Abstract
Cellular analysis is a central concept for both biology and medicine. Over the past two decades, acoustofluidic technologies, which marry acoustic waves with microfluidics, have significantly contributed to the development of innovative approaches for cellular analysis. Acoustofluidic technologies enable precise manipulations of cells and the fluids that confine them, and these capabilities have been utilized in many cell analysis applications. In this review article, we examine various applications where acoustofluidic methods have been implemented, including cell imaging, cell mechanotyping, circulating tumor cell phenotyping, sample preparation in clinics, and investigation of cell-cell interactions and cell-environment responses. We also provide our perspectives on the technological advantages, limitations, and potential future directions for this innovative field of methods.
Keywords: Acoustofluidics, Microfluidics, Surface acoustic waves (SAW), Bulk acoustic waves (BAW), Cell analysis
1. Introduction
Cell analysis is an overarching process that describes the goal of assessing specific properties and/or dynamics of cells, either in isolation or as part of the tissue in multicellular or unicellular organisms [1]. Ever since cells were identified as the basic structural, functional, and biological unit of all known living organisms in 1800s [2], their analysis has served as the central concept for fundamental biology research and provided the basis for clinical diagnosis and therapeutics. As such, researchers have regarded the improvement of cell analysis techniques as a critical pathway to the advancement of biology and medicine. To this end, technical innovations have provided a consistent source of improvement for cell analysis techniques. Historically, the invention of the modern microscope led to the discovery of cells by Robert Hooke in 1665 [3]. This was the first instance of cell imaging, and this feat directly accelerated the establishment of cell theory [2]. Fast forward several hundred years, where advances in flow cytometry and fluorescence activated cell sorting (FACS) [4,5] in 1950–1960s allowed researchers to investigate and analyze the heterogeneity within cell clusters based on individual surface biomarkers. In the 1980s, the development of the polymerase chain reaction (PCR) technique by Kary Mullis et al enabled precision diagnostics based on the sequencing of unknown etiologies in cells [6]. As a more recent example, the maturation of droplet microfluidic technology has contributed to the achievement of single-cell RNA sequencing [7,8], which has been used to measure critical differences between individual cells at the transcription level within a cell population. It is clear that the future of precision cell analysis will depend upon technological innovations; it is just unclear of which area will provide the catalyst for this advancement.
Acoustic-based methods for manipulating particles and fluids in a microfluidic environment, termed acoustofluidics [9,10], have significantly advanced the field of cell analysis just as the numerous techniques that came before have done. In the 19th century, microparticles were used to visualize the distribution of acoustic waves, known as Kundt’s tube [11,12], and ever since, researchers have relentlessly sought to harness acoustic waves as a tool to manipulate cells and extracellular fluidic environments. Acoustofluidic devices have become critical elements in the construction of cell analysis platforms; combining acoustofluidic manipulation techniques with cell characterization methods (e.g. PCR or imaging) enables precise manipulation and analysis in the microfluidic environment. In the last ten years, acoustofluidic techniques have been demonstrated for the patterning [13–17], focusing [18–21], separation [22–25], accumulation [26,27], sorting [28], and rotation of cells [29,30], and they have also been successfully applied in cellular analysis applications such as cell imaging [31], cell mechanotyping [32], circulating tumor cell (CTC) phenotyping [33], flow cytometry [34,35], and investigations into the interactions of cell-cell [36] and cell-environment [37,38] systems. It should also be mentioned that the development of acoustofluidic manipulation technologies is supported by (and not independent from) acoustic sensing, where changes in the frequency, amplitude, and phase of acoustic signals are used to analyze biochemical molecules and cells [39–41].
Acoustofluidic methods feature unique advantages for cell analysis applications, including operation with gentle force that will not disrupt normal cell physiology, and the ability to operate in a continuous manner with adjustable throughputs for both fundamental research and clinical applications on specimen ranging from bacteria to cells, and even multicellular organisms (ranging in size from nanometers to millimeters) in media including aqueous solutions, blood, and sputum. Acoustofluidic platforms also avoid the use of special working media, like those required in optical tweezers [42–44] or dielectrophoretic manipulation methods [45,46], which might damage cells. With these technical advantages in mind, we believe that acoustofluidic methods have the potential to provide many powerful tools and functionalities required for the next generation of cell analysis.
This review article aims to survey recent advances of acoustofluidic methods for cell analysis. Unlike other review articles in the field of acoustofluidics [10,47,56–60,48–55], this article focuses on acoustofluidic methods for cell analysis. Our discussion is categorized by the cell analysis applications achieved (e.g. imaging or mechanotyping) rather than the acoustic operation (e.g. rotation or deformation) demonstrated. The scope of this work covers both surface acoustic waves (SAW) and bulk acoustic waves (BAW) as they share similar working mechanisms. Particularly, we will focus on whole, live cell analysis, instead of acoustofluidic cell lysis [61–63] or sonoporation [64–71]; we will focus on acoustic actuation rather than acoustic sensing [72,73]. To keep the discussion concise, acoustofluidic cell sorting are excluded, as they were discussed in earlier review articles [60]. A brief perspective and outlook on the future implications of acoustofluidic technology is also provided at the end of the article. We begin our discussion with applications of acoustofluidics in cell imaging.
2. Cell imaging
Microscopic cell imaging is one of the most inexpensive and effective methods to understand biological systems; it also set the fundamentals for many down-streaming technologies, such as cytometers. Simple bright-field analysis at the microscopic level can discern characteristics within cells and microorganisms that are sufficient for many types of analyses. Fluorescent capabilities greatly expand the applications and analysis that can be completed, where selective staining of biological components effectively enhances the specificity and intensity of the signal in observation. Furthermore, confocal microscopy provides benefits such as ultra-high spatial resolution in addition to capturing 3D images, which is crucial to the study of cell structures (such as the cytoskeleton); 2D slices of transparent samples also provide both internal and surface characteristics of the cell/model animals [74,75]. While modern microscopic methods may be powerful, they often suffer from two inherent limitations: (1) the throughput of observation is limited; and (2) the equipment for 3D imaging is expensive and bulky, prohibitive to many research groups. To overcome these limitations, acoustofluidic methods with rotational 3D imaging and parallel imaging have been developed.
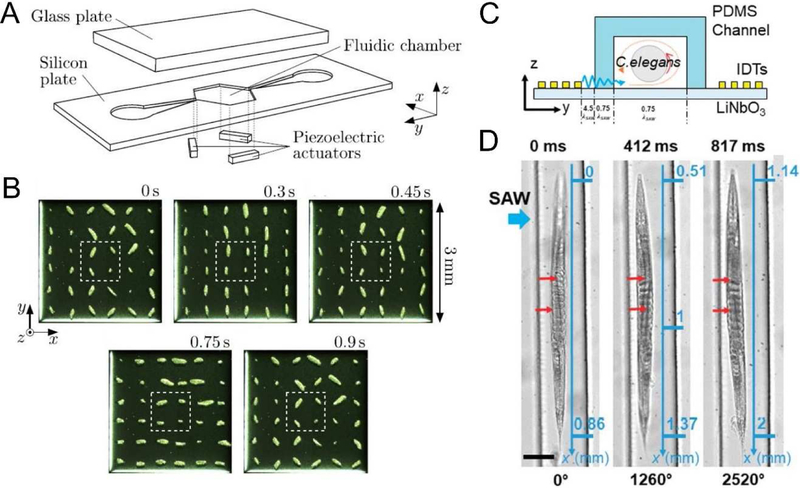
The precise manipulation capabilities of acoustofluidic systems made them excellent candidates for rotational 3D imaging, where the body of interest rotates with acoustic streaming; capturing planar images throughout rotation can uncover features that may have been hidden from a single, static view. Ahmed et al leveraged the acoustic streaming created by an oscillating bubble that has been excited by BAW to rotate cells and model organisms [31]. In this work, the authors were able to achieve rotation of cells about a variable axis dependent upon bubble size and excitation frequency. Additionally, they achieved precise rotation of a model organism, Caenorhabditis elegans (C. elegans), with a rotational step size of 4° with a 5 ms pulse of the transducer. This rotation enabled the visualization of individual dendrites along the length of the worm using a fluorescent microscope. Ozcelik et al also leveraged BAWs to interact with the fluid domain [76]. In their device, the oscillations of solid sharp-edge structures, as opposed to trapped bubbles, were used to create acoustic streaming that rotated cells and organism. They also rotated C. elegans to achieve a 3D visualization using a 2D microscope. Schwarz et al [77] used three separated piezoelectric transducers to modulate the frequency, phase, amplitude, and directions of BAWs and rotated particle clusters parallel in microfluidic chamber (Fig. 1 A–B). Recently, Zhang et al used SAWs to achieve rotation of C. elegans (Fig. 1C) [29]. They achieved a 4° rotation angle with a signal pulse of 1.5 ms, and even demonstrated rotation in a continuous flow (Fig. 1D). Trapping and rotation of cells and micro-objects using ultrahigh-frequency signals [30] or by leveraging acoustic potential fields [29] have also been reported.
