and Lazartigues 2019). Channelrhodopsin 2 (ChR2) is the most widely used tool for cellular activation and is stimulated by exposure to blue light. Halorhodopsin (NpHR) is used for cellular inhibition and is stimulated by exposure to yellow light. Based on variants in the light spectrum, different opsins can be co-expressed in the same cell to induce or silence cell activities.
Microbial opsins are also limited by light intensity and sensitivity. Variant wavelengths of light penetrate and scatter differently in tissue. Additionally, only high-intensity light can meet the requirements of optogenetic stimulation. This leads to the accumulation of heat and potentially, neuronal injury (Mahmoudi et al. 2017). In the past several years, a new family of microbial opsins has quickly advanced through engineered alterations to compensate for these limitations (Govorunova et al. 2015; Kim SS 2016; Muhlhauser et al. 2017; Pathak et al. 2017; Li G et al. 2018). Kim et al. adapted the wavelength activation peak for ChR2 from blue to red (Kim CK et al. 2017). With less absorption and scattering, red light stimulated a deeper region of the tissue and increased spectral width. Opsins have also been engineered to alter the function and increase sensitivity to light. For example, ChRs generally depolarize the cell under the influence of weak blue light. A protein modification that chimerizes a ChR1 and ChR2 creates a protein called iC1C2 that is 200 times more sensitive to blue light and hyperpolarizes the cell (Berndt et al. 2014). Another modification addressing the limitations of microbial opsins is the step-function opsin (SFO), which is a modified ChR that reduces the damage caused by consecutive high-power laser pulses (Igarashi et al. 2018). It can activate the cell with exposure to blue light and maintain depolarization up to several minutes without light. This method allows discontinuous light to activate the cell. SFO can also be immediately deactivated by yellow light if necessary.
Physiology of opsins
Opsins exposed to light regulate cell activities by ion transport through the cell membrane. Opsin is composed of retinal and a seven-transmembrane, light-sensitive protein (Duebel et al. 2015). Generally, 11-cis-retinal is covalently bound to the light-sensitive protein and the ion channel in the trans-membrane protein is closed. Photons in the correct wavelength can transform 11-cis-retinal to all-trans-retinal and open the ion channel. When light is discontinued, all-trans-retinal returns to 11-cis retinal and the ion channel closes (Josselyn 2018; Kato et al. 2018).
When depolarizing ChRs that are sensitive to blue light are stimulated, retinal undergoes a conformational change and the ChR-related ion channel opens. This channel is a nonspecific cation channel that allows various cations (K+, Na+, H+) to go into the cell, resulting in depolarization (Mukerjee and Lazartigues 2019). Hyperpolarizing microbial opsins include bacteriorhodopsins and halorhodopsins, both of which can inhibit the activation of a specific cell depending on the path of light through different ion pumps. Bacteriorhodopsins transport hydrogen ions to the outside of the cell when stimulated, resulting in hyperpolarization. Halorhodopsins allow chloride to go into the cell when exposed to yellow light (Deisseroth 2015; Mukerjee and Lazartigues 2019). Variable ion conductance levels and wavelength activation peaks of opsins have been engineered to facilitate future optogenetic experiments (Maimon, Sparks, et al. 2018; Hare et al. 2019; Kovalev et al. 2019; Mederos et al. 2019; Nguyen et al. 2019).
Optogenetic stimulation
In recent years, optogenetics has been regarded as an ideal tool in neuroscience. Effective optogenetic stimulation consists of light that is sufficiently intense to reach the designated location and provide energy to activate the expression of opsin, which uses the energy from the light to trigger a cellular signaling cascade that modulates cell activity and cell fate (Deisseroth 2015). The expression of opsin requires genetically transduced opsin genes in cell genomes to be expressed. Transduction occurs via three main approaches: transgenesis, viral transduction, and electroporation (Barnett et al. 2018). The selection of the method depends on the tissue and design of the experiment. Currently, viral transduction is a widely used and effective method for optogenetic stimulation in preclinical studies and is regarded as a potential approach for future clinical application (Deisseroth 2015; Barnett et al. 2018).
The device and system of light delivery are complex. In general, the most popular light source is laser or light-emitting diodes (LEDs), which require complex resistance and power transmission components, and are often too large or heavy. The typical device of light delivery contains external light sources, controller equipment, optical fibers, and probes. However, the physical tethers unavoidably restrict the activity of animals and affect their natural behavior, which affects application and repeatability of the experiments (Gutruf and Rogers 2018). To overcome these limitations, the devices have become wireless modification of light stimulation and the technology of miniaturization has evolved rapidly. Wireless stimulation implemented with miniaturized circuitry and specialized antenna designs allow researchers to observe behavior, physiology and pathology of animals in their natural condition (Gutruf and Rogers 2018). Moreover, new schemes using magnetic resonant coupling as an alternative will solve the shortcomings of attenuation and directivity of electromagnetic waves in wireless stimulation (Khan et al. 2019). To improve the spatial accuracy of optogenetic stimulation, microscale light-emitting diode (μLED) arrays and tapered optical fibers that reduce injury and imprecise positioning of the sources have been widely applied in recent years (Ji et al. 2018; Pisanello et al. 2018; Sileo et al. 2018). Understanding the causal relationship between neural activity and behavior has been a persistent scientific problem. Closed-loop functional optogenetic stimulation is an avenue for scientists to begin to understand this relationship. Closed-loop functional optogenetic stimulation can dynamically control neuronal activity patterns in awake animals and demonstrate the function of neural circuits. It can also induce specific behaviors, such as control of the joint angle, feeding, and bladder activity (Srinivasan et al. 2018; Zhang Z et al. 2018; Mickle et al. 2019). In summary, wireless and microscale light-emitting diode (μLED) arrays and tapered optical fibers may have translational potential in optogenetic stimulation. Future development of new devices that cause less damage, are easier to install and are lighter would greatly promote the translational value of these techniques.
The application of optogenetics in the CNS
Thousands of studies about optogenetic stimulation in the CNS have been published in recent years. Optogenetic stimulation, which has significant temporal and spatial accuracy, has greatly revolutionized neuroscience, especially the understanding of special zone neurons in neural circuits (Forcelli 2017; Juarez et al. 2019). Optogenetics has generated a more comprehensive understanding of the brain’s role in behavior, physiology, pathology, cognition, action and neurological disease. Most studies on the application of optogenetic technology related to disorders of the CNS have focused on the excitation or inhibition of a specific population of neural cells involved in the pathophysiology of a disease. For example, several in vivo studies have produced evidence suggesting a potentially therapeutic role of optogenetics in the treatment of refractory epilepsy (Chang WJ et al. 2017; Berglind et al. 2018; Chang M et al. 2018; Magloire et al. 2018; Tung et al. 2018; Zhang H et al. 2018). Procedures thus far have predominately used two microbial opsins or similar variants: ChR2 and Natronomonas pharaonic (NpHR). Epilepsy subtypes, brain regions, and neuronal cell lines chosen for studies have been diverse, but findings generally indicate that optogenetic application to ictal implicated brain areas reduces epileptiform activity during light exposure by inhibiting burst neuron firing. (Chang WJ et al. 2017; Chang M et al. 2018; Tung et al. 2018; Zhang H et al. 2018). Furthermore, animal studies have examined the application of optogenetics to address depression symptoms. Studies using excitatory opsins in the prefrontal cortex and mesolimbic reward system have had mixed evidence, but the area of research is growing (Covington et al. 2010; Warden et al. 2012; Kumar et al. 2013). Further research into specific cell subtypes and neuronal circuits could lead to a groundbreaking treatment plan for patients suffering from depression.
Optogenetic methods have been used to understand the potency and function of cells involved in sleeping (Goldstein et al. 2018), memory (Barnett et al. 2018), movement regulation (Tyree and de Lecea 2017), and behavioral pathways (Adhikari et al. 2015). Optogenetics studies have also identified the pathophysiological function of cells in diseases or tumors (Venkatesh et al. 2015; Camporeze et al. 2018; Watanabe et al. 2018; Prsa et al. 2019) and offer novel strategies for treatment. Prsa et al. showed that optogenetic inhibition of the rat entopeduncular nucleus increases thalamic neuronal activity and improves parkinsonian motor behavior, which provides a basic method for therapy (Prsa et al. 2019). The medial prefrontal cortex (mPFC) plays an important role in pain processing (Huang S et al. 2018). Lee et al. reported that optogenetic activation of the mPFC produces strong antinociceptive effects in a rat model of persistent neuropathic pain and reduces the affective symptoms of pain (Lee GH and Kim 2016). Another study showed that the optogenetic inhibition of GABAergic interneurons in mPFC reduces pain responses, which could be regarded as a potential target for treatment (Melchior et al. 2015). In addition, Venkatesh et al. found that the optogenetic activation of neurons can promote secretion of the synaptic protein neuroligin-3, which can induce PI3K-mTOR pathway activity and proliferation of glioma cells (Venkatesh et al. 2015). As a result, the activated neurons contributed to cancer growth. This study could generate a new indication for cancer treatment. Table 1 summarizes studies about optogenetics in the CNS. Such applications are clearly robust and growing, but there has been little research into the application of optogenetics in the CNS for the purpose of neural regeneration.
