Abstract
Dimethylaminoethanol (DMAE) and its salts have been used to treat numerous disorders in humans and hence safety of its use is a concern. DMAE is a close structural analog of choline, an essential nutrient. Exposure to DMAE may affect choline uptake and synthesis. The current investigation characterizes :1) the absorption, distribution, metabolism, and excretion (ADME) of DMAE in Wistar Han rats and B6C3F1 mice following a single gavage or intravenous (IV) administration of 10, 100 or 500 mg/kg [14C]DMAE, and 2) the ADME of [14C]choline (160 mg/kg) and the effect on its disposition following pre-treatment with DMAE (100 or 500 mg/kg). In both rats and mice, following gavage administration, DMAE was excreted in urine (16–69%) and as exhaled CO2 (3–22%). The tissue retention was moderate (21–44%); however, the brain concentrations were low and there was no accumulation. Serum choline levels were not elevated following administration of DMAE. The DMAE metabolites in urine were DMAE N-oxide and N,N-dimethylglycine; the carcinogen, N-N-dimethylnitrosamine, was not detected. The pattern of disposition of [14C]choline following gavage administration was similar to that of [14C]DMAE. Prior treatment with DMAE had minimal effects on choline disposition. The pattern of disposition of [14C]DMAE and [14C]choline following IV administration was similar to gavage administration. There were minimal dose-, sex- or species-related effects following gavage or IV administration of [14C]DMAE or [14C]choline. Data from the current study did not support previous reports that: 1) DMAE alters choline uptake and distribution, or 2) that DMAE is converted into choline in vivo.
INTRODUCTION
Dimethylaminoethanol (DMAE) is a high production volume chemical used in a variety of consumer and industrial applications (OECD, 2004) with a national production volume of 100–250 million pounds in 2015 (US EPA, 2016). Occupational exposure to DMAE is believed to primarily occur in workers in the spray painting and beverage can lacquering industries. There is also some concern for the release of DMAE into the environment from these industrial sources (Pitts et al., 1981). Air concentrations of DMAE near new polyurethane foam insulation have been measured at 6.7 mg DMAE/m3, and were shown to persist at levels of 4.0 mg DMAE/m3 up to two months post-insulation (summarized in NTP, 2002).
Occupational exposure to DMAE has been associated with cardiovascular, neurological, and psychological health effects; however, a direct correlation is unclear (summarized in NTP, 2002). Non-occupational exposure to DMAE can occur primarily via intake of pharmaceuticals and dietary supplements. DMAE and DMAE salts (e.g. p-acetamidobenzoate) have been used to treat central nervous system disorders, particularly those associated with decreases in cholinergic neuron function in humans (Schlenk, 1990). Some specific uses include management of learning and behavioral problems, chronic fatigue, neurasthenia, and to treat symptoms of attention deficit hyperactivity disorder in children (De Silva, 1977; Hendler and Rorvik, 2001; HSDB, 2015; Source Naturals, 2017a, b; Nature’s Plus, 2017). The DMAE substructure is a part of numerous complex pharmaceutical products, including antihistamines, antiemetics, local anesthetics, and tamoxifen (summarized in NTP, 2002). The recommended dosage for these supplements varies greatly between products, with child doses as high as 100 mg DMAE/day and adult doses ranging from 100–500 mg DMAE/day (Source Naturals, 2017a, b; Nature’s Plus, 2017).
DMAE is a close structural analog of choline (N,N,N-trimethylaminoethanol), an essential nutrient. Mammals are limited in their ability to synthesize choline, and therefore acquire much of it through the diet. Healthy levels of choline in adults range from 7–20 mmol/L (2), and the recommended adequate intake for adults is 550 and 425 mg/day for males and females, respectively (Institute of Medicine, 1998). Choline is required for the synthesis of phosphatidylcholine and sphingomyelin, the phospholipids vital for the integrity of cell membranes. Choline is also necessary for production of the neurotransmitter acetylcholine, which mediates neurological signals controlling memory, mood, and muscle control, along with other activities of the central nervous system (Institute of Medicine, 1998; Zeisel et al., 2010; 2012). Choline deficiency has been associated with the development of neural tube defects (Fisher et al., 2001; 2002).
Homeostasis of brain choline is maintained by a complex system that interrelates choline net movements into and out of the brain and choline incorporation into and release from phospholipids (Klein et al., 1992). Choline is a charged hydrophilic cation at physiological pH and thus cannot appreciably diffuse across cellular membranes, requiring mechanisms for it to cross biological membranes. Choline transport is characterized by sodium-dependent high-affinity, sodium-independent low-affinity, and sodium-independent blood–brain barrier transport mechanisms (Crowe et al., 2002; Lockman and Allen, 2002). The choline transporters of the blood brain barrier are inhibited by close structural analogs of choline and quaternary amines (Ferguson and Collier, 1994; Geldenhuys et al., 2005; Cai et al., 2007). Changes in the activity of these transporters induced by close analogues of choline has direct impact on levels of brain acetylcholine (Sterling et al., 1986). Therefore, there is potential for DMAE to disrupt choline uptake and metabolism and interfere with biological processes such as development. It has been hypothesized that DMAE crosses the blood-brain barrier and may be methylated to choline where it is then acetylated by choline acetylase to form acetylcholine; however, the extent of mechanism is not well-understood (De Silva, 1977; HSDB, 2015).
Comprehensive absorption, distribution, metabolism and excretion (ADME) data are not available for DMAE or choline following routes of exposure relevant to humans. Following oral or intravenous (IV) administration in rodents, DMAE is rapidly transported to the liver, where the majority of it is metabolized (Zahniser et al., 1977; Hendler and Rorvik, 2001). Dormard et al. (1975) reported that 0.16% of an IV dose of [14C]DMAE (11 mg/kg) was present in the plasma of male Wistar rats 6 minutes post-dosing. Approximately 13.5% of the dose was eliminated by 24 hours post-dosing with N-oxide of DMAE detected in urine as the major urinary metabolite. The authors concluded that the majority of the dose was incorporated into phospholipid pathways. Rats or mice administered [14C]DMAE via intracerebral injection were observed with rapid clearance of DMAE and increases in brain levels of phosphatidylethanolamine, phosphotidylethanolamine, or other acid-soluble and lipid cholines (Schlenk, 1990). In female mice administered 0.1 mg/kg [1,2-14C]DMAE, [1,2-14C]choline, or [methyl-14C]choline, excretion of CO2 and radioactivity levels in urine were higher following choline exposure compared to DMAE exposure (Groth et al., 1958). In the same study, it was reported that approximately 15% of the administered dose of [1,2-14C]DMAE was converted to choline by 12 hours post-dose; however, DMAE was not formed following demethylation of [14C]choline. Administration of a higher dose (150 mg/kg) of the three compounds resulted in similar distribution and excretion patterns. Other studies in mice and rats have shown increased levels of free choline in the blood and kidneys (mice) and the brain and plasma (rats) following DMAE administration (Jope and Jenden, 1979; HSDB, 2015).
Due to the potential for widespread human exposure through its use in industrial and consumer products, DMAE was nominated to the National Toxicology Program (NTP) for toxicological characterization in rodent models. ADME data in the same animal models are essential to put toxicological findings in to context. However, the current body of literature on the ADME of DMAE following routes of exposure relevant to humans is incomplete. Therefore, the current studies were undertaken to: 1) investigate the ADME of DMAE in male and female rats and mice following a single oral gavage administration of [14C]DMAE, and 2) As DMAE is structurally similar to choline and may impact choline disposition, the ADME of choline was evaluated following gavage administration of [14C]choline with or without pre-treatment with single or multiple dose of DMAE. In addition, limited studies were conducted following a single IV administration of DMAE to better understand the absorption following oral administration. The study designs are given in Tables 1 (DMAE) and 2 (choline). The highest DMAE dose of 500 mg/kg (~ 0.1 × of LD50) was selected based on the oral LD50 of DMAE in rodents (3000 to 3400 mg/kg) (summarized in NTP 2002); the two lower doses were selected as 1/5th (100 mg/kg) and 1/50th (10 mg/kg) of the highest dose. The choline dose of 160 mg/kg (~ 0.05 × of LD50) was selected based on the oral LD50 of choline in rodents (3400 to 3900 mg/kg) (summarized in NTP 2002).
Table 1.
Single gavage and intravenous administration study design of [14C]DMAE in Wistar Han rats, Sprague Dawley rats, and B6C3F1 mice.
| Species (Sex) | Dose (mg/kg) | Study Duration (h) | Endpoint |
|---|---|---|---|
| Rat (M) | 10,100,500 | 24 or 72 | Dose response |
| Rat (M) | 500 | 2, 24,72,168 | Tissue distribution |
| Rat (F) | 500 | 24 | Sex difference |
| Rat (M) | 100 | 2 | Lipid incorporation |
| Rat (M)a | 10 | 24 | Route difference |
| Rat (M)b | 500 | 24 | Strain comparison |
| Rat (M) | 500 | 0, immediate, 0.25, 0.5, 1, 2, 4, 6, 1, 24 | Effect on endogenous choline; metabolic conversion of DMAE to choline |
| Mouse (F) | 10,100,500 | 24, 72 | Dose response |
| Mouse (M) | 500 | 24 | Sex difference |
Intravenous groups
Sprague Dawley rats
Table 2.
Single gavage and intravenous administration study design of [14C]choline in Wistar Han rats and B6C3F1 mice.
| Species (Sex) |
Choline Dose (mg/kg) | DMAE Pre-treatment (mg/kg) |
No. of Doses (DMAE) | Study Duration (h) |
|---|---|---|---|---|
| Rat (M) | 160 | --- | --- | 24 |
| Rat (F) | 160 | --- | --- | 24 |
| Rat (M)a | 16 | --- | --- | 24 |
| Rat (F)a | 16 | --- | --- | 24 |
| Rat (M) | 160 | 100,500 | 1 (1 h)b | 24 |
| Rat (M) | 160 | 100 | 3 (48, 24, 1 h)b | 24 |
| Rat (F) | 160 | 100,500 | 1 | 24 |
| Mouse (F) | 160 | --- | --- | 24 |
| Mouse (F) | 160 | 500 | 1 | 24 |
Intravenous groups
Time before administration of choline dose is shown in parentheses
MATERIALS AND METHODS
Chemicals and reagents
Dimethylaminoethanol (DMAE, CAS RN 108-01-0, vendor purity > 99.5%) and choline chloride (vendor purity ≥ 99%) were obtained from Sigma-Aldrich, Inc. (St. Louis, MO). The identity of both DMAE and choline were confirmed by proton nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) using electrospray ionization. Radiolabeled hydrochloride salt of DMAE with the label on ethanol carbons, [1,2-14C]DMAE ([14C]DMAE, specific activity 58 mCi/mmol; 0.5 mCi/mL radiochemical purity 100%) was obtained as an ethanolic solution from Moravek Biochemicals, Inc. (Brea, CA). Radiolabeled choline chloride with the label on the methyl groups, [methyl-14C]choline ([14C]choline), was purchased from either Sigma-Aldrich, Inc. (specific activity 9.8 mCi/mmol, radiochemical purity ≥ 95%) as an 2% aqueous ethanolic solution (1.0 mCi/mL) or from MP Biomedicals (Irvine, CA) (specific activity 55 mCi/mmol, radiochemical purity > 99%) in ethanol (0.25 mCi/mL). Structures of [14C]DMAE and [14C]choline with the position of the label are shown in Figure 1. The identity of [14C]DMAE was confirmed by MS analysis and [14C]choline chloride by either MS analysis or co-elution with non-labeled choline by high performance liquid chromatography (HPLC).