Figure 1.
(A) A BAW-based acoustofluidic device that rotates particles with acoustic radiation torques; (B) A 180 rotation of 36 particle clumps formed out of 17 μm particles achieved using a phase modulation. (C) A SAW-based device for C. elegans rotation; and (D) rotation of a C. elegans in a continuous flow via acoustofluidics. Images reproduced with permission from references [29,77].
Acoustofluidic technologies have also been utilized in high-throughput cell imaging platforms where acoustic forces are used to pre-focus the cells or conduct parallel cell manipulations. Zmijan et al [78] developed a BAW based method for acoustically focusing particles into a single flat layer, which mitigated issues associated with image blur due to a shallow imaging field of view. The device was capable of imaging in rapid flows with linear speeds up to 104 mm/s, resulting in a throughput of 208,000 beads per second. They also demonstrated the imaging of ATDC5 cells at ~60,000 cell per second and proposed their technology as a tool for CTC characterization. Another technique, known as “Imaging FlowCytobot (IFCB),” [79,80] utilized BAWs to focus cells into a line before passing the camera for imaging. This work successfully captured high-resolution (1 m) images and measured chlorophyll fluorescence of nano- and micro-plankton sized particles.
Thus far, rotational 3D imaging and high-throughput imaging enabled by the acoustofluidics has successfully expanded the capability of conventional microscopic imaging techniques. In order to take full advantage of these technologies, it is critical that researchers leverage the compatible nature of acoustofluidic technology; that is, these acoustofluidic tools need to be combined with additional microfluidic or micro-imaging technologies that will help to unlock their full potential. For example, integrating these acoustofluidic rotation platforms with any of the low-cost, or portable imaging platforms (such as cell phones) that have been developed recently [81] would remove the need for a standard benchtop microscope, making these tools more accessible in resource-limited environments. Additionally, combining acoustofluidic rotation with real-time image and analysis could allow researchers to screen specific cells/organisms which show desirable characteristics, and separate them for further analysis. For example, acoustofluidic rotation-based 3D imaging may be used to identify a C. elegans with a positive drug response. After identification, this worm could be isolated for downstream genetic analysis to identify markers that would justify the varied drug response. In summary, while the acoustofluidic-based 3D imaging tools are powerful, they can be taken further and integrated with other technology to improve their relevance.
3. Cell mechanotyping
The characterization of cell mechanical properties (i.e. cell mechanotyping) is valuable for both diagnostic and cell biology research efforts. Changes in cells’ mechanical properties are usually associated with multiple diseases such as cancer, malaria, arthritis, atherosclerosis, hypertension, cerebral edema, stroke, and asthma [82–86]. Several biomechanical characteristics, including deformability, Young’s modulus, and compressibility are used to represent cell mechanical properties. These mechanical properties are conventionally measured using either an atomic force microscope (AFM) or optical tweezers (OT). When using an AFM, cells are linked with the tip of AFM probes and stretched precisely; using OTs, cells are conjugated with microparticles and deformed using optical radiation forces. Although these methods provide exceptional resolution in displacement and force measurement, AFM and OT methods suffer from low throughputs (~1 minute for a single cell). Therefore, while these methods may be sufficient for some fundamental biological studies, they are less applicable to many other applications such as clinical diagnoses. Acoustofluidic devices provide an alternative approach to AFMs or OTs for cell mechanotyping.
Cell deformability can be measured by stretching cells in acoustic field. As an example, Xie et al (Fig. 2A–B) developed a method that utilizes an acoustically oscillating bubble to deform cells suspended in an acoustic streaming field and assess their deformability [32]. This method measured mechanical biomarkers from multiple cells in a single experiment. Specifically, using this technique, the mean deformability of tens of HeLa, HEK, and HUVEC cells were assessed to distinguish their mechanical features. The method was easily integrated with other bioanalysis and drug-screening platforms; for example, the mechanical response of Hela cells after treatment with Cytochalasin was demonstrated. This acoustofluidic technique was also able to uncover the deformability of each subpopulation in a mixed, heterogeneous cell sample by using both fluorescent markers and mechanical biomarkers. Furthermore, a recent work demonstrated that the stiffness (i.e. elastic modulus) of single suspended cells can be measured by the shift of resonance frequency of a suspended microchannel resonator; this method is dependent upon acoustic scattering [87].
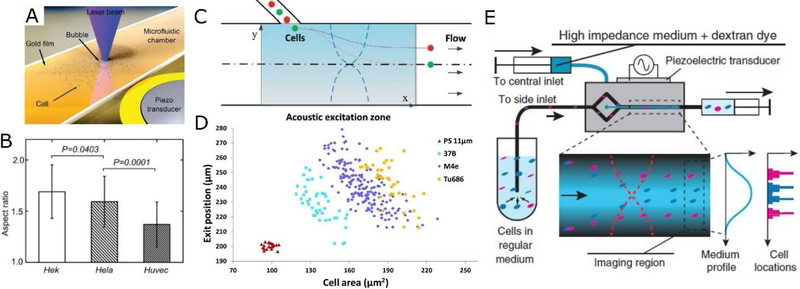
Figure 2.
(A-B) Probing cell deformability with acoustic streaming near an oscillating bubble. (C-D) Probing cell compressibility with deflection in a BAW-based device. (E) Probing cell impedance with acoustic contrast gradients in a BAW-based acoustofluidic device. Images reproduced with permission from references [32,89,90].
Acoustofluidics also provides a unique mechanism to measure cell compressibility by using the acoustic radiation force. Theoretically, a particle/cell exposed to an acoustic field experiences an acoustic radiation force given by [33]:
where p0 and Vp are the acoustic pressure and the volume of the particle, respectively; λ and k are the wavelength and the wave number of the acoustic waves, respectively; and φ is the acoustic contrast factor, which is dependent on the compressibility and density of the particle (βp and ρp, respectively ) and the medium (βm and ρp , respectively). In a typical mechanotyping experiment, the medium properties, excitation frequency, and pressure amplitude are known; Vp is assessed with microscopic observation; Fa is calculated using cell trajectories in the acoustic field; ρp is estimated from literature. Knowing all of the other factors, the cell compressibility (βp) can be readily calculated. For example, Hartono et al [88] recorded cell trajectories in a BAW field and obtained the compressibility of various cell types. They found that the compressibility of cancer cells is higher than that of normal breast cells, indicating that compressibility could be used as a diagnostic marker for cancer. To simplify the experiments, Wang et al [89] infused cells from the same initial positions and recorded a deflection-cell size relationship in a BAW field that indicated cell compressibility. They revealed differences in multiple cell lines with a throughput of 10 cells per second (Fig. 2C–D). In another work, iodixanol was introduced to alter the acoustic properties of the cell-culture medium such that cells can have positive, zero, or negative acoustic contrast depending on the molecular concentration. Manipulated by the BAW field, cells migrate to their iso-acoustic point, where their positions are indicative of their compressibility (Fig. 2E) [90]. In addition to cells, acoustofluidic mechanotyping was also applied to larger animals. Baasch et al [91] measured the compressibility of C. elegans in a BAW field, and correlated it with the volume fraction of lipids and proteins that would mainly make up the body of C. elegans. The above-mentioned cases exemplify that acoustofluidic methods have unique advantages in cell mechanotyping. They are contactless and can more readily achieve higher throughputs compared with traditional techniques such as OT and AFM. Acoustofluidic methods can also measure cell compressibility and the bulk modulus (i.e. inverse of compressibility) using the acoustic radiation force, in addition to the conventional deformability measurements.
In addition to measuring deformability and compressibility of individual cells, the strength of cell adhesion can be characterized with acoustofluidic methods as well. In a technique named “Acoustic Force Spectroscopy”, cells that have been bound to a microfluidic surface are levitated using a BAW toward the position of a pressure node. The displacement of cells and input power required to achieve the displacement were correlated and used to calculate adhesion strength. The authors demonstrated exertion of controlled forces, up to 1 nN, onto hundreds of individual cells in a parallel fashion [92]. It was determined that CD4 adhesion is accelerated by interleukin-7, remaining CD4 binding strength the same [92]. “Acoustic Force Spectroscopy” was also used to uncover protein-DNA interactions [93,94], and red blood cell deformability [95]. In these cases, the displacements of cells/DNAs that were linked to a microparticle were recorded when exposed to an external BAW field.