Table 1:
The application of Optogenetics in CNS
| Classification | Application | Reference |
|---|---|---|
| Physiology | Arousal | Kirszenblat L et al. (Kirszenblat et al. 2019) Gerashchenko et al. (Gerashchenko et al. 2018) Cisse Y et al. (Cisse et al. 2018) |
| Memory | de Sousa AF et al. (de Sousa et al. 2019) Oishi N et al. (Oishi et al. 2019) Meira T et al. (Meira et al. 2018) |
|
| Sensor and pain | Liu S et al. (Liu et al. 2019) Tashima R et al. (Tashima et al. 2018) DeBerry JJ et al. (DeBerry et al. 2018) |
|
| Respiration | Burke PG et al. (Burke et al. 2015) Basting TM et al. (Basting et al. 2015) |
|
| Circadian Rhythm | Todd WD et al. (Todd et al. 2018) | |
| Pathology | Huntington’s Disease | Perez-Rosello T et al. (Perez-Rosello et al. 2019) Barry J et al. (Barry et al. 2018) |
| Parkinsonian | Lee EJ et al. (Lee EJ et al. 2018) Moon HC et al. (Moon et al. 2018) |
|
| Alzheimer’s Disease | Perusini JN et al. (Perusini et al. 2017) | |
| Epilepsy | Magloire V et al. (Magloire et al. 2018) Berglind F et al. (Berglind et al. 2018) Chang WJ et al. (Chang WJ et al. 2017) |
|
| Drug Addiction | de Guglielmo G et al. (de Guglielmo et al. 2019) Gioia DA et al. (Gioia et al. 2018) |
|
| Depression | Covington, H.E., et al. (Covington et al. 2010) Kumar, S., et al. (Kumar et al. 2013) Warden, M.R. et al. (Warden et al. 2012) |
|
| Stroke | Shah A.M et al. (Shah et al. 2017) Wahl A.S et al. (Wahl et al. 2017) Cheng M.Y et al. (Cheng et al. 2014) |
|
| Spinal Cord Injury | Alilain W.J et al. (Alilain et al. 2008) Awad Basem I et al. (Awad et al. 2013) |
The application of optogenetics in spinal cord injury (SCI)
Optogenetics has recently gained recognition in SCI research as an emerging technology. One application is in the C2 hemisection model, in which ChR2 can be expressed in interneurons and motor neurons at the C3-C6 level of the spinal cord by sindbis virus transfection (Alilain et al. 2008). Further, optogenetic stimulation enhances the communication of denervated phrenic motor neurons to rostral ventral respiratory groups through sub-threshold influence of spared, crossed pathways and restores breathing activity in SCI rats (Alilain et al. 2008). Similarly, Basem et. al show that the spinal cord can express ChR2 by virus transfection. Lastly, it was reported that intermittent light stimulation (0.5Hz, 1s on, 1s off) can restore the function of the bladder in rats that have undergone SCI (Awad et al. 2013). These outcomes posit that optogenetics can be a useful strategy to treat SCI, and they provide direction in applications to other diseases.
Optogenetic stimulation relieves neuropathic pain
Neuropathic pain is a common, debilitating disease. Recently, studies have reported that optogenetics can be used to relieve neuropathic pain (Zhang et al. 2014). Uhlski ML et al. showed that the function of afferent fibers can be determined in transgenic mice that express light-sensitive proteins. In the experiment, C-fiber nociceptors expressed ChR2 that responded to heat, mechanical, and light stimulation (Uhelski et al. 2017). Similarly, Tashima et al. found that non-nociceptive Aβ Fibers expressing ChR2 induce a pain reaction when illuminated by a blue light in rats (Tashima et al. 2018). Optogenetics not only promotes the understanding of neuropathic pain, but also directly relieves it. For example, transgenically modified primary afferent nerves of mice express inhibitory archaerhodopsin-3 (Arch) proton pumps; yellow light stimulation inhibits the afferent nerve, blocking the action of the dorsal root ganglion (DRG) and relieving the neuropathic pain (Daou et al. 2016). Further, Li et al. showed that a 532-nm green laser light can inhibit the activity of the DRG that expresses virally constructed Arch T, relieving neuropathic pain (Li et al. 2015). These outcomes suggest that optogenetics can play an important role in neuropathic pain. Identifying associated afferent fibers may be an effective clinical strategy to relieve neuropathic pain.
Optogenetic stimulation promotes functional recovery in CNS
In addition to neural circuit research, many studies have confirmed that optogenetic manipulation can promote nerve regeneration (Pendharkar et al. 2016; Ortiz et al. 2019). The focus of neuron regeneration in the CNS is for stroke treatment (Pendharkar et al. 2016; Wahl et al. 2017). Cheng et al. revealed that selectively activating ipsilesional primary motor cortex neurons with optogenetics can promote functional and sensory recovery in a mouse model of transient middle cerebral artery occlusion (Cheng et al. 2014). Biochemically, the level of brain-derived neuro-trophic factor (BDNF), nerve growth factor (NGF), neurotrophin-3 (NTF3) and GAP-43 plasticity marker significantly increases with neuronal activation. The data provides proof of concept that optogenetic neuronal stimulation can enhance recovery after stroke (Cheng et al. 2014). Similarly, the optogenetic inhibition of striatal GABAergic neuronal activity improves outcomes after 60-minute transient middle cerebral artery occlusion of mice. In this process, inhibiting striatal GABAergic neuronal activity increases microvessel density and basic fibroblast growth factor (bFGF) expression. Further experiments demonstrated that the photoinhibition of GABAergic neuronal activity could upregulate bFGF expression in endothelial cells, depending on the presence of astrocytes (Jiang, Li, et al. 2017). Interestingly, recent research showed that optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery for 2 weeks after 30 minutes of middle cerebral artery occlusion (Shah et al. 2017). This discovery provides a basis for clinical application.
Functional circuit formation caused by the application of optogenetic stimulation could promote functional recovery after brain injury caused by stroke (Jin et al. 2015; Tennant et al. 2017; Wahl et al. 2017). Optogenetically activated corticospinal neurons promote sprouting from the intact nerve to the denervated cervical hemi-cord in the photothrombotic stroke mice. Correspondingly, the inhibition of corticospinal neurons causes the loss of the restored grasping function. These results provide the anatomical location of functional reestablishment and a method for clinical therapy (Wahl et al. 2017). Another study observed that optogenetic stimulation in thalamocortical neurons promoted the formation of new and stable thalamocortical synaptic boutons and enhanced the recovery of sensory function in a photothrombotic stroke mice (Tennant et al. 2017). In this study, rewired thalamocortical circuits and restored function have been induced by optogenetic stimulation (Tennant et al. 2017).
As is noted previously, optogenetic stimulation promotes nerve regeneration by regulating the special neurons and rewiring the new neural circuit. When exposed to light, the secretion of various factors and changes in cell activity in the neuron are the main mechanisms of nerve regeneration. Lanshakov et al. showed that optogenetic activation of the hippocampus in rats, which expressed ChR2 by virus infection, significantly increased the level of antiapoptotic protein Bcl-xL in neurons and protected neurons and surrounding cells (Lanshakov et al. 2017). Optogenetic inhibition of GABAergic neurons could improve the therapy of transplanted neural stem cells after stroke. In vitro, optogenetic excitation of GABAergic neurons increased apoptosis and decreased the migration of stem cells by promoting the secretion of glial cell line-derived neurotrophic factors and nerve growth factors (Lu et al. 2017). Recently, Ping Yu S et al. showed that optogenetic stimulation of transplanted induced pluripotent stem cell-derived neural progenitor cells (iPS-NPCs) can improve recovery after ischemic stroke (Ping Yu et al. 2019). In vitro, researchers modified iPS-NPCs expressing light-sensitive protein VChR1 by virus infection and observed that light stimulation promotes neurite growth and synaptic protein upregulation. Meanwhile, transplanting iPS-NPCs into the ischemic cortex can enhance synaptic transmission, neuroplasticity and functional recovery in stroke mice with light stimulation. These outcomes give us a direction for future therapy. The role of optogenetic stimulation in central nerve regeneration is being studied, but remains largely unelucidated. Further studies about the mechanism of optogenetic stimulation’s role in the promotion of neuron regeneration are necessary.
