Abstract
Background:
During surgery, trauma to musculoskeletal tissue induces a systemic reaction known as the acute phase response (APR). When excessive or prolonged, the APR has been implicated as an underlying cause of surgical complications. The purpose of this study was to determine the typical APR following total joint arthroplasty in a healthy population defined by the Charlson Comorbidity Index (CCI).
Methods:
This retrospective study identified 180 healthy patients (CCI < 2) who underwent total joint arthroplasty by a single surgeon for primary osteoarthritis from 2013 to 2015. Serial measurements of C-reactive protein (CRP) and fibrinogen were obtained preoperative, perioperative, and at 2 and 6 weeks postoperative.
Results:
Postoperative CRP peaked during the inpatient period and returned to baseline by 2 weeks. Fibrinogen peaked after CRP and returned to baseline by 6 weeks. Elevated preoperative CRP correlated with a more robust postoperative APR for both total hip arthroplasty and total knee arthroplasty, suggesting that a patient’s preoperative inflammatory state correlates with the magnitude of the postoperative APR.
Conclusion:
Measurement of preoperative acute phase reactants may provide an objective means to predict a patient’s risk of postoperative dysregulation of the APR and complications.
The acute phase response (APR) is a systemic reaction to tissue injury; it is mediated by the liver and characterized by the modulation of more than 1000 genes. This response results in the production of cytokines that prevent hemorrhage, combat infection, and stimulate wound healing. Although the APR is essential for normal coagulation and immunity, dysregulation leads to complications such as life-threatening hemorrhage, poor wound healing, and venous thromboembolism [1]. These thromboembolic complications stem from Virchow’s triad of venous stasis, endothelial injury, and hypercoagulability. Prior studies have demonstrated that the magnitude and duration of this response directly correlates with the level of initial tissue injury and predicts poor outcomes in injured patients [2–4].
Surgical trauma during total joint arthroplasty (TJA) evokes a profound APR [5–7]. Prior studies have shown that C-reactive protein (CRP), an acute phase reactant, and erythrocyte sedimentation rate (ESR), an indirect marker of the APR, follow general trends post-TJA [5–7]. Yet, these early studies from the 1990s were conducted under different conditions than current standards of practice and have high variability for maximum inpatient values and time to resolution for CRP and ESR [6,8]. Moreover, this variability is also seen in a recent study by Yombi et al [9] following conventional and minimally invasive total knee arthroplasty (TKA). These studies did not address the APR in the context of an objectively defined healthy population and a large sample size [6,8,9]. Therefore, this study aims at measuring the APR following arthroplasty in a healthy population with current implants, techniques, and standards of practice. Furthermore, this investigation proposes to use fibrinogen to study the APR rather than ESR. ESR quantifies inflammation through an indirect measurement of fibrinogen [10,11], an acute phase reactant that is upregulated following injury [12]. Prior investigations showed that ESR does not return to normal within 1 year following TJA [6]. This imprecision has lead others to conclude that while ESR advanced medical science when introduced in the 1920s, it is often misleading because it is greatly influenced by immunoglobulins, plasma constituents, and changes to erythrocyte morphology and number [13]. Thus, ESR is a less-sensitive marker for the APR than fibrinogen, an acute phase reactant.
This study aims at describing the typical APR exhibited by healthy patients following uncomplicated TJA with current surgical techniques through serial measurement of CRP and fibrinogen. The authors hypothesize that the APR following uncomplicated primary TJA in healthy patients is both consistent and predictable.
Methods
Sample Population and Inclusion Criteria
This IRB-approved retrospective cohort study (level of evidence III) was conducted on patients undergoing primary total hip arthroplasty (THA) and primary TKA at Vanderbilt University Medical Center from 2013 to 2015; 207 patients were enrolled in the study; 180 patients met both the inclusion and exclusion criteria. Adult patients aged between 17 and 88 years with a Charlson Comorbidity Index (CCI) <2 were included in the study. The CCI is a widely accepted and tested method for predicting the influence of comorbidities on functional outcomes [14], implant survival [15], length of stay [16,17], and mortality [18]. The score ranges from 0 to 33 with an increasing score indicating the presence of multiple comorbidities [19]. The CCI was calculated for each patient through review of comorbidities in the electronic medical records.
Surgical Approach and Exclusion Criteria
All surgeries included in the study were performed by a single surgeon (G.G.P.). The surgeon used a posterior surgical approach for the THA and a medial parapatellar arthrotomy with tourniquet use for the TKA. All patients in the study were placed on Celebrex during the perioperative period unless contraindicated. Contraindications included known hypersensitivity to celecoxib, sulfon-amides, aspirin, or other nonsteroidal anti-inflammatory drugs as well as patients with a history of gastrointestinal sensitivity or peptic ulcer disease. Atypical or complex primary arthroplasty procedures for postinfectious arthrosis, posttraumatic osteoarthritis, or arthroplasty were excluded from the study. Patients requiring THA or TKA revision were also excluded. The electronic medical record, containing both office and hospital records, was reviewed for complications in the 6-week postoperative period.
Laboratory Methods
CRP and fibrinogen laboratory values were collected at preoperative, perioperative, and postoperative 2-week and 6-week time points as part of regular standard of care for tracking acute inflammation. Laboratory tests were drawn on the floor and processed by our institution’s clinical laboratory, which is Clinical Laboratory Improvement Amendments (CLIA) certified, accredited by the College of American Pathologists, and licensed by the state of Tennessee. Fibrinogen was tested in accordance with the Clauss clotting method using the STA-R Evolution Analyzer with reagents from Diagnostica Stago, Inc. Interassay coefficients of variation have been reported to range from 3.0% to 4.8% [20]. CRP levels were measured using an immunoturbidimetric assay with the Architect C1600 by Abbott Laboratories with reported interassay coefficients of variation of 0.44% to 1.25% [21].
Temporal Criteria for Laboratory Values
The preoperative period was defined as 40 days before surgery; perioperative period was defined as the end of surgery to discharge; week 2 was defined as postoperative day 10 to 20; and week 6 was defined as postoperative day 35 to day 50. The wide time intervals for postoperative laboratory values were secondary to variability in clinic scheduling. Laboratory values not taken during these time frames were excluded from analysis. Percent resolution of max for CRP and fibrinogen was defined as the change from maximum at 2 and 6 weeks divided by the maximum change from baseline.
Statistical Analysis
Mann-Whitney 2-tailed unpaired t tests were performed to compare the non-Gaussian THA and TKA cohorts. Unpaired t tests using the Holm-Sidak method were performed to compare the 25th and 75th percentile CRP and fibrinogen values of the patient cohorts and to compare the THA and TKA APR time courses.
Estimates of sample size were calculated based on the evaluation of peak CRP and fibrinogen following TKA and THA. For a study of alpha 0.05 and 1-beta 0.80, 13 patients were required for each group based on a difference in population means of 49.7 mg/L and standard deviation of difference of 59.25 mg/L for CRP. This was consistent with prior studies [8]. For fibrinogen with alpha = 0.05 and 1-beta = 0.80, 54 patients were required for each group based on a difference in population means of 42.5 mg/dL and standard deviation of difference of 109.8 mg/dL. This study was adequately powered with 118 TKA and 62 THA patients.
Determination of Consistency and Predictability
To test the hypothesis that the APR is consistent and predictable, these terms must first be established. Consistency is defined as the ability to significantly distinguish between the THA and TKA cohorts with respect to inpatient maximum value and time to inpatient maximum. Predictability is defined as the ability for preoperative acute phase reactants to predict the postoperative APR. This will be tested by a subset analysis of the THA and TKA cohorts in the 25th and 75th quartiles for preoperative CRP values. If there is a significant difference between the quartiles for postoperative CRP and fibrinogen, then the null hypothesis will be rejected.
Results
The sample demographics for the study cohort are reported in Table 1. The sample population had few comorbidities, with a median CCI of 0 with the 25th and 75th interquartile range of 0–1 for both TKAs and THAs. There were no complications recorded in either of the populations for 6 weeks postoperatively.
Table 1.
Sample Demographics of Patients Undergoing Total Knee Arthroplasty and Total Hip Arthroplasty.
| Demographics | Total Knee Arthroplasty | Total Hip Arthroplasty |
|---|---|---|
| Number (n) | 118 | 62 |
| Age | 64 (55.8–86.0) | 58 (46.5–65.0) |
| Gender | 64 Female/54 male | 30 Female/34 male |
| Mean Charlson Comorbidity Index | 0 (0–1) | 0 (0–1) |
| Postoperative complications at 6 wk | 0 | 0 |
Values presented as median and 25th and 75th interquartile range or absolute number. Unpaired, nonparametric Mann-Whitney t tests were used to calculate P values.
The Acute Phase Response Was Consistent for THA and TKA Cohorts
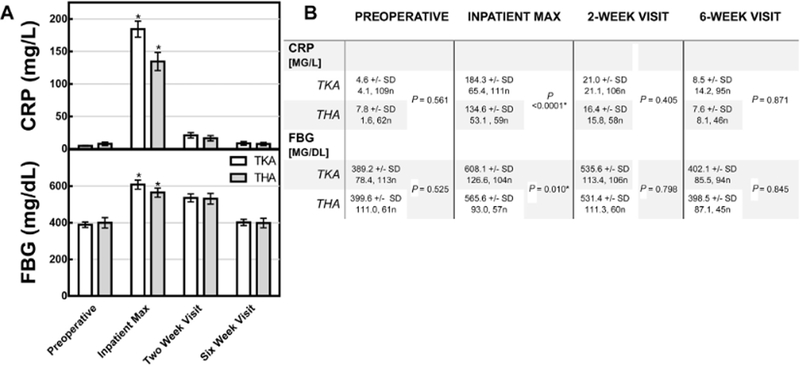
The CRP and fibrinogen responses to TJA were consistent for both TKA and THA. In both procedures, postoperative CRP and fibrinogen peaked during the perioperative period and returned to baseline at 2 and 6 weeks, respectively (Fig. 1). The peak CRP for TKA and THA was 184.3 mg/L and 134.6 mg/L, respectively. The peak CRP was significantly higher for TKA than THA (P < .0001). The peak fibrinogen for TKA and THA was 608.1 mg/dL and 565.6 mg/dL (P .010), respectively.
Fig. 1.
The perioperative rise and fall of 2 acute phase markers. (A) Mean circulating levels of CRP and FBG in response to TKA and THA in the study populations. Y-error bars depict a CI of 95%, and asterisks indicate significant difference between cohorts. (B) Unpaired t tests were performed to compare groups. Statistical significance denoted by * and defined as P < .05. CRP, C-reactive protein; FBG, fibrinogen; TKA, total knee arthroplasty; THA, total hip arthroplasty; CI, confidence interval; SD, standard deviation.
CRP has a narrow peak (in comparison to fibrinogen) around postoperative day 2 with 93%–95% resolution by week 2 (Table 2). Fibrinogen has a broad peak (with respect to CRP), as indicated by the wide interquartile range for time to max, and has 87%−96% resolution by week 6.
Table 2.
Median (25th–75th IQR) Time to Peak Acute Phase Reactant and Resolution Following Total Knee Arthroplasty (TKA) and Total Hip Arthroplasty (THA).
| Acute Phase Reactants | Time to Max (d) | Resolution of Max at 2 wk (%) | Resolution of Max at 6 wk (%) | |||
|---|---|---|---|---|---|---|
| CRP | ||||||
| TKA | 2.5 (1.7–2.7), 102n | P < .0001* | 93.4 (87.3–97.5), 87n | P < .0001* | 99.5% (97.5–100.6), 74n | P = .247 |
| THA | 1.7 (1.6–1.8), 58n | 95.2 (92.5–99.2), 52n | 99.5% (97.7–101.6), 41n | |||
| FBG | ||||||
| TKA | 2.8 (2.5–12.2), 91n | P = .267 | 41.0 (0.0–72.5), 83n | P = .015* | 95.6% (80.1–116.1), 72n | P = .155 |
| THA | 2.5 (1.7–13.8), 54n | 25.8 (0.0–56.6), 53n | 86.7% (58.0–110.0), 38n | |||
Nonparametric Mann-Whitney unpaired t tests were used to determine significance.
Statistical significance denoted by and defined as P < .05. IQR, interquartile range; CRP, C-reactive protein; FBG, fibrinogen; n, number.
The Acute Phase Response Was Predictable for THA and TKA Cohorts
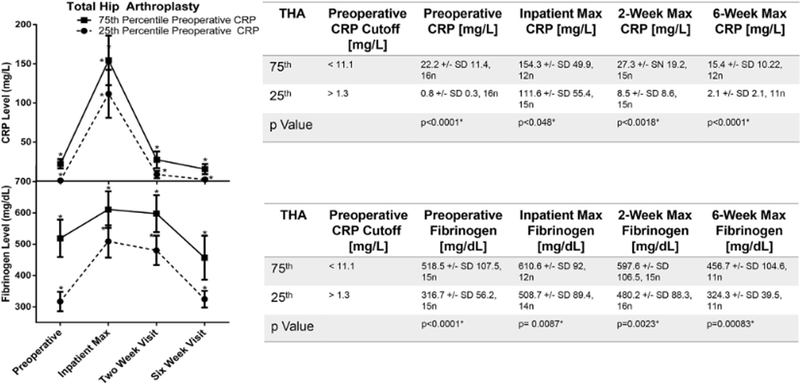
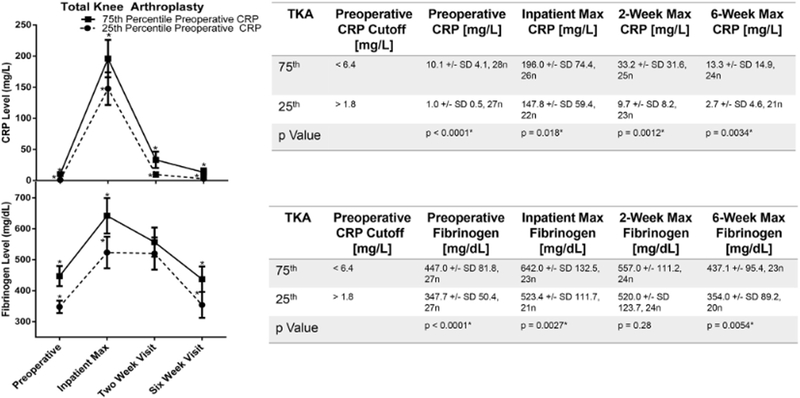
An elevated preoperative APR correlated with higher post-operative acute phase reactant measurements. For patients undergoing THA, postoperative fibrinogen and CRP were higher for patients with preoperative CRP in the 75th percentile compared to those in the 25th percentile at each time point (Fig. 2). This was also true for TKA for all measurements except the fibrinogen 2-week time point (Fig. 3).
Fig. 2.
The influence of preoperative CRP on the rise and fall of mean fibrinogen following THA. Cohorts defined by the 25th or 75th percentile of preoperative CRP. Y-error bars depict a CI of 95%, and * indicates significant difference between the 25th and 75th percentile. Unpaired t tests were performed to compare groups. Statistical significance denoted by * and defined as P < .05.
Fig. 3.
The influence of preoperative CRP on the rise and fall of mean fibrinogen following TKA. Cohorts defined by the 25th or 75th percentile of preoperative CRP. Y-error bars depict a CI of 95%, and * indicates significant difference between the 25th and 75th percentile. Unpaired t tests were performed to compare groups. Statistical significance denoted by * and defined as P < .05.
Discussion
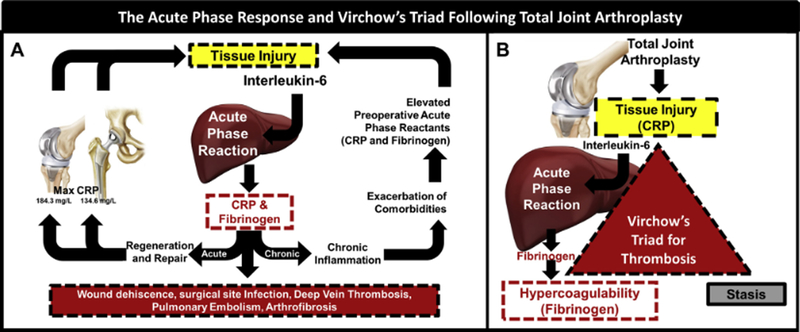
This study demonstrates that the APR following THA and TKA is both predictable and consistent in healthy patients operated on by a single surgeon. Furthermore, there is a different response to TKA and THA (Fig. 4A). We hypothesize that the elevated APR in patients undergoing TKA might be attributed to the use of a tourniquet and the involvement of an additional long bone when compared to the APR for THA. Interestingly, the results of this study demonstrate that an elevated preoperative APR predisposes patients to a more robust postoperative APR.
Fig. 4.
The acute phase response (APR) and Virchow’s triad following total joint arthroplasty. (A) Damage induced by surgery initiates the acute phase response, a necessary component of wound healing. An unresolved acute phase response can result in chronic inflammation which further elevates acute phase reactants. While the APR is necessary for tissue regeneration, the lack of resolution can lead to life-threatening coagulopathies and poor wound healing. (B) The intersection of the Virchow’s triad and the acute phase response. CRP, a short half-life acute phase reactant, peaks close to the time of injury. As such, CRP is highly associated with injury. Fibrinogen, a long half-life acute phase reactant, is an integral factor in hemostasis and can lead to hypercoagulable states if elevated. Thus, CRP and fibrinogen are directly related to 2 components of Virchow’s triad: tissue injury and hypercoagulability.
Knowledge of how the APR resolves may be useful in guiding postoperative thromboprophylaxis. The 3 aspects of Virchow’s triad, tissue injury, hypercoagulability, and stasis exist in the perioperative period following TJA. CRP and fibrinogen represent 2 components of the triad, namely tissue injury [22] and hypercoagulability [23], respectively (Fig. 4B). In this study, CRP and fibrinogen return to their preoperative baselines by 2 and 6 weeks, respectively, with the difference in time to resolution due to their relative half-lives. Therefore, it would appear that the tissue injury aspect of Virchow’s triad resolves 2 weeks postoperatively. Given that the third aspect of Virchow’s triad, stasis, ends with early mobilization and ambulation within the hospitalization period [24–26], two-thirds of the triad have returned to baseline at 2 weeks. Therefore, we hypothesize that in a healthy population, discontinuation of thromboprophylaxis theoretically may be indicated by 2 weeks postoperation.
In comparison with prior investigations [5,6,8,9], this study attempted to limit variability in outcomes through selection of patients who underwent TJA by a single high-volume joint replacement specialist, thereby providing consistent surgical trauma and better standardization of postoperative care. In addition, the study population included only healthy patients objectively defined by a CCI < 2. The CCI has been validated to predict comorbidity and complications in many fields, including TJA [27–31]. Moreover, the exclusion of patients with multiple comorbidities markedly reduced the distribution and spread of maximum inpatient laboratory values of CRP in comparison to the studies of White et al and Yombi et al. Although our study includes a highly specific patient cohort, the consistency of median laboratory values with prior studies that include different patient populations and surgical techniques (Table 3) suggests that the results are generalizable to a broader population [5,7–9]. This was not true with all prior studies; most notably, our study had a 29.2% and 41.9% reduction in maximum CRP for TKA and THA, respectively, in comparison with the results of Bilgen et al [6], conducted with a limited cohort in Turkey during the late 90s. In general, the authors note that despite surgical advances, our data in the context of prior studies suggest that the APR to both TKA and THA does not vary significantly and that differences in surgical technique have a minimal effect on the APR following TJA. This conclusion is consistent with Yombi et al’s investigation of the time course of CRP following minimally invasive and conventional TKA, in which the study found no difference in inpatient CRP between the 2 surgical approaches.
Table 3.
Prior Studies on CRP Following Total Joint Arthroplasty.
| Study | Year | Country | Procedure | Number of Patients | Average Maximum CRP (mg/L) | Days to Maximum CRP | Time to Resolution |
|---|---|---|---|---|---|---|---|
| Aalto et al | 1983 | Finland | Uncomplicated primary TDA | 40 | 134 | 2 | <3 wk |
| Shih et al | 1986 | Republic of China | Uncomplicated primary THA | 50 | 136 | 2 | <3 wk |
| White et al | 1998 | United Kingdom | Uncomplicated primary THA | 13 | 100.4 | 3 | >2 wk |
| Uncomplicated primary TKA | 13 | 154.9 | 2 | >2 wk | |||
| Bilgen et al | 2001 | Turkey | Uncomplicated primary THA | 12 | 232 | 2 | <3 wk |
| Uncomplicated primary TKA | 16 | 260 | 2 | <2 mo | |||
| Yombi et al | 2015 | Belgium | Minimally invasive TKA | 807 | 162 | 2 | <3 wk |
| Conventional TKA | 249 | 156 | 2 | <6 wk |
CRP, C-reactive protein; THA, total hip arthroplasty; TKA, total knee arthroplasty.
This study was limited in its ability to determine the precise time-to-maximum value for the acute phase reactants measured due to intermittent monitoring. The same limitation prevented precise measurement of the time to resolution for CRP and fibrinogen, as levels were only recorded at 2-week and 6-week post-operative follow-up visits. While the strength of this study was in the objective exclusion of multiple comorbidities with a CCI < 2, the authors believe that the inclusion of a wider range of pathologies would have allowed for the expansion of the interquartile comparisons of preoperative acute phase reactants. Additionally, this study is somewhat limited by its relatively small sample size as only 180 patients were enrolled and these patients were split between 2 cohorts. Previous studies describing the APR to TKA and THA have similarly small samples, so a future large multi-institutional study would be helpful in validating these findings among a broader population.
Overall, the APRs to TKA and THA are predictable and reproducible in healthy patients. Knowledge of the normal APR to TJA in healthy patients helps identify patients with pathologic deviations from the trend. In this study’s cohort of patients, an elevated preoperative CRP predicted an increase in postoperative CRP and fibrinogen. No prior study has reported the relationship between preoperative CRP and postoperative acute phase reactants following TJA. These data suggest that an elevated preoperative APR may have the potential to predict a robust APR and subsequent complications such as infection [32,33], systemic inflationary response syndrome state [34], and mortality [35]. This relationship is important in the context of patients undergoing surgery with chronic conditions that persistently upregulate the APR, including coronary artery disease, diabetes, and chronic pulmonary disease [36–38]. Early identification of patients at risk of postoperative complications will allow for preoperative mitigation of modifiable risk factors and alterations to postoperative protocols, such as augmented thromboprophylaxis and therapies aimed at resolving the APR. Taken as a whole, these results provide the foundation for future studies investigating the impact of elevated preoperative inflammatory markers due to trauma, infection, or chronic inflammatory conditions on postoperative outcomes.
Acknowledgments
The authors would like to thank the Caitlin Lovejoy Fund and the NIH for sponsoring this work with training grant NIH T35DK007383 and NIH T32DK007061. Furthermore, the authors would like to recognize Dr. Bill Sales, Mrs. Sally A. Schoenecker, and Dr. Perry L. Schoenecker for their generous contributions to the laboratory.
References
- 1.Stutz CM, O’Rear LD, O’Neill KR, et al. Coagulopathies in orthopaedics: links to inflammation and the potential of individualizing treatment strategies. J Orthop Trauma 2013;27:236. [DOI] [PubMed] [Google Scholar]
- 2.Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85:109. [DOI] [PubMed] [Google Scholar]
- 3.Gebhard F, Pfetsch H, Steinbach G, et al. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg 2000;135:291. [DOI] [PubMed] [Google Scholar]
- 4.Huang TJ, Hsu RW, Li YY, et al. Less systemic cytokine response in patients following microendoscopic versus open lumbar discectomy. J Orthop Res 2005;23:406. [DOI] [PubMed] [Google Scholar]
- 5.Aalto K, Osterman K, Peltola H, et al. Changes in erythrocyte sedimentation rate and C-reactive protein after total hip arthroplasty. Clin Orthop Relat Res 1984;184:118. [PubMed] [Google Scholar]
- 6.Bilgen O, Atici T, Durak K, et al. C-reactive protein values and erythrocyte sedimentation rates after total hip and total knee arthroplasty. J Int Med Res 2001;29:7. [DOI] [PubMed] [Google Scholar]
- 7.Shih LY, Wu JJ, Yang DJ. Erythrocyte sedimentation rate and C-reactive protein values in patients with total hip arthroplasty. Clin Orthop Relat Res 1987;225: 238. [PubMed] [Google Scholar]
- 8.White J, Kelly M, Dunsmuir R. C-reactive protein level after total hip and total knee replacement. J Bone Joint Surg Br 1998;80:909. [DOI] [PubMed] [Google Scholar]
- 9.Yombi JC, Schwab PE, Thienpont E. Serum C-reactive protein distribution in minimally invasive total knee arthroplasty do not differ with distribution in conventional total knee arthroplasty. PLoS One 2015;10:e0124788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain BJ. Some influences on the ESR and the fibrinogen level in healthy subjects. Clin Lab Haematol 1983;5:45. [DOI] [PubMed] [Google Scholar]
- 11.Talstad I, Haugen HF. The relationship between the erythrocyte sedimentation rate (ESR) and plasma proteins in clinical materials and models. Scand J Clin Lab Invest 1979;39:519. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990;265:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448. [DOI] [PubMed] [Google Scholar]
- 14.Reininga IH, Wagenmakers R, van den Akker-Scheek I, et al. Effectiveness of computer-navigated minimally invasive total hip surgery compared to conventional total hip arthroplasty: design of a randomized controlled trial. BMC Musculoskelet Disord 2007;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnsen SP, Sørensen HT, Lucht U, et al. Patient-related predictors of implant failure after primary total hip replacement in the initial, short-and long-terms. A nationwide Danish follow-up study including 36,984 patients. J Bone Joint Surg Br 2006;88:1303. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613. [DOI] [PubMed] [Google Scholar]
- 17.Rius C, Perez G, Martinez JM, et al. An adaptation of Charlson comorbidity index predicted subsequent mortality in a health survey. J Clin Epidemiol 2004;57:403. [DOI] [PubMed] [Google Scholar]
- 18.Streubel PN, Ricci WM, Wong A, et al. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res 2011;469:1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol 2010;11:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bie P, Schornagel WJ, Van Den Dool EJ, et al. Laboratory evaluation of the Coasys® Plus C coagulation analyzer. Thromb Res 2013;131:357. [DOI] [PubMed] [Google Scholar]
- 21.Meynaar IA, Droog W, Batstra M, et al. In critically ill patients, serum procalcitonin is more useful in differentiating between sepsis and SIRS than CRP, Il-6, or LBP. Crit Care Res Pract 2011;2011:594645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goölbasi Z, Uçar O, Keles T, et al. Increased levels of high sensitive C-reactive protein in patients with chronic rheumatic valve disease: evidence of ongoing inflammation. Eur J Heart Fail 2002;4:593. [DOI] [PubMed] [Google Scholar]
- 23.Machlus KR, Cardenas JCJ, Church FC, et al. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood 2011;117:4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger RA, Jacobs JJ, Meneghini RM, et al. Rapid rehabilitation and recovery with minimally invasive total hip arthroplasty. Clin Orthop Relat Res 2004;429: 239. [DOI] [PubMed] [Google Scholar]
- 25.White RH, Gettner S, Newman JM, et al. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med 2000;343:1758. [DOI] [PubMed] [Google Scholar]
- 26.Wenz JF, Gurkan I, Jibodh SR. Mini-incision total hip arthroplasty: a comparative assessment of perioperative outcomes. Orthopedics 2002;25:1031. [DOI] [PubMed] [Google Scholar]
- 27.Whitmore RG, Stephen JH, Vernick C, et al. ASA grade and Charlson Comorbidity Index of spinal surgery patients: correlation with complications and societal costs. Spine J 2014;14:31. [DOI] [PubMed] [Google Scholar]
- 28.St-Louis E, Iqbal S, Feldman LS, et al. Using the age-adjusted Charlson comorbidity index to predict outcomes in emergency general surgery. J Trauma Acute Care Surg 2015;78:318. [DOI] [PubMed] [Google Scholar]
- 29.Crooks CJ, West J, Card TR. A comparison of the recording of comorbidity in primary and secondary care by using the Charlson Index to predict short-term and long-term survival in a routine linked data cohort. BMJ Open 2015;5: e007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suidan RS, Leitao MM, Zivanovic O, et al. Predictive value of the Age-Adjusted Charlson Comorbidity Index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol 2015;138:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meessen JM, Pisani S, Gambino ML, et al. Assessment of mortality risk in elderly patients after proximal femoral fracture. Orthopedics 2014;37:e194. [DOI] [PubMed] [Google Scholar]
- 32.Haupt W, Hohenberger W, Mueller R, et al. Association between preoperative acute phase response and postoperative complications. Eur J Surg 1997;163:39. [PubMed] [Google Scholar]
- 33.Fransen EJ, Maessen JG, Elenbaas TW, et al. Increased preoperative C-reactive protein plasma levels as a risk factor for postoperative infections. Ann Thorac Surg 1999;67:134. [DOI] [PubMed] [Google Scholar]
- 34.Giannoudis PV, Harwood PJ, Loughenbury P, et al. Correlation between IL-6 levels and the systemic inflammatory response score: can an IL-6 cutoff pre-dict a SIRS state? J Trauma 2008;65:646. [DOI] [PubMed] [Google Scholar]
- 35.Chapman G, Holton J, Chapman A. A threshold for concern? C-reactive protein levels following operatively managed neck of femur fractures can detect infectious complications with a simple formula. Clin Biochem 2016;49(3):219. [DOI] [PubMed] [Google Scholar]
- 36.Festa A, D’Agostino R, Tracy R, et al. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 2002;51:1131. [DOI] [PubMed] [Google Scholar]
- 37.Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:26. [DOI] [PubMed] [Google Scholar]
- 38.Koenig W, Rosenson RS. Acute-phase reactants and coronary heart disease. Semin Vasc Med 2002;02:417. [DOI] [PubMed] [Google Scholar]