Despite its advantages in versatility for cell mechanotyping, the choice of which biophysical parameter to measure, and the conditions under which the measurement is should be carefully considered. First, one must consider whether the compressibility or deformability is a more accurate biomechanical marker for their application. Most reports indicate that cancer cells are both more compressible and more deformable compared with benign cells [85]; from this information, it would appear that the cell compressibility and deformability are positively correlated. However, Ravetto et al [96] revealed that monocytic cells become less compressible but more deformable upon exposure to inflammatory chemokines, confounding the simple relationship that researchers might have taken for granted. Researchers must also consider whether measurements should be taken in situ or when cells are suspended in a microfluidic channel. Unlike AFM or OT, current acoustofluidic mechanotyping procedures are not conducive to in situ experimentation; this means that cell handling and medium exchanges might alter the mechanical properties of cells before testing. These are just two of the factors that need to be considered when choosing to implement an acoustofluidic strategy in a cell mechanotyping experiment; nonetheless, acoustofluidics provides an excellent, biocompatible, high-throughput method for cell mechanotyping experiments.
4. Circulating tumor cell phenotyping
CTCs are an extremely rare type of cancerous cells which escape from a primary tumor and are carried around the body with blood circulation [97–100]. The CTCs induce metastasis, a major cause of cancer-related death, by acting as a seed for subsequent growth of tumors on distant organs. Despite the importance of analyzing CTCs for cancer prognosis and research, one major barrier for CTC phenotyping is their extremely rare nature in blood. Researchers have estimated the number of CTCs in the blood of patients with metastatic diseases to be on the order of 0–100 CTCs per mL. Therefore, developing methods to isolate CTCs from the vast number of other blood cells is imperative for successful CTC analysis. Cellsearch, a macro-scale immunomagnetic-based approach, is currently the first and only method approved by the U.S. Food and Drug Administration to isolate CTCs. Aiming to circumvent the limitation surrounding the use of surface biochemical markers (e.g. EpCAM) and obtain live CTCs, acoustofluidic methods have been developed to isolate, enrich, and phenotype CTCs based on differences in size and/or compressibility between CTCs and other cell types in blood.
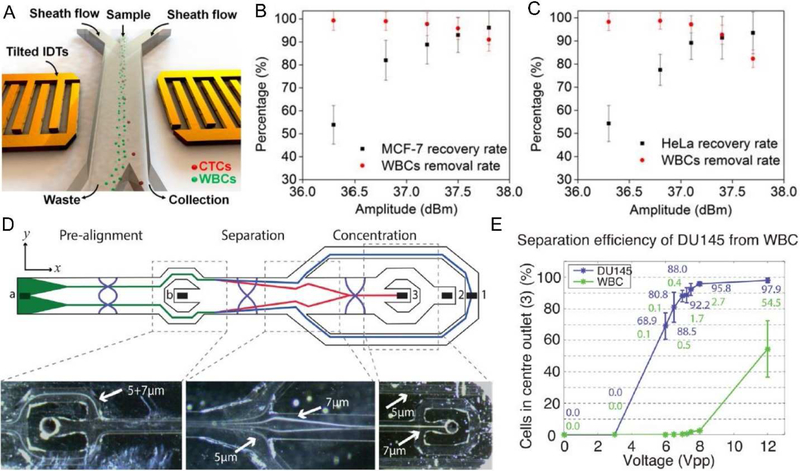
In the last five years, acoustofluidics have been proven as one of the most promising methods for CTC phenotyping. Li et al [33] developed a SAW-based method that was capable of separating CTCs in peripheral blood samples obtained from cancer patients. They used a tilted-angle standing SAW field to deflect cells to different outlets based on their size difference. This method can separate low concentrations (~100 cells/mL blood) of a variety of cultured cancer cell lines from white blood cells (WBCs) with a recovery rate better than 83% and a flow rate of 1.2 mL/h. The isolation of CTCs in blood samples obtained from patients with breast cancer was demonstrated as well (Fig. 3A–C). Recent work from the same group improved the throughput of SAW-based CTC separation [101]; the revised design employed a poly-dimethylsiloxane (PDMS)-glass hybrid channel, as an acoustic resonator, to increase the energy density and the resulting throughput. It also featured a divider in the channel to decrease the velocity of cells, thereby increasing the travel time of the cells within the SAW field and improving CTC separation efficiency. The separation throughput was increased to 7.5 mL/h, comparable to current clinical CTC assays (i.e. Cellsearch). In addition to SAW-based methods, BAW techniques have also been exploited extensively to separate cancer cells from blood cells [102–105]. For example, with a BAW-assisted cell focusing, separation, and enrichment protocol, Antfolk et al [102] demonstrated that breast cancer cells (MCF7 cells) spiked into red blood cell-lysed human blood were separated with an efficiency of 91.8 ± 1.0% at a flow rate of 100 L/min. The recovery rate of prostate cancer cells (DU145 cells) spiked into whole blood was 84.1 ± 2.1% (Fig. 3 D–E); the same group improved their device design by eliminating the need for a secondary medium to hydrodynamically pre-position cells before the separation, and reached an 86.5 ± 6.7% recovery rate for the cancer cells (DU145) spiked into blood [103].
Figure 3.
(A) SAW-based CTC separation and phenotyping. Performance for separation of (B) MCF-7 cells from WBCs, and (C) HeLa cells from WBCs at different power inputs. (D) Schematics and trajectories of 5 and 7 μm polystyrene particles in a BAW-based CTC separation device. (E) Performance for separation of DU 145 cells from WBCs at different power inputs. Images reproduced with permission from references [33,102]
There is great promise, as well as many challenges faced when using acoustofluidics for CTC phenotyping. BAW and SAW based devices both provide contactless, biocompatible, and compact methods for isolating CTCs from blood samples [100]. However, the CTC separation performance is depended upon the size and compressibility of the individual cells. This might not be a technical limitation, but it is necessary to define what type of CTCs are suitable for acoustofluidic separation/analysis, which requires extensive background knowledge of specific cancers and their CTCs. Additionally, current acoustofluidic methods are expected to integrate with downstream molecular characterization of CTCs. Mere enumeration, as current results of CTCs phenotyping, has had yet to enhance clinical prognostication or diagnostics in a meaningful way; these devices should be explored in conjunction with molecular analyses like transcriptomic or proteomic analysis [106] to truly impact cancer diagnosis, prognosis, and treatment.
5. Cell interaction
Cells form organized tissues and organs through precisely regulated interactions including cell-cell and cell-environment associations. Understanding physiological and pathological cell interactions is of great importance for diagnosis and therapeutics of diseases such as cancer and diabetes [107,108]. Uncovering this information requires techniques that are capable of manipulating cells and/or the surrounding fluidic environment with high resolution both spatially and temporally [109,110]. Recently, researchers have made great efforts in developing acoustofluidic devices to probe cell-cell interactions and cell-environment responses [56].
With regards to cell-cell interactions, Li et al [111] developed a SAW-based cell co-culture platform to alternatively seed and culture two cell lines; in this platform, one type of cells was patterned on the chamber surface using SAWs; then, a phase-shift of the SAW field was implemented to move the pattern of the second cell type in between the node-lines of the first cell type. Co-culturing HeLa and HMVEC-d cells led to the discovery of increased cancer cell mobility, which might be attributed to the cross-talk initiated by endothelial cells which enhances cancer cells through STAT3/Akt/ERK, α5β1 integrin, and GTPases signaling pathways (Fig. 4A–B). Kang et al [112] used a SAW-based method to fabricate therapeutic vascular tissue containing a three-dimensional collateral distribution of vessels. Co-aligned human umbilical vein endothelial cells and human adipose stem cells were arranged in a biodegradable catecholconjugated hyaluronic acid hydrogel; this arrangement enhanced cell-cell contact, gene expression, and secretion of angiogenic and anti-inflammatory paracrine factors (Fig. 4C). SAW-based methods have also been used to investigate cell-cell interactions at the single cell level. Guo et al [36] demonstrated a SAW-based approach to control the distance and spatial arrangements of a few suspended cells, and quantitatively investigated the gap junctional intercellular communication in several homotypic and heterotypic populations by visualizing the transfer of fluorescent dye between cells (Fig. 4D–E). The SAW-based method was also applied towards understanding how individual human lymphocytes and red blood cells are effected by the malarial parasite Plasmodium falciparum (Fig. 4F) [113]. Recently, investigation of cell interactions in more complex, 3D spheroids, was demonstrated with SAWs in order to investigate tumor growth and treatment [114–116]. Chen et al [114] discovered that the existence of HepG2 cells on the outer surface of spheroids protected the inner cells from the anti-cancer drug 5-fluorouracil. The work on spheroids mimics cancerous tissues in humans and elucidates that tumor spheroids feature distinct drug resistance due to their 3D structure (Fig. 4G).