Figure 1:
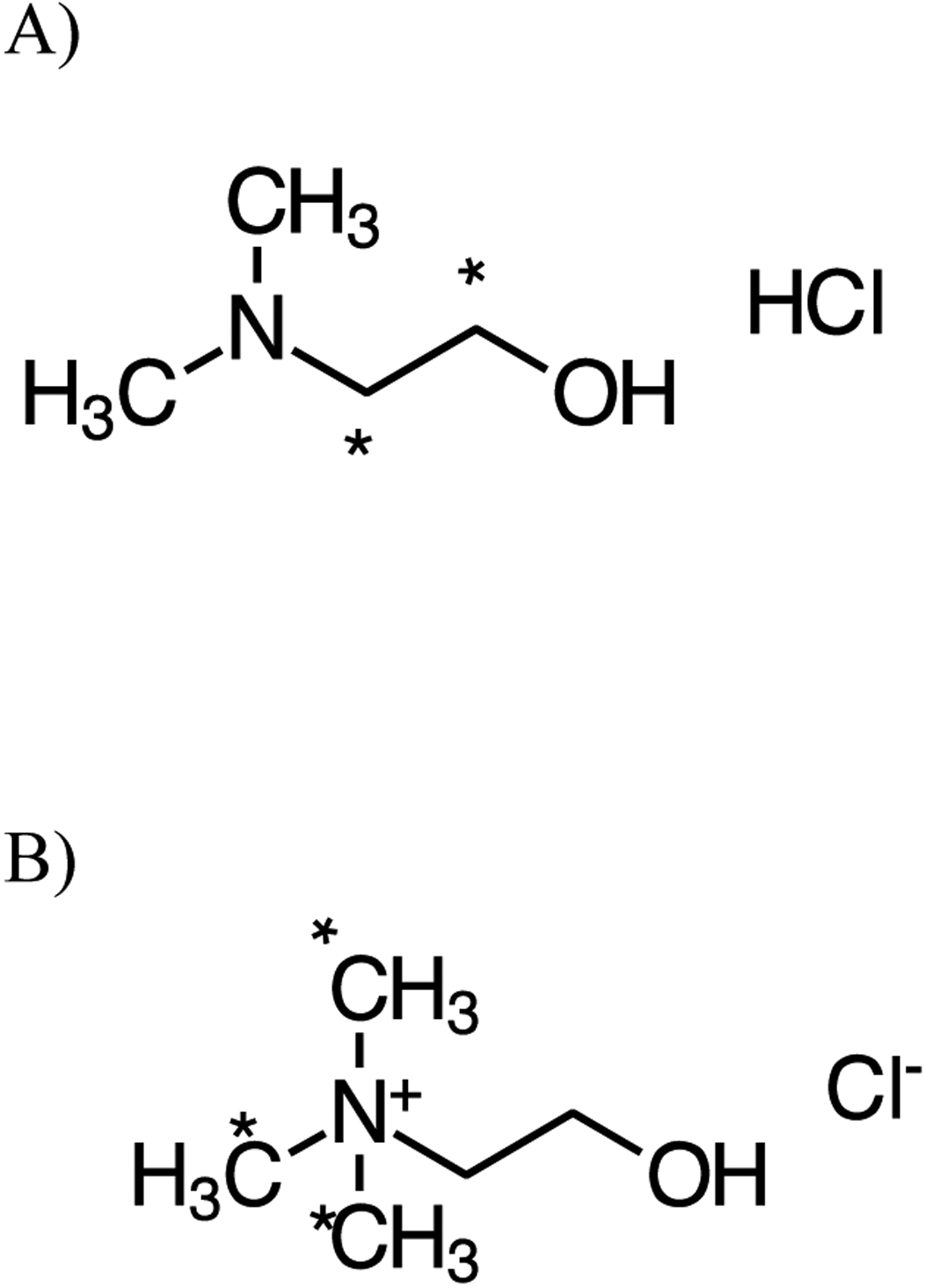
Chemical structure of A) DMAE and B) choline. * Denotes the position of radioactivity.
Radiolabeled betaine (N,N,N-trimethylglycine), a potential metabolite, with the label on the methyl groups ([14C]betaine, specific activity 37.7 mCi/mmol, radiochemical purity 98.7%) was purchased from Moravek Biochemicals, Inc. (Brea, CA) as a 50% aqueous ethanolic solution (0.1 mCi/mL, 313.9 μg/mL). Euthasol® was purchased from Delmarva Laboratories, Inc. (Midlothian, VA). Ultima Gold scintillation cocktail, Permafluor E+, and Carbo-Sorb E were purchased from PerkinElmer (Boston, MA). All other reagents were purchased from commercial sources.
High performance liquid chromatography
HPLC was performed using an Agilent model 1100 system (Agilent, Santa Clara, CA) with a variable wavelength detector and a β-RAM-LS radioactivity detector (IN/US Systems; Tampa, FL) with a 500-μL lithium glass, flow-through, solid-flow cell or a 500-μL liquid cell.
HPLC Method A:
The column used was a Microsorb 100–5 silica column (250 × 4.6 mm, Varian; Palo Alto, CA). Mobile phase [acetonitrile:water:ethanol:acetic acid:0.83 M ammonium acetate (0.78:0.14:0.07:0.04:0.007, v/v, pH 3.6)] was used in isocratic conditions at a flow rate of 2.7 mL/min. [14C]choline eluted at 3.6 min.
HPLC Method B:
The column used was a Phenomenex Luna C18 column (150 × 4.6 mm, 5 μm; Torrance, CA). Mobile phases A [potassium phosphate:sodium heptanesulphonate:tetramethylammonium chloride (0.05 M:0.025 M:0.0025 M, pH 3.0)] and B (acetonitrile) were used with a gradient run from 0% B to 10% B over 10 min, then to 95% B over 20 min at a flow rate of 1.0 mL/min. [14C]choline was eluted at 8.1 min. [14C]DMAE eluted at 8.8 min.
HPLC Method C:
The column, mobile phases and flow rate used were the same as in Method B, except that a gradient was run from 0% B to 10% B over 15 min, then to 30% B over 10 min at a flow rate of 1.0 mL/min.
HPLC Method D:
The column used was a Phenomenex Luna C18(2) column (150 × 2.0 mm, 3 μm; Torrance, CA). Mobile phases A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile) were used with a gradient run from 5% B, held for 1.5 min, to 60% B at 7 min at a flow rate of 0.2 mL/min.
Liquid chromatography-mass spectrometry (LC-MS)
The LC used was an Agilent model 1100 system (Agilent, Santa Clara, CA). The mass spectrometer used was an Applied Biosystems API 5000 (Waltham, MA). The Turbo Spray ion source was used operated in the positive mode with ion spray voltage of 5500 V and a source temperature of 400 °C.
LC-MS Method A:
A Phenomenex Luna 3-μ HILIC column (150 × 4.6 mm) was used with an isocratic mobile phase (10 nM ammonium formate in 75:25 (v:v) acetonitrile:water) and a flow rate of 0.8 mL/min. The multiple reaction monitoring (MRM) transition of m/z 103.9 −> 60.2 was used to quantify choline; the retention time was ~ 3.4 min. Standard curves of choline were run in triplicate ranging from 5 to 100 ng/mL. For the detection of choline derived from [14C]DMAE, MRM transition of m/z 105.9 −> 60.2 was also monitored.
LC-MS Method B:
The column and mobile phases used were the same as in HPLC Method D with a gradient run from 5% B held for 0.5 min, to 80% B in 3.9 min at a flow rate of 0.25 mL/min. MRM transition 75 −> 43 was used for the quantitation N-N-dimethylnitrosamine.
Animals
All studies were conducted at Lovelace Biomedical and Environmental Research Institute (LBERI). All animal procedures were approved by the LBERI Institutional Animal Care and Use Committee (IACUC) and were in accordance with applicable local, state, and federal regulations. Animals were housed in a facility that was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Adult male and female Wistar Han rats, including jugular vein cannulated (JVC) animals, were purchased from Charles River Laboratories, Inc (Hollister, CA). Adult male and female B6C3F1 mice were obtained from Taconic Laboratories (New Hyde Park, NY). Rats were 6–8 weeks old and mice were 7–9 weeks old upon receipt and were quarantined for up to 14 days prior to the study start. During quarantine and prior to the study start, rats were housed up to 2 animals per cage and mice were housed up to 4 animals per cage in shoebox cages with heat-treated hardwood chip bedding (Sani-Chip®; P.J. Murphy Forest Products Corp., Montville, NJ). Fresh bedding and clean, sanitized cages were supplied at least twice weekly. Animal rooms were maintained at 18–26 °C and 30–70% relative humidity with a 12-hour light/dark cycle. Air circulations were 100% fresh filtered air with 10–15 air changes/hour. All animals were uniquely identified with tail markings. Rats and mice were individually acclimated to the all-glass metabolism chambers approximately 24 h prior to dosing. Following dosing, animals were returned to the metabolism chamber to allow for separate collection of urine, feces, and expired CO2 and volatile organic chemicals (VOCs). Animals were fed NTP-2000 rodent diet (Ziegler Brothers, Inc.; Gardeners, PA) and provided access to municipal water (Kirtland Air Force Base, Albuquerque, NM) ad libitum. Feed was used within 120 days of milling. Feed was analyzed by the manufacturer and the water was analyzed annually at LBERI; no contaminant is known to be in the diet or water in quantities that would interfere with the outcome of this study.
Formulation and administration of [14C]DMAE or [14C]choline in rats and mice
Animals were weighed and randomized into groups prior to dosing and individual animal weights were used to determine dose volumes. Male or female rats or mice (up to 5 animals/group) were given a single oral or IV dose of [14C]DMAE as given in Table 1. Oral dose formulations were prepared in water one day prior to dosing and contained [14C]DMAE (~100 μCi/kg in mice and ~50 μCi/kg in rats), and an appropriate amount of unlabeled DMAE to achieve the dose formulation concentration required. Male or female rats or mice (up to 5 animals/group) were given a single oral dose of [14C]choline with or without pretreatment with unlabeled DMAE as given in Table 2. Oral dose formulations were prepared in water one day prior to dosing and contained [14C]choline (~100 μCi/kg in mice and ~50 μCi/kg in rats), and an appropriate amount of unlabeled choline to achieve the dose formulation concentration required. Oral doses were administered by intragastric gavage at a volume of 5 mL/kg using a syringe equipped with a gavage needle (16-gauge with a ball tip). IV dose formulations for DMAE were prepared one day prior to dosing similar oral formulations but in 0.9% saline. IV doses were administered in a volume of 1 mL/kg via a tail vein catheter (Surflo 24 G, Terumo Medical Corporation; Tokyo, Japan) using a syringe. The catheter was flushed with approximately 0.2 mL of 0.85% saline to ensure that the entire dose was delivered. Following dosing, animals were returned to the metabolism cages.
The radiochemical content of [14C]DMAE or [14C]choline in the dose formulations was determined before, during, and after dosing for a total of five aliquots sampled on the day of dosing. Samples were analyzed by HPLC Method A (choline only), B or C. The radiochemical purity of [14C]DMAE and [14C]choline in dose formulations ranged from 93.9–98.3% and 95–98.5%, respectively.
Collection of biological samples and analysis for radioactivity in disposition studies
Urine and feces were collected separately from each animal into receivers cooled over dry ice. Urine was collected at 4, 8, 12, and 24 h, and at 24-h intervals thereafter, following the last dose administration. Feces was collected at 8 and 24 h, and at 24-h intervals thereafter, depending on study duration. Urine from the bladder at sacrifice was added to the last urine collection. At the end of each collection interval, the metabolism cages were rinsed with water (or water and ethanol after the terminal collection). Rinsates were collected separately from urine. The weight of urine, rinses, and/or feces collected for each sample interval was measured. Samples were stored in the dark at −20 °C until analysis.
Expired VOCs and CO2 were collected by passing the air from the metabolism chamber (200–600 mL/min) through a series of traps. The first two traps, which contained approximately 60 mL of isopropanol, were used for trapping VOCs. The second two traps, which contained approximately 350 mL of 1 N NaOH in H2O, were in-line after the isopropanol traps and used to trap CO2. A trap containing water was placed between the last trap and the vacuum line to catch any overflow. All traps were cooled in wet ice. For the 24 h studies, traps were changed at 1, 2, 3, 4, 6, 8, 12, and 24 h. For the 72 h studies, traps were changed at 4, 8, 12, 24, and 32 h.
At the end of all studies animals were administered Euthasol® (390 mg sodium pentobarbital/kg and 50 mg phenytoin sodium/kg) by intraperitoneal injection to induce surgical-level anesthesia.
Blood was collected into a heparinized syringe by cardiac puncture following which animals were exsanguinated and euthanized by section of the diaphragm. At necropsy, the following tissues were excised and weighed: adipose (perirenal, reproductive), muscle (hind leg, trapezius), and skin (ears), and the following organs in their entirety: brain, lung, heart, spleen, kidney, testes or uterus and ovaries, liver, thyroid, small and large intestine with contents, cecum with contents, stomach with contents, and urinary bladder. The remaining carcasses were weighed.
Duplicate aliquots of urine, cage rinse, and breath traps were mixed with Ultima Gold scintillation cocktail for analysis of total radioactivity. Feces samples were homogenized with an approximately equal mass of water. Triplicate aliquots of fecal homogenates, blood, and tissues except gastrointestinal (GI) tissues were combusted in a Packard 307 biological sample oxidizer (Shelton, CT) using Carbo-Sorb E for trapping CO2 and Permaflour E+ as the scintillation cocktail, and collected into vials for analysis. All GI tissues (with contents) and residual carcass were digested in 2 N ethanolic sodium hydroxide. Once dissolved, the samples were neutralized with nitric acid, bleached with hydrogen peroxide, weighed into scintillation vials containing Ultimate Gold scintillation cocktail. All samples were analyzed for total radioactivity using a Packard Model 2500TR or 3100TR (Meridian, CT) liquid scintillation spectrophotometer (LSS). For determination of total [14C]DMAE or [14C]choline in dispersed tissues, the total weight of rats was assumed to be comprised of 7.4% blood, 7.0% adipose, 40.4% muscle, and 19% skin and the total weight of mice was assumed to be comprised of 4.9% blood, 7.0% adipose, 38.4% muscle, and 16.5% skin (Brown, 1997).
Evaluation of [14C] DMAE incorporation in phospholipids
Incorporation of [14C]DMAE-derived radioactivity in phospholipids was evaluated in spleen, brain, lung, kidney, testes, skin, heart, and liver of male Wistar Han rats administered 100 mg/kg [14C]DMAE via oral gavage and sacrificed 2 h after administration (Table 1). Lipids were extracted using the method of Folch et al., 1956. Briefly, approximately 1 g of tissue was homogenized in chloroform for 5 min. The homogenate was filtered and the extract was dried under N2 to obtain total lipid consisting of neutral lipids, fatty acids, and phospholipids. The total lipid was then dissolved in a chloroform (0.5–1.0 mL) and fractionated by solid phase extraction (SPE) (Kaluzny et al., 1985) using 4 mL each of choloroform:2-propanol (2:1), 2% acetic acid in diethyl ether, and methanol, respectively to elute neutral lipids, fatty acids, and phospholipids. Each fraction was analyzed by LSS for radioactivity.
Effect of DMAE on serum choline levels
The effect of DMAE on endogenous choline levels was evaluated in JVC Wistar Han rats following a single gavage dose of 500 mg/kg [14C]DMAE. Blood (0.3 μL) was collected in heparin at 0 (pre-dose), immediate, 0.25, 0.5, 1, 2, 4, 6, 12, and 24 h post-administration. Choline levels in serum were quantified using LC-MS Method A, a previously published LC-MS method (Yue et al., 2008).
In order to determine whether DMAE is converted to choline, a group of male and female Wistar Han rats was administered a single gavage dose of 500 mg/kg [14C]DMAE with a higher radioactivity (1.5 mCi/kg) and blood was collected at 0.25, 0.5, 1, 2 and 4h post administration. Serum was analyzed using LC-MS Method A monitoring for [14C]choline.
Metabolite profiling and identification
Urine composites were prepared for dose groups and time points by combining in a ratio equal to their total collected volumes. To improve the mass of analyte and subsequently the sensitivity, urine samples were either concentrated via lyophilization up to 75 times their original concentration or cleaned up by SPE using C18 cartridges to concentrate and isolate analytes. Cartridges were equilibrated with 0.1% trifluoracetic acid (TFA) in water, samples were loaded onto the column, washed with water, and analyzed using HPLC Method C or Method D.
RESULTS
1. Disposition and metabolism of [14C]DMAE
Disposition of [14C]DMAE in male and female rats following gavage or IV administration.
The disposition of [14C]DMAE 24 h after a single gavage dose (10, 100, or 500 mg/kg) in Wistar Han rats is presented in Table 3. Dose response in disposition was investigated only in male rats with limited studies in females. Following oral administration in male Wistar Han rats, 57, 59, and 62% of the 10, 100, and 500 mg/kg administered dose, respectively, was recovered in urine 24 h after administration. Of the administered dose, approximately 4–5% was found as expired CO2 with minimal excretion in the feces (≤ 0.5%) at all dose levels. Excretion as VOCs was low (≤ 0.2%) in the 10 and 500 mg/kg groups. Radioactivity was not detected in VOCs from the 100 mg/kg dose group above the background which is likely due to a combination of higher background levels and overall lower excretion of [14C]DMAE-derived radioactivity as VOCs. The urinary and fecal excretion was also investigated up to 72 h following a single oral dose of 500 mg/kg in male rats and was similar to that of 24 h (Table 3). For male oral dose groups, the cumulative percentage of dose excreted in urine and as exhaled VOCs and CO2 is shown in Figure 2. Excretion of [14C]DMAE-derived radioactivity in urine was rapid for up to 24 h; excretion only slightly increased between 24 and 72 h. The percentage of dose exhaled as CO2 steadily increased up to the last collection time point of 24 h (Figure 2). The disposition of [14C]DMAE in female Wistar Han rats administered a single oral dose of 500 mg/kg [14C]DMAE was similar to males administered the same dose, with approximately 64% recovered in the urine and 6% as expired CO2 (Table 3 and S1).
Table 3.
Disposition of [14C]DMAE in male and female Wistar Han rats 24 or 72 h following a single gavage or intravenous administration.
| % Dose Recovereda | |||||||
|---|---|---|---|---|---|---|---|
| Tissue | Male, Gavage 10 mg/kg |
Male, Gavage 100 mg/kg |
Male, Gavage 500 mg/kg |
Male, IV 10 mg/kg |
Female, Gavage 500 mg/kg |
Male, Gavageb 500 mg/kg |
Male, Gavagec 500 mg/kg |
| Urined | 57.0 ± 4.10 | 58.6 ± 5.10 | 62.1 ± 1.50 | 39.2 ± 7.80 | 63.5 ± 1.80 | 68.6 ± 2.00 | 60.4 ± 3.80 |
| Feces | 0.206 ± 0.242 | 0.302 ± 0.061 | 0.518 ± 0.472 | 0.330 ± 0.092 | 0.549 ± 0.147 | 1.11 ± 1.01 | 0.538 ± 0.335 |
| VOCs | 0.153 ± 0.037 | --- | 0.198 ± 0.038 | 0.230 ± 0.029 | 0.818 ± 0.456 | --- | 0.220 ± 0.047 |
| CO2 | 3.67 ± 0.440 | 4.84 ± 0.700 | 4.78 ± 0.810 | 3.52 ± 0.780 | 6.09 ± 0.810 | 7.94 ± 1.72e | 2.94 ± 0.230 |
| Tissues and GI Tractf | 33.9 ± 2.20 | 30.1 ± 3.80 | 24.3 ± 2.10 | 38.5 ± 2.30 | 27.4 ± 1.40 | 14.5 ± 1.20 | 34.1 ± 1.80 |
| Total Recovered | 94.9 ± 3.20 | 93.9 ± 2.90 | 92.1 ± 1.90 | 81.8 ± 7.10 | 98.4 ± 0.800 | 92.0 ± 2.20 | 98.3 ± 3.50 |
Mean ± SD, N= 3 animals;
72 h, N = 5
Sprague Dawley rats
Includes urine present in the bladder at study termination
Collection was terminated at 39 h
Includes contents
Figure 2:
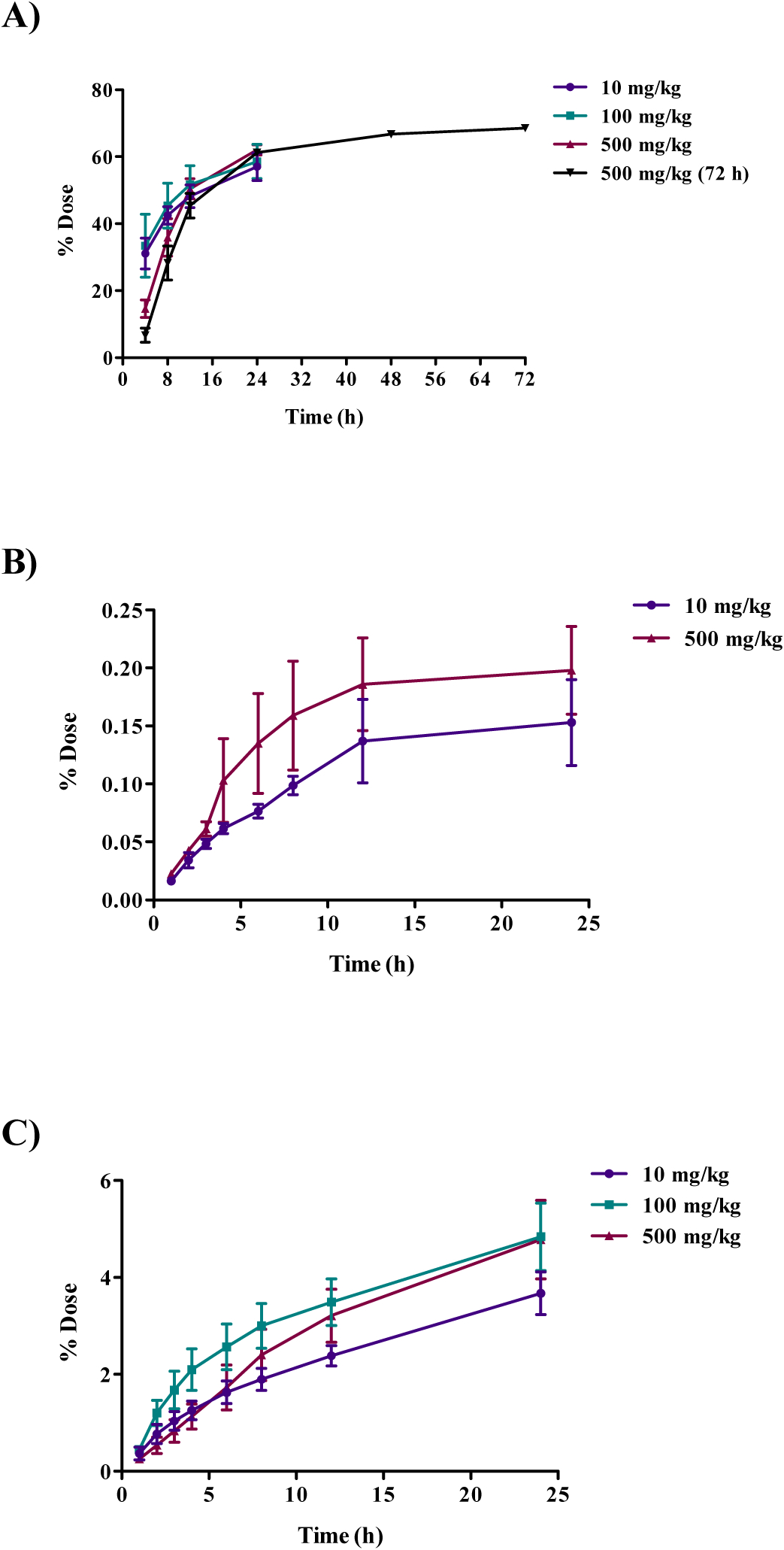
Cumulative excretion of radioactivity following oral administration of 10, 100 or 500 mg/kg [14C]DMAE in male Wistar Han rats. Cumulative percent dose A) excreted in urine, B) excreted as exhaled volatile organic compounds (VOCs), and C) excreted as expired CO2. VOCs were below the limit of detection in the 100 mg/kg group.
The concentration of [14C]DMAE-derived radioactivity in tissues, in general, increased with dose (Table S1). The approximate percent radioactivity remaining in all tissues, excluding GI tract, (note: GI tract tissues were excluded due to the presence of GI content) at 24 h post-administration was 28, 25, and 21% for 10, 100, and 500 mg/kg, respectively (Table S1). The tissues with the highest levels of radioactivity were the liver, kidney, thyroid, lung, and spleen. The time course in tissue distribution was investigated in male rats following a single oral dose of 500 mg/kg [14C]DMAE and sacrificed 2, 24, 72 or 168 h post-administration. [14C]DMAE-derived radioactivity was highest at 24 h and declined over time with the lowest detected at 168 h (Table S2 and Figure 3). The total administered dose recovered following oral administration of [14C]DMAE in male and female Wistar Han rats ranged from 92–98% (Table 3).
Figure 3:
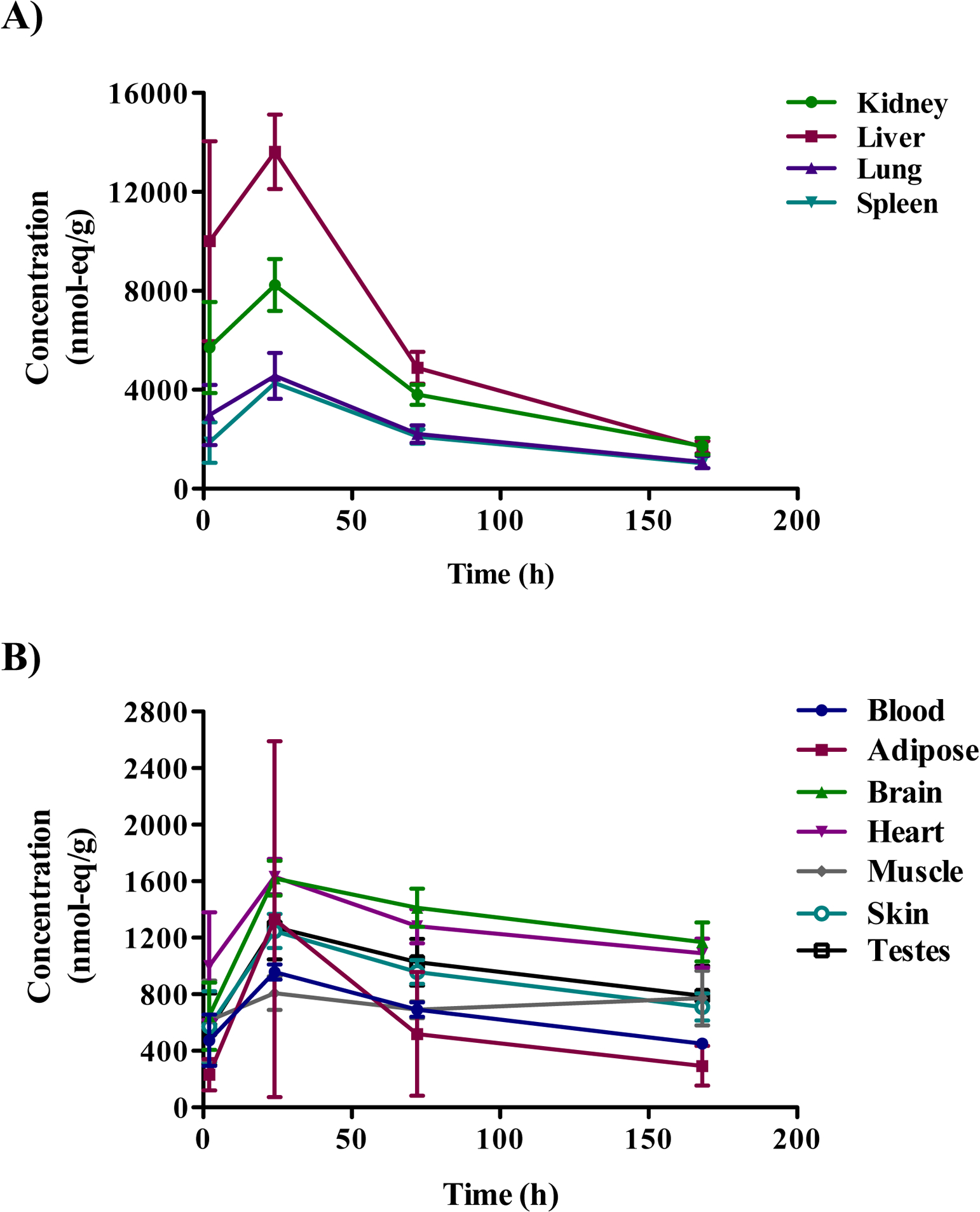
Tissue distribution of radioactivity 2, 24, 72, and 168 h in selected tissues following oral administration of [14C]DMAE (500 mg/kg) in male Wistar Han rats. A) Distribution in the kidney, liver, lung, and spleen B) Distribution in the blood, adipose, brain, heart, muscle, skin, and testes.
In order to investigate the strain difference in disposition, male Sprague Dawley rats were orally administered 500 mg/kg [14C]DMAE and sacrificed 24 h following administration. The disposition of radioactivity was similar to Wistar Han rats given a similar dose (Tables 3 and S1).
Following IV administration of 10 mg/kg [14C]DMAE in male Wistar Han rats, the pattern of disposition was similar to oral administration (Tables 3 and S1). However, the total dose recovered in oral group (95%) was higher than the IV group (82%), and the difference likely reflects the lower recovery in urine in IV dose group (~ 39%) compared to oral dose group (~ 57%).
Incorporation of [14C]DMAE-derived radioactivity in lipid in Wistar Han rats following gavage administration of [14C]DMAE.
Male Wistar Han rats were orally administered 100 mg/kg [14C]DMAE and sacrificed 2 h following administration. Residual radioactivity associated with lipids in selected tissues was estimated (Table 4). The highest level of radioactivity incorporated into lipids in any tissues was ~ 1% of the administered dose. Of the three lipids evaluated (neutral lipids, fatty acids, and phospholipids), the phospholipids contained the highest amount of radioactivity across all tissues examined, suggesting that DMAE is preferentially incorporated into the phospholipids. The highest levels of radioactivity in phospholipids were measured in the lung, kidney, and liver, while the highest levels in fatty acids were determined in the lung and the kidney. The highest levels of radioactivity in neutral lipids were found in the lung and the brain.
Table 4.
Incorporation of [14C]DMAE-derived radioactivity in tissue lipids in male Wistar Han rats following a single gavage administration of 100 mg/kg [14C]DMAE.
| Total radioactivity (% dose administered) | |||
|---|---|---|---|
| Tissue | Neutral Lipids | Fatty Acids | Phospholipids |
| Spleen | 7 (0.004) | 7 (0.004) | 95 (0.055) |
| Brain | 107 (0.075) | 34 (0.020) | 167 (0.097) |
| Lung | 395 (0.276) | 127 (0.074) | 1587 (0.926) |
| Kidney | 41 (0.029) | 81 (0.047) | 1322 (0.771) |
| Testes | 11 (0.008) | 23 (0.013) | 167 (0.097) |
| Skin | 4 (0.003) | 12 (0.007) | 430 (0.251) |
| Heart | 40 (0.028) | 17 (0.010) | 323 (0.188) |
| Liver | NDa | ND | 1915 (1.117) |
ND = Not determined
Disposition of [14C]DMAE in male and female B6C3F1 mice following gavage administration.
The disposition of [14C]DMAE 24 h after a single gavage dose (10, 100, or 500 mg/kg) is presented in Table 5. Dose response in disposition was investigated only in female mice with limited studies in male mice. Following oral administration in female B6C3F1 mice, the excretion in urine increased with the increasing dose (16, 18, and 43% recovered at 10, 100, and 500 mg/kg, respectively) while that recovered as CO2 decreased with increasing dose (22, 16, and 13% recovered at 10, 100, and 500 mg/kg, respectively) suggesting potential change in metabolism and excretion pathways with increasing dose. Radioactivity in feces and VOCs was generally low (<2%) across all dose groups. Excretion was also investigated up to 72 h following a single oral administration of 500 mg/kg [14C]DMAE in female mice (Table 5) and cumulative percentages of dose excreted are shown in Figure 4 for urine, CO2, and VOCs for all dose groups. Excretion of [14C]DMAE-derived radioactivity in urine was rapid for up to 24 h but increased only slightly between 24 and 72 h. However, the percentage of dose recovered as expired CO2 and VOCs increased with time up to 72 h (Figure 4).
Table 5.
Disposition of [14C]DMAE in male and female B6C3F1 mice 24 or 72 h following a single gavage administration.
| % Dose Recovereda | |||||
|---|---|---|---|---|---|
| Tissue | Female 10 mg/kg |
Female 100 mg/kg |
Female 500 mg/kg |
Male 500 mg/kg |
Femaleb 500 mg/kg |
| Urinec | 15.8 ± 4.00 | 17.9 ± 3.90 | 43.4 ± 11.5 | 27.1 ± 20.0 | 53.8 ± 3.90 |
| Feces | 1.94 ±0.490 | 1.10 ± 0.410 | 0.713 ± 0.273 | 13.7 ± 14.9 | 2.24 ± 1.31 |
| VOCs | 1.66 ± 0.340 | 1.27 ± 0.160 | 1.51 ± 0.210 | 1.75 ± 0.319 | 1.08 ± 0.190 |
| CO2 | 22.3 ± 3.68 | 15.5 ± 0.600 | 13.2 ± 1.80 | 17.8 ± 1.10 | 21.0 ± 2.20 |
| Tissues and GI Tractd | 57.6 ± 3.00 | 44.5 ± 1.70 | 38.4 ± 16.1 | 31.8 ± 1.50 | 19.0 ± 3.70 |
| Total Recovered | 99.3 ± 1.60 | 81.6 ± 4.40 | 97.1 ± 15.2 | 92.1 ± 13.6 | 97.2 ± 6.40 |
Mean ± SD for 3 animals;
72 h, N = 4
Includes urine present in the bladder at study termination
Includes contents
Figure 4:
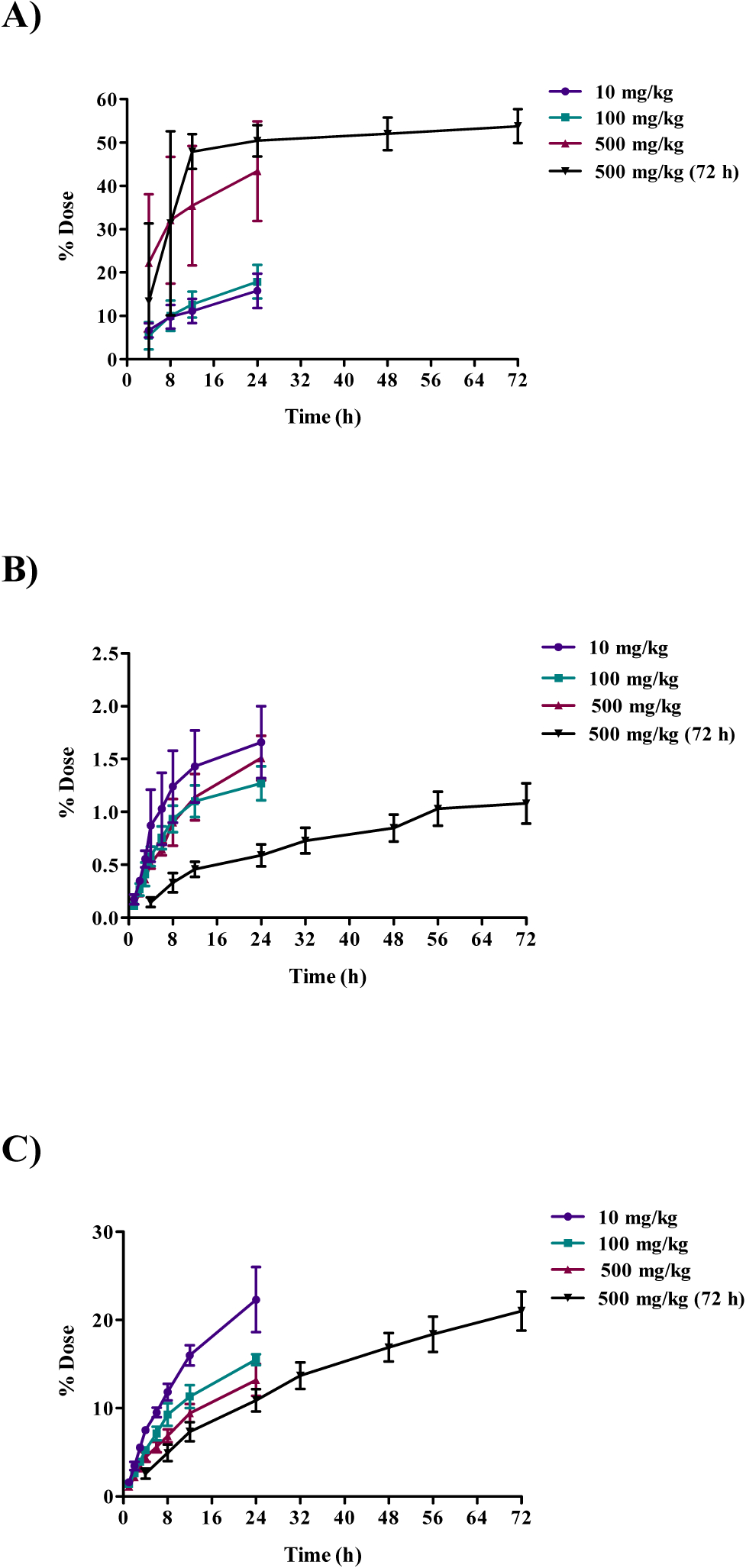
Cumulative excretion of radioactivity following oral administration of 10, 100, or 500 mg/kg [14C]DMAE in female B6C3F1 mice. Cumulative percent dose A) excreted in urine, B) excreted as exhaled volatile organic compounds (VOCs), and C) excreted as expired CO2.
The concentration of [14C]DMAE-derived radioactivity in tissues, 24 h after administration, increased with dose (Table S3). The approximate percent radioactivity remaining in all tissues, excluding GI tract, at 24 h post-administration was 44, 30 and 32% for 10, 100, and 500 mg/kg, respectively. The tissues with the highest levels of radioactivity were the liver, kidney, thyroid, lung, spleen, adipose, and uterus (Table S3). In general, the concentration of total radioactivity in tissues was higher at 24 h compared to 72 h with 32% and 16% of the total administered dose recovered at 24 and 72 h, respectively (Table S3). The pattern of disposition of [14C]DMAE in male B6C3F1 mice administered a single oral dose of 500 mg/kg [14C]DMAE was similar to females administered the same dose. The total percentage of administered dose recovered in mouse studies ranged from 82–99% (Table 5).
Effect of [14C]DMAE on serum choline levels in male or female rats.
Blood was collected at multiple timepoints following oral gavage administration of 500 mg/kg [14C]DMAE in male rats. Serum choline levels were measured to determine if administration of DMAE would increase serum choline levels. Mean choline levels at pre-dose were approximately 17 ng/mL; the levels increased approximately to 23 ng/mL serum at 1 h post-dose, and remained elevated at these levels up to 12 h post-administration. In serum from male and female rats administered a gavage dose of 500 mg/kg [14C]DMAE with a higher radioactivity (1.5 mCi/kg), [14]C choline was not detected, suggesting the absence of metabolic conversion of DMAE to choline under the experimental conditions used (data not shown).
Metabolite profiling and identification.
Urine from the 500 mg/kg group was extracted using C18 SPE and analyzed by LC-MS. The analysis detected DMAE (m/z 90) and two metabolites with m/z at 104 and 106 (Figure 5A). The m/z 104 was tentatively identified by MS/MS as N,N-dimethylglycine (Figure 5B) and m/z 106 as DMAE oxide (Figure 5C).
Figure 5:
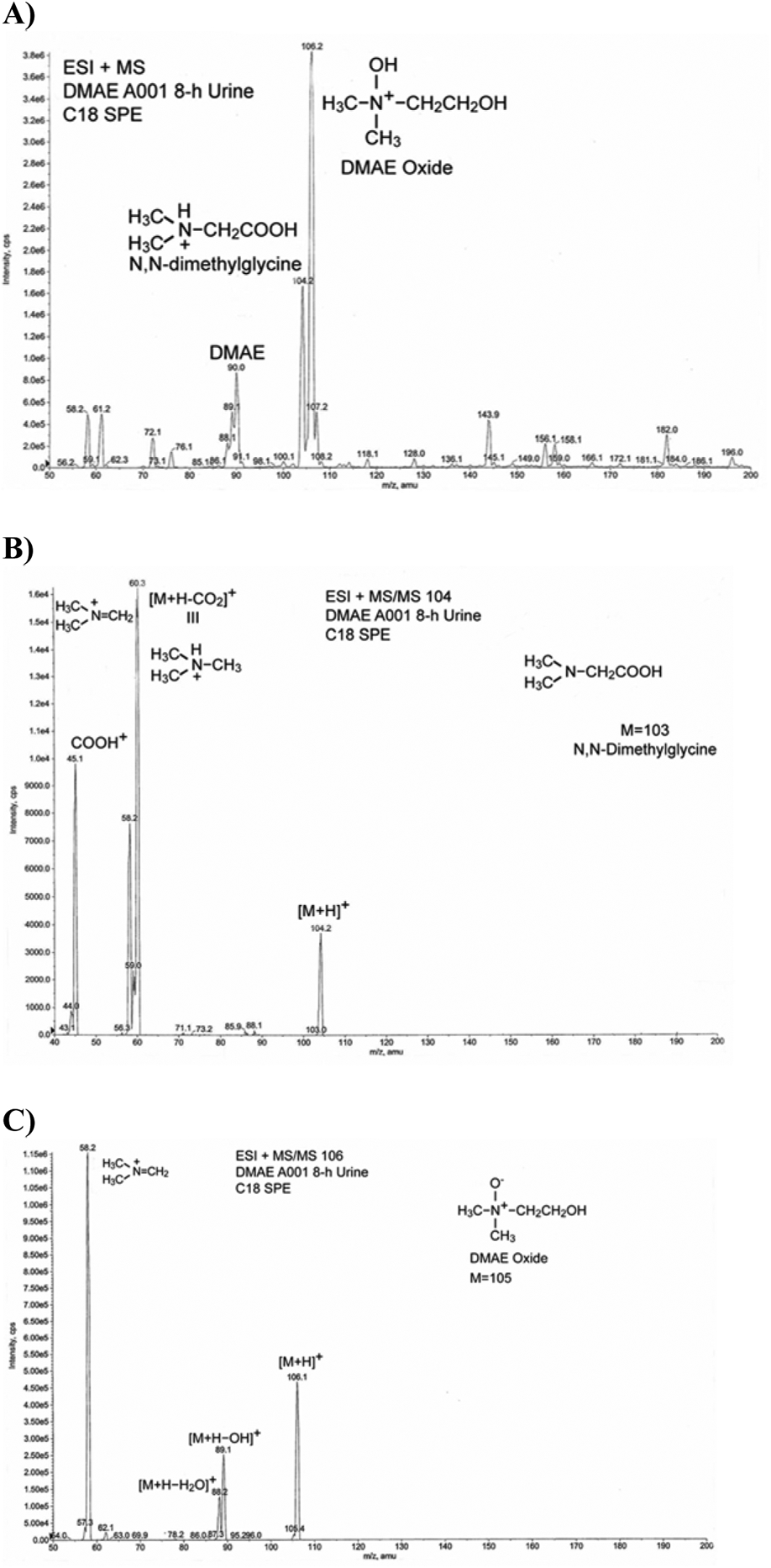
Identification of metabolites of DMAE in rat urine by LC-MS following a single oral administration of 500 mg/kg [14C]DMAE. A) Total ion chromatogram. B) m/z 104. C) m/z 106.
Urine from all DMAE dose groups was profiled using HPLC (Method C) using radiochemical profiling. Chromatograms showed up to two peaks; early eluting peak co-eluted with DMAE (~ 8.6 min) (data not shown). Estimated percentages of area represented by DMAE and DMAE oxide in chromatograms in urine from Harlan Sprague Dawley rats and B6C3F1 mice following administration of [14C]DMAE are shown in Table 6. Following oral administration, there was a trend in increasing DMAE oxide levels with increasing dose in male rats with moderate differences between males and females. Following an IV administration in male rats (10 mg/kg), levels of DMAE oxide were much higher (oral, 5%; IV, 97%) than an oral administration. In female mice, the levels of DMAE oxide decreased with dose with 92% detected at 10 mg/kg and 18–34% detected at 100–500 mg/kg. In male mice, under the conditions of the analysis, a peak where DMAE oxide was eluting was absent and the reason for this is not clear. These data suggest potential route-, species, and sex-dependent metabolism of DMAE to DMAE oxide. The potential formation of a carcinogen, N,N-dimethylnitrosamine, was investigated using LC-MS Method B following oral administration of DMAE in rats. N,N-dimethylnitrosamine was not detected in rats following administration of 10 to 500 mg/kg dose group at or above the limit of quantitation (5 pg/mL) of the analytical method.
Table 6.
Estimated percentagea of area represented by each peak in chromatogram in urine from Harlan Sprague Dawley rats and B6C3F1 mice following administration of [14C]DMAEa
| Species/Sex | Dose (mg/kg) | Route | DMAE (%) | DMAE Oxide (%) |
|---|---|---|---|---|
| Rat/Male | 10 | Oral | 95 | 5 |
| Rat/Male | 100 | Oral | 56 | 44 |
| Rat/Male | 500 | Oral | 34 | 66 |
| Rat/Female | 500 | Oral | 55 | 45 |
| Rat/Male | 10 | IV | 3 | 97 |
| Mouse/Female | 10 | Oral | 8 | 92 |
| Mouse/Female | 100 | Oral | 82 | 18 |
| Mouse/Female | 500 | Oral | 66 | 34 |
| Mouse/Male | 500 | Oral | 100 | 0 |
Number of animals per group varies between 3 and 5
2. Disposition of [14C]choline with or without DMAE pre-treatment
Disposition of [14C]choline in male and female rats following gavage administration with or without DMAE pre-treatment and following intravenous administration.
The disposition of [14C]choline in male Wistar Han rats 24 h after a single gavage (160 mg/kg) dose with or without DMAE pre-treatment (100 or 500 mg/kg) is presented in Table 7. The cumulative excretion data are shown in Figure 6 and tissue data are given in Figure 7 and Table S4. Administration of [14C]choline alone resulted in approximately 33% of the dose recovered in urine, 22% in feces, 13% in CO2, and ~18% in tissues (excluding GI tract); only trace amounts of radioactivity were recovered in VOCs (Tables 7 and S4). Pre-treatment with one (100 or 500 mg/kg, 1 h prior to) or three doses (100 mg/kg, 48, 24, and 1 h prior to) of DMAE prior to [14C]choline administration resulted in a similar disposition pattern to that of [14C]choline alone with 32–41%, 12–17%, and 15–22% of the administered dose recovered in urine, CO2 and tissues (excluding GI tract), respectively (Tables 7 and S4 and Figures 6 and 7). The [14C]choline dose recovered in feces following a single pre-treatment of 100 mg/kg DMAE (21%) was similar to [14C]choline (22%) alone. However, compared to choline administration alone (22%), the dose recovered in feces was lower (10–12%) following pre-treatment with multiple doses of 100 mg/kg or a single dose of 500 mg/kg DMAE (Table 7). Tissue concentrations were highest in the liver, kidney, thyroid, lung, and spleen and lowest in the brain and adipose, regardless of treatment (Table S4, Figure 7). The total dose recovered in male rat groups ranged from 86–88%.
Table 7.
Disposition of [14C]choline in male and female Wistar Han rats 24 h following a single gavage (160 mg/kg) or IV (16 mg/kg) administration with or without DMAE pre-treatment.
| % Dose Recovereda | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DMAE dose (mg/kg) | 0 | 100 | 500 | 100(3)b | 0 | 100 | 500 | 0 | 0 |
| Tissue | Male, Gavage 160 mg/kg |
Male, Gavage 160 mg/kg |
Male, Gavage 160 mg/kg |
Male, Gavage 160 mg/kg |
Female, Gavage 160 mg/kg |
Female, Gavage 160 mg/kg |
Female, Gavage 160 mg/kg |
Male, IV 16 mg/kg |
Female, IV 16 mg/kg |
| Urinec | 32.6 ± 2.30 | 31.9 ± 4.60 | 40.7 ± 6.30 | 39.9 ± 3.80 | 34.2 ± 7.90 | 34.7 ± 5.70 | 43.3 ± 5.60 | 3.92 ± 0.850 | 7.45 ± 2.58 |
| Feces | 21.5 ± 5.10 | 21.3 ± 13.8 | 11.2 ± 8.40 | 10.2 ± 10.2 | 12.9 ± 10.0 | 10.8 ± 6.00 | 2.52 ± 2.85 | 0.107 ± 0.052 | 0.303 ± 0.132 |
| VOCs | 0.123 ± 0.034 | 0.018 ± 0.003 | 0.065 ± 0.002 | --- | 0.325 ± 0.025 | 0.268 ± 0.026 | 0.227 ± 0.057 | 0.139 ± 0.035 | 0.284 ± 0.038 |
| CO2 | 12.5 ± 2.20 | 12.4 ± 1.70 | 15.1 ± 4.00 | 17.0 ± 1.70 | 17.8 ± 1.70 | 22.5 ± 4.40 | 22.4 ± 3.60 | 17.4 ± 2.40 | 28.1 ± 2.90 |
| Tissues and GI Tractd | 21.4 ± 2.80 | 20.4 ± 5.60 | 20.0 ± 3.80 | 26.6 ± 5.30 | 26.1 ± 3.80 | 24.7 ± 2.70 | 25.9 ± 4.70 | 56.2 ± 9.90 | 54.0 ± 5.50 |
| Total Recovered | 88.2 ± 5.50 | 86.1 ± 6.20 | 87.1 ± 1.90 | 93.7 ± 2.70 | 91.3 ± 9.60 | 92.9 ± 4.80 | 94.1 ± 4.80 | 77.7 ± 12.0 | 90.1 ± 6.10 |
Mean ± SD for 3 animals
Males were administered three DMAE pre-treatments 48, 24, and 1 h prior to [14C]-choline administration.
Includes urine present in the bladder at study termination
Includes contents
Figure 6:
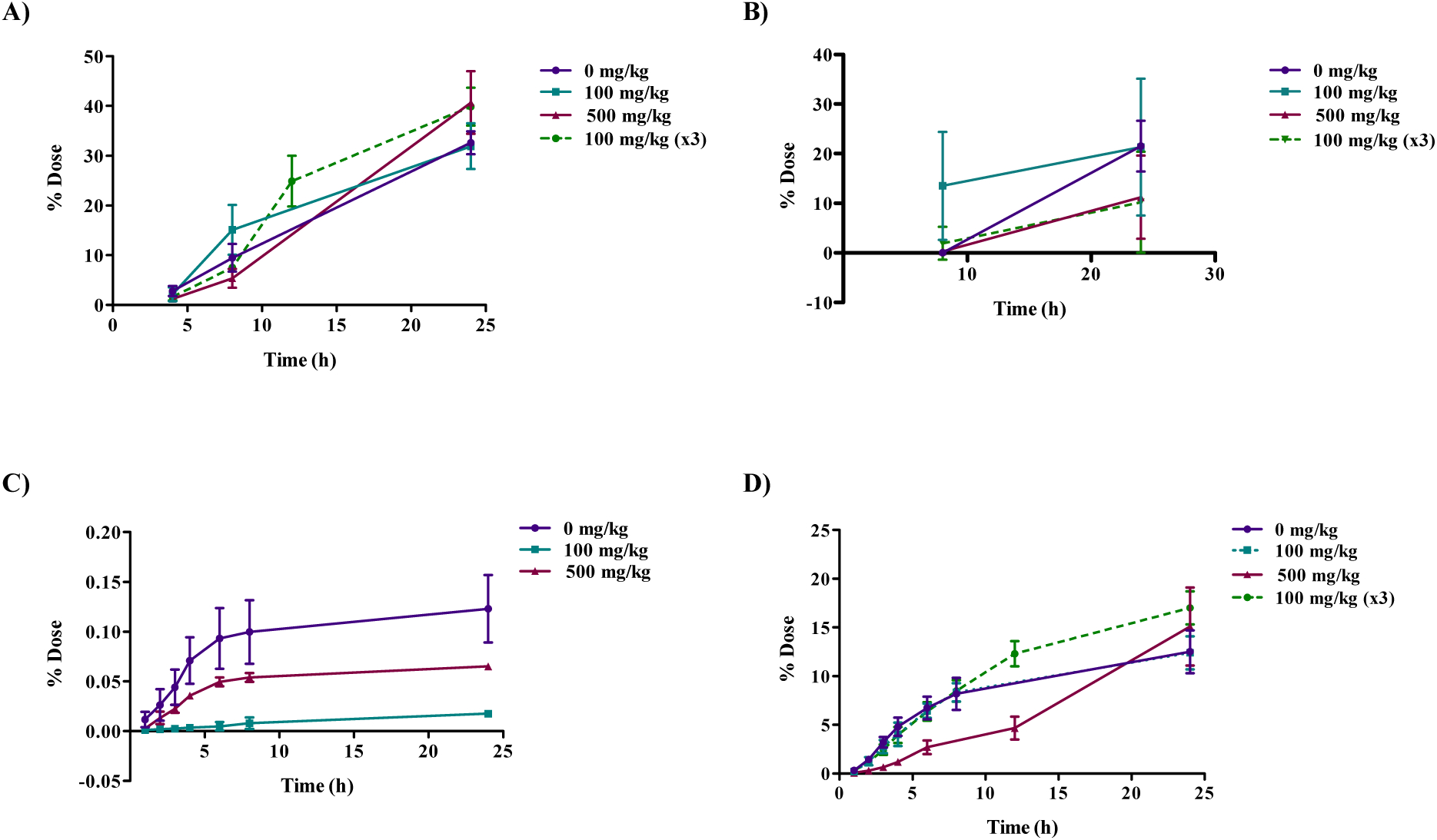
Cumulative excretion of radioactivity 24 h following oral administration of 160 mg/kg [14C]choline without or with DMAE pre-treatment of 100 (1 or 3 doses) or 500 mg/kg in male Wistar Han rats. Cumulative percent dose A) excreted in urine, B) excreted in feces, C) excreted as volatile organic compounds (VOCs), D) excreted as expired CO2. Rats in the DMAE pre-treatment groups received a single dose of 100 or 500 mg/kg DMAE 1 h prior to or 3 doses of 100 mg/kg DMAE 48, 24, and 1 h prior to [14C]choline administration. VOCs were below the limit of detection in the 100 mg/kg (x3) group.
Figure 7:
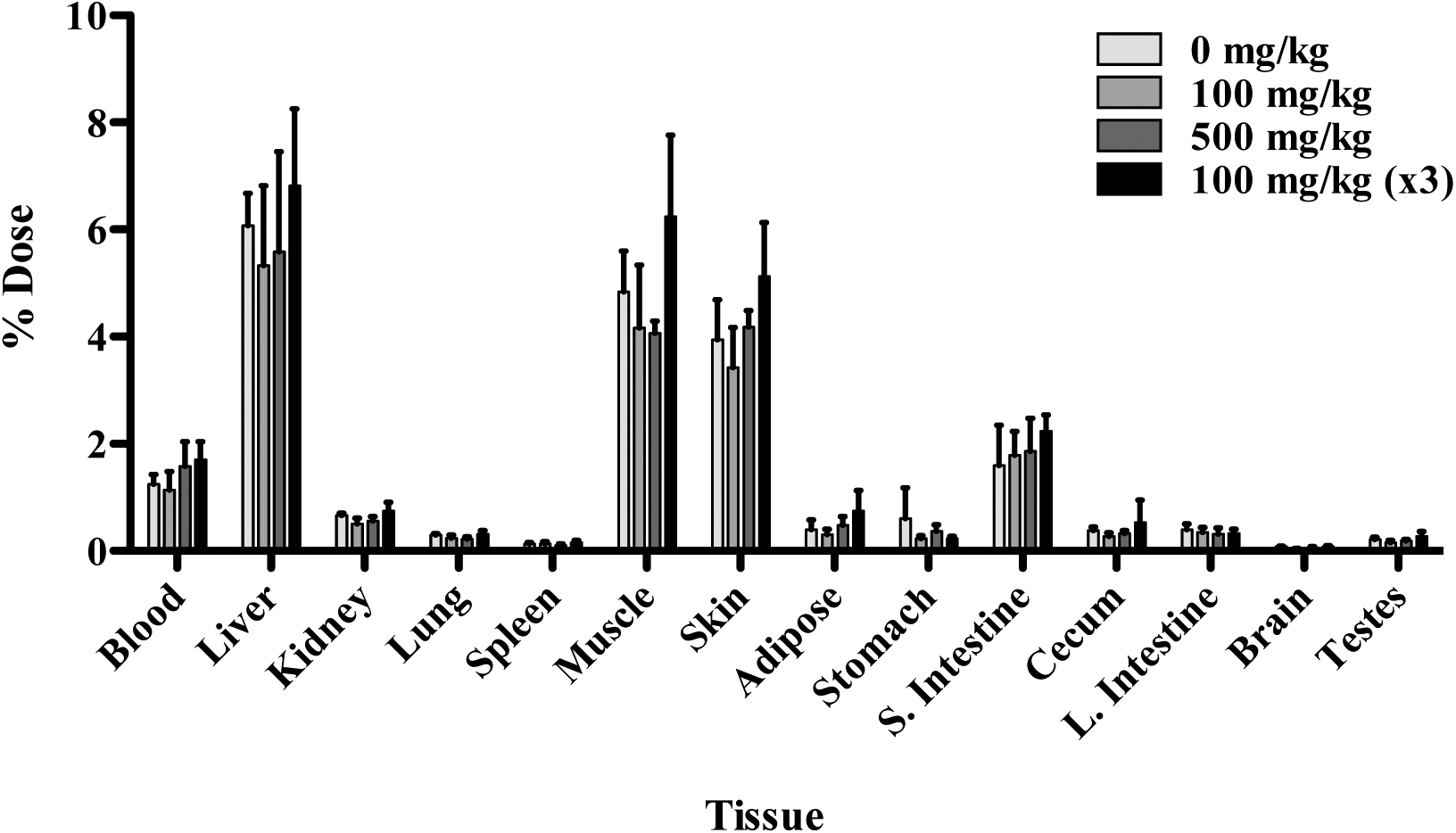
Tissue distribution of radioactivity 24 h following oral administration of 160 mg/kg [14C]choline without or with DMAE pre-treatment of 100 (1 or 3 doses) or 500 mg/kg in male Wistar Han rats. Rats in the DMAE pre-treatment groups received a single dose of 100 or 500 mg/kg DMAE 1 h prior to or 3 doses of 100 mg/kg DMAE 48, 24, and 1 h prior to [14C]choline administration. Data for selected tissues are presented.
The disposition of [14C]choline in female Wistar Han rats 24 h after a single gavage (160 mg/kg) dose with or without DMAE pre-treatment (a single 100 or 500 mg/kg) is presented in Table 7. Administration of [14C]choline alone resulted in 34% recovery in urine, 13% in feces, 18% in CO2, and ~21% in tissues other than the GI tract (Table 7 and S4). Pre-treatment with a single dose of 100 mg/kg DMAE prior to [14C]choline administration resulted in a similar disposition pattern to that of [14C]choline alone with 35%, 11%, 23%, and 21% of the administered [14C]choline dose recovered in urine, feces, CO2 and tissues (excluding GI tract), respectively (Tables 7 and S4). However, a single pretreatment with a 500 mg/kg DMAE dose prior to [14C]choline administration resulted in decreased fecal excretion (2.5%) relative to no pre-treatment or 100 mg/kg DMAE pre-treatment (11–13%) (Table 7). Similar to male rats, tissue concentrations were highest in the liver, kidney, thyroid, lung, and spleen and lowest in the brain and adipose, regardless of treatment (Table S4). The total dose recovered in female rats ranged from 91–94% (Table 7).
When comparing across route of administration, IV administration of 16 mg/kg [14C]choline resulted in less excretion in the urine of male and female rats (4 and 7%, respectively) (Table 7). The residual radioactivity remaining in tissues (excluding GI tract) was higher following IV administration (48–49%) (Table S5) relative to oral gavage (17–21%) (Table S4). Tissue radioactivity concentrations were highest in the liver, kidney, lung, spleen, and thyroid (Table S5). When comparing between sex, similar disposition patterns were observed in both male and female Wistar Han rats (Table 7). The total percentage of administered dose recovered was approximately 78% in males and 90% in females (Table 7).
Disposition of [14C]choline in female mice following gavage administration with or without DMAE pre-treatment.
The disposition of [14C]choline in female B6C3F1 mice 24 h after a single gavage (160 mg/kg) dose with or without a single DMAE pre-treatment of 500 mg/kg is presented in Table 8. The cumulative excretion data are shown in Figure 8 and tissue data are given in Table S6. Administration of [14C]choline alone resulted in 32% recovery in urine, 3% in feces, 4% in VOCs, 25% in CO2, and 15% in tissues (excluding GI tract). Pre-treatment with 500 mg/kg DMAE resulted in an excretion pattern very similar to that of [14C]choline alone, with 32% recovery in urine. The total dose recovered in feces was 11% with DMAE pre-treatment, compared to 3% without pre-treatment; however, this could potentially be an artifact due to higher total dose recovered in the DMAE-pre-treated group)91%) compared to untreated (81%). The total percentage recovered in tissues was not affected by DMAE pre-treatment and was approximately 14% in both groups (Table S6). Tissue concentrations were highest in the liver, kidney, and lung regardless of pre-treatment. Radioactivity levels were also high in the thyroid and uterus of mice administered [14C]choline alone (Table S6).
Table 8.
Disposition of [14C]choline in female B6C3F1 mice 24 h following a single gavage administration with or without DMAE pre-treatment.
| % Dose Recovereda | ||
|---|---|---|
| DMAE dose (mg/kg) | 0 | 500 |
| Tissue | Female 160 mg/kg |
Female 160 mg/kg |
| Urineb | 31.8 ± 11.1 | 32.4 ± 7.20 |
| Feces | 2.62 ± 3.34 | 11.2 ± 5.90 |
| VOCs | 3.61 ± 3.75 | 2.41 ± 1.77 |
| CO2 | 24.5 ± 5.40 | 26.4 ± 8.60 |
| Tissues and GI Tractc | 18.2 ± 2.90 | 18.4 ± 9.80 |
| Total Recovered | 80.8 ± 16.7 | 90.8 ± 8.60 |
Mean ± SD for 3 animals
Includes urine present in the bladder at study termination
Includes contents
Figure 8:
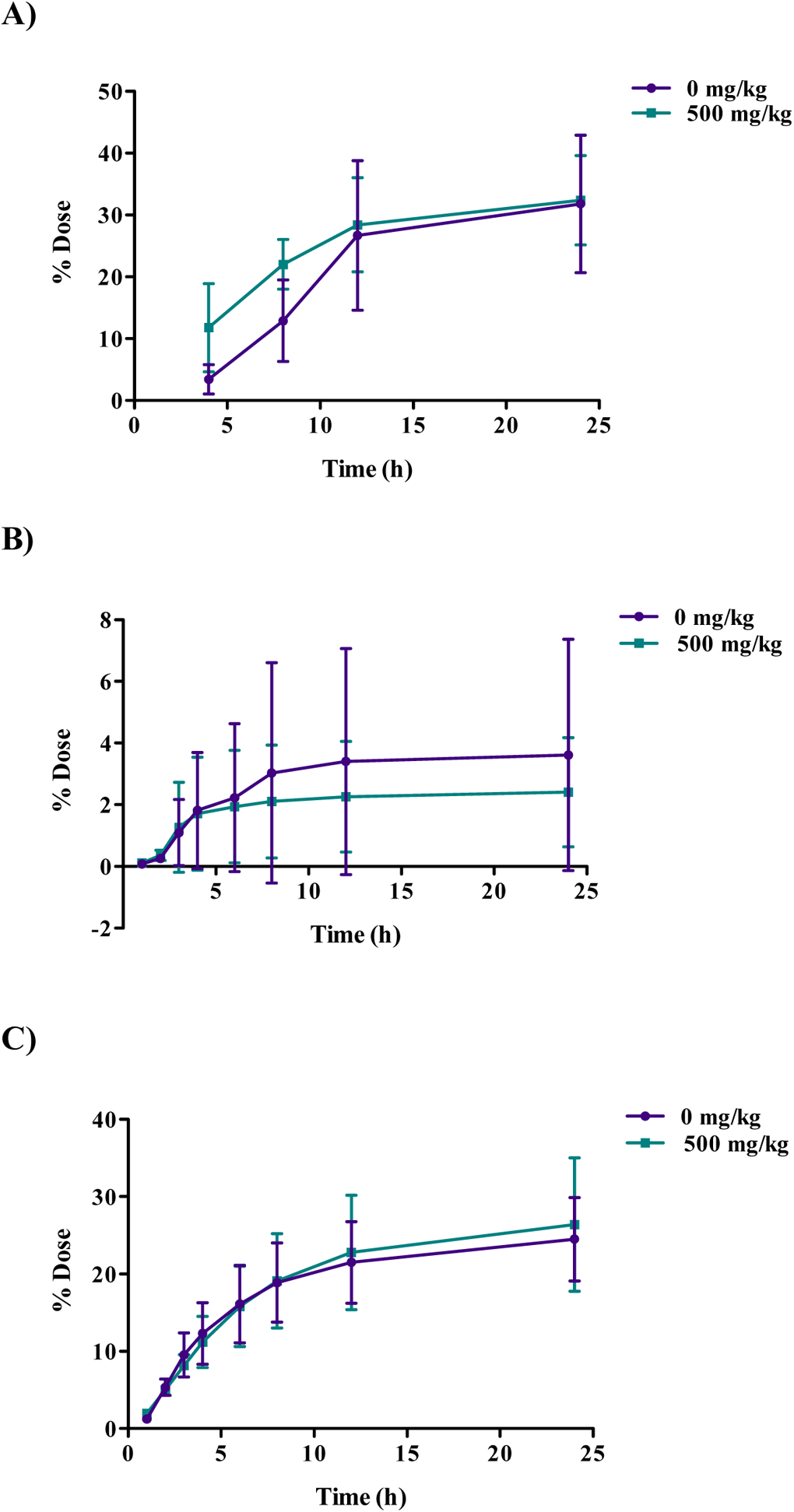
Cumulative excretion of radioactivity 24 h following oral administration of [14C]choline (160 mg/kg) with or without DMAE pre-treatment (500 mg/kg) in female B6C3F1 mice. Mice in DMAE pre-treatment groups received a single dose of 500 mg/kg DMAE 1 h prior to [14C]choline administration Cumulative percent dose A) excreted in urine, B) excreted as volatile organic compounds (VOCs), C) excreted as expired CO2.
DISCUSSION
DMAE and DMAE salts (e.g. p-acetamidobenzoate) have been used to treat central nervous system disorders in humans (Stenbäck et al., 1988). Due to a paucity of toxicity data for these compounds, the NTP is testing the toxicity of DMAE in rodents. ADME data are important to designing toxicology studies and interpreting study data, and there is a significant data gap for ADME of DMAE following routes of exposure relevant to humans. Choline, an essential nutrient, is required for many biological processes, including nervous system development. Choline deficiency has been associated with neural tube defects in both rodents and humans (Fisher et al., 2001; 2002; Shaw et al., 2004; 2009; Mills et al., 2014). DMAE is a close structural analog of choline, and hence, there is potential for DMAE to disrupt choline uptake and metabolism and interfere with biological processes such as development. Therefore, it is important to understand the disposition choline in the presence of DMAE. This research: 1) addressed sex-, species-, and dose-dependent effects on DMAE ADME, 2) addressed the potential for DMAE to form reactive metabolites 3) compared the in vivo fate of DMAE and choline, and 4) evaluated the effect of DMAE pre-treatment on the uptake and de novo synthesis of choline.
DMAE was well-absorbed following oral administration to both Wistar Han rats and B6C3F1 mice, with approximately 21–29% (rats) and 26–44% (mice) of the administered dose recovered in tissues (excluding GI tract) 24 h post-exposure. The pattern of tissue recovery after DMAE administration is similar to that reported for another alkanolamine, diethanolamine (DEA), where significant tissue accumulation was noted following oral or IV administration (Mathews et al., 1997). In those studies, the authors hypothesized that the high tissue accumulation of DEA was due to metabolic incorporation of DEA into phospholipid headgroups and the slow elimination kinetics of phospholipid catabolism. We hypothesized a similar mechanism may be responsible for the high recovery of DMAE in tissues in the studies reported here. In our studies, DMAE was incorporated into lipids, in particular phospholipids, which is consistent with previous studies showing that alkanolamines may incorporate into phospholipids (Matthews et al., 1997).
The primary route of DMAE excretion was in urine, with approximately 57–64% excretion in rats and 16–43% excretion in mice following gavage administration. There were no dose- or sex-specific differences in rats; however, in mice, excretion in urine was highest in females administered 500 mg/kg [14C]DMAE. A high percentage of administered DMAE was also excreted as exhaled CO2 in mice (13–22%) compared to rats (≤ 6%). The overall excretion pattern in both urine and CO2 was similar in rats regardless of dose; however, this was not true for mice. In mice, there was a clear switch in excretion pattern for urine and CO2, with excretion in urine increasing with dose and excretion in CO2 decreasing with dose. In addition, the percent excretion in urine plateaued by 24 h post-administration, while the percent excretion in CO2 continued to increase through 72 h. Excretion in urine was much lower in rats following IV administration (39%) relative to oral administration. Minimal excretion in feces or as VOCs occurred in both rats and mice regardless of route of administration.
One of the concerns with DMAE exposure is effects on the central nervous system. Concentrations of [14C]DMAE equivalents in the brains of exposed rats and mice were fairly low relative to other tissues (kidney, liver, lung, spleen). In male Wistar Han rats, brain concentrations peaked at approximately 24 h and decreased slightly over time out to 168 h post-administration; brain to blood ratios were 1.36 to 2.60 suggesting limited entry of DMAE to the brain (Table S2). The lipid incorporation study showed that the radioactivity recovered in lipids in the brain was relatively low compared to the other tissues. This observed low incorporation could partly be due to the fact that the radiolabel was on ethanolic carbons and not methyl carbons as these carbon groups are likely metabolized differently. These observations point to limited effects on the brain following DMAE administration.
It has been proposed that DMAE may interfere with choline synthesis and subsequently effect acetylcholine levels and downstream CNS function. In order to evaluate this, rats and mice were administered [14C]choline with or without pre-treatment with unlabeled DMAE. Following oral administration, the pattern of [14C]choline disposition was similar to [14C]DMAE, and the primary route of excretion was urine. There were no sex- or species-specific differences in [14C]choline uptake and excretion. The absorption, tissue distribution, and excretion patterns of [14C]choline were not significantly affected by DMAE pre-treatment, regardless of pre-treatment dose (100 or 500 mg/kg) or number of doses (1 or 3). The only exception to this was a slight delay in the excretion of [14C]choline as expired CO2 as the DMAE pre-treatment dose increased.
The absence of a significant effect of DMAE on choline uptake and distribution is a significant finding and suggests that DMAE and choline interaction in vivo may be minimal. As an analog of choline, one of the primary concerns for DMAE toxicity is the potential for it to affect acetylcholine levels, either by inhibiting choline transporters or being methylated itself to form choline (Sterling et al., 1986; De Silva, 1977; HSDB, 2015). It has been suggested that DMAE may be a precursor for acetylcholine (Pfeiffer et al., 1957). However, other reports have failed to support that hypothesis (Zahniser et al., 1977). It has also been reported that DMAE may impact choline metabolism and excretion in the kidney, but not the liver, and that DMAE inhibits choline oxidase and modifies choline metabolism (Haubrich, et al., 1981; Lohr and Acara, 1990). The majority of these previous studies on DMAE and choline interaction were conducted in vitro or ex vivo. The data reported here do not support a direct interaction between DMAE and choline, indicating that there may in fact be little concern for toxicity, at least related to acetylcholine levels, associated with this compound. Taken collectively, these findings suggest that observations in vitro or at the organ level may not translate to in vivo.
Following administration of [14C]choline to rats, two metabolites were identified in urine: trimethylamine oxide (major metabolite) and betaine (minor metabolite) (Figure 9). Urine levels of these metabolites were higher in rats pre-treated with DMAE and administered [14C]choline relative to the [14C]choline-only control. This suggests a possible effect of DMAE on renal handling of choline metabolites.
Figure 9:
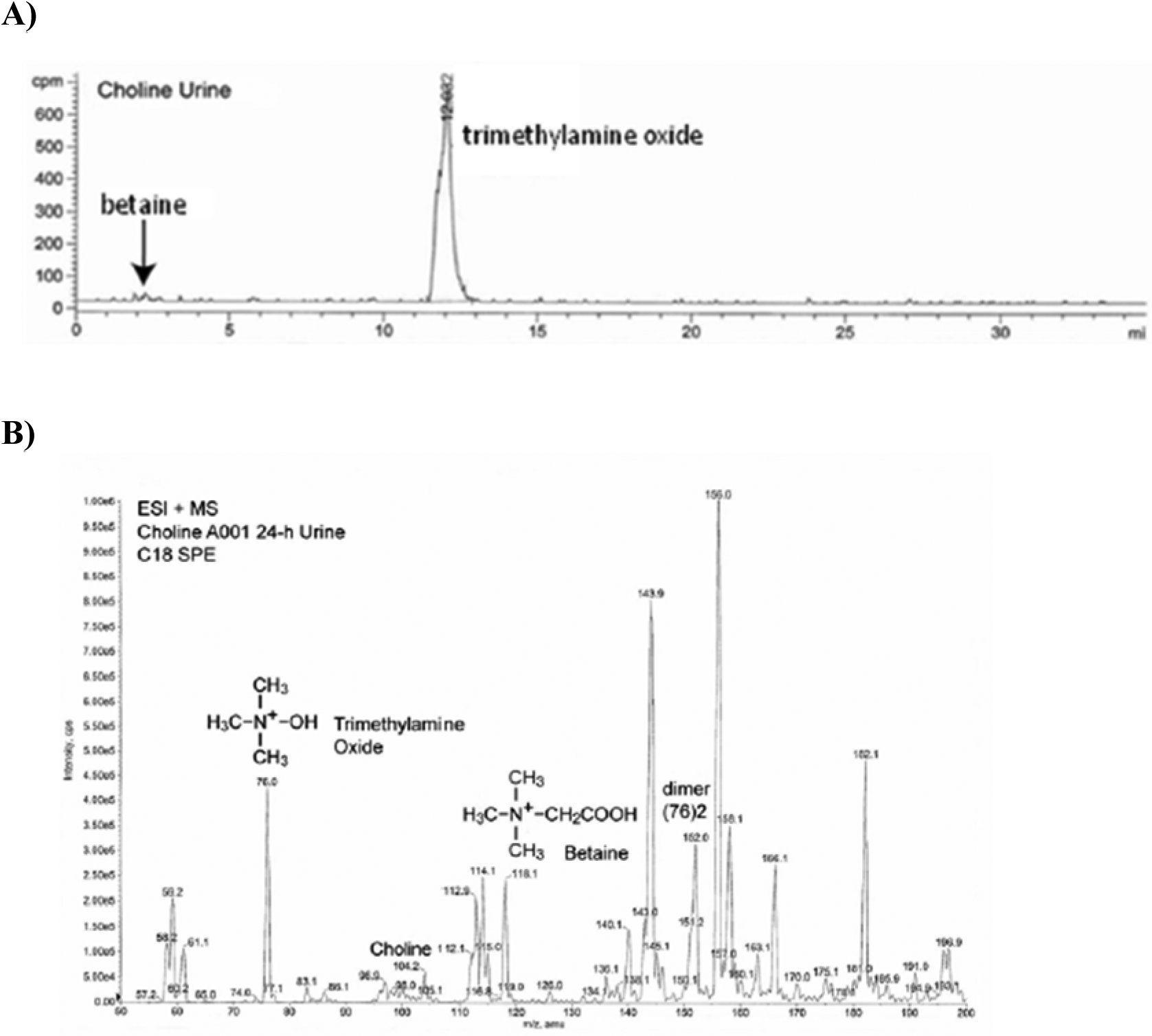
Identification of choline metabolites in male rat urine following oral administration of 160 mg/kg [14C]choline. A) Total ion chromatogram, B) ESI(+)-MS mass spectrum
It has been suggested that DMAE may be methylated to choline in vivo; however, the work presented here does not support that hypothesis. Serum choline levels in male rats were not significantly affected by administration of [14C]DMAE and there was also no evidence for the formation of [14C]choline in serum from Sprague Dawley rats administered 500 mg/kg [14C]DMAE. These studies in rats demonstrated that [14C]DMAE equivalents were not metabolized directly to [14C]choline. However, incorporation of [14C]DMAE into phospholipid headgroups and subsequent methylation to phosphatidyl[14C]choline may be one potential overlap of DMAE with choline metabolic pools.
The DMAE and DMAE oxide levels in urine samples were variable depending on dose, sex, and species. Male rats administered 500 mg/kg [14C]DMAE had high levels of DMAE oxide formation, while male mice administered the same dose produced very little DMAE oxide. Generally, levels of DMAE oxide increased in a dose-dependent manner in male rats and decreased in a dose-dependent manner in female mice. The exact reason for this difference is not clear but points to species differences in metabolism pathways.
One of the concerns for DMAE exposure in humans is the potential for DMAE to undergo oxidative dealkylation to dimethylamine, which can then be further metabolized to N,N-dimethylnitrosamine, a potential carcinogen (Preusmann, 1984; Guengerich et al., 1996; Chowdhury et al., 2010). The data from these studies did not support this hypothesis as N,N-dimethylnitrosamine was not detected in the urine of rats or mice. There was also no evidence of the formation of oxalate in urine following [14C]DMAE administration, suggesting that oxidative cleavage of DMAE to dimethylamine is an unlike metabolism pathway of DMAE in rodents. A proposed metabolic pathway for DMAE is presented in Figure 10.
Figure 10:
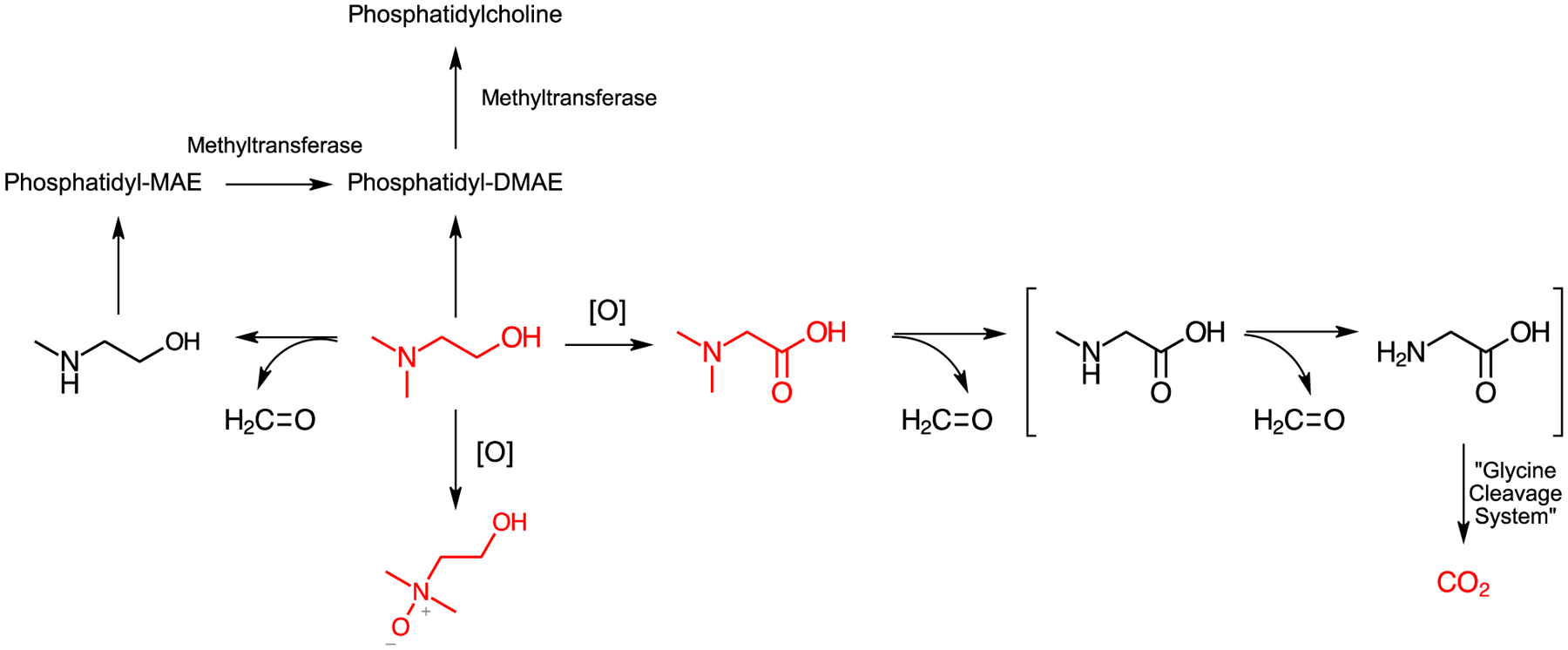
Proposed metabolic pathway for DMAE. Structures in red were identified in these studies. Bracketed structures are hypothesized intermediates.
CONCLUSIONS
These studies demonstrated that DMAE was well-absorbed and highly retained in tissues. DMAE is excreted primarily as exhaled CO2 and through the urine, either unchanged or as DMAE oxide. Under the conditions of our studies, DMAE was not metabolized to choline or the carcinogenic N,N-dimethylnitrosamine. In addition, choline disposition was not affected by DMAE pre-treatment. Data from the current study did not support previous reports that: 1) DMAE alters choline uptake and distribution, or 2) that DMAE is converted into choline in vivo.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Drs. Madelyn Huang and Gabriel Knudsen for their review of this manuscript. This work was performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contract No. N01-ES-75562 (HHSN29120077562).
Footnotes
Declaration of Interest
The authors report no declarations of interest.
REFERENCES
- 1.Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP 1997. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 13, 407–84. [DOI] [PubMed] [Google Scholar]
- 2.Cai S, Mukherjee J, Tillekeratne LM, Hudson RA, Kirchhoff JR 2007. Inhibition of choline transport by redox-active cholinomimetic bis-catechol reagents. Bioorg. Med. Chem 15, 7042–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowe AP, Lockman PR, Abbruscato TJ, Allen DD 2002. Novel choline transport characteristics in Caco-2 cells. Drug. Dev. Ind. Pharm 28, 773–781. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury G, Calcutt M, Guengerich FP 2010. Oxidation of N-nitrosoalkylamines by human cytochrome P450 2A6. J. Biol. Chem 285, 8031–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Silva L 1977. Biochemical mechanisms and management of choreiform movement disorders. Drugs. 14, 300–310. [DOI] [PubMed] [Google Scholar]
- 6.Dormard Y, Levron JC, le Fur JM 1975. Pharmacokinetic study of maleate acid of 2-(N,N-dimethylaminoethanol-14C1)-cyclohexylpropionate (cyprodenate) and of N,N-dimethylaminoethanol-14C1 in animals. Arzneimittelforschung. 25, 201–207. [PubMed] [Google Scholar]
- 7.Ferguson SS, Collier B 1994. Stereoselectivity of the inhibition of [3H]hemicholinium-3 binding to the sodium-dependent high-affinity choline transporter by the enantiomers of alpha- and beta-methylcholine. J. Neurochem 62, 1449–57. [DOI] [PubMed] [Google Scholar]
- 8.Fisher MC, Zeisel SH, Mar M-H, Sadler TW 2001. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology. 64, 114–122. [DOI] [PubMed] [Google Scholar]
- 9.Fisher MC, Zeisel SH, Mar M-H, Sadler TW 2002. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB. J 16, 619–621. [DOI] [PubMed] [Google Scholar]
- 10.Folch J, Lees M, Sloan-Stanley GH 1956. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem 226, 497–509. [PubMed] [Google Scholar]
- 11.Geldenhuys WJ, Lockman PR, Philip AE, McAfee JH, Miller BL, McCurdy CR, Allen DD 2005. Inhibition of choline uptake by N-cyclohexylcholine, a high affinity ligand for the choline transporter at the blood-brain barrier. J. Drug. Target 13, 259–66. [DOI] [PubMed] [Google Scholar]
- 12.Guengerich FP, Yun CH, Macdonald TL 1996. Evidence for a 1-electron oxidation mechanism in N-dealkylation of N,N-dialkyanilines by cytochrome P450 2B1. Kinetic hydrogen isotope effects, linear free energy relationships, comparisons with horseradish peroxidase, and studies with oxygen surrogates. J. Biol. Chem 271, 27321–27329. [DOI] [PubMed] [Google Scholar]
- 13.Groth DP, Bain JA, Pfeiffer CC 1958. The comparative distribution of C14-labeled 2-dimethylaminoethanol and choline in the mouse. J. Pharmacol. Exp. Ther 124, 290–295. [PubMed] [Google Scholar]
- 14.Haubrich DR, Gerber NH, Pflueger AB 1981. Deanol affects choline metabolism in peripheral tissues of mice. J. Neurochem 37, 476–482. [DOI] [PubMed] [Google Scholar]
- 15.Hazardous Substances Data Bank (HSDB). 2015. 2-Dimethylaminoethanol. CASRN: 108-01-0. HSDB No. 1329 National Library of Medicine, Bethesda, MD: Profile updated 10/19/2015 <https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+1329> [Google Scholar]
- 16.Hendler SS, Rorvik D, Eds. 2001. Deanol In: PDR for Nutritional Supplements, Thomson Healthcare, Montvale, NJ: pp. 123–124. [Google Scholar]
- 17.Institute of Medicine: Food and Nutrition Board. 1998. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- 18.Jope RS, Jenden DJ 1979. Dimethylaminoethanol (deanol) metabolism in rat brain and its effect on acetylcholine synthesis. J. Pharmacol. Exp. Ther 211, 472–479. [PubMed] [Google Scholar]
- 19.Kaluzny MA, Duncan LA, Merritt MV, Epps DE 1985. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J. Lipid. Res 26, 135–140. [PubMed] [Google Scholar]
- 20.Klein J, Köppen A, Löffelholz K, Schmitthenner J 1992. Uptake and metabolism of choline by rat brain after acute choline administration. J. Neurochem 58, 870–6. [DOI] [PubMed] [Google Scholar]
- 21.Lockman PR, Allen DD 2002. The transport of choline. Drug. Dev. Ind. Pharm 28, 749–771. [DOI] [PubMed] [Google Scholar]
- 22.Lohr J, Acara M 1990. Effect of dimethylaminoethanol, an inhibitor of betaine production, on the disposition of choline in the rat kidney. J. Pharmacol. Exp. Ther 252, 154–158. [PubMed] [Google Scholar]
- 23.Matthews JM, Garner CE, Black SL, Matthews HB 1997. Diethanolamine absorption, metabolism and disposition in rat and mouse following oral, intravenous and dermal administration. Xenobiotica. 27, 733–746. [DOI] [PubMed] [Google Scholar]
- 24.Mills JL, Fan R, Brody LC, Liu A, Ueland PM, Wang Y, Kirke PN, Shane B, Molloy AM 2014. Maternal choline concentrations during pregnancy and choline-related genetic variants as risk factors for neural tube defects. Am. J. Clin. Nutr 100, 1069–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Toxicology Program (NTP). 2002. Dimethylethanolamine (DMAE) [108-01-0] and selected salts and esters; review of toxicological literature. Retrieved from: http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/Dimethylethanolamine.pdf
- 26.Natures Plus. 2017. Pedi-Active (Phosphatidylserion, DMAE Complex). <http://naturesplus.com/products/productdetail.php?productNumber=3000> Accessed June 22, 2017.
- 27.Organisation for Economic Cooperation and Development (OECD). 1996. Screening Information Data Set. N,N-Dimethylamino-2-ethanol Processed by International Register of Potentially Toxic Chemicals. A contribution to the International Programme on Chemical Safety, United Nations, New York and Geneva. [Google Scholar]
- 28.Pfeiffer CC, Jenney EH, Gallacher W, Smith RP, Bevan J, Killam KF, Killam EK, Blackmore W 1957. Stimulant effect of 2-dimethylaminoethanol–possible precursor of brain acetylcholine. Science. 126, 610–611. [DOI] [PubMed] [Google Scholar]
- 29.Pitts JN Jr., Winer AM, Carter WPL 1981. Chemical Consequences of Air Quality Standards and of Control Implementation Programs, Report No. ARB-R-80/131, NTIS Order No. PB81–137697, p.408 Statewide Air Pollution Research Center, University of California, Riverside, CA. [Google Scholar]
- 30.Preusmann R, Stewart BW 1984. In: Searle CE (Ed), Chemical Carcinogens. American Chemical Society, Washington DC, pp. 643–828. [Google Scholar]
- 31.Schlenk DK 1990. Dimethylaminoethanol. In: Buhler DR, Reed DJ (Eds). Ethel Browning’s Toxicity and Metabolism of Industrial Solvents. Elsevier Science Publishers, New York: pp. 417–422. [Google Scholar]
- 32.Shaw GM, Carmichael SL, Yang W, Schaffer DM 2004. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol 160, 102–109. [DOI] [PubMed] [Google Scholar]
- 33.Shaw GM, Finnell RH, Blom HJ, Carmichael SL, Vollset SE, Yang W, Ueland PM 2009. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology. 20, 714–719. [DOI] [PubMed] [Google Scholar]
- 34.Source Naturals®. 2017a. Dimethylaminoethanol Bitartrate (DMAE). <http://www.sourcenaturals.com/products/GP1101> Accessed June 22, 2017.
- 35.Source Naturals®. 2017b. Attentive ChildTM. <http://www.sourcenaturals.com/products/GP1028> Accessed June 22, 2017.
- 36.Stenbäck F, Weisburger JH, Williams GM 1988. Effect of lifetime administration of dimethylaminoethanol on longevity, aging changes, and cryptogenic neoplasms in C3H mice. Mech. Ageing Dev 42, 129–138. [DOI] [PubMed] [Google Scholar]
- 37.Sterling GH, Doukas PH, Ricciardi FJ Jr., Biedrzycka DW, O’Neill JJ 1986. Inhibition of high-affinity choline uptake and acetylcholine synthesis by quinuclidinyl and hemicholinium derivatives. J. Neurochem 46, 1170–1175. [DOI] [PubMed] [Google Scholar]
- 38.United States Environmental Protection Agency (US EPA). 2016. Chemical Data Reporting Results: 2-Dimethylaminoethanol (CAS No. 108-01-0) < https://chemview.epa.gov/chemview> Accessed October 12, 2018.
- 39.Yue B, Pattison E, Roberts WL, Rockwood AL, Danne O, Lueders C, Möckel M 2008. Choline in whole blood and plasma: sample preparation and stability. Clin. Chem 54, 590–593. [DOI] [PubMed] [Google Scholar]
- 40.Zahniser NR, Chou D, Hanin I 1977. Is 2-dimethyaminoethanol (Deanol) indeed a precursor of brain acetylcholine? A gas chromatographic evaluation. J. Pharmacol. Exp. Ther 200, 545–559. [PubMed] [Google Scholar]
- 41.Zeisel SH 2010. Choline In: Coates PM, Betz JM, Blackman MR, et al. (Eds.), Encyclopedia of Dietary Supplements. Informa Healthcare, London and New York, pp. 136–43. [Google Scholar]
- 42.Zeisel SH, Corbin KD 2012. Choline In: Erdman JW, Macdonald IA, Zeisel SH (Eds.), Present Knowledge in Nutrition. Wiley-Blackwell, Washington, DC, pp. 405–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