Ortiz et al. reveals that optogenetic stimulation can enhance myelin repair in a model of multiple sclerosis (Ortiz et al. 2019). Optogenetic stimulation with a 3-hour protocol [30 seconds delivered at 20Hz (10 ms on/40 ms off pulses) and 4.5 minutes of rest] 7 to 13 days after injection can increase the activation of axons and enhance the differentiation and maturation of oligodendrocyte precursor cells (Ortiz et al. 2019). This change promotes myelin repair and functional recovery of neurons in repeated optogenetic stimulation 7 to 13 days after injection. If repeated with no optogenetic stimulation, these changes are absent. The activity of neurons induced by optogenetic stimulation enhances axon-oligodendroglia interaction, but the mechanism of this interaction remains unclear.
The application of optogenetics in the PNS
Unlike the burgeoning research field of optogenetic applications to the CNS, research into the application of optogenetics in the PNS is limited. Reports have shown that optogenetic applications to the PNS may promote axonal growth and nerve regeneration (Park et al. 2015; Ward et al. 2016; Ward et al. 2018). However, more research about the function of Schwann cells, macrophages and other cells affected by optogenetic stimulation that promote PNS regeneration should be conducted. In addition, signaling pathways that promote axon growth and secretion of growth factors in activated neurons by optogenetic stimulation remain unknown. Studies thus far are summarized in Table 2.
Table 2:
The application of Optogenetics in PNS
| Application | Parameter | Opsins | Reference | Function |
|---|---|---|---|---|
| Axon Growth | 20Hz, 5ms pulse, 1s stimulation/1s rest,1h | ChR2 | Park S et al. (Park et al. 2015) | Optogenetic stimulation can promote axon growth and neurotrophic factors (NGF, BDNF) release in dorsal root ganglia |
| N/A | blue-light activatable adenylyl cyclase | Xiao Y et al. (Xiao et al. 2015) | Promotes axon growth | |
| 5 s / 5 min | the light-dependent activation of a guidance receptor, Deleted in Colorectal Cancer (DCC) | Endo M et al. (Endo et al. 2016) | Controls direction of neurite outgrowth | |
| Peripheral Nerve Control | 50Hz, 5ms pulse N/A 20mW, 5ms , 1Hz |
ChR2 | Kang-II Song et al. (Kang-Il et al. 2015; Song et al. 2018) Michoud F et al. (Michoud et al. 2018) Towne C et al. (Towne et al. 2013) |
Peripheral nerve control |
| 20 Hz, 2 ms pulse (ChR2) 17 Hz, 50 μs pulse (Jaws) |
ChR2 Jaws |
Maimin BE et al. (Maimon, Sparks, et al. 2018) | Two-colour optogentic stimulation in PNS | |
| Peripheral Nerve Regeneration | 175 W/mm, 10s on/ 50s off, 24h | ChR2 | Sun L et al. (Sun et al. 2014) | Promotes neuronal regeneration via calcium release |
| 20 Hz,3 ms and 5ms pulse, continuous, 1h | ChR2 | Ward P J et al. (Ward et al. 2018) | Promotes motor and sensor neuron regeneration | |
| 20 Hz,1 ms pulse, continuous, 1h | ChR2 | Ward P J et al. (Ward et al. 2016) | Functional motor axon regeneration | |
| 10Hz, 1ms pulse, 0.5s on/1.5s off,1h | ChR2 | Lee SY et al. (Lee SY et al. 2019) | Control differentiation of induced pluripotent stem cell-derived neuron | |
| 20Hz, 5ms pulse, 1s stimulation/1s rest,1h | ChR2 | Jung K et al. (Jung et al. 2019) | Promotes Schwann cell proliferation, differentiation, and myelination | |
| Neuropathic Pain | 0.3-0.45 mW/mm2,3s on/1s off N/A |
Archaerhodopsin-3 | Daou I et al. (Daou et al. 2016) Li B et al. (Li et al. 2015) |
Relieves neuropathic pain |
| N/A 12mW, 500ms pulse, 10s on/10s off |
ChR2 | Uhelski M L et al. (Uhelski et al. 2017) Tashima R et al. (Tashima et al. 2018) |
Determines the functional properties of cutaneous nociceptors reveal the role of non-nociceptive Aβ fibers in sensor and pain |
Optogenetics and the neuron in peripheral nerve regeneration
Peripheral nerve regeneration after injury is a complex process and includes the activation of neurons, Wallerian degeneration, inflammatory response, revascularization, axonal growth and myelination in different phases (Cattin and Lloyd 2016). Whereas ES stimulates all types of cells (neurons, Schwann cells and other cells), optogenetics can selectively stimulate special cells in different phases with different kinds of light (Gordon 2016). Park et al. first confirmed that optogenetic stimulation of the neuron can increase neurite outgrowth (Park et al. 2015). The researchers in this study isolated dorsal root ganglion cells expressing ChR2 from P0 neonatal transgenic Thy1-ChR2-YFP mice. When exposed to blue light, these dorsal root ganglia cells enhanced neurite outgrowth three-fold compared to unstimulated or wild-type (WT) controls. Furthermore, the same results were observed in WT dorsal root ganglia cells when co-culturing with ChR2-expressing dorsal root ganglia cells (Park et al. 2015). Further research confirmed that neurite growth caused by optogenetic stimulation is accompanied by an increased expression of NGFs and BDNFs. The parameters of light stimulation for maximum neurite outgrowth were determined to be 1 h of 20Hz, 5ms pulse, 1s of stimulation followed by 1s of rest (Park et al. 2015). Similar outcomes can be found in the article published by Xiao and his colleagues (Xiao et al. 2015). In this study, neurons expressing ChR2 that combined with the second messenger cyclic adenosine monophosphate (cAMP) activated more axon regrowth than neurons that do not express ChR2. Ward et al. first showed that 20Hz of 1ms pulses continuously for 1h to optically-induced neuronal activity can promote functional motor regeneration in vivo (Ward et al. 2016). Many more ChR2+ axons successfully re-innervated the gastrocnemius muscle in thy-1-ChR2/YFP transgenic mice that received optical treatment compared to the non-treatment group. Additionally, the number of Synaptic Vesicle Protein 2 (SVP2) endplates was greater in mice that received optical treatment, which led to more reinnervation of the gastrocnemius muscles. In genetic mouse models of sciatic nerve transection and repair, both sensory and motor axons selectively activated by light stimulation had regenerated successfully (Ward et al. 2018).
Further data showed that optogenetic stimulation induced axon growth, which was demonstrated by the activation of the neuronal cell and secretion of NGFs and BDNFs. Park et al. shows that optogenetic stimulation promotes the secretion of related growth factors that contribute to neurite growth (Park et al. 2015). However, the intracellular signaling pathways involved have not been reported. Prior optogenetic research revealed that calcium signaling and cAMP are essential for activation of the neuron and neurite growth (Sun et al. 2014; Xiao et al. 2015; Endo et al. 2016), possibly indicating a connection between the two (Huang CY et al. 2017). More research about this mechanism investigating the role of optogenetic stimulation to promote secretion of related growth factors is needed.
Optogenetics and Schwann cells
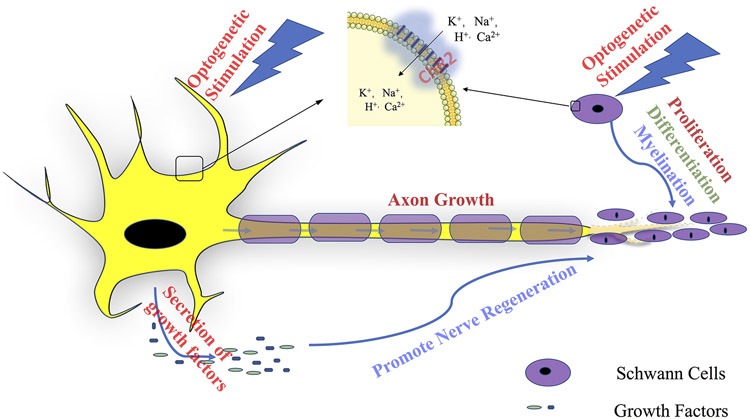
Schwann cells (SCs) are crucial elements of the PNS and play an important role in peripheral nerve regeneration. NGFs and BDNFs induced by activation of neurons by optical stimulation have a positive effect on the neuronal cell body and SCs (Willand et al. 2016). Recent research showed that optogenetic stimulation with an intensity of 5 mW/mm2 at 20 Hz for 1 h, 1s stimulation/1s rest with a constant pulse width of 5 ms LED irradiator can promote SC proliferation, differentiation, and myelination in vitro (Jung et al. 2019). In a transfected SCs monoculture, the number of BrdU+-S100ß+-SCs and the expression of both Krox20 and myelin, which indicates the differentiation and myelination of SCs, increases with light stimulation. The authors observed that the intracellular Ca+ level increased in optogenetic induced SCs. By blocking inositol 1,4,5-trisphosphate(IP3) formation and ryanodine receptors in the experiment, Jung et al. confirmed that optical stimulation induces an elevated intracellular Ca+ level by promoting flow through the T-type voltage-gated calcium channel (VGCC) and mobilizing Ca2+ from both inositol 1,4,5-trisphosphate(IP3)-sensitive and caffeine/ryanodine-sensitive stores (Jung et al. 2019). Furthermore, optogenetic stimulation promotes differentiation of SCs in SC-motor neuron co-culture (Jung et al. 2019). These in vitro study results explain the effect of optogenetic stimulation on SCs and point out the direction for future in vivo studies (Figure 2).
Figure 2:
Optogenetic stimulation promotes nerve regeneration in the peripheral nervous system.
Current Challenges and future perspective
Despite great potential, optogenetics has limitations and challenges that need to be considered and addressed before further, broader development. Within the brain, light delivery is usually achieved by inserting an optic fiber into a specific area through surgery. Invasive operations inevitably may cause brain damage and illicit side effects, infections, and bleeding.
Although transdermal illumination has been shown to be effective in the optical stimulation of peripheral nerve endings and muscles, the weak tissue penetration of light limits the use of this method (Bruegmann et al. 2015; Maimon et al. 2017). In order to solve this problem, new methods have emerged in recent years. Tapered optical fiber reduces damage and allows the light to reach a specific zone (Pisanello et al. 2018; Sileo et al. 2018). The development of epidural flexible uLED arrays, which cause less harm and illuminate more effectively, also provides an alternative solution (Ji et al. 2018). In addition, the combination of light stimulation and microelectrode arrays that detect the change of cell signals and closed-loop systems have greatly promoted the development of knowledge in neuroscience (Ji et al. 2018; Srinivasan et al. 2018; Zhang Z et al. 2018).
The physical tethers, cable connection of the power supply and heavy weight of light illumination are other important challenges. The limitations of these technologies restrict the activity of animals and affect their natural behavior, which affects application and repeatability in neuroscience experiments. Recent development of wireless technology and miniaturization of the equipment have provided alternative solutions (Gutruf and Rogers 2018). Moreover, new schemes using magnetic resonant coupling as an alternative will provide a steadier and more effective optogenetic stimulus (Mirbozorgi et al. 2016; Khan et al. 2019).
Heat accumulation from light illumination also elicits concern. Temperature increase will cause cell damage and other side effects. Recently, it was demonstrated that thermal energy from optogenetic stimulation can inhibit the activity of neurons and confound the outcomes of optogenetic experiments (Owen et al. 2019). Commonly, the temperature from light illumination increased by 0.2-2 °C. New technological advances may minimize this effect.
The expression of opsin is the key factor of optogenetic stimulation. It involves opsin selection, opsin gene transduction, and expression persistence. ChR2 is the most commonly used opsin for cells and tissues in optogenetic stimulation because of its superior light sensitivity and fast activation and relaxation kinetics (Mukerjee and Lazartigues 2019). At the same time, the application of ChR2, which is activated by low penetration blue light, faces challenges. In the past several years, a variety of new or modified microbial opsins have been developed to achieve better kinetics, light sensitivity, and trafficking efficiency (Govorunova et al. 2015; Muhlhauser et al. 2017; Pathak et al. 2017). For example, Klapoetke et al. discovered a new opsin (Chronos) with super-fast on and off kinetics from Stigeoclonium helveticum (Klapoetke et al. 2014). Kim et al. adapted the wavelength activation peak for ChR2 from blue to red (Kim CK et al. 2017).
Opsin gene transduction is the basis of opsin expression. Transgene and virus transduction are the most commonly used methods of gene expression and have their own advantages and disadvantages. Transgene can allow more efficient gene expression, but control of transgene expression requires a sophisticated technology and cannot be applied to humans (Montgomery et al. 2016). Currently, virus transduction is a widely used and effective method in optogenetic stimulation. However, many challenges remain and limit the application of this technology. Adeno-associated virus (AAV) is regarded as an ideal viral vector and can be applied in gene therapy for many diseases (Donadon et al. 2019; Gardner et al. 2019; Leng et al. 2019). However, AAV only has 4 kilobases (kb), which limits the potential to insert genes and promoters. Lentivirus (LV) has a greater genome capacity (8-10 kb) to insert the genes and promoters, but with a relatively large particle size (100 nm), which restricts its diffusion through neural tissue (Barnett et al. 2018). Furthermore, AAV serotypes and LV glycoproteins also influence transduction efficiency, tropism and axonal transport in different tissues (Aschauer et al. 2013; Parr-Brownlie et al. 2015). In particular, overexpression of virus may induce a greater immune response that causes cell sickness or death. Meanwhile, opsins in the neurons themselves may produce the potential immune response and cause the death of neurons (Maimon, Diaz, et al. 2018).
Stable, extended expression of opsin is very important for optogenetic applications in chronic diseases and congenital diseases. Although it has been reported that ChR2 can still be detected by AAV2 transduction at 64 weeks after AAV injection in rat retinal ganglion cells, there is growing evidence that opsin expression can decrease within a few weeks, especially outside of the CNS and in nonhuman primates (Sugano et al. 2011; Mingozzi and High 2013; Mendoza et al. 2017).
Despite its limitations, optogenetic stimulation has advantages over traditional stimulation methods (electrical stimulation, transcranial magnetic stimulation and pharmacological stimulation) because optogenetic stimulation has better spatial and temporal accuracy. For example, electrical stimulation does not determine tissue volume of activity and activates all cells in the region (Merrill et al. 2005). Similarly, transcranial magnetic stimulation can only excite or inhibit a small area of the brain below the coil, reducing cellular selectivity. It also has no effect on deep tissue (Hallett 2007). Although some selectivity is possible, pharmacological stimulation cannot identify special cells and has a persistent effect for hours after the procedure is over (van Duuren et al. 2007). Optogenetics has the distinct advantage of being able to bidirectionally modulate specific cellular activity (Pathak et al. 2017; Musso et al. 2019).
In the CNS, optogenetics can activate specific cells in special zones and reveal neural circuits. (Li G et al. 2018; de Sousa et al. 2019; Kirszenblat et al. 2019). The development of novel applications in the CNS, including engineered variants of existing opsins, will help us understand the brain in the future. Recently, Rajendran et al. showed that optogenetic stimulation can be applied to identify peripheral neural circuits (Rajendran et al. 2019). This study indicates that control of heart function elucidates the activity of the heart. Similarly, optogenetic stimulation has applications in many other fields, including respiration, taste, and the secretory function of glands. Several studies have demonstrated that optogenetic stimulation can have a positive effect on stroke outcomes, spinal cord injury outcomes and various aspects of the PNS (Zhang et al. 2014; Jiang, Li, et al. 2017; Wahl et al. 2017). Optogenetic stimulation can promote the activation of neurons, the growth of axons and the regeneration of nerves. Recently, Zheng H et al. showed that optogenetic stimulation can precisely control different parts of nerve bundles by a light-emitting device (Zheng et al. 2019). Optogenetic stimulation may have potential translational value to be a novel strategy to promote nerve regeneration in the future. Also, peripheral nerve repair is a complex and self-sustaining process, which involves many different cells. Optogenetics can activate different cells at different times and spaces, which provides the possibility of a deeper understanding of the repair process of peripheral nerves. Currently, the treatment outcomes of critical-sized nerve defects are suboptimal. Some research indicates that peripheral nerve regeneration requires the activation of different cells or different processes in the same cell in different phases (Cattin and Lloyd 2016). Optogenetic stimulation may be able to strengthen the progress of specific cells and promote peripheral nerve regeneration. For example, SCs need to proliferate in the early stages of nerve regeneration but differentiate later (Jung et al. 2019). The selectivity of optogenetic stimulation satisfies these demands (Maimon et al. 2017; Maimon, Sparks, et al. 2018). Researchers have showed that optogenetic stimulation can regulate the differentiation of stem cells and monitor transient calcium concentration to evaluate the differentiation state of neurons in vitro in 2D and 3D systems (Lee SY et al. 2019). It may provide precise control for stem cell transplantation therapy in peripheral nerve regeneration. Also, prior research has reported that optogenetic stimulation can restore the function of the lungs and bladder. These findings give us insight into the direction and future of optogenetic stimulation in the treatment of other diseases such as hemiplegia, brachial plexus injury and more. More technology and strategies for optogenetic stimulation of the PNS will emerge.
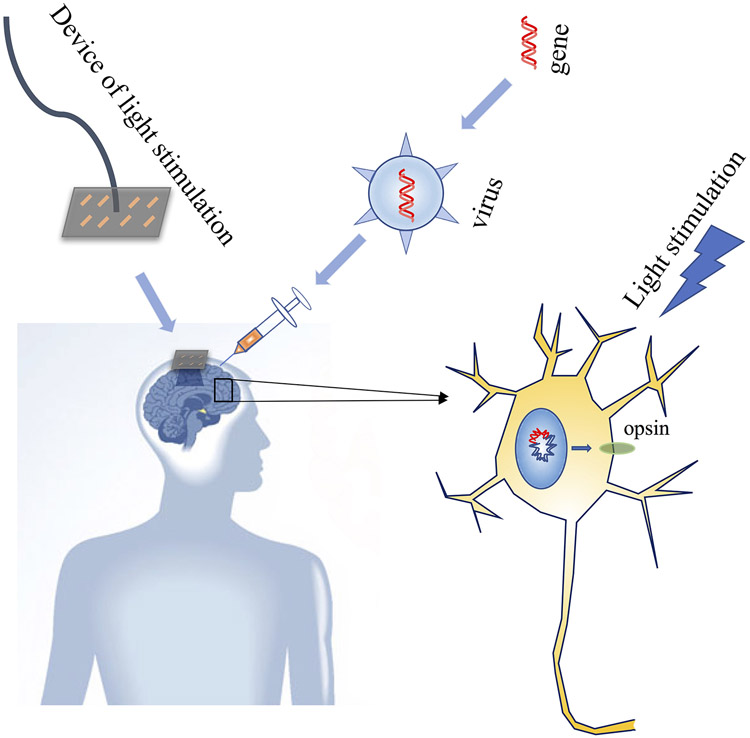
The research on optogenetic stimulation in rodents has developed rapidly and produced remarkable observations. However, the research is still in its infancy in non-human primates (NHP). NHPs have a stronger immune system, more complex cell types and a larger brain size than rodents, all of which pose challenges for the application of optogenetic stimulation (Galvan et al. 2017). Excitingly, optogenetic stimulation in the CNS and PNS of NHPs has developed in recent years. For example, optogenetic stimulation with modified LV conduction refined our understanding of the mechanisms of visual information processing in the lateral geniculate nucleus and primary visual cortex (Chernov et al. 2018). By using adeno-associated viruses (AAVs) with a tetracycline- inducible gene expression (TET-inducible) system that amplifies neuronal expression of induced genes and a ChR2 variant (E123T/T159C) with fast kinetics, Ebina et. al showed that optogenetic stimulation of the motor cortex of a common marmoset can induce arm movement (Ebina et al. 2019). In the PNS, the first optogenetic stimulation in NHPs has been reported recently (Williams et al. 2019). Rhesus macaques were injected intramuscularly with either one or both of two optogenetic constructs (AAV6-hSyn-ChR2-eYFP and/or AAV6-hSyn-Chronos-eYFP) to transduce opsin expression in the corresponding nerves. Expression of ChR2 in the nerve and muscle contraction stimulated by a 400 μm diameter core multimode fiber connected to a 150 mW, 472 nm fiber-coupled laser (15–20 ms, 2.5 Hz, 100 mW) was detected at 5-9 weeks, while Chronos (an opsin discovered by Stigeoclonium helveticum that has faster kinetics than ChR2) was briefly detected at 10 weeks. Together, they represent an important step in translating these optogenetic techniques to a clinically viable gene therapy. In the future, it may be possible to treat disease with optogenetic stimulation by injecting opsin gene-carrying viruses in clinical settings. When injected, patients’ neurons will express opsins (ChR2), and the device of light delivery will be installed in the patient’s brain or other organs. With light illumination, the neurons will be activated or silenced to promote nerve regeneration and treat some diseases. Another clinical application could consist of injecting cells expressing opsins, similar to injecting viruses. Low transgene burden, high opsin photosensitivity, reduced immune response, and long persistent expression of opsins in the viral construct will be key to successful clinical application of optogenetics (Figure 3).
Figure 3:
Potential future translation of optogenetics.
Conclusion
In conclusion, this review summarizes recent reports of optogenetic applications in the nervous system, exploring and discussing the potential for optogenetics to revolutionize treatment. The potential of optogenetic stimulation to optimize therapy for peripheral nerve injuries (PNIs) was also highlighted. Optogenetic stimulation is a novel tool in neuroscience and may potentially improve the outcomes of nerve regeneration. It may be useful in clinical treatment in the future pending further studies.
Acknowledgments
Funding
This work was supported in part by Maryland Stem Cell Research Fund, USA (2018-MSCRFD-4271) (to XJ). XJ was partially supported by R01HL118084 and R01NS110387 from United States National Institutes of Health (both to XJ).
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, Ferenczi E, Gunaydin LA, Mirzabekov JJ, Ye L et al. 2015. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 527(7577):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. 2008. Light-induced rescue of breathing after spinal cord injury. J Neurosci. 28(46):11862–11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HE, Weir RFF. 2019. On the development of optical peripheral nerve interfaces. Neural Regen Res. 14(3):425–436. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S. 2013. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 8(9):e76310. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad BI, Gutierrez DV, Alilain W, Steinmetz MP. 2013. Optogenetic Photostimulation to Control Bladder Function After Experimental Spinal Cord Injury. The Spine Journal. 13(9). [Google Scholar]
- Barnett SC, Perry BAL, Dalrymple-Alford JC, Parr-Brownlie LC. 2018. Optogenetic stimulation: Understanding memory and treating deficits. Hippocampus. 28(7):457–470. [DOI] [PubMed] [Google Scholar]
- Barry J, Akopian G, Cepeda C, Levine MS. 2018. Striatal Direct and Indirect Pathway Output Structures Are Differentially Altered in Mouse Models of Huntington's Disease. J Neurosci. 38(20):4678–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basting TM, Burke PG, Kanbar R, Viar KE, Stornetta DS, Stornetta RL, Guyenet PG. 2015. Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosis. J Neurosci. 35(2):527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind F, Andersson M, Kokaia M. 2018. Dynamic interaction of local and transhemispheric networks is necessary for progressive intensification of hippocampal seizures. Sci Rep. 8(1):5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. 2014. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 344(6182):420–424. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruegmann T, van Bremen T, Vogt CC, Send T, Fleischmann BK, Sasse P. 2015. Optogenetic control of contractile function in skeletal muscle. Nat Commun. 6:7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PG, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL, Guyenet PG. 2015. State-dependent control of breathing by the retrotrapezoid nucleus. J Physiol. 593(13):2909–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camporeze B, Manica BA, Bonafe GA, Ferreira JJC, Diniz AL, de Oliveira CTP, Mathias Junior LR, de Aguiar PHP, Ortega MM. 2018. Optogenetics: the new molecular approach to control functions of neural cells in epilepsy, depression and tumors of the central nervous system. American journal of cancer research. 8(10):1900–1918. eng. [PMC free article] [PubMed] [Google Scholar]
- Cattin AL, Lloyd AC. 2016. The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol. 39:38–46. [DOI] [PubMed] [Google Scholar]
- Chang M, Dian JA, Dufour S, Wang L, Moradi Chameh H, Ramani M, Zhang L, Carlen PL, Womelsdorf T, Valiante TA. 2018. Brief activation of GABAergic interneurons initiates the transition to ictal events through post-inhibitory rebound excitation. Neurobiol Dis. 109(Pt A):102–116. eng. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Chang WP, Shyu BC. 2017. Suppression of cortical seizures by optic stimulation of the reticular thalamus in PV-mhChR2-YFP BAC transgenic mice. Mol Brain. 10(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG, Arac A, Fenno LE, Deisseroth K, Steinberg GK. 2014. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci U S A. 111(35):12913–12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernov MM, Friedman RM, Chen G, Stoner GR, Roe AW. 2018. Functionally specific optogenetic modulation in primate visual cortex. Proc Natl Acad Sci U S A. 115(41):10505–10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse Y, Toossi H, Ishibashi M, Mainville L, Leonard CS, Adamantidis A, Jones BE. 2018. Discharge and Role of Acetylcholine Pontomesencephalic Neurons in Cortical Activity and Sleep-Wake States Examined by Optogenetics and Juxtacellular Recording in Mice. eNeuro. 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA et al. 2010. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 30(48):16082–16090. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou I, Beaudry H, Ase AR, Wieskopf JS, Ribeiro-da-Silva A, Mogil JS, Seguela P. 2016. Optogenetic Silencing of Nav1.8-Positive Afferents Alleviates Inflammatory and Neuropathic Pain. eNeuro. 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis LVM, Moraes MN, Magalhaes-Marques KK, Castrucci AML. 2018. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: Unravelling the photosensitive system of the skin. European journal of cell biology. 97(3):150–162. eng. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, Koob GF, Messing RO, George O. 2019. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun. 10(1):1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa AF, Cowansage KK, Zutshi I, Cardozo LM, Yoo EJ, Leutgeb S, Mayford M. 2019. Optogenetic reactivation of memory ensembles in the retrosplenial cortex induces systems consolidation. Proc Natl Acad Sci U S A. 116(17):8576–8581. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerry JJ, Samineni VK, Copits BA, Sullivan CJ, Vogt SK, Albers KM, Davis BM, Gereau RI. 2018. Differential Regulation of Bladder Pain and Voiding Function by Sensory Afferent Populations Revealed by Selective Optogenetic Activation. Front Integr Neurosci. 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K 2015. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 18(9):1213–1225. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgikh DA, Malyshev AY, Salozhin SV, Nekrasova OV, Petrovskaya LE, Roshchin MV, Borodinova AA, Feldman TB, Balaban PM, Kirpichnikov MP et al. 2015. Anion-selective channelrhodopsin expressed in neuronal cell culture and in vivo in murine brain: Light-induced inhibition of generation of action potentials. Dokl Biochem Biophys. 465:424–427. [DOI] [PubMed] [Google Scholar]
- Donadon I, Bussani E, Riccardi F, Licastro D, Romano G, Pianigiani G, Pinotti M, Konstantinova P, Evers M, Lin S et al. 2019. Rescue of spinal muscular atrophy mouse models with AAV9-Exon-specific U1 snRNA. Nucleic Acids Res. 47(14):7618–7632. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Chen H, Qing L, Yang X, Jia X. 2018. Biomimetic neural scaffolds: a crucial step towards optimal peripheral nerve regeneration. Biomater Sci. 6(6):1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhen G, Chen H, Zhang S, Qing L, Yang X, Lee G, Mao HQ, Jia X. 2018. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials. 181:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duebel J, Marazova K, Sahel JA. 2015. Optogenetics. Current opinion in ophthalmology. 26(3):226–232. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina T, Obara K, Watakabe A, Masamizu Y, Terada SI, Matoba R, Takaji M, Hatanaka N, Nambu A, Mizukami H et al. 2019. Arm movements induced by noninvasive optogenetic stimulation of the motor cortex in the common marmoset. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed]
- Emiliani V, Cohen AE, Deisseroth K, Hausser M. 2015. All-Optical Interrogation of Neural Circuits. J Neurosci. 35(41):13917–13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Hattori M, Toriyabe H, Ohno H, Kamiguchi H, Iino Y, Ozawa T. 2016. Optogenetic activation of axon guidance receptors controls direction of neurite outgrowth. Sci Rep. 6:23976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquiva G, Avivi A, Hannibal J. 2016. Non-image Forming Light Detection by Melanopsin, Rhodopsin, and Long-Middlewave (L/W) Cone Opsin in the Subterranean Blind Mole Rat, Spalax Ehrenbergi: Immunohistochemical Characterization, Distribution, and Connectivity. Front Neuroanat. 10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA. 2017. Applications of optogenetic and chemogenetic methods to seizure circuits: Where to go next? Journal of neuroscience research. 95(12):2345–2356. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Stauffer WR, Acker L, El-Shamayleh Y, Inoue KI, Ohayon S, Schmid MC. 2017. Nonhuman Primate Optogenetics: Recent Advances and Future Directions. J Neurosci. 37(45):10894–10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MR, Fellinger CH, Kattenhorn LM, Davis-Gardner ME, Weber JA, Alfant B, Zhou AS, Prasad NR, Kondur HR, Newton WA et al. 2019. AAV-delivered eCD4-Ig protects rhesus macaques from high-dose SIVmac239 challenges. Science translational medicine. 11(502). eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Schmidt MA, Zielinski MR, Moore ME, Wisor JP. 2018. Sleep State Dependence of Optogenetically evoked Responses in Neuronal Nitric Oxide Synthase-positive Cells of the Cerebral Cortex. Neuroscience. 379:189–201. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia DA, Xu M, Wayman WN, Woodward JJ. 2018. Effects of drugs of abuse on channelrhodopsin-2 function. Neuropharmacology. 135:316–327. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein N, Levine BJ, Loy KA, Duke WL, Meyerson OS, Jamnik AA, Carter ME. 2018. Hypothalamic Neurons that Regulate Feeding Can Influence Sleep/Wake States Based on Homeostatic Need. Curr Biol. 28(23):3736–3747 e3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T 2016. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics. 13(2):295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. 2015. NEUROSCIENCE. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science. 349(6248):647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Bansal H, Roy S. 2019. Theoretical optimization of high-frequency optogenetic spiking of red-shifted very fast-Chrimson-expressing neurons. Neurophotonics. 6(2):025002. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutruf P, Rogers JA. 2018. Implantable, wireless device platforms for neuroscience research. Curr Opin Neurobiol. 50:42–49. [DOI] [PubMed] [Google Scholar]
- Hallett M 2007. Transcranial magnetic stimulation: a primer. Neuron. 55(2):187–199. eng. [DOI] [PubMed] [Google Scholar]
- Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS. 2019. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun. 10(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Lien CC, Cheng CF, Yen TY, Chen CJ, Tsaur ML. 2017. K(+) Channel Kv3.4 Is Essential for Axon Growth by Limiting the Influx of Ca(2+) into Growth Cones. J Neurosci. 37(17):4433–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Borgland SL, Zamponi GW. 2018. Dopaminergic modulation of pain signals in the medial prefrontal cortex: Challenges and perspectives. Neurosci Lett. eng. [DOI] [PubMed]
- Igarashi H, Ikeda K, Onimaru H, Kaneko R, Koizumi K, Beppu K, Nishizawa K, Takahashi Y, Kato F, Matsui K et al. 2018. Targeted expression of step-function opsins in transgenic rats for optogenetic studies. Sci Rep. 8(1):5435. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Guo Z, Wang M, Yang B, Wang X, Li W, Liu J. 2018. Flexible polyimide-based hybrid opto-electric neural interface with 16 channels of micro-LEDs and electrodes. Microsyst Nanoeng. 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Jones S, Jia X. 2017. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int J Mol Sci. 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Li W, Mamtilahun M, Song Y, Ma Y, Qu M, Lu Y, He X, Zheng J, Fu Z et al. 2017. Optogenetic Inhibition of Striatal GABAergic Neuronal Activity Improves Outcomes After Ischemic Brain Injury. Stroke. 48(12):3375–3383. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu Y, Sun F, Wang X, Liu X, He Z. 2015. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat Commun. 6:8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A, Meng F et al. 2015. 3D Printed Anatomical Nerve Regeneration Pathways. Adv Funct Mater. 25(39):6205–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA. 2018. The past, present and future of light-gated ion channels and optogenetics. Elife. 7. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, Liu Y, Zhang L, Han MH. 2019. Optogenetic investigation of neural mechanisms for alcohol-use disorder. Alcohol (Fayetteville, NY). 74:29–38. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Park JH, Kim SY, Jeon NL, Cho SR, Hyung S. 2019. Optogenetic stimulation promotes Schwann cell proliferation, differentiation, and myelination in vitro. Sci Rep. 9(1):3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Il S, Park SE, Myoung-Soo K, Chulmin J, Yong-Jun K, Suh JK, Dosik H, Inchan Y. 2015. An implantable wireless optogenetic stimulation system for peripheral nerve control. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference 2015:1033–1036. eng. [DOI] [PubMed] [Google Scholar]
- Kato HE, Kim YS, Paggi JM, Evans KE, Allen WE, Richardson C, Inoue K, Ito S, Ramakrishnan C, Fenno LE et al. 2018. Structural mechanisms of selectivity and gating in anion channelrhodopsins. Nature. 561(7723):349–354. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W, Jia Y, Madi F, Weber A, Ghovanloo M, Li W. 2019. Inductively coupled, mm-sized, single channel optical neuro-stimulator with intensity enhancer. Microsyst Nanoeng. 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Adhikari A, Deisseroth K. 2017. Integration of optogenetics with complementary methodologies in systems neuroscience. Nature reviews Neuroscience. 18(4):222–235. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS. 2016. Manipulation of P2X Receptor Activities by Light Stimulation. Mediators Inflamm. 2016:7852168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirszenblat L, Yaun R, van Swinderen B. 2019. Visual experience drives sleep need in Drosophila. Sleep. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z et al. 2014. Independent optical excitation of distinct neural populations. Nat Methods. 11(3):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalev K, Polovinkin V, Gushchin I, Alekseev A, Shevchenko V, Borshchevskiy V, Astashkin R, Balandin T, Bratanov D, Vaganova S et al. 2019. Structure and mechanisms of sodium-pumping KR2 rhodopsin. Science advances. 5(4):eaav2671. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak CA, Kung TA, Brown DL, Cederna PS, Kemp SWP. 2018. State-of-the-Art Techniques in Treating Peripheral Nerve Injury. Plast Reconstr Surg. 141(3):702–710. [DOI] [PubMed] [Google Scholar]
- Kuhne J, Vierock J, Tennigkeit SA, Dreier MA, Wietek J, Petersen D, Gavriljuk K, El-Mashtoly SF, Hegemann P, Gerwert K. 2019. Unifying photocycle model for light adaptation and temporal evolution of cation conductance in channelrhodopsin-2. Proc Natl Acad Sci U S A. eng. [DOI] [PMC free article] [PubMed]
- Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J, Katz BM, Feng G, Covington HE, Dzirasa K. 2013. Cortical control of affective networks. J Neurosci. 33(3):1116–1129. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanshakov DA, Drozd US, Dygalo NN. 2017. Optogenetic Stimulation Increases Level of Antiapoptotic Protein Bcl-xL in Neurons. Biochemistry (Mosc). 82(3):340–344. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Yoon HH, Park ES, Min J, Jeon SR. 2018. A Novel Animal Model of Parkinson's Disease Using Optogenetics: Representation of Various Disease Stages by Modulating the Illumination Parameter. Stereotact Funct Neurosurg. 96(1):22–32. [DOI] [PubMed] [Google Scholar]
- Lee GH, Kim SS. 2016. Therapeutic Strategies for Neuropathic Pain: Potential Application of Pharmacosynthetics and Optogenetics. Mediators Inflamm. 2016:5808215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, George JH, Nagel DA, Ye H, Kueberuwa G, Seymour LW. 2019. Optogenetic control of iPS cell-derived neurons in 2D and 3D culture systems using channelrhodopsin-2 expression driven by the synapsin-1 and calcium-calmodulin kinase II promoters. J Tissue Eng Regen Med. 13(3):369–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Li P, Zhou L, Xiao L, Liu Y, Zheng Z, Qin F, Hao Q, Xu H, Yao S et al. 2019. Long-term correction of copper metabolism in WD mice with AAV8 vector delivering truncated ATP7b. Human gene therapy. eng. [DOI] [PubMed]
- Li B, Yang XY, Qian FP, Tang M, Ma C, Chiang LY. 2015. A novel analgesic approach to optogenetically and specifically inhibit pain transmission using TRPV1 promoter. Brain research. 1609:12–20. eng. [DOI] [PubMed] [Google Scholar]
- Li G, Yang J, Wang Y, Wang W, Liu L. 2018. Development of a novel optogenetic indicator based on cellular deformations for mapping optogenetic activities. Nanoscale. 10(45):21046–21051. [DOI] [PubMed] [Google Scholar]
- Li R, Li Y, Wu Y, Zhao Y, Chen H, Yuan Y, Xu K, Zhang H, Lu Y, Wang J et al. 2018. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials. 168:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Tang Y, Xing Y, Kramer P, Bellinger L, Tao F. 2019. Potential Application of Optogenetic Stimulation in the Treatment of Pain and Migraine Headache: A Perspective from Animal Studies. Brain Sci. 9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Jiang L, Li W, Qu M, Song Y, He X, Zhang Z, Yang GY, Wang Y. 2017. Optogenetic Inhibition of Striatal Neuronal Activity Improves the Survival of Transplanted Neural Stem Cells and Neurological Outcomes after Ischemic Stroke in Mice. Stem Cells Int. 2017:4364302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloire V, Mercier MS, Kullmann DM, Pavlov I. 2018. GABAergic Interneurons in Seizures: Investigating Causality With Optogenetics. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry.1073858418805002. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi P, Veladi H, Pakdel FG. 2017. Optogenetics, Tools and Applications in Neurobiology. Journal of medical signals and sensors. 7(2):71–79. eng. [PMC free article] [PubMed] [Google Scholar]
- Maimon BE, Diaz M, Revol ECM, Schneider AM, Leaker B, Varela CE, Srinivasan S, Weber MB, Herr HM. 2018. Optogenetic Peripheral Nerve Immunogenicity. Sci Rep. 8(1):14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimon BE, Sparks K, Srinivasan S, Zorzos AN, Herr HM. 2018. Spectrally distinct channelrhodopsins for two-colour optogenetic peripheral nerve stimulation. Nat Biomed Eng. 2(7):485–496. [DOI] [PubMed] [Google Scholar]
- Maimon BE, Zorzos AN, Bendell R, Harding A, Fahmi M, Srinivasan S, Calvaresi P, Herr HM. 2017. Transdermal optogenetic peripheral nerve stimulation. J Neural Eng. 14(3):034002. [DOI] [PubMed] [Google Scholar]
- Mederos S, Hernandez-Vivanco A, Ramirez-Franco J, Martin-Fernandez M, Navarrete M, Yang A, Boyden ES, Perea G. 2019. Melanopsin for precise optogenetic activation of astrocyte-neuron networks. Glia. 67(5):915–934. [DOI] [PubMed] [Google Scholar]
- Meira T, Leroy F, Buss EW, Oliva A, Park J, Siegelbaum SA. 2018. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat Commun. 9(1):4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior JR, Ferris MJ, Stuber GD, Riddle DR, Jones SR. 2015. Optogenetic versus electrical stimulation of dopamine terminals in the nucleus accumbens reveals local modulation of presynaptic release. J Neurochem. 134(5):833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SD, El-Shamayleh Y, Horwitz GD. 2017. AAV-mediated delivery of optogenetic constructs to the macaque brain triggers humoral immune responses. J Neurophysiol. 117(5):2004–2013. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DR, Bikson M, Jefferys JG. 2005. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 141(2):171–198. eng. [DOI] [PubMed] [Google Scholar]
- Michoud F, Sottas L, Browne LE, Asboth L, Latremoliere A, Sakuma M, Courtine G, Woolf CJ, Lacour SP. 2018. Optical cuff for optogenetic control of the peripheral nervous system. J Neural Eng. 15(1):015002. [DOI] [PubMed] [Google Scholar]
- Mickle AD, Won SM, Noh KN, Yoon J, Meacham KW, Xue Y, McIlvried LA, Copits BA, Samineni VK, Crawford KE et al. 2019. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature. 565(7739):361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, High KA. 2013. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 122(1):23–36. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbozorgi SA, Jia Y, Canales D, Ghovanloo M. 2016. A Wirelessly-Powered Homecage With Segmented Copper Foils and Closed-Loop Power Control. IEEE Trans Biomed Circuits Syst. 10(5):979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery KL, Iyer SM, Christensen AJ, Deisseroth K, Delp SL. 2016. Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system. Science translational medicine. 8(337):337rv335. eng. [DOI] [PubMed] [Google Scholar]
- Moon HC, Won SY, Kim EG, Kim HK, Cho CB, Park YS. 2018. Effect of optogenetic modulation on entopeduncular input affects thalamic discharge and behavior in an AAV2-alpha-synuclein-induced hemiparkinson rat model. Neurosci Lett. 662:129–135. [DOI] [PubMed] [Google Scholar]
- Muhlhauser WW, Horner M, Weber W, Radziwill G. 2017. Light-Regulated Protein Kinases Based on the CRY2-CIB1 System. Methods Mol Biol. 1596:257–270. [DOI] [PubMed] [Google Scholar]
- Mukerjee S, Lazartigues E. 2019. Next-Generation Tools to Study Autonomic Regulation In Vivo. Neurosci Bull. 35(1):113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso PY, Junca P, Jelen M, Feldman-Kiss D, Zhang H, Chan RC, Gordon MD. 2019. Closed-loop optogenetic activation of peripheral or central neurons modulates feeding in freely moving Drosophila. Elife. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Upadhyay H, Murphy M, Borja G, Rozsahegyi EJ, Barnett A, Brookings T, McManus OB, Werley CA. 2019. Simultaneous voltage and calcium imaging and optogenetic stimulation with high sensitivity and a wide field of view. Biomed Opt Express. 10(2):789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D, Stoeckenius W. 1971. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nature: New biology. 233(39):149–152. eng. [DOI] [PubMed] [Google Scholar]
- Oishi N, Nomoto M, Ohkawa N, Saitoh Y, Sano Y, Tsujimura S, Nishizono H, Matsuo M, Muramatsu SI, Inokuchi K. 2019. Artificial association of memory events by optogenetic stimulation of hippocampal CA3 cell ensembles. Mol Brain. 12(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz FC, Habermacher C, Graciarena M, Houry PY, Nishiyama A, Nait-Oumesmar B, Angulo MC. 2019. Neuronal activity in vivo enhances functional myelin repair. JCI Insight. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Liu MH, Kreitzer AC. 2019. Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci. 22(7):1061–1065. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Koppes RA, Froriep UP, Jia X, Achyuta AK, McLaughlin BL, Anikeeva P. 2015. Optogenetic control of nerve growth. Sci Rep. 5:9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Bosch-Bouju C, Schoderboeck L, Sizemore RJ, Abraham WC, Hughes SM. 2015. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Frontiers in molecular neuroscience. 8:14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak GP, Spiltoir JI, Hoglund C, Polstein LR, Heine-Koskinen S, Gersbach CA, Rossi J, Tucker CL. 2017. Bidirectional approaches for optogenetic regulation of gene expression in mammalian cells using Arabidopsis cryptochrome 2. Nucleic Acids Res. 45(20):e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendharkar AV, Levy SL, Ho AL, Sussman ES, Cheng MY, Steinberg GK. 2016. Optogenetic modulation in stroke recovery. Neurosurg Focus. 40(5):E6. [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T, Gelman S, Tombaugh G, Cachope R, Beaumont V, Surmeier DJ. 2019. Enhanced striatopallidal gamma-aminobutyric acid (GABA)A receptor transmission in mouse models of huntington's disease. Movement disorders : official journal of the Movement Disorder Society. 34(5):684–696. eng. [DOI] [PubMed] [Google Scholar]
- Perusini JN, Cajigas SA, Cohensedgh O, Lim SC, Pavlova IP, Donaldson ZR, Denny CA. 2017. Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer's disease mice. Hippocampus. 27(10):1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Yu S, Tung JK, Wei ZZ, Chen D, Berglund K, Zhong W, Zhang JY, Gu X, Song M, Gross RE et al. 2019. Optochemogenetics Stimulation of Transplanted iPS-NPCs Enhances Neuronal Repair and Functional Recovery after Ischemic Stroke. J Neurosci. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanello M, Pisano F, Sileo L, Maglie E, Bellistri E, Spagnolo B, Mandelbaum G, Sabatini BL, De Vittorio M, Pisanello F. 2018. Tailoring light delivery for optogenetics by modal demultiplexing in tapered optical fibers. Sci Rep. 8(1):4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prsa M, Morandell K, Cuenu G, Huber D. 2019. Feature-selective encoding of substrate vibrations in the forelimb somatosensory cortex. Nature. 567(7748):384–388. eng. [DOI] [PubMed] [Google Scholar]
- Qing L, Chen H, Tang J, Jia X. 2018. Exosomes and Their MicroRNA Cargo: New Players in Peripheral Nerve Regeneration. Neurorehabil Neural Repair. 32(9):765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran PS, Challis RC, Fowlkes CC, Hanna P, Tompkins JD, Jordan MC, Hiyari S, Gabris-Weber BA, Greenbaum A, Chan KY et al. 2019. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat Commun. 10(1):1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost BR, Schneider-Warme F, Schmitz D, Hegemann P. 2017. Optogenetic Tools for Subcellular Applications in Neuroscience. Neuron. 96(3):572–603. [DOI] [PubMed] [Google Scholar]
- Shah AM, Ishizaka S, Cheng MY, Wang EH, Bautista AR, Levy S, Smerin D, Sun G, Steinberg GK. 2017. Optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery after stroke. Sci Rep. 7:46612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sileo L, Bitzenhofer SH, Spagnolo B, Pöpplau JA, Holzhammer T, Pisanello M, Pisano F, Bellistri E, Maglie E, De Vittorio M et al. 2018. Tapered Fibers Combined With a Multi-Electrode Array for Optogenetics in Mouse Medial Prefrontal Cortex. Frontiers in Neuroscience. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KI, Park SE, Lee S, Kim H, Lee SH, Youn I. 2018. Compact Optical Nerve Cuff Electrode for Simultaneous Neural Activity Monitoring and Optogenetic Stimulation of Peripheral Nerves. Sci Rep. 8(1):15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SS, Maimon BE, Diaz M, Song H, Herr HM. 2018. Closed-loop functional optogenetic stimulation. Nat Commun. 9(1):5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano E, Isago H, Wang Z, Murayama N, Tamai M, Tomita H. 2011. Immune responses to adeno-associated virus type 2 encoding channelrhodopsin-2 in a genetically blind rat model for gene therapy. Gene therapy. 18(3):266–274. eng. [DOI] [PubMed] [Google Scholar]
- Sun L, Shay J, McLoed M, Roodhouse K, Chung SH, Clark CM, Pirri JK, Alkema MJ, Gabel CV. 2014. Neuronal regeneration in C. elegans requires subcellular calcium release by ryanodine receptor channels and can be enhanced by optogenetic stimulation. J Neurosci. 34(48):15947–15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashima R, Koga K, Sekine M, Kanehisa K, Kohro Y, Tominaga K, Matsushita K, Tozaki-Saitoh H, Fukazawa Y, Inoue K et al. 2018. Optogenetic Activation of Non-Nociceptive Abeta Fibers Induces Neuropathic Pain-Like Sensory and Emotional Behaviors after Nerve Injury in Rats. eNeuro. 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Taylor SL, White ER, Brown CE. 2017. Optogenetic rewiring of thalamocortical circuits to restore function in the stroke injured brain. Nat Commun. 8:15879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd WD, Fenselau H, Wang JL, Zhang R, Machado NL, Venner A, Broadhurst RY, Kaur S, Lynagh T, Olson DP et al. 2018. A hypothalamic circuit for the circadian control of aggression. Nat Neurosci. 21(5):717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL. 2013. Optogenetic control of targeted peripheral axons in freely moving animals. PLoS One. 8(8):e72691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung JK, Shiu FH, Ding K, Gross RE. 2018. Chemically activated luminopsins allow optogenetic inhibition of distributed nodes in an epileptic network for non-invasive and multi-site suppression of seizure activity. Neurobiol Dis. 109(Pt A):1–10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree SM, de Lecea L. 2017. Optogenetic Investigation of Arousal Circuits. Int J Mol Sci. 18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhelski ML, Bruce DJ, Seguela P, Wilcox GL, Simone DA. 2017. In vivo optogenetic activation of Nav1.8(+) cutaneous nociceptors and their responses to natural stimuli. J Neurophysiol. 117(6):2218–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duuren E, van der Plasse G, van der Blom R, Joosten RN, Mulder AB, Pennartz CM, Feenstra MG. 2007. Pharmacological manipulation of neuronal ensemble activity by reverse microdialysis in freely moving rats: a comparative study of the effects of tetrodotoxin, lidocaine, and muscimol. The Journal of pharmacology and experimental therapeutics. 323(1):61–69. eng. [DOI] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS et al. 2015. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 161(4):803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana Magno LA, Tenza-Ferrer H, Collodetti M, Felipe Guimaraes Aguiar M, Paula Carneiro Rodrigues A, Souza da Silva R, do Prado Silva J, Ferreira Nicolau N, Valadao Freitas Rosa D, Birbrair A et al. 2019. Optogenetic stimulation of the M2 cortex reverts motor dysfunction in a mouse model of Parkinson's Disease. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl AS, Buchler U, Brandli A, Brattoli B, Musall S, Kasper H, Ineichen BV, Helmchen F, Ommer B, Schwab ME. 2017. Optogenetically stimulating intact rat corticospinal tract post-stroke restores motor control through regionalized functional circuit formation. Nat Commun. 8(1):1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walocko FM, Khouri RK Jr., Urbanchek MG, Levi B, Cederna PS. 2016. The potential roles for adipose tissue in peripheral nerve regeneration. Microsurgery. 36(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PJ, Clanton SL 2nd, English AW. 2018. Optogenetically enhanced axon regeneration: motor versus sensory neuron-specific stimulation. Eur J Neurosci. 47(4):294–304. [DOI] [PubMed] [Google Scholar]
- Ward PJ, Jones LN, Mulligan A, Goolsby W, Wilhelm JC, English AW. 2016. Optically-Induced Neuronal Activity Is Sufficient to Promote Functional Motor Axon Regeneration In Vivo. PLoS One. 11(5):e0154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. 2012. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 492(7429):428–432. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Narita M, Hamada Y, Yamashita A, Tamura H, Ikegami D, Kondo T, Shinzato T, Shimizu T, Fukuchi Y et al. 2018. Activation of ventral tegmental area dopaminergic neurons reverses pathological allodynia resulting from nerve injury or bone cancer. Molecular pain. 14:1744806918756406. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert JS, Mahn M, Prigge M, Printz Y, Yizhar O. 2017. Silencing Neurons: Tools, Applications, and Experimental Constraints. Neuron. 95(3):504–529. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willand MP, Nguyen MA, Borschel GH, Gordon T. 2016. Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabil Neural Repair. 30(5):490–496. [DOI] [PubMed] [Google Scholar]
- Williams JJ, Watson AM, Vazquez AL, Schwartz AB. 2019. Viral-Mediated Optogenetic Stimulation of Peripheral Motor Nerves in Non-human Primates. Front Neurosci. 13:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Tian W, Lopez-Schier H. 2015. Optogenetic stimulation of neuronal repair. Curr Biol. 25(22):R1068–1069. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Zeng C, Van Dort C, Faingold CL, Taylor NE, Solt K, Feng HJ. 2018. Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol Dis. 110:47–58. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yue J, Ai M, Ji Z, Liu Z, Cao X, Li L. 2014. Channelrhodopsin-2-expressed dorsal root ganglion neurons activates calcium channel currents and increases action potential in spinal cord. Spine (Phila Pa 1976). 39(15):E865–869. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Russell LE, Packer AM, Gauld OM, Hausser M. 2018. Closed-loop all-optical interrogation of neural circuits in vivo. Nat Methods. 15(12):1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zhang Z, Jiang S, Yan B, Shi X, Xie Y, Huang X, Yu Z, Liu H, Weng S et al. 2019. A shape-memory and spiral light-emitting device for precise multisite stimulation of nerve bundles. Nat Commun. 10(1):2790. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]