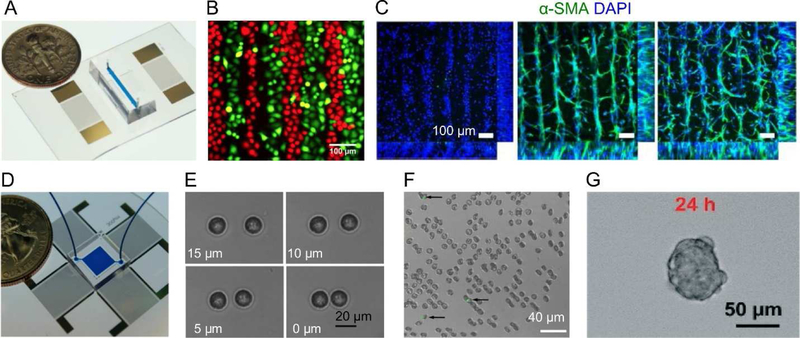
Figure 4.
Acoustofluidic methods to probe cell interactions. With (A) two parallel interdigital transducers, (B) two cell types can be co-cultured in adjacent lines, (C) co-culturing HUVEC/hADSC in ratios of 1:0, 5:1, and 2:1 demonstrated enhanced cell–cell contact in the fabricated cell-hydrogel construct for studying vessel maturation. (D) With four transducers in a square configuration, cells can be manipulated individually to investigate (E) cell-cell interactions, (F) cell-malarial parasite interactions, and (G) cells in a 3D spheroid. Images reproduced with permission from references [36,111–114].
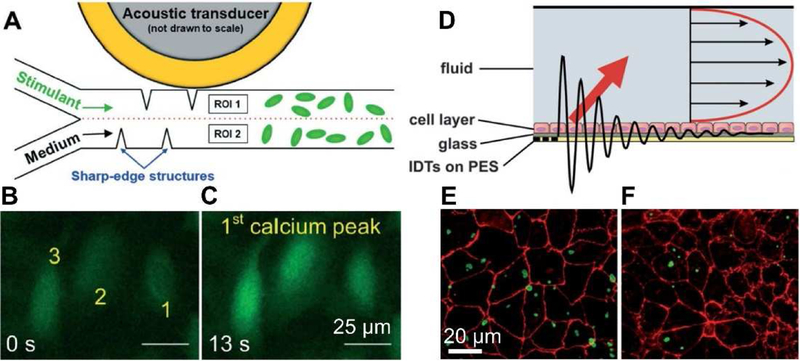
With regards to cell-environment responses, progress has been made toward the spatial modulation of biochemical and/or biophysical stimuli with BAWs and SAWs through acoustic streaming-based fluid manipulation. For example, Ahmed et al [38] demonstrated an oscillating bubble-based, BAW approach to generate programmable chemical waveforms that permitted continuous modulation of the signal characteristics including the amplitude (i.e. concentration of chemical stimuli), shape, frequency, and duty cycle, with frequencies reaching up to 30 Hz. Using this method, they demonstrated a frequency-dependent activation and internalization of G-protein coupled receptor internalization on HEK 293 cells. To overcome the instability of the oscillating bubble, Huang et al [37] employed a sharp-edge-based BAW method to generate chemical signals. Chemical signals were precisely controlled with periods from 100 ms up to several hours. This method was applied later in cell signaling studies by probing the dynamics of calcium release stimulated by ionomycin signals in HMVEC-d, HeLa, and U-251 cells. They found that a short single-pulse ionomycin (100 ms) allowed cells to dynamically adjust the intracellular level of Ca2+ through constantly releasing and accepting Ca2+ to the cytoplasm and from the extracellular environment, respectively (Fig. 5A–C). In addition to BAW methods, SAW-induced acoustic streaming has also been used to quantify the interaction of protein-coated particles with cells, in order to evaluate performance of drug delivery vehicles under physiological flow conditions (Fig. 5D–F) [117]. In terms of cellular responses to biophysical stimuli, Zhang et al showed F-actin cytoskeletal rearrangement in MC3T3-E1 cells and an increase in intracellular calcium concentration with application of an acoustic radiation force generated by SAWs [118]. Greco et al [119] reported that U-937 monocyte cell proliferation under acoustic streaming from SAWs was enhanced by 36% with respect to those of standard static cultures. Stamp et al [120] demonstrated that SAW-treated, osteoblast-like SaOs-2 cells exhibit a significantly increased migration, promoted cell growth, and stimulated wound healing, as compared to the control samples. The above-mentioned examples clearly demonstrated the contributions from acoustofluidic devices in understanding cellular response to extracellular biochemical/biophysical signals [121–123], which have great potential in developing future therapeutics [124–129].
Figure 5.
Acoustofluidic methods to investigate cellular responses to the environment. (A) acoustic-actuated sharp-edge-based BAW methods to produce chemical waveforms by acoustic streaming. (B-C) Change in fluorescence intensity of three representative HMVEC-d cells with periodic ionomycin stimulation. (D) SAW-based method to generate acoustic streaming. (E-F) Detachment of wheat germ agglutinin coated microparticles from cell surfaces under stationary conditions and flow conditions. Images reproduced with permission from references [37,117].
6. Streamlined cell analysis from clinical samples
Owing to the complex, potentially viscous nature of many biological samples (e.g. whole blood, stool, or sputum), many acoustofluidic devices require external equipment to process the sample before it can be successfully injected into the microfluidic domain and function properly. This dependence on benchtop equipment negates many of the benefits, including continuous operation and biosafety, gained when using an all-in-one, integrated microfluidic platform. This has led researchers to develop methods that enable the handling of these complex samples.
The development, commercialization, and application of acoustic focusing to flow cytometry efforts exemplifies this process [130]. Although research started with simple acoustic focusing of particles in a cylindrical tube, commercial implementations have integrated hydrodynamic and acoustic focusing together with 4-laser optical detection and mechanical-electric systems to develop complete cytometry systems [34,131]. This has enabled high precision analysis of cells with volumetric sample input rates up to an order of magnitude higher than systems that forgo acoustic-assisted focusing for hydrodynamic focusing alone [132,133].
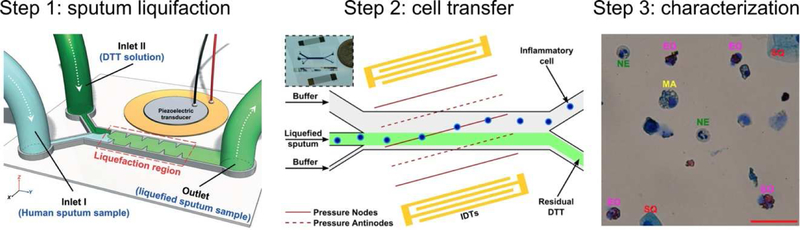
As another example, recent publications from Huang and his colleagues demonstrated a series of acoustofluidic devices that can perform sputum liquefaction, cell transfer, and inflammatory cell analysis using human sputum samples. They first demonstrated a sharp-edge-based BAW device capable of liquefying sputum samples to facilitate further cell handling [134]. Their device combined dithiothreitol, a mucus liquifying reagent, with sputum samples using acoustic streaming that was induced by oscillating sharp-edge structures; the strong acoustic streaming produced by the device was able to liquefy sputum effectively at a throughput of 30 μL/min, while cell viability and integrity were well maintained (Fig. 6A). After liquefaction, the cells remain mixed with the potentially harmful dithiothreitol, which necessitates a centrifugation step to remove the cells. In order to avoid this step, and keep the process solely dependent on compact microfluidic tools, the same group created a SAW-based platform to transfer the cells from the liquefied sputum sample to culture media or a buffer solution [135] to avoid the toxicity of residual dithiothreitol, and prepare for cell cytometry and immunostaining analysis (Fig. 6B). These works demonstrated the capability of acoustofluidic devices in dealing with complex, highly viscous, and delicate biological samples. It also showed that acoustofluidic devices can prove advantageous when integrating up/down-stream cell handling and analysis platforms. The application of this method was later expanded to liquify and purify stool samples for bacterial identification [136]. The combination of multiple, functionalized acoustofluidic units greatly expands the capabilities for handling complex clinical samples, and provides numerous benefits compared to traditional, benchtop analysis. Nonetheless, current designs have only been shown to work as discontinuous sub-units, meaning that future innovation and integration is needed to achieve a completely cohesive system that realizes the full potential of acoustofluidic technologies in cell analysis.
Figure 6.
Acoustofluidic devices are used to streamline the processing and analysis of human sputum samples. (A) In the 1st step, an acoustofluidic device is used to liquify sputum sample with acoustic streaming. (B) In the 2nd step, an acoustofluidic device is used to transfer cells from liquified sputum to cell culture medium for analysis. (C) A histology study was conducted to characterize cell types in the 3rd step. Images reproduced with permission from references [134,135]
7. Perspectives
Acoustofluidic methods feature several advantages for live cell analysis. Acoustofluidic methods are able to maintain excellent cell viability during manipulation. Both SAWs and BAWs work with low power intensity (e.g. ~0.1 W/cm2 ) [10], similar to clinically applied ultrasound tests, inducing minimal heating or shear forces that might cause cell damage [63]. They work effectively with almost any biofluids such as cell culture medium [111], sputum [134], stool [136], blood [23,138], and milk [50], in contrast to the requirement of special medias in many traditional methods such as optical tweezers and dielectrophoresis; this feature help to maintain normal cell functions during cell analysis. In addition, acoustofluidic methods can manipulate both cells and extracellular environments. Acoustic radiation forces can be used to [48,139] deflect cells for separation, or trap cells for patterning, contacting, and forming spheroids [13]; on the other hand, acoustic streaming [49,140] can be used to deform cells for mechanotyping [32], rotate cells for imaging [29–31,76,141,142], or actuate fluids to manipulate extracellular environments [37,143]. Acoustic waves feature proper resolutions and a suitable wavelength size range for cell manipulations. For example, an acoustic wave with a 10–100 MHz frequency produces a wavelength of 15–150 micrometers in water. This allows researchers to manipulate whole cells, and precisely move cells by distances of about 1–1000 micrometers, which is suitable for many applications such as cell separation, mechanotyping, and interaction. The biocompatibility, versatility, resolution, and simplicity make these acoustofluidic methods superior platforms capable of handling a wide range of applications in biology and medicine.
Despite the rapid growth and intriguing applications, there are a few considerations for future developments of acoustofluidic-based cell analysis methods in fundamental and clinical research efforts. First, the complexity of cells, diseases, and biological processes must be properly recognized. Similar diseases may yield distinct cell analysis results. Take CTC analysis as an example, there is no single characteristics that could be applied for all types of CTCs. In the Cellsearch method, the application of EpCAM positive selections is valid for the monitoring of patients with metastatic breast, colorectal, or prostate cancer, but has limited performance in detecting lung cancer [144,145]. Similarly, acoustofluidic methods separate cells based on size, compressibility, or other physical properties, where the best performance might only be achieved for certain types of cancer. Thus, it is essential to define the scope of applications and standardize the operation protocol accordingly. In addition, similar results of cell analysis might be caused by distinct biological mechanisms. Take cell mechanotyping as an example, changes in cell deformability and compressibility might be associated with altered compositions of plasma membrane, cytoskeleton structure, cell-phase, or parasite invasions [85,86,146]. In another case, cell-cell communication can be attributed from physical contact, diffusion of soluble factors, transmission of electrical signal, and transduction of mechanical cues within the extracellular matrix [147]. In both cases, it may be difficult to correctly assign the reason for the cellular responses; correct interpretation of the results requires integration of multiple analytical methods, proper design of control experiments, and development of approaches to separate targeted signals from the background noise. Here, both BAW and SAW devices are promising for use in combination with other molecular biology or analytical chemistry methods to enhance the accuracy of data interpretation. Furthermore, the complexity of cells must be considered when studies involve vast types of cell specimens. Current acoustofluidic methods deal with single cells, 2D/3D cell cultures, and small animals (i.e. C. elegans); however, more specimen types such as tissues, organoids, organ slices, and cells in vivo remain mostly unexplored. Additionally, most research efforts are carried out with cells merged in liquid; however, a large portion of cells function only at liquid-air interfaces (e.g. skin, eye, and lung) [148,149] which have yet to be investigated with acoustofluidic methods.
The idea of integrating multiple methods for accurate cell analysis brings about the second consideration: to develop acoustofluidic system for fundamental cell biology studies. In this regard, special emphasis should be paid to the logical and physical interfaces between acoustofluidic units and other analytical methods up- and down-stream. Since cells are usually analyzed with multiple approaches, the ability to integrate with other biochemical characterization methods is desirable. For example, in a typical cell biology study, cells that are exposed to external stimuli are often investigated by considering transcription (via RT-PCR), proteomics (via mass spectra), histology (via fixation and staining), and cell cultures. Other than real-time in situ observations, most procedures require collecting the whole or a selective portion of cells from the microfluidic chamber, which is not readily feasible for traditional PDMS-based microfluidic devices. To overcome this limitation, recent acoustofluidic devices have been fabricated using disposable chips [150,151], glass capillaries [152], flexible substrates [153], as well as open droplets [14]. These devices share similar mechanisms and performance standards to early acoustofluidic platforms but successfully remove PDMS barriers. We expect that future cell analyses will be conducted on fully integrated platforms where the acoustofluidic fraction plays an essential, but not exclusive, role in the procedure.
Finally, to develop acoustofluidic systems capable of functioning outside of centralized labs, the device design, fabrication, and peripheral equipment must be modified to suit the application environment. Despite the remarkable research-laboratory performance, there are few commercially available acoustofluidic products for cell analysis [34]. Part of the reason for this disconnect might be that current acoustofluidic devices still heavily rely on expensive, complicated equipment such as function generators, amplifiers, and infusion pumps. Reducing the dependence of acoustofluidic devices on these pieces of equipment based on specific applications will broaden the range of settings where acoustofluidics can be relevant. For point-of-care diagnostics that occur at homes, nurse stations, or other resource-limited settings, microscope-free, pump-free, and function-generator-free systems are desirable. Although challenging, efforts have been taking to simplify the supportive equipment. For instance, Huang et al [154] invented a programmable acoustofluidic pump, to replace conventional syringe pumps, that utilized the acoustic streaming effects generated by the oscillation of tilted sharp-edge structures. Later, with the use of a cell phone, a modified Bluetooth® speaker, a sharp-edge-based acoustofluidic device, and a simple portable microscope, Bachman et al [155] developed an on-demand acoustofluidic pump and mixer, and prototyped with commercial available Arduino platforms [156] that was promising for point-of-care applications. With efforts from academia and industries, we expect that a broader application of acoustofluidic methods will boost both fundamental and clinical cell studies.
Acoustofluidics provides benefits comparing to conventional cell analysis methods. Surface acoustic waves and bulk acoustic waves are two major acoustofluidic methods. Examining applications that utilize acoustofluidics to analyze whole, living cells. Discussing advantages, limitations, and outlooks for acoustofluidic cell analysis.
Acknowledgements:
We gratefully acknowledge financial support from the National Institutes of Health (R01GM132603, R01HD086325, R44GM125439, R33CA223908, and R01GM127714), United States Army Medical Research Acquisition Activity (W81XWH-18-1-0242), and National Science Foundation (ECCS-1807601).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sibbitts J, Sellens KA, Jia S, Klasner SA, Culbertson CT, Cellular Analysis Using Microfluidics, Anal. Chem 90 (2018) 65–85. doi: 10.1021/acs.analchem.7b04519. [DOI] [PubMed] [Google Scholar]
- [2].Mazzarello P, A unifying concept: the history of cell theory, Nat. Cell Biol. 1 (1999) E13–E15. doi: 10.1038/8964. [DOI] [PubMed] [Google Scholar]
- [3].Gest H, The discovery of microorganisms by Robert Hooke and Antoni van Leeuwenhoek, Fellows of The Royal Society, Notes Rec. R. Soc. Lond 58 (2004) 187–201. doi: 10.1098/rsnr.2004.0055. [DOI] [PubMed] [Google Scholar]
- [4].Fulwyler MJ, Electronic Separation of Biological Cells by Volume, Science (80-. ). 150 (1965) 910–911. doi: 10.1126/science.150.3698.910. [DOI] [PubMed] [Google Scholar]
- [5].Julius MH, Masuda T, Herzenberg LA, Demonstration That Antigen-Binding Cells Are Precursors of Antibody-Producing Cells After Purification with a Fluorescence-Activated Cell Sorter, Proc. Natl. Acad. Sci 69 (1972) 1934–1938. doi: 10.1073/pnas.69.7.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saiki R, Scharf S, Faloona F, Mullis K, Horn G, Erlich H, Arnheim N, Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia, Science (80-. ). 230 (1985) 1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- [7].Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW, Droplet Barcoding for Single-Cell Transcriptomics Applied to Embryonic Stem Cells, Cell. 161 (2015) 1187–1201. doi: 10.1016/j.cell.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA, Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets, Cell. 161 (2015) 1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bruus H, Acoustofluidics 1: Governing equations in microfluidics, Lab Chip. 11 (2011) 3742. doi: 10.1039/c1lc20658c. [DOI] [PubMed] [Google Scholar]
- [10].Ozcelik A, Rufo J, Guo F, Gu Y, Li P, Lata J, Huang TJ, Acoustic tweezers for the life sciences, Nat. Methods 15 (2018) 1021–1028. doi: 10.1038/s41592-018-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kundt A, Ueber eine neue Art akustischer Staubfiguren und über die Anwendung derselben zur Bestimmung der Schallgeschwindigkeit in festen Körpern und Gasen, Ann. Der Phys. Und Chemie 203 (1866) 497–523. doi: 10.1002/andp.18662030402. [DOI] [Google Scholar]
- [12].Barnes RB, Burton CJ, Visual methods for studying ultrasonic phenomena, J. Appl. Phys 20 (1949) 286–294. doi: 10.1063/1.1698357. [DOI] [Google Scholar]
- [13].Shi J, Ahmed D, Mao X, Lin S-CS, Lawit A, Huang TJ, Acoustic tweezers: patterning cells and microparticles using standing surface acoustic waves (SSAW), Lab Chip. 9 (2009) 2890. doi: 10.1039/b910595f. [DOI] [PubMed] [Google Scholar]
- [14].Zhang SP, Lata J, Chen C, Mai J, Guo F, Tian Z, Ren L, Mao Z, Huang P-H, Li P, Yang S, Huang TJ, Digital acoustofluidics enables contactless and programmable liquid handling, Nat. Commun 9 (2018) 2928. doi: 10.1038/s41467-018-05297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tian Z, Yang S, Huang P-H, Wang Z, Zhang P, Gu Y, Bachman H, Chen C, Wu M, Xie Y, Huang TJ, Wave number–spiral acoustic tweezers for dynamic and reconfigurable manipulation of particles and cells, Sci. Adv 5 (2019) eaau6062. doi: 10.1126/sciadv.aau6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Habibi R, Devendran C, Neild A, Trapping and patterning of large particles and cells in a 1D ultrasonic standing wave, Lab Chip. 17 (2017) 3279–3290. doi: 10.1039/C7LC00640C. [DOI] [PubMed] [Google Scholar]
- [17].Beyeler F, Neild A, Oberti S, Bell DJ, Sun Y, Dual J, Nelson BJ, Monolithically Fabricated Microgripper With Integrated Force Sensor for Manipulating Microobjects and Biological Cells Aligned in an Ultrasonic Field, J. Microelectromechanical Syst 16 (2007) 7–15. doi: 10.1109/JMEMS.2006.885853. [DOI] [Google Scholar]
- [18].Shi J, Yazdi S, Steven Lin S-C, Ding X, Chiang I-K, Sharp K, Huang TJ, Three-dimensional continuous particle focusing in a microfluidic channel via standing surface acoustic waves (SSAW), Lab Chip. 11 (2011) 2319. doi: 10.1039/c1lc20042a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shi J, Mao X, Ahmed D, Colletti A, Huang TJ, Focusing microparticles in a microfluidic channel with standing surface acoustic waves (SSAW), Lab Chip. 8 (2008) 221–223. doi: 10.1039/B716321E. [DOI] [PubMed] [Google Scholar]
- [20].Agrawal P, Gandhi PS, Neild A, Continuous Focusing of Microparticles in Horizontally Actuated Rectangular Channels, Phys. Rev. Appl 10 (2018) 1. doi: 10.1103/PhysRevApplied.10.024036. [DOI] [Google Scholar]
- [21].Antfolk M, Muller PB, Augustsson P, Bruus H, Laurell T, Focusing of submicrometer particles and bacteria enabled by two-dimensional acoustophoresis, Lab Chip. 14 (2014) 2791–2799. doi: 10.1039/C4LC00202D. [DOI] [PubMed] [Google Scholar]
- [22].Ding X, Peng Z, Lin S-CS, Geri M, Li S, Li P, Chen Y, Dao M, Suresh S, Huang TJ, Cell separation using tilted-angle standing surface acoustic waves, Proc. Natl. Acad. Sci 111 (2014) 12992–12997. doi: 10.1073/pnas.1413325111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu M, Ouyang Y, Wang Z, Zhang R, Huang P-H, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ, Isolation of exosomes from whole blood by integrating acoustics and microfluidics, Proc. Natl. Acad. Sci 114 (2017) 10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Collins DJ, Alan T, Neild A, Particle separation using virtual deterministic lateral displacement (vDLD), Lab Chip. 14 (2014) 1595–1603. doi: 10.1039/C3LC51367J. [DOI] [PubMed] [Google Scholar]
- [25].Thévoz P, Adams JD, Shea H, Bruus H, Soh HT, Acoustophoretic Synchronization of Mammalian Cells in Microchannels, Anal. Chem 82 (2010) 3094–3098. doi: 10.1021/ac100357u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shilton R, Tan MK, Yeo LY, Friend JR, Particle concentration and mixing in microdrops driven by focused surface acoustic waves, J. Appl. Phys 104 (2008) 014910. doi: 10.1063/1.2951467. [DOI] [Google Scholar]
- [27].Xie Y, Zhao C, Zhao Y, Li S, Rufo J, Yang S, Guo F, Huang TJ, Optoacoustic tweezers: a programmable, localized cell concentrator based on opto-thermally generated, acoustically activated, surface bubbles., Lab Chip. 13 (2013) 1772–9. doi: 10.1039/c3lc00043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Franke T, Braunmüller S, Schmid L, Wixforth A, Weitz DA, Surface acoustic wave actuated cell sorting (SAWACS), Lab Chip. 10 (2010) 789. doi: 10.1039/b915522h. [DOI] [PubMed] [Google Scholar]
- [29].Zhang J, Yang S, Chen C, Hartman JH, Huang P-H, Wang L, Tian Z, Zhang P, Faulkenberry D, Meyer JN, Huang TJ, Surface acoustic waves enable rotational manipulation of Caenorhabditis elegans, Lab Chip. (2019). doi: 10.1039/C8LC01012A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].He M, Cui W, Zhang H, Yang Y, Pang W, Qu H, Duan X, In-line trapping and rotation of bio-particles via 3-D micro-vortices generated by localized ultrahigh frequency acoustic resonators, in: 2017 19th Int. Conf. Solid-State Sensors, Actuators Microsystems, IEEE, 2017: pp. 1789–1792. doi: 10.1109/TRANSDUCERS.2017.7994416. [DOI] [Google Scholar]
- [31].Ahmed D, Ozcelik A, Bojanala N, Nama N, Upadhyay A, Chen Y, Hanna-Rose W, Huang TJ, Rotational manipulation of single cells and organisms using acoustic waves, Nat. Commun 7 (2016) 11085. doi: 10.1038/ncomms11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xie Y, Nama N, Li P, Mao Z, Huang P-H, Zhao C, Costanzo F, Huang TJ, Probing Cell Deformability via Acoustically Actuated Bubbles, Small. 12 (2016) 902–910. doi: 10.1002/smll.201502220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li P, Mao Z, Peng Z, Zhou L, Chen Y, Huang P, Truica CI, Drabick JJ, El-Deiry WS, Dao M, Suresh S, Huang TJ, Acoustic separation of circulating tumor cells, Proc. Natl. Acad. Sci 112 (2015) 4970–4975. doi: 10.1073/pnas.1504484112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Goddard G, Martin JC, Graves SW, Kaduchak G, Ultrasonic particle-concentration for sheathless focusing of particles for analysis in a flow cytometer, Cytom. Part A 69A (2006) 66–74. doi: 10.1002/cyto.a.20205. [DOI] [PubMed] [Google Scholar]
- [35].Hiramatsu K, Ideguchi T, Yonamine Y, Lee S, Luo Y, Hashimoto K, Ito T, Hase M, Park J-W, Kasai Y, Sakuma S, Hayakawa T, Arai F, Hoshino Y, Goda K, High-throughput label-free molecular fingerprinting flow cytometry, Sci. Adv 5 (2019) eaau0241. doi: 10.1126/sciadv.aau0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Guo F, Li P, French JB, Mao Z, Zhao H, Li S, Nama N, Fick JR, Benkovic SJ, Huang TJ, Controlling cell–cell interactions using surface acoustic waves, Proc. Natl. Acad. Sci 112 (2015) 43–48. doi: 10.1073/pnas.1422068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Huang P-H, Chan CY, Li P, Wang Y, Nama N, Bachman H, Huang TJ, A sharp-edge-based acoustofluidic chemical signal generator, Lab Chip. 18 (2018) 1411–1421. doi: 10.1039/C8LC00193F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ahmed D, Muddana HS, Lu M, French JB, Ozcelik A, Fang Y, Butler PJ, Benkovic SJ, Manz A, Huang TJ, Acoustofluidic Chemical Waveform Generator and Switch, Anal. Chem 86 (2014) 11803–11810. doi: 10.1021/ac5033676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mitsakakis K, Tserepi A, Gizeli E, SAW device integrated with microfluidics for array-type biosensing, Microelectron. Eng 86 (2009) 1416–1418. doi: 10.1016/j.mee.2008.12.063. [DOI] [Google Scholar]
- [40].Gizeli E, Design considerations for the acoustic waveguide biosensor, Smart Mater. Struct 6 (1997) 700–706. doi: 10.1088/0964-1726/6/6/006. [DOI] [Google Scholar]
- [41].Sonato A, Agostini M, Ruffato G, Gazzola E, Liuni D, Greco G, Travagliati M, Cecchini M, Romanato F, A surface acoustic wave (SAW)-enhanced grating-coupling phase-interrogation surface plasmon resonance (SPR) microfluidic biosensor, Lab Chip. 16 (2016) 1224–1233. doi: 10.1039/C6LC00057F. [DOI] [PubMed] [Google Scholar]
- [42].Ashkin A, Dziedzic J, Optical trapping and manipulation of viruses and bacteria, Science (80-. ). 235 (1987) 1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- [43].MacDonald MP, Spalding GC, Dholakia K, Microfluidic sorting in an optical lattice, Nature. 426 (2003) 421–424. doi: 10.1038/nature02144. [DOI] [PubMed] [Google Scholar]
- [44].Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S, Observation of a single-beam gradient force optical trap for dielectric particles, Opt. Lett 11 (1986) 288. doi: 10.1364/OL.11.000288. [DOI] [PubMed] [Google Scholar]
- [45].Pethig R, Dielectrophoresis: Using Inhomogeneous AC Electrical Fields to Separate and Manipulate Cells, Crit. Rev. Biotechnol 16 (1996) 331–348. doi: 10.3109/07388559609147425. [DOI] [Google Scholar]
- [46].Wang Xiao-Bo, Huang Ying, Gascoyne PRC, Becker FF, Dielectrophoretic manipulation of particles, IEEE Trans. Ind. Appl 33 (1997) 660–669. doi: 10.1109/28.585855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bruus H, Acoustofluidics 1: Governing equations in microfluidics, Lab Chip. 11 (2011) 3742. doi: 10.1039/c1lc20658c. [DOI] [PubMed] [Google Scholar]
- [48].Bruus H, Acoustofluidics 7: The acoustic radiation force on small particles, Lab Chip. 12 (2012) 1014. doi: 10.1039/c2lc21068a. [DOI] [PubMed] [Google Scholar]
- [49].Wiklund M, Green R, Ohlin M, Acoustofluidics 14: Applications of acoustic streaming in microfluidic devices, Lab Chip. 12 (2012) 2438. doi: 10.1039/c2lc40203c. [DOI] [PubMed] [Google Scholar]
- [50].Lenshof A, Magnusson C, Laurell T, Acoustofluidics 8: Applications of acoustophoresis in continuous flow microsystems, Lab Chip. 12 (2012) 1210. doi: 10.1039/c2lc21256k. [DOI] [PubMed] [Google Scholar]
- [51].Bruus H, Dual J, Hawkes J, Hill M, Laurell T, Nilsson J, Radel S, Sadhal S, Wiklund M, Forthcoming Lab on a Chip tutorial series on acoustofluidics: Acoustofluidics— exploiting ultrasonic standing wave forces and acoustic streaming in microfluidic systems for cell and particle manipulation, Lab Chip. 11 (2011) 3579. doi: 10.1039/c1lc90058g. [DOI] [PubMed] [Google Scholar]
- [52].Marx V, Biophysics: using sound to move cells, Nat. Methods 12 (2015) 41–44. doi: 10.1038/nmeth.3218. [DOI] [PubMed] [Google Scholar]
- [53].Yeo LY, Friend JR, Surface Acoustic Wave Microfluidics, Annu. Rev. Fluid Mech 46 (2014) 379–406. doi: 10.1146/annurev-fluid-010313-141418. [DOI] [Google Scholar]
- [54].Ding X, Li P, Lin S-CS, Stratton ZS, Nama N, Guo F, Slotcavage D, Mao X, Shi J, Costanzo F, Huang TJ, Surface acoustic wave microfluidics., Lab Chip. 13 (2013) 3626–49. doi: 10.1039/c3lc50361e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Destgeer G, Sung HJ, Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves, Lab Chip. 15 (2015) 2722–2738. doi: 10.1039/C5LC00265F. [DOI] [PubMed] [Google Scholar]
- [56].AlHasan L, Qi A, Al-Aboodi A, Rezk A, Shilton RR, Chan PPY, Friend J, Yeo L, Surface acoustic streaming in microfluidic system for rapid multicellular tumor spheroids generation, in: Friend J, Tan HH (Eds.), Micro/Nano Mater. Devices, Syst, 2013: p. 89235C. doi: 10.1117/12.2034050. [DOI] [Google Scholar]
- [57].Wu M, Ozcelik A, Rufo J, Wang Z, Fang R, Jun Huang T, Acoustofluidic separation of cells and particles, Microsystems Nanoeng. 5 (2019) 32. doi: 10.1038/s41378-019-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Meng L, Cai F, Li F, Zhou W, Niu L, Zheng H, Acoustic tweezers, J. Phys. D. Appl. Phys 52 (2019) 273001. doi: 10.1088/1361-6463/ab16b5. [DOI] [Google Scholar]
- [59].Connacher W, Zhang N, Huang A, Mei J, Zhang S, Gopesh T, Friend J, Micro/nano acoustofluidics: materials, phenomena, design, devices, and applications, Lab Chip. 18 (2018) 1952–1996. doi: 10.1039/C8LC00112J. [DOI] [PubMed] [Google Scholar]
- [60].Li P, Huang TJ, Applications of Acoustofluidics in Bioanalytical Chemistry, Anal. Chem 91 (2019) 757–767. doi: 10.1021/acs.analchem.8b03786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Taller D, Richards K, Slouka Z, Senapati S, Hill R, Go DB, Chang H-C, On-chip surface acoustic wave lysis and ion-exchange nanomembrane detection of exosomal RNA for pancreatic cancer study and diagnosis, Lab Chip. 15 (2015) 1656–1666. doi: 10.1039/C5LC00036J. [DOI] [PubMed] [Google Scholar]
- [62].Salehi-Reyhani A, Gesellchen F, Mampallil D, Wilson R, Reboud J, Ces O, Willison KR, Cooper JM, Klug DR, Chemical-Free Lysis and Fractionation of Cells by Use of Surface Acoustic Waves for Sensitive Protein Assays, Anal. Chem 87 (2015) 2161–2169. doi: 10.1021/ac5033758. [DOI] [PubMed] [Google Scholar]
- [63].Nee Tan J, Ma C, Sivanantha N, Neild A, Bubble inducing cell lysis in a sessile droplet, Appl. Phys. Lett 104 (2014) 103704. doi: 10.1063/1.4868407. [DOI] [Google Scholar]
- [64].Deng CX, Sieling F, Pan H, Cui J, Ultrasound-induced cell membrane porosity, Ultrasound Med. Biol 30 (2004) 519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- [65].Fan Z, Liu H, Mayer M, Deng CX, Spatiotemporally controlled single cell sonoporation, Proc. Natl. Acad. Sci 109 (2012) 16486–16491. doi: 10.1073/pnas.1208198109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang Z, Wang Y, Zhang H, Tang Z, Liu W, Lu Y, Wang Z, Yang H, Pang W, Zhang H, Zhang D, Duan X, Hypersonic Poration: A New Versatile Cell Poration Method to Enhance Cellular Uptake Using a Piezoelectric Nano-Electromechanical Device, Small. 13 (2017) 1602962. doi: 10.1002/smll.201602962. [DOI] [PubMed] [Google Scholar]
- [67].Lu Y, Huskens J, Pang W, Duan X, Hypersonic poration of supported lipid bilayers, Mater. Chem. Front 3 (2019) 782–790. doi: 10.1039/C8QM00589C. [DOI] [Google Scholar]
- [68].Lu Y, de Vries WC, Overeem NJ, Duan X, Zhang H, Zhang H, Pang W, Ravoo BJ, Huskens J, Controlled and Tunable Loading and Release of Vesicles by Using Gigahertz Acoustics, Angew. Chemie Int. Ed 58 (2019) 159–163. doi: 10.1002/anie.201810181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dijkmans P, Juffermans L, Musters R, Vanwamel A, Tencate F, Vangilst W, Visser C, Dejong N, Kamp O, Microbubbles and ultrasound: from diagnosis to therapy, Eur. J. Echocardiogr 5 (2004) 245–256. doi: 10.1016/j.euje.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [70].Tomizawa M, Sonoporation: Gene transfer using ultrasound, World J. Methodol 3 (2013) 39. doi: 10.5662/wjm.v3.i4.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liang H-D, Tang J, Halliwell M, Sonoporation, drug delivery, and gene therapy, Proc. Inst. Mech. Eng. Part H J. Eng. Med 224 (2010) 343–361. doi: 10.1243/09544119JEIM565. [DOI] [PubMed] [Google Scholar]
- [72].Länge K, Rapp BE, Rapp M, Surface acoustic wave biosensors: a review, Anal. Bioanal. Chem 391 (2008) 1509–1519. doi: 10.1007/s00216-008-1911-5. [DOI] [PubMed] [Google Scholar]
- [73].Go DB, Atashbar MZ, Ramshani Z, Chang H-C, Surface acoustic wave devices for chemical sensing and microfluidics: a review and perspective, Anal. Methods 9 (2017) 4112–4134. doi: 10.1039/C7AY00690J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Minsky M, Memoir on inventing the confocal scanning microscope, Scanning. 10 (1988) 128–138. doi: 10.1002/sca.4950100403. [DOI] [Google Scholar]
- [75].Hoffman A, Goetz M, Vieth M, Galle P, Neurath M, Kiesslich R, Confocal laser endomicroscopy: technical status and current indications, Endoscopy. 38 (2006) 1275–1283. doi: 10.1055/s-2006-944813. [DOI] [PubMed] [Google Scholar]
- [76].Ozcelik A, Nama N, Huang P-H, Kaynak M, McReynolds MR, Hanna-Rose W, Huang TJ, Acoustofluidic Rotational Manipulation of Cells and Organisms Using Oscillating Solid Structures, Small. 12 (2016) 5120–5125. doi: 10.1002/smll.201601760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Schwarz T, Hahn P, Petit-Pierre G, Dual J, Rotation of fibers and other non-spherical particles by the acoustic radiation torque, Microfluid. Nanofluidics 18 (2014) 65–79. doi: 10.1007/s10404-014-1408-9. [DOI] [Google Scholar]
- [78].Zmijan R, Jonnalagadda US, Carugo D, Kochi Y, Lemm E, Packham G, Hill M, Glynne-Jones P, High throughput imaging cytometer with acoustic focussing, RSC Adv. 5 (2015) 83206–83216. doi: 10.1039/C5RA19497K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Olson RJ, Shalapyonok A, Kalb DJ, Graves SW, Sosik HM, Imaging FlowCytobot modified for high throughput by in-line acoustic focusing of sample particles, Limnol. Oceanogr. Methods 15 (2017) 867–874. doi: 10.1002/lom3.10205. [DOI] [Google Scholar]
- [80].Lambert BS, Olson RJ, Sosik HM, A fluorescence-activated cell sorting subsystem for the Imaging FlowCytobot, Limnol. Oceanogr. Methods 15 (2017) 94–102. doi: 10.1002/lom3.10145. [DOI] [Google Scholar]
- [81].Ozcan A, Mobile phones democratize and cultivate next-generation imaging, diagnostics and measurement tools, Lab Chip. 14 (2014) 3187–3194. doi: 10.1039/C4LC00010B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Adamo A, Sharei A, Adamo L, Lee B, Mao S, Jensen KF, Microfluidics-Based Assessment of Cell Deformability, Anal. Chem 84 (2012) 6438–6443. doi: 10.1021/ac300264v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Grover WH, Bryan AK, Diez-Silva M, Suresh S, Higgins JM, Manalis SR, Measuring single-cell density, Proc. Natl. Acad. Sci 108 (2011) 10992–10996. doi: 10.1073/pnas.1104651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cross SE, Jin Y-S, Rao J, Gimzewski JK, Nanomechanical analysis of cells from cancer patients, Nat. Nanotechnol 2 (2007) 780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- [85].Suresh S, Biomechanics and biophysics of cancer cells, Acta Biomater. 3 (2007) 413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lee GYH, Lim CT, Biomechanics approaches to studying human diseases, Trends Biotechnol. 25 (2007) 111–118. doi: 10.1016/j.tibtech.2007.01.005. [DOI] [PubMed] [Google Scholar]
- [87].Kang JH, Miettinen TP, Chen L, Olcum S, Katsikis G, Doyle PS, Manalis SR, Noninvasive monitoring of single-cell mechanics by acoustic scattering, Nat. Methods 16 (2019) 263–269. doi: 10.1038/s41592-019-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hartono D, Liu Y, Tan PL, Then XYS, Yung L-YL, Lim K-M, On-chip measurements of cell compressibility via acoustic radiation, Lab Chip. 11 (2011) 4072. doi: 10.1039/c1lc20687g. [DOI] [PubMed] [Google Scholar]
- [89].Wang H, Liu Z, Shin DM, Chen ZG, Cho Y, Kim Y-J, Han A, A continuous-flow acoustofluidic cytometer for single-cell mechanotyping, Lab Chip. 19 (2019) 387–393. doi: 10.1039/C8LC00711J. [DOI] [PubMed] [Google Scholar]
- [90].Augustsson P, Karlsen JT, Su H-W, Bruus H, Voldman J, Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping, Nat. Commun 7 (2016) 11556. doi: 10.1038/ncomms11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Baasch T, Reichert P, Lakämper S, Vertti-Quintero N, Hack G, Casadevall i Solvas X, DeMello A, Gunawan R, Dual J, Acoustic Compressibility of Caenorhabditis elegans, Biophys. J 115 (2018) 1817–1825. doi: 10.1016/j.bpj.2018.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kamsma D, Bochet P, Oswald F, Alblas N, Goyard S, Wuite GJL, Peterman EJG, Rose T, Single-Cell Acoustic Force Spectroscopy: Resolving Kinetics and Strength of T Cell Adhesion to Fibronectin, Cell Rep. 24 (2018) 3008–3016. doi: 10.1016/j.celrep.2018.08.034. [DOI] [PubMed] [Google Scholar]
- [93].Sitters G, Kamsma D, Thalhammer G, Ritsch-Marte M, Peterman EJG, Wuite GJL, Acoustic force spectroscopy, Nat. Methods 12 (2015) 47–50. doi: 10.1038/nmeth.3183. [DOI] [PubMed] [Google Scholar]
- [94].Kamsma D, Creyghton R, Sitters G, Wuite GJL, Peterman EJG, Tuning the Music: Acoustic Force Spectroscopy (AFS) 2.0, Methods. 105 (2016) 26–33. doi: 10.1016/j.ymeth.2016.05.002. [DOI] [PubMed] [Google Scholar]
- [95].Sorkin R, Bergamaschi G, Kamsma D, Brand G, Dekel E, Ofir-Birin Y, Rudik A, Gironella M, Ritort F, Regev-Rudzki N, Roos WH, Wuite GJL, Probing cellular mechanics with acoustic force spectroscopy, Mol. Biol. Cell 29 (2018) 2005–2011. doi: 10.1091/mbc.E18-03-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ravetto A, Wyss HM, Anderson PD, den Toonder JMJ, Bouten CVC, Monocytic Cells Become Less Compressible but More Deformable upon Activation, PLoS One. 9 (2014) e92814. doi: 10.1371/journal.pone.0092814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, V Doyle G, Allard WJ, Terstappen LWMM, Hayes DF, Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer, N. Engl. J. Med 351 (2004) 781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- [98].Murlidhar V, Zeinali M, Grabauskiene S, Ghannad-Rezaie M, Wicha MS, Simeone DM, Ramnath N, Reddy RM, Nagