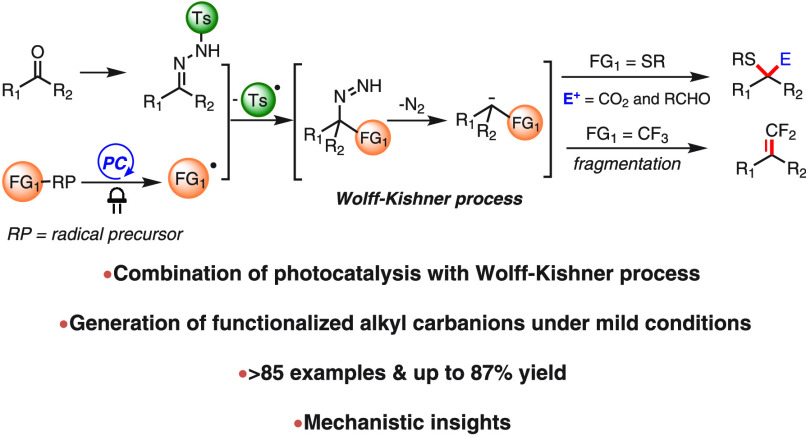
Abstract
The combination of photoredox catalysis with the Wolff–Kishner (WK) reaction allows the difunctionalization of carbonyl groups by a radical-carbanion relay sequence (photo-Wolff–Kishner reaction). Photoredox initiated radical addition to N-sulfonylhydrazones yields α-functionalized carbanions following the WK-type mechanism. With sulfur-centered radicals, the carbanions are further functionalized by reaction with electrophiles including CO2 and aldehydes, whereas CF3 radical addition furnishes a wide range of gem-difluoroalkenes through β-fluoride elimination of the generated α-CF3 carbanions. More than 80 substrate examples demonstrate the broad applicability of this reaction sequence. A series of investigations including radical inhibition, deuterium labeling, fluorescence quenching, cyclic voltammetry, and control experiments support the proposed radical-carbanion relay mechanism.
1. Introduction
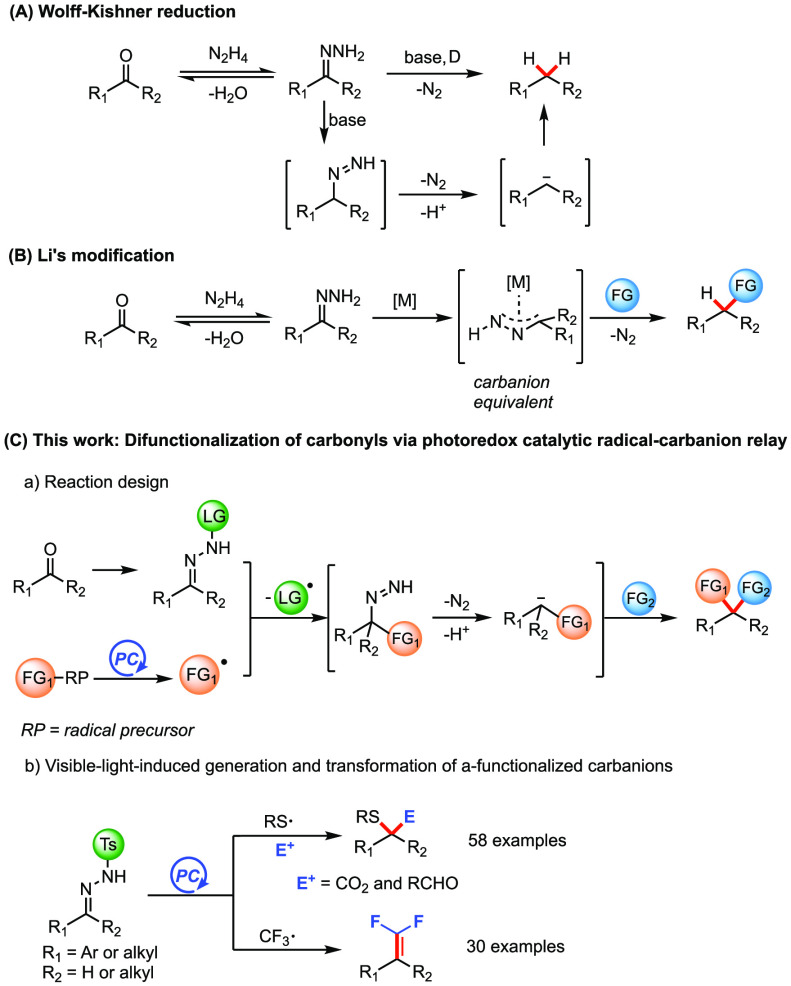
The inversion of the inherent polarity of organic functionalities, termed as umpolung, is a key bond-forming strategy in organic synthesis.1 The umpolung of a carbonyl group places a negative charge on the carbon atom, making it nucleophilic and prone to attack electrophiles. Carbonyl umpolung is achieved in many ways: Acyl anion equivalents are obtained by the umpolung of electrophilic aldehydes in stoichiometric dithiane chemistry2 and catalytic N-heterocyclic carbene (NHC) chemistry.3 Synthetically important alkyl carbanion intermediates can be obtained from carbonyl groups using the Wolff–Kishner (WK) reduction. The polarity inversion is accomplished by sequential hydrazone formation, tautomerization, and N2-extrusion to generate a nucleophilic alkyl carbanionic species (Scheme 1A). With elegant modifications from Huang Minlon4 and others,5 the WK process has evolved over the past century into a powerful carbonyl deoxygenation tool in the synthesis of complex molecules.5 Despite being a very effective way of producing carbanions, synthetic applications of this chemistry have long been underexplored considering that the alkyl carbanion in such an umpolung can, in principle, react with many electrophiles other than a proton. Few examples based on the modified WK process have been developed for the construction of C–C bonds, wherein highly reactive alkyllithium reagents were employed to react with sulfonylhydrazones.6 More recently, pioneering work by Li and co-workers demonstrated the direct functionalization of the carbanion in a Wolff–Kishner reaction by nucleophilic addition to carbonyl compounds,7 imines,8 CO2,9 and Michael acceptors10 under ruthenium catalysis. The same group utilized such carbanions in metal-catalyzed Negishi-type coupling,11 Heck-type coupling,12 Tsuji-Trost alkylation,13 and olefination reactions14 or metal-free C–C bond-forming reactions.15 In these cases, the functional groups are installed through metal-assisted nucleophilic trapping of nonfunctionalized alkyl carbanions (Scheme 1B). Inspired by the facile generation of carbanions in the classic WK process, we questioned if functionalized carbanions can be produced catalytically for a subsequent nucleophilic reaction allowing the simultaneous installation of two functional groups at a geminal position. The scope of such a reaction sequence has remained unexplored although its realization represents a desirable synthetic tool for carbonyl group functionalization.
Scheme 1. Umpolung Generation of Alkyl Carbanions from Carbonyls.
As part of our ongoing research activities in photoredox catalytic generation of functionalized carbanions from carbonyls,16 we envisioned that a combination of a conventional WK process with photoredox catalysis might furnish functionalized alkyl carbanions for a subsequent derivatization. In the anticipated radical-carbanion relay sequence, radicals generated by the photoredox catalytic system would be captured by N-sulfonylhydrazone,17 thus installing the first functional group. Subsequently, the diazene intermediate, resulting from radical fragmentation,17a,17c,18 enters a similar reaction sequence as involved in the WK reduction to give functionalized carbanions, which offers a second opportunity for further transformations (Scheme 1C,a). Herein we report the successful implementation of this radical-carbanion relay functionalization concept. The combination of photoredox catalysis with a Wolff–Kishner process allows the facile generation of α-sulfenyl and α-CF3 carbanions that undergo further nucleophilic attack or fragmentation, respectively (Scheme 1C,b).
2. Results and Discussion
2.1. Generation of α-Sulfenyl Carbanions and Their Reactions with Electrophiles
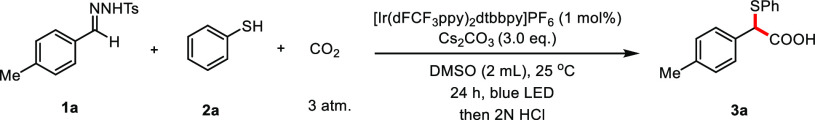
Carbon–sulfur bonds are found in pharmaceuticals or natural products and are widely used in synthesis. Recent years have witnessed increasing attention to develop an efficient approach to forge C–S bonds.19 We postulate that photogenerated thiyl radicals20 from various thiols can engage in the radical-carbanion relay functionalization sequence. Such a process would yield synthetically useful α-sulfenyl carbanions, which are traditionally produced through deprotonation of sulfides with strong bases such as nBuLi and NaNH2.21 Building on the facile carbanion trapping by CO222 and our continued interest in utilization of CO2 as the C1 feedstock for photocatalytic carboxylation reactions,22e,23 we selected N-tosylhydrazone as the radical acceptor in the anticipated sequence based on the following considerations: (1) they can be easily prepared through condensation of carbonyl compounds with TsNHNH2; (2) after radical addition to N-tosylhydrazone, rapid β-sulfone elimination was anticipated to produce a sulfinyl radical which should undergo single-electron transfer with the photocatalyst.17,24 We commenced our study by utilizing aldehyde hydrazone 1a, thiophenol 2a, and CO2 as model substrates for the optimization of the reaction conditions.
After systematic screening of all reaction parameters (see the SI for details), we were delighted to obtain the desired functionalized carboxylic acid 3a in 81% yield using [Ir(dFCF3ppy)2dtbbpy]PF6 (1 mol %) under 3 atm of CO2 in DMSO (Table 1, entry 1). Polar solvents like DMSO and DMF were effective for this thiocarboxylation reaction (see the SI, Table S2). Moreover, we successfully converted p-tolualdehyde into the desired product 3a in one pot by means of a condensation and photocatalytic sequence with similar efficiency (Table 1, entry 2). Rigorous control experiments revealed that photocatalyst, base, and light were crucial for the transformation to occur (Table 1, entries 6–8).
Table 1. Screening of Reaction Conditions for Thiocarboxylation of N-Tosylhydrazonea.
| entry | change from standard conditions | yieldb |
|---|---|---|
| 1 | none | 81% |
| 2 | one-pot process | 80%c |
| 3 | MeCN instead of DMSO | n.d. |
| 4 | THF instead of DMSO | n.d. |
| 5 | 4CzIPN instead of Ir–F | n.d. |
| 6 | without Cs2CO3 | n.d. |
| 7 | without PC | n.d. |
| 8 | in the dark | n.d. |
Reaction conditions: compound 1a (0.2 mmol), 2a (0.3 mmol), Cs2CO3 (0.6 mmol), [Ir(dFCF3ppy)2dtbbpy]PF6 (1 mol %), and 3 atm of CO2 in 2 mL of solvent, irradiation with blue LED (455 nm) at 25 °C for 24 h. n.d. = not detected.
Yields were determined by 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethoxybenzene as the internal standard.
1a was formed in one pot starting from p-tolualdehyde and used directly without purification. 4CzIPN = 2,4,5,6-tetra(carbazol-9-yl)isophthalonitrile. Ir–F = [Ir(dFCF3ppy)2dtbbpy]PF6. PC = photocatalyst.
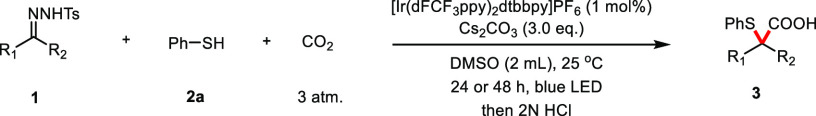
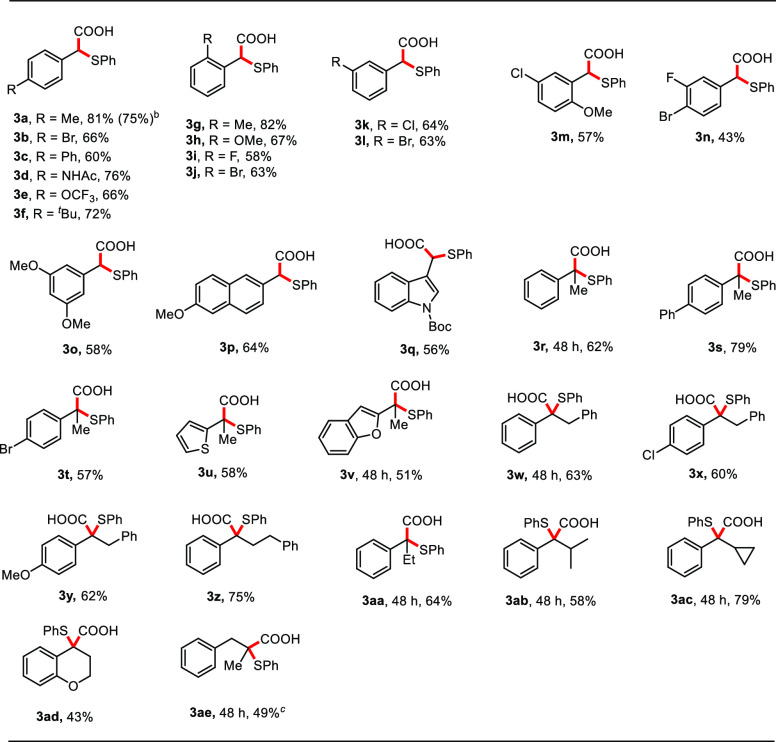
With the optimized reaction conditions in hand, we examined the scope of the method (Table 2). The reaction gave good yields of the corresponding products with a series of aromatic aldehyde-derived N-tosylhydrazones bearing electron-neutral (3a–3c, 3f, 3g, 3j, and 3l), electron-donating (3d, 3h, and 3o), or electron-withdrawing (3i, 3k, and 3n) groups at para-, meta-, or ortho- positions. The reaction was compatible with N-tosylhydrazones containing two substituents on the aromatic ring, affording the desired carboxylic acids (3m–3o) in reasonable yields (43–58%). Heterocyclic and naphthalene-containing substituents were also well tolerated by the catalytic system (3p, 3q).
Table 2. Scope of N-Tosylhydrazones for Thiocarboxylationa.
Reaction conditions: unless otherwise noted, all reactions were carried out with 1 (0.2 mmol), 2a (0.3 mmol), Cs2CO3 (0.6 mmol), [Ir(dFCF3ppy)2dtbbpy]PF6 (1 mol %), and 3 atm of CO2 in 2 mL of DMSO, irradiation with blue LED (455 nm) at 25 °C for 24 h, and isolated yields were shown.
6 mmol scale, CO2 was bubbled into the reaction continuously.
Reaction was conducted at 0 °C in DMF (2 mL)
The reaction system could also be extended to N-tosylhydrazones derived from ketones, affording a wide range of carboxylic acids with quaternary carbon-centers (3r–3ae). Gratifyingly, functional groups including phenyl (3s), halogen (3t and 3x), thiophene (3u), benzofuran (3v), and methoxy (3y) on the aromatic rings of substrates were well tolerated. The reaction proceeded with similar efficiencies for electron-rich or electron-poor substrates. Moreover, N-tosylhydrazones bearing more sterically hindered substituents at the α-position such as ethyl (3aa), isopropyl (3ab), and cyclopropyl (3ac) gave the desired products in good yields, but longer reaction times were required. The reaction could be utilized for the thiocarboxylation of N-tosylhydrazone derived from 4-chromanone, yielding the heterocyclic product 3ad in 43% yield. To our delight, N-tosylhydrazone derived from an aliphatic ketone reacted at 0 °C yielding product 3ae in moderate yield. The decreased efficiency and required low reaction temperature were rationalized by the instability of the aliphatic α-sulfenly carbanion. Importantly, this reaction is easily scalable, as demonstrated by the gram scale synthesis of 3a in 75% yield.
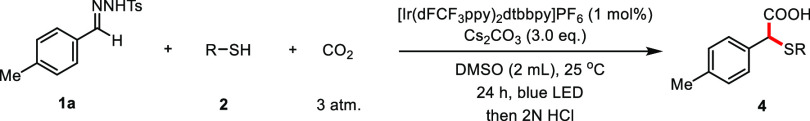
Next, we explored the scope of the reaction with respect to thiols. As shown in Table 3, thiophenols bearing either electron-donating (4a–4c) or electron-withdrawing groups (4e) on the para position of the aromatic ring reacted smoothly to generate the expected products in mostly good yields. Both ortho- and meta-substituted thiophenols were suitable substrates, affording the products in high yields (71–85%). However, 4-nitro-thiolphenol failed to give the desired product. Notably, besides aromatic thiophenols, our method could be extended to primary, secondary, and tertiary aliphatic thiols (4j–4l), albeit with moderate efficiencies.
Table 3. Scope of the Thiols for Thiocarboxylationa.
Reaction conditions: unless otherwise noted, all reactions were carried out with 1a (0.2 mmol), 2 (0.3 mmol), Cs2CO3 (0.6 mmol), [Ir(dFCF3ppy)2dtbbpy]PF6 (1 mol %), and 3 atm of CO2 in 2 mL of DMSO, irradiation with blue LED (455 nm) at 25 °C for 24 h, and isolated yields were shown.
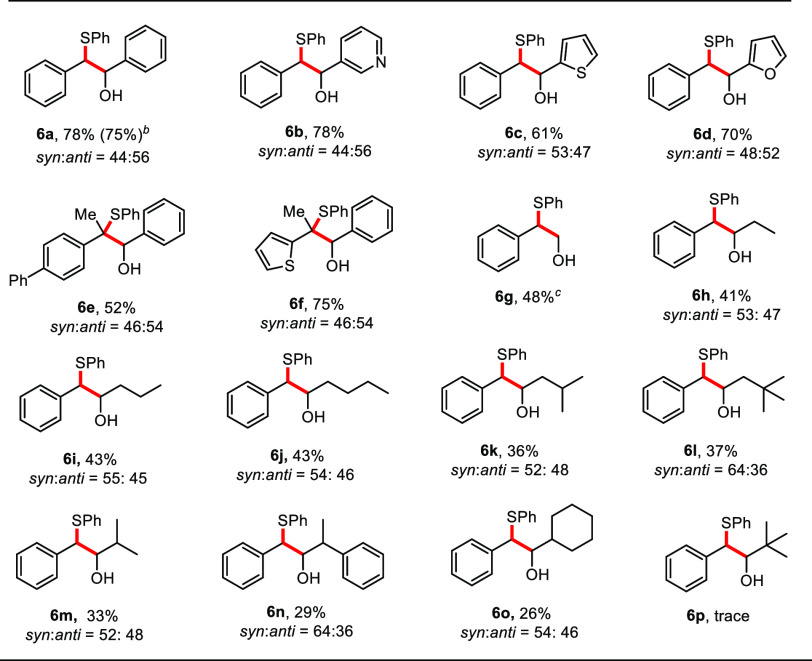
After successful application of this radical-carbanion relay sequence for carboxylation, we tested other electrophiles, like aldehydes or ketones, to realize a visible-light driven Barbier-type reaction.22b,25 Barbier-type reactions are well-known carbon–carbon forming reactions utilizing the nucleophilic attack of organometallic species to carbonyl compounds.26 Using slightly modified reaction conditions, we discovered that photo-Wolff–Kishner generated carbanions can be efficiently trapped with a wide range of aldehydes (Table 4). Benzaldehyde reacted smoothly to give the desired alcohol 6a in 78% yield. We were delighted to find that heteroaryl aldehydes readily participated in the coupling reaction to give products 6b–6d. When ketone-derived N-tosylhydrazones were employed, densely functionalized sulfides (6e–6f) were constructed in synthetically useful yields. Besides aromatic aldehydes, aliphatic aldehydes bearing short or long chains were suitable electrophiles in our system, giving the desired products in moderate yields (6g–6o).27 Notably, solid paraformaldehyde reacted to provide the desired product (6g) in 48% yield. This transformation was however sensitive to steric hindrance. The presence of additional substituents at the α-carbon on the trapping aldehyde decreased the yield considerably (6m–6o), and only a trace amount of the product was detected when pivalaldehyde was employed. Moreover, ketones failed to trap the generated carbanion in the catalytic system, which may be explained by the undesired deprotonation of the α-protons to the carbonyl yielding benzyl phenyl sulfide.28
Table 4. Scope of the Aldehydes for Thiohydroxyalkylationa.
Reaction conditions: unless otherwise noted, all reactions were carried out with 1 (0.2 mmol), 2a (0.3 mmol), 5 (0.8 mmol), Cs2CO3 (0.3 mmol), [Ir(dFCF3ppy)2dtbbpy]PF6 (1 mol %) in 2 mL of DMSO, irradiation with blue LED (455 nm) at 25 °C for 24 h, and isolated yields were shown.
6 mmol scale.
Paraformaldehyde (0.8 mmol) and DMSO (4 mL) were used.
2.2. Generation of α-CF3 Carbanions and Their Fragmentation Reactions
Organic molecules containing a fluorine moiety generally exhibit improved reactivity, bioactivity, and metabolic stability compared to their nonfluorinated counterparts.29 An important privileged fluoro-containing group is the gem-difluoroethylene moiety based on their unique property in medicinal chemistry.30 Moreover, gem-difluoroalkenes are versatile building blocks for the synthesis of other fluorine-containing molecules.31 Traditional methods such as Wittig32 and Julia33 reactions for the synthesis of 1,1-difloroalkenes generally suffer from limited scope, modest efficiency, or harsh conditions. Another efficient pathway is the gem-difluorination of diazo compounds under metal catalytic34 or metal-free reaction conditions.35 This strategy is generally restricted to aromatic diazo compounds or diazo esters. Recently, several elegant defluorination strategies starting from α-trifluoromethyl alkenes based on metal catalysis36 or photoredox catalysis37 have been developed for the synthesis of gem-difluoroalkenes. Nevertheless, this route requires the presence of trifluoromethyl groups on the alkene moieties, and the product scope is limited by the accessibility of such trifluoromethylated alkenes.
Following the proposal shown in Scheme 1C and encouraged by the success in the generation of α-sulfenyl carbanions as described in section 2.1, we wondered whether difluoroalkenes could be produced involving CF3 radicals in the radical-carbanion relay sequence. The feasibility of this approach was supported by the facile E1cB elimination of α-CF3 carbanions to yield the difluoroalkenes.37a,37f,38 We used sodium triflinate (Langlois reagent, CF3SO2Na), a bench-stable and commercially available trifluoromethylation reagent, as the CF3 radical precursor and N-tosylhydrazone 7a as the model substrate.39 The optimized conditions (see the SI, Tables S5–S7), which include the use of [Ir(dFCF3ppy)2dtbbpy]PF6 (2 mol %) as photocatalyst and Cs2CO3 (1.5 equiv) as the base in 1 mL of solvent (DMSO/acetone = 1/1), delivered the desired 1,1-difluoroalkene 9a in 77% yield (Table 5, entry 2). Likewise, this transformation demonstrated retained efficiency when the reaction was carried out in a one-pot process (Table 5, entry 3). Control experiments indicated that the base, photocatalyst, and light irradiation were essential for this reaction (Table 5, entries 4–6).
Table 5. Screening of Reaction Conditions for the 1,1-Difluoroolefination of N-Tosylhydrazonea.
| entry | change from standard conditions | yieldb |
|---|---|---|
| 1 | none | 70% |
| 2 | PC (2 mol %) | 77% (73%)c |
| 3 | one-pot, PC (2 mol %) | 75%d |
| 4 | without Cs2CO3 | n.d. |
| 5 | without PC | n.d. |
| 6 | in the dark | n.d. |
Reaction conditions: compound 7a (0.2 mmol), 8 (0.3 mmol), Cs2CO3 (0.3 mmol), [Ir(dFCF3ppy)2dtbbpy]PF6 (1 mol %) in 1 mL of solvent, irradiation with blue LED (455 nm) at 25 °C for 24 h. n.d. = not detected.
Yields were determined by 19F NMR analysis of the crude reaction mixture using 4,4′-difluorobenzophenone as the internal standard.
Isolated yield.
7a was formed in one pot starting from corresponding ketone and used directly without purification. PC = photocatalyst.
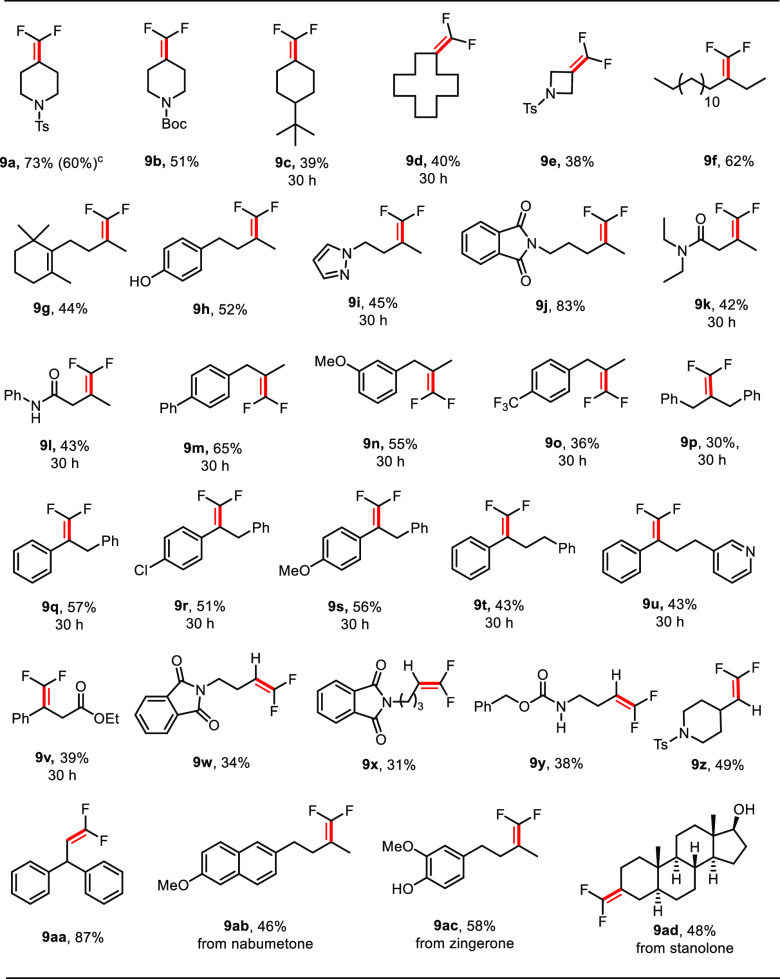
Using the optimized reaction conditions for the gem-difluoroolefination, the scope of this methodology was evaluated. As summarized in Table 6, the reaction proceeded smoothly with a variety of N-tosylhydrazones, affording the expected gem-difluoroalkenes in moderate to good yields. The reactions of sodium triflinate with cycloketone-derived N-tosylhydrazones led to the corresponding products 9a–9e in 38–73% yields. Interestingly, a strained substrate like azetidinone-derivatized N-tosylhydrazone could be successfully functionalized yielding difluoroalkene 9e in modest yield.40 Our method could be also extended to acyclic N-tosylhydrazones. For instance, tosylhydrazone derived from 3-hexadecanone performed well in our reaction affording the desired product 9f in 62% yield. Moreover, the mild reaction conditions were compatible with ketone-based tosylhydrazones bearing a wide range of functional groups including alkene (9g), phenol (9h), and amide (9j–9l). With N-tosylhydrazones derived from phenylacetones, functional groups such as phenyl, methoxy, and trifluoromethyl on the aromatic ring were well tolerated (9m–9o). A sterically hindered substrate 7p participated in the reaction well to yield the gem-difluoroalkene. Tosylhydrazones derived from aromatic ketones were also applicable affording the desired products (9q–9u) in reasonable yields. The reactions proceeded smoothly with heterocycle-containing substrates (e.g., pyrazole 7i and pyridine 7u). Notably, ester groups on the carbon chain remained untouched (9v). This catalytic system was also suitable for aliphatic aldehyde-based N-tosylhydrazones, delivering the corresponding products in moderate to excellent yields (9w–9aa). The utility of this method was further demonstrated by applying it to functionalize structurally and functionally complex natural products like nabumetone, zingerone, and stanolone, providing the desired products (9ab–9ad) in good yields.
Table 6. Scope of the gem-Difluoroolefination of N-Tosylhydrazonesa.
Reaction conditions: unless otherwise noted, all reactions were carried out with 7 (0.2 mmol), 8 (0.3 mmol), Cs2CO3 (0.3 mmol), [Ir(dFCF3ppy)2dtbbpy]PF6 (2 mol %) in (DMSO/acetone = 1/1) 1 mL, irradiation with blue LED (455 nm) at 25 °C for 24 or 30 h, and isolated yields were shown.
8 mmol scale, reaction time: 48 h.
2.3. Reaction Mechanism
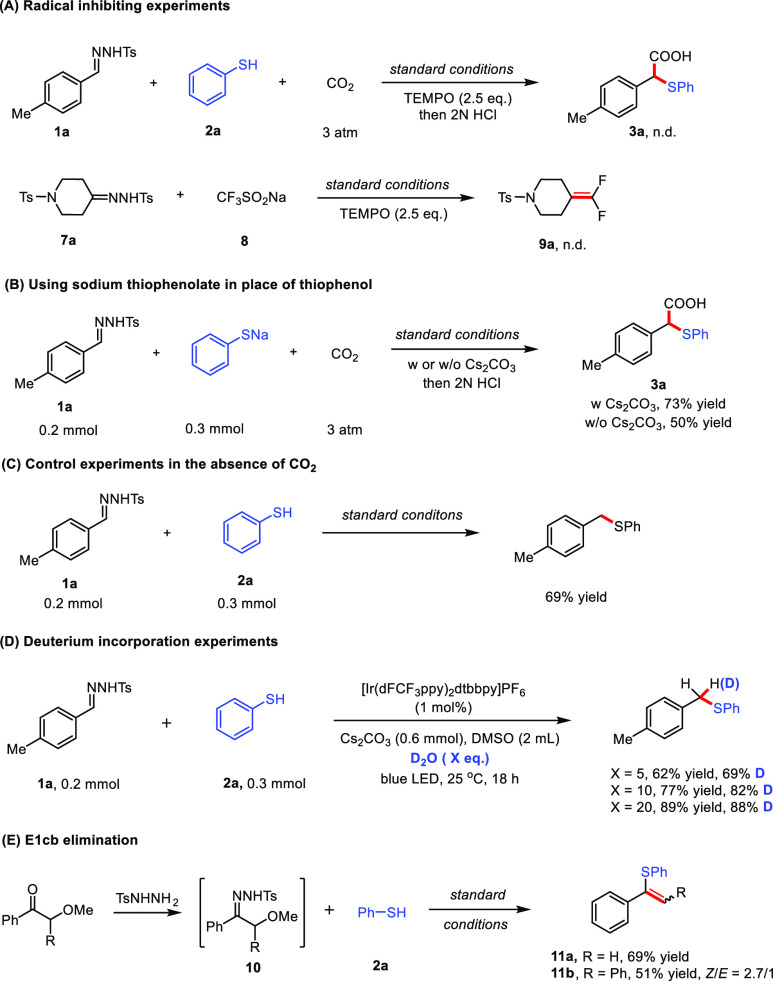
To gain insights into the reaction mechanism, a series of spectroscopic investigations and control experiments were conducted. First, no desired products were detected when the radical scavenger TEMPO (2.5 equiv) was added to the thiocarboxylation or gem-difluoroolefination reaction. The radical nature of this type of reaction was further confirmed by the formation of TEMPO–SPh and TEMPO–CF3 adducts, which were detected by the HRMS (Scheme 2A and the SI). Based on our results and literature reports about the radical functionalization of N-sulfonylhydrazones,17 we postulate that the sulfur-centered radical and CF3 radical follow a similar mechanism to react with N-tosylhydrazones to give the carbanions. We chose the thiocarboxylation reaction as a model reaction to study the mechanism more closely.
Scheme 2. Mechanistic Studies.
A control experiment using sodium thiophenolate in place of the corresponding thiophenol 2a yielded the desired carboxylic acid 3a in 73% yield. Moreover, we found that the product could be generated in 50% yield with sodium thiophenolate even in the absence of Cs2CO3, suggesting that the base (Cs2CO3) merely serves to deprotonate the thiols (Scheme 2B). The Stern–Volmer luminescence quenching experiments revealed that sodium thiophenolate quenches the excited state of the photocatalyst much more efficiently than N-tosylhydrazone 1a and thiophenol 2a (see the SI, Figures S8–S11). Light “on–off” experiments indicated that continuous light irradiation was essential for the reaction to proceed (see the SI). Additionally, the quantum yield of this transformation was determined to be 2.1%. Hence, a radical chain process is unlikely for this reaction. The combined results suggest a transient sulfur-centered radical, generated by single-electron oxidation of thiophenolate by the excited state of photocatalyst in a reductive quenching photocatalytic cycle.
Further control experiments showed that (4-methylbenzyl)(phenyl)sulfide could be obtained in 69% yield in the absence of CO2 (Scheme 2C). This finding suggests that the sulfur-centered radical could interact with N-tosylhydrazone 1a irrespective of the existence of CO2. The result is in accordance with the hypothesis that a transient α-sulfenyl carbanion might occur in the reaction process. On this basis, we conducted isotope-labeling experiments. Indeed, when D2O was added in the absence of CO2, up to 88% deuterium incorporation into sulfide was observed (Scheme 2D). In addition, a carbanion intermediate should in principle undergo E1cB elimination when the adjacent carbon atom bears an appropriate leaving group.41 Therefore, N-tosylhydrazones bearing a methoxyl group at the vicinal carbon were prepared and subjected to the standard reaction conditions in the absence of CO2 giving the corresponding alkenes 11a and 11b in good yields (Scheme 2E).
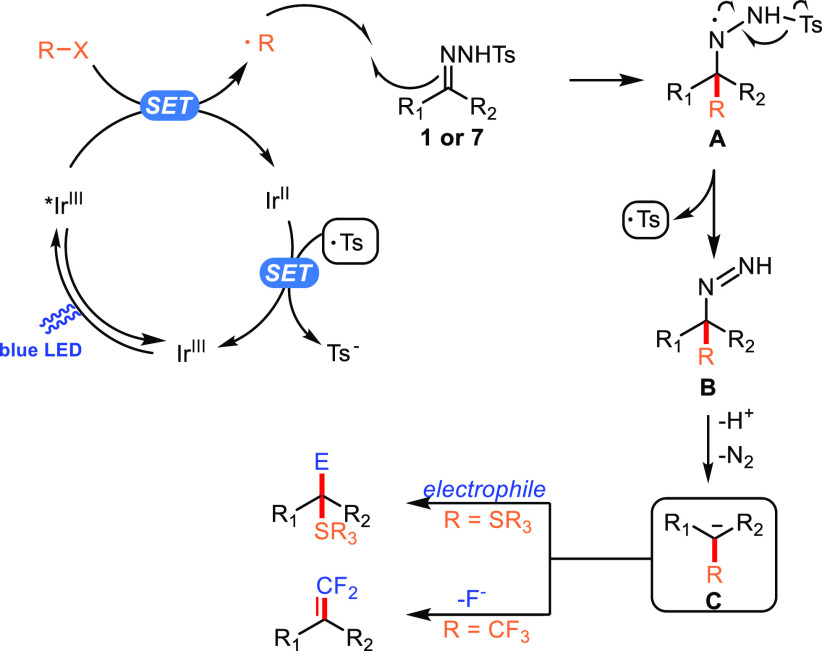
Based on the above experimental evidence and mechanistic pathways reported in the literature, we propose a plausible mechanism as depicted in Scheme 3 for the reported photocatalytic generation of functionalized carbanions. Initially, the photoexcited state of [IrIII(dFCF3ppy)2dtbbpy]+ (E1/2[*IrIII/II] = +1.21 V vs SCE)42 is reductively quenched by sodium triflinate (Eox = +1.05 V vs SCE)22a or thiophenolate (Eox = ∼0.75 V vs SCE),43 formed through the deprotonation of thiophenol by base, affording a sulfur-centered radical and a CF3 radical, respectively. Subsequent radical addition to the C=N bond of N-tosylhydrazone generates the aminyl radical species A.44 Fragmentation of the arenesulfonyl radical from intermediate A leads to a functionalized diazene intermediate B,18 and the following Wolff–Kishner type N2 extrusion process proceeds to give α-CF3 or sulfur carbanion C for further reactions. In the case of the α-sulfenyl carbanion, subsequent nucleophilic attack to CO2 or aliphatic aldehydes give carboxylic acids or alcohols. When α-CF3 carbanions were produced, β-fluoride elimination occurred to furnish the gem-difluoroalkenes. Finally, single-electron transfer (SET) from the reduced photoredox catalyst IrII (E1/2[IrIII/II] = −1.37 V vs SCE)42 to the arenesulfonyl radical (Ered = +0.50 V vs SCE)24b yields a sulfinate anion and regenerates the photocatalyst.
Scheme 3. Proposed Mechanism of the Photo-Wolff–Kishner Carbanion Generation.
3. Conclusion
In summary, we have established a new reaction sequence for the generation of α-functionalized alkyl carbanions through the merger of photoredox catalytic radical generation with the classic Wolff–Kishner (WK) reaction. This radical-carbanion relay for carbonyl functionalization involves the radical addition to N-sulfonylhydrazones, which enables the formation of α-substituted carbanion intermediates. Subsequent reaction with electrophiles including CO2 and aldehydes or fragmentation results in thiocarboxylation, thiohydroxyalkylation, and gem-difluoroolefination with broad substrate scope and good tolerance of many functional groups. Mechanistic studies support the hypothesis that a tandem photocatalytic radical addition/Wolff–Kishner process starting from N-sulfonylhydrazones facilitates the formation of the carbanion. This strategy greatly expands the synthetic potential of Wolff–Kishner reaction. Further studies aiming to generate nonstabilized carbanions by this strategy are currently under investigation.
Acknowledgments
This work was supported by the German Science Foundation (DFG) (KO 1537/18-1). This project has received funding from the European Research Council (ERC) under the European Unions Horizon 2020 research and innovation programme (grant agreement 741623). S.W. (CSC student number 201606280052) and B.-Y.C. thank the China Scholarship Council (CSC) for a predoctoral fellowship. We thank Dr. Rudolf Vasold (University of Regensburg) for his assistance in GC-MS measurements and Ms. Regina Hoheisel (University of Regensburg) for her assistance in cyclic voltammetry measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c00629.
Experiments and spectral details for all new compounds and all reactions reported (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Seebach D. Methods of Reactivity Umpolung. Angew. Chem., Int. Ed. Engl. 1979, 18, 239–258. 10.1002/anie.197902393. [DOI] [Google Scholar]
- a Gröbel B.-T.; Seebach D. Umpolung of the Reactivity of Carbonyl Compounds through Sulfur-Containing Reagents. Synthesis 1977, 1977, 357–402. 10.1055/s-1977-24412. [DOI] [Google Scholar]; b Seebach D.; Corey E. J. Generation and Synthetic Applications of 2-lithio-1,3-dithianes. J. Org. Chem. 1975, 40, 231–237. 10.1021/jo00890a018. [DOI] [Google Scholar]; c Smith A. B.; Adams C. M. Evolution of Dithiane-Based Strategies for the Construction of Architecturally Complex Natural Products. Acc. Chem. Res. 2004, 37, 365–377. 10.1021/ar030245r. [DOI] [PubMed] [Google Scholar]
- a Bugaut X.; Glorius F. Organocatalytic Umpolung: N-heterocyclic Carbenes and Beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. 10.1039/c2cs15333e. [DOI] [PubMed] [Google Scholar]; b Flanigan D. M.; Romanov-Michailidis F.; White N. A.; Rovis T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. 10.1021/acs.chemrev.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Marion N.; Díez-González S.; Nolan S. P. N-Heterocyclic Carbenes as Organocatalysts. Angew. Chem., Int. Ed. 2007, 46, 2988–3000. 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]
- Huang-Minlon A Simple Modification of the Wolff-Kishner Reduction. J. Am. Chem. Soc. 1946, 68, 2487–2488. 10.1021/ja01216a013. [DOI] [Google Scholar]
- Lewis D. E.The Wolff-Kishner Reduction and Related Reactions: Discovery and Development; Elsevier, 2019. [Google Scholar]
- a Vedejs E.; Stolle W. T. Reductive alkylation of aldehyde tosylhydrazones with organolithium reagents. Tetrahedron Lett. 1977, 18, 135–138. 10.1016/S0040-4039(01)92569-9. [DOI] [Google Scholar]; b Vedejs E.; Dolphin J.; Stolle W. A new olefin synthesis: condensation of aldehyde tosylhydrazones with stabilized carbanions. J. Am. Chem. Soc. 1979, 101, 249–251. 10.1021/ja00495a057. [DOI] [Google Scholar]; c Myers A. G.; Kukkola P. J. Stereoselective synthesis of olefins from silylated sulfonylhydrazones. J. Am. Chem. Soc. 1990, 112, 8208–8210. 10.1021/ja00178a078. [DOI] [Google Scholar]; d Myers A. G.; Movassaghi M. Highly Efficient Methodology for the Reductive Coupling of Aldehyde Tosylhydrazones with Alkyllithium Reagents. J. Am. Chem. Soc. 1998, 120, 8891–8892. 10.1021/ja981918h. [DOI] [Google Scholar]
- Wang H.; Dai X.-J.; Li C.-J. Aldehydes as Alkyl Carbanion Equivalents for Additions to Carbonyl Compounds. Nat. Chem. 2017, 9, 374–378. 10.1038/nchem.2677. [DOI] [PubMed] [Google Scholar]
- Chen N.; Dai X.-J.; Wang H.; Li C.-J. Umpolung Addition of Aldehydes to Aryl Imines. Angew. Chem., Int. Ed. 2017, 56, 6260–6263. 10.1002/anie.201610578. [DOI] [PubMed] [Google Scholar]
- Yan S.-S.; Zhu L.; Ye J.-H.; Zhang Z.; Huang H.; Zeng H.; Li C.-J.; Lan Y.; Yu D.-G. Ruthenium-Catalyzed Umpolung Carboxylation of Hydrazones with CO2. Chem. Sci. 2018, 9, 4873–4878. 10.1039/C8SC01299G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X.-J.; Wang H.; Li C.-J. Carbonyls as Latent Alkyl Carbanions for Conjugate Additions. Angew. Chem., Int. Ed. 2017, 56, 6302–6306. 10.1002/anie.201700059. [DOI] [PubMed] [Google Scholar]
- Tang J.; Lv L.; Dai X.-J.; Li C.-C.; Li L.; Li C.-J. Nickel-Catalyzed Cross-Coupling of Aldehydes with Aryl Halides via Hydrazone Intermediates. Chem. Commun. 2018, 54, 1750–1753. 10.1039/C7CC09290C. [DOI] [PubMed] [Google Scholar]
- Lv L.; Zhu D.; Li C.-J. Direct Dehydrogenative Alkyl Heck-Couplings of Vinylarenes with Umpolung Aldehydes Catalyzed by Nickel. Nat. Commun. 2019, 10, 715. 10.1038/s41467-019-08631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D.; Lv L.; Li C.-C.; Ung S.; Gao J.; Li C.-J. Umpolung of Carbonyl Groups as Alkyl Organometallic Reagent Surrogates for Palladium-Catalyzed Allylic Alkylation. Angew. Chem., Int. Ed. 2018, 57, 16520–16524. 10.1002/anie.201809112. [DOI] [PubMed] [Google Scholar]
- Wei W.; Dai X.-J.; Wang H.; Li C.; Yang X.; Li C.-J. Ruthenium(II)-Catalyzed Olefination via Carbonyl Reductive Cross-Coupling. Chem. Sci. 2017, 8, 8193–8197. 10.1039/C7SC04207H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H.; Luo Z.; Han X.; Li C.-J. Metal-Free Construction of the C(sp3)-CF3 Bond: Trifluoromethylation of Hydrazones with Togni’s Reagent under Mild Conditions. Org. Lett. 2019, 21, 5948–5951. 10.1021/acs.orglett.9b02072. [DOI] [PubMed] [Google Scholar]
- Wang S.; Lokesh N.; Hioe J.; Gschwind R. M.; König B. Photoinitiated Carbonyl-Metathesis: Deoxygenative Reductive Olefination of Aromatic Aldehydes via Photoredox Catalysis. Chem. Sci. 2019, 10, 4580–4587. 10.1039/C9SC00711C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kim S.; Cho J. R. Radical Cyclization of Mesitylsulfonylhydrazones. Synlett 1992, 1992, 629–630. 10.1055/s-1992-21435. [DOI] [Google Scholar]; b Dao H. T.; Li C.; Michaudel Q.; Maxwell B. D.; Baran P. S. Hydromethylation of Unactivated Olefins. J. Am. Chem. Soc. 2015, 137, 8046–8049. 10.1021/jacs.5b05144. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Campbell N. E.; Sammis G. M. Single-Electron/Pericyclic Cascade for the Synthesis of Dienes. Angew. Chem., Int. Ed. 2014, 53, 6228–6231. 10.1002/anie.201403234. [DOI] [PubMed] [Google Scholar]
- a Baldwin J. E.; Bottaro J. C.; Kolhe J. N.; Adlington R. M. Azo Anions in Synthesis. Use of Trityl- and Diphenyl-4-Pyridylmethyl-Hydrazones for Reductive C-C Bond Formation from Aldehydes and Ketones. J. Chem. Soc., Chem. Commun. 1984, 22–23. 10.1039/C39840000022. [DOI] [Google Scholar]; b Baldwin J. E.; Adlington R. M.; Bottaro J. C.; Kolhe J. N.; Newington I. M.; Perry M. W. D. Azo Anions in Synthesis: Use of Trityl- and Diphenyl-4-pyridylmethylhydrazones for Reductive C-C Bond Formation. Tetrahedron 1986, 42, 4235–4246. 10.1016/S0040-4020(01)87648-1. [DOI] [Google Scholar]; c Reyes J. R.; Rawal V. H. Reductive Chlorination and Bromination of Ketones via Trityl Hydrazones. Angew. Chem. 2016, 128, 3129–3132. 10.1002/ange.201510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Chauhan P.; Mahajan S.; Enders D. Organocatalytic Carbon-Sulfur Bond-Forming Reactions. Chem. Rev. 2014, 114, 8807–8864. 10.1021/cr500235v. [DOI] [PubMed] [Google Scholar]; b Shen C.; Zhang P.; Sun Q.; Bai S.; Hor T. S. A.; Liu X. Recent Advances in C-S Bond Formation via C-H Bond Functionalization and Decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. 10.1039/C4CS00239C. [DOI] [PubMed] [Google Scholar]; c Feng M.; Tang B.; Liang S. H.; Jiang X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Wang N.; Saidhareddy P.; Jiang X. Construction of Sulfur-Containing Moieties in the Total Synthesis of Natural Products. Nat. Prod. Rep. 2020, 37, 246–275. 10.1039/C8NP00093J. [DOI] [PubMed] [Google Scholar]
- a Guo W.; Tao K.; Tan W.; Zhao M.; Zheng L.; Fan X. Recent Advances in Photocatalytic C-S/P-S Bond Formation via the Generation of Sulfur Centered Radicals and Functionalization. Org. Chem. Front. 2019, 6, 2048–2066. 10.1039/C8QO01353E. [DOI] [Google Scholar]; b Wimmer A.; König B. Photocatalytic Formation of Carbon-Sulfur Bonds. Beilstein J. Org. Chem. 2018, 14, 54–83. 10.3762/bjoc.14.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de la Pradilla R.; Viso A. Alkylation of α-Sulfur-Containing Carbanions. Comprehensive Organic Synthesis: Second Edition 2014, 3, 157–208. 10.1016/B978-0-08-097742-3.00304-9. [DOI] [Google Scholar]
- a Yatham V. R.; Shen Y.; Martin R. Catalytic Intermolecular Dicarbofunctionalization of Styrenes with CO2 and Radical Precursors. Angew. Chem., Int. Ed. 2017, 56, 10915–10919. 10.1002/anie.201706263. [DOI] [PubMed] [Google Scholar]; b Liao L.-L.; Cao G.-M.; Ye J.-H.; Sun G.-Q.; Zhou W.-J.; Gui Y.-Y.; Yan S.-S.; Shen G.; Yu D.-G. Visible-Light-Driven External-Reductant-Free Cross-Electrophile Couplings of Tetraalkyl Ammonium Salts. J. Am. Chem. Soc. 2018, 140, 17338–17342. 10.1021/jacs.8b08792. [DOI] [PubMed] [Google Scholar]; c Hou J.; Ee A.; Cao H.; Ong H.-W.; Xu J.-H.; Wu J. Visible-Light-Mediated Metal-Free Difunctionalization of Alkenes with CO2 and Silanes or C(sp3)-H Alkanes. Angew. Chem., Int. Ed. 2018, 57, 17220–17224. 10.1002/anie.201811266. [DOI] [PubMed] [Google Scholar]; d Yoo W.-J.; Kondo J.; Rodríguez-Santamaría J. A.; Nguyen T. V. Q.; Kobayashi S. Efficient Synthesis of α-Trifluoromethyl Carboxylic Acids and Esters through Fluorocarboxylation of gem-Difluoroalkenes. Angew. Chem., Int. Ed. 2019, 58, 6772–6775. 10.1002/anie.201902779. [DOI] [PubMed] [Google Scholar]; e Meng Q.-Y.; Schirmer T. E.; Berger A. L.; Donabauer K.; König B. Photocarboxylation of Benzylic C-H Bonds. J. Am. Chem. Soc. 2019, 141, 11393–11397. 10.1021/jacs.9b05360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Meng Q.-Y.; Wang S.; König B. Carboxylation of Aromatic and Aliphatic Bromides and Triflates with CO2 by Dual Visible-Light-Nickel Catalysis. Angew. Chem., Int. Ed. 2017, 56, 13426–13430. 10.1002/anie.201706724. [DOI] [PubMed] [Google Scholar]; b Meng Q.-Y.; Wang S.; Huff G. S.; König B. Ligand-Controlled Regioselective Hydrocarboxylation of Styrenes with CO2 by Combining Visible Light and Nickel Catalysis. J. Am. Chem. Soc. 2018, 140, 3198–3201. 10.1021/jacs.7b13448. [DOI] [PubMed] [Google Scholar]; c Sahoo B.; Bellotti P.; Juliá-Hernández F.; Meng Q.-Y.; Crespi S.; König B.; Martin R. Site-Selective, Remote sp3 C-H Carboxylation Enabled by the Merger of Photoredox and Nickel Catalysis. Chem. - Eur. J. 2019, 25, 9001–9005. 10.1002/chem.201902095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Noble A.; MacMillan D. W. C. Photoredox α-Vinylation of α-Amino Acids and N-Aryl Amines. J. Am. Chem. Soc. 2014, 136, 11602–11605. 10.1021/ja506094d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Heitz D. R.; Rizwan K.; Molander G. A. Visible-Light-Mediated Alkenylation, Allylation, and Cyanation of Potassium Alkyltrifluoroborates with Organic Photoredox Catalysts. J. Org. Chem. 2016, 81, 7308–7313. 10.1021/acs.joc.6b01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. L.; Donabauer K.; König B. Photocatalytic Barbier Reaction - Visible-Light Induced Allylation and Benzylation of Aldehydes and Ketones. Chem. Sci. 2018, 9, 7230–7235. 10.1039/C8SC02038H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Barbier P. Synthesis of dimethylheptenol. C. R. Acad. Sci. 1899, 128, 110–111. [Google Scholar]; b Yamamoto Y.; Asao N. Selective Reactions Using Allylic Metals. Chem. Rev. 1993, 93, 2207–2293. 10.1021/cr00022a010. [DOI] [Google Scholar]
- In the examples of 6h and 6i, benzyl phenyl sulfide was formed in 29% and 32% yield, respectively.
- Nakamura S.; Nakagawa R.; Watanabe Y.; Toru T. Highly Enantioselective Reactions of Configurationally Labile α-Thioorganolithiums Using Chiral Bis(oxazoline)s via Two Different Enantiodetermining Steps. J. Am. Chem. Soc. 2000, 122, 11340–11347. 10.1021/ja0025191. [DOI] [Google Scholar]
- a Müller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; b Hagmann W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]; c Gillis E. P.; Eastman K. J.; Hill M. D.; Donnelly D. J.; Meanwell N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- a Gouverneur V., Müller K., Eds. Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications; Imperial College Press: London, 2012. [Google Scholar]; b Ojima I., Ed. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: West Sussex, UK, 2009. [Google Scholar]
- a Chelucci G. Synthesis and Metal-Catalyzed Reactions of gem-Dihalovinyl Systems. Chem. Rev. 2012, 112, 1344–1462. 10.1021/cr200165q. [DOI] [PubMed] [Google Scholar]; b Zhang X.; Cao S. Recent advances in the synthesis and C-F functionalization of gem-difluoroalkenes. Tetrahedron Lett. 2017, 58, 375–392. 10.1016/j.tetlet.2016.12.054. [DOI] [Google Scholar]; c Koley S.; Altman R. A.. Recent Advances in Transition Metal-catalyzed Functionalization of gem-Difluoroalkenes. Isr. J. Chem. 2020, in press. 10.1002/ijch.201900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zheng J.; Cai J.; Lin J.-H.; Guo Y.; Xiao J.-C. Synthesis and Decarboxylative Wittig Reaction of Difluoromethylene Phosphobetaine. Chem. Commun. 2013, 49, 7513–7515. 10.1039/c3cc44271c. [DOI] [PubMed] [Google Scholar]; b Burton D. J.; Yang Z.-Y.; Qiu W. Fluorinated Ylides and Related Compounds. Chem. Rev. 1996, 96, 1641–1716. 10.1021/cr941140s. [DOI] [PubMed] [Google Scholar]
- a Zhao Y.; Huang W.; Zhu L.; Hu J. Difluoromethyl 2-Pyridyl Sulfone: A New gem-Difluoroolefination Reagent for Aldehydes and Ketones. Org. Lett. 2010, 12, 1444–1447. 10.1021/ol100090r. [DOI] [PubMed] [Google Scholar]; b Gao B.; Zhao Y.; Hu M.; Ni C.; Hu J. gem-Difluoroolefination of Diaryl Ketones and Enolizable Aldehydes with Difluoromethyl 2-Pyridyl Sulfone: New Insights into the Julia-Kocienski Reaction. Chem. - Eur. J. 2014, 20, 7803–7810. 10.1002/chem.201402183. [DOI] [PubMed] [Google Scholar]
- a Zhang Z.; Zhou Q.; Yu W.; Li T.; Wu G.; Zhang Y.; Wang J. Cu(I)-Catalyzed Cross-Coupling of Terminal Alkynes with Trifluoromethyl Ketone N-Tosylhydrazones: Access to 1,1-Difluoro-1,3-enynes. Org. Lett. 2015, 17, 2474–2477. 10.1021/acs.orglett.5b00980. [DOI] [PubMed] [Google Scholar]; b Hu M.; He Z.; Gao B.; Li L.; Ni C.; Hu J. Copper-Catalyzed gem-Difluoroolefination of Diazo Compounds with TMSCF3 via C-F Bond Cleavage. J. Am. Chem. Soc. 2013, 135, 17302–17305. 10.1021/ja409941r. [DOI] [PubMed] [Google Scholar]; c Yang Z.; Möller M.; Koenigs R. M. Synthesis of gem-Difluoro Olefins through C-H Functionalization and β-Fluoride Elimination Reactions. Angew. Chem., Int. Ed. 2020, 59, 5572–5576. 10.1002/anie.201915500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M.; Ni C.; Li L.; Han Y.; Hu J. gem-Difluoroolefination of Diazo Compounds with TMSCF3 or TMSCF2Br: Transition-Metal-Free Cross-Coupling of Two Carbene Precursors. J. Am. Chem. Soc. 2015, 137, 14496–14501. 10.1021/jacs.5b09888. [DOI] [PubMed] [Google Scholar]
- a Ding D.; Lan Y.; Lin Z.; Wang C. Synthesis of gem-Difluoroalkenes by Merging Ni-Catalyzed C-F and C-C Bond Activation in Cross-Electrophile Coupling. Org. Lett. 2019, 21, 2723–2730. 10.1021/acs.orglett.9b00692. [DOI] [PubMed] [Google Scholar]; b Lan Y.; Yang F.; Wang C. Synthesis of gem-Difluoroalkenes via Nickel-Catalyzed Allylic Defluorinative Reductive Cross-Coupling. ACS Catal. 2018, 8, 9245–9251. 10.1021/acscatal.8b02784. [DOI] [Google Scholar]; c Lin Z.; Lan Y.; Wang C. Reductive Allylic Defluorinative Cross-Coupling Enabled by Ni/Ti Cooperative Catalysis. Org. Lett. 2019, 21, 8316–8322. 10.1021/acs.orglett.9b03102. [DOI] [PubMed] [Google Scholar]; d Lin Z.; Lan Y.; Wang C. Synthesis of gem-Difluoroalkenes via Nickel-Catalyzed Reductive C-F and C-O Bond Cleavage. ACS Catal. 2019, 9, 775–780. 10.1021/acscatal.8b04348. [DOI] [Google Scholar]; e Lu X.; Wang X.-X.; Gong T.-J.; Pi J.-J.; He S.-J.; Fu Y. Nickel-Catalyzed Allylic Defluorinative Alkylation of Trifluoromethyl Alkenes with Reductive Decarboxylation of Redox-Active Esters. Chem. Sci. 2019, 10, 809–814. 10.1039/C8SC04335C. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Zhao X.; Li C.; Wang B.; Cao S. Copper-Catalyzed Synthesis of gem-Difluoroallylboronates from α-Trifluoromethyl Alkenes and B2 pin2. Tetrahedron Lett. 2019, 60, 129–132. 10.1016/j.tetlet.2018.11.073. [DOI] [Google Scholar]
- a Lang S. B.; Wiles R. J.; Kelly C. B.; Molander G. A. Photoredox Generation of Carbon-Centered Radicals Enables the Construction of 1,1-Difluoroalkene Carbonyl Mimics. Angew. Chem., Int. Ed. 2017, 56, 15073–15077. 10.1002/anie.201709487. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Xiao T.; Li L.; Zhou L. Synthesis of Functionalized gem-Difluoroalkenes via a Photocatalytic Decarboxylative/Defluorinative Reaction. J. Org. Chem. 2016, 81, 7908–7916. 10.1021/acs.joc.6b01620. [DOI] [PubMed] [Google Scholar]; c Chen H.; Anand D.; Zhou L. Photoredox Defluorinative Alkylation of 1-Trifluoromethyl Alkenes and 1,3-Butadienes with 1,4-Dihydropyridines as Alkylation Reagents. Asian J. Org. Chem. 2019, 8, 661–664. 10.1002/ajoc.201900026. [DOI] [Google Scholar]; d Phelan J. P.; Lang S. B.; Sim J.; Berritt S.; Peat A. J.; Billings K.; Fan L.; Molander G. A. Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis. J. Am. Chem. Soc. 2019, 141, 3723–3732. 10.1021/jacs.9b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Wiles R. J.; Phelan J. P.; Molander G. A. Metal-free Defluorinative Arylation of Trifluoromethyl Alkenes via Photoredox Catalysis. Chem. Commun. 2019, 55, 7599–7602. 10.1039/C9CC04265B. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Wu L.-H.; Cheng J.-K.; Shen L.; Shen Z.-L.; Loh T.-P. Visible Light-Mediated Trifluoromethylation of Fluorinated Alkenes via C-F Bond Cleavage. Adv. Synth. Catal. 2018, 360, 3894–3899. 10.1002/adsc.201800740. [DOI] [Google Scholar]; g Guo Y.-Q.; Wang R.; Song H.; Liu Y.; Wang Q. Visible-Light-Induced Deoxygenation/Defluorination Protocol for Synthesis of γ,γ-Difluoroallylic Ketones. Org. Lett. 2020, 22, 709–713. 10.1021/acs.orglett.9b04504. [DOI] [PubMed] [Google Scholar]
- Uneyama K.; Katagiri T.; Amii H. α-Trifluoromethylated Carbanion Synthons. Acc. Chem. Res. 2008, 41, 817–829. 10.1021/ar7002573. [DOI] [PubMed] [Google Scholar]
- a Zhang C. Application of Langlois’ Reagent in Trifluoromethylation Reactions. Adv. Synth. Catal. 2014, 356, 2895–2906. 10.1002/adsc.201400370. [DOI] [Google Scholar]; b Koike T.; Akita M. New Horizons of Photocatalytic Fluoromethylative Difunctionalization of Alkenes. Chem. 2018, 4, 409–437. 10.1016/j.chempr.2017.11.004. [DOI] [Google Scholar]; c Alonso C.; Martínez de Marigorta E.; Rubiales G.; Palacios F. Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem. Rev. 2015, 115, 1847–1935. 10.1021/cr500368h. [DOI] [PubMed] [Google Scholar]; d Pan X.; Xia H.; Wu J. Recent Advances in Photoinduced Trifluoromethylation and Difluoroalkylation. Org. Chem. Front. 2016, 3, 1163–1185. 10.1039/C6QO00153J. [DOI] [Google Scholar]
- In this case, the protonated byproduct was isolated in 24% yield.
- Donabauer K.; Maity M.; Berger A. L.; Huff G. S.; Crespi S.; König B. Photocatalytic Carbanion Generation - Benzylation of Aliphatic Aldehydes to Secondary Alcohols. Chem. Sci. 2019, 10, 5162–5166. 10.1039/C9SC01356C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry M. S.; Goldsmith J. I.; Slinker J. D.; Rohl R.; Pascal R. A.; Malliaras G. G.; Bernhard S. Single-Layer Electroluminescent Devices and Photoinduced Hydrogen Production from An Ionic Iridium (III) Complex. Chem. Mater. 2005, 17, 5712–5719. 10.1021/cm051312+. [DOI] [Google Scholar]
- a Jiang M.; Li H.; Yang H.; Fu H. Room-Temperature Arylation of Thiols: Breakthrough with Aryl Chlorides. Angew. Chem., Int. Ed. 2017, 56, 874–879. 10.1002/anie.201610414. [DOI] [PubMed] [Google Scholar]; b Liu D.; Ma H.-X.; Fang P.; Mei T.-S. Nickel-Catalyzed Thiolation of Aryl Halides and Heteroaryl Halides through Electrochemistry. Angew. Chem., Int. Ed. 2019, 58, 5033–5037. 10.1002/anie.201900956. [DOI] [PubMed] [Google Scholar]
- Xu P.; Li W.; Xie J.; Zhu C. Exploration of C-H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond. Acc. Chem. Res. 2018, 51, 484–495. 10.1021/acs.accounts.7b00565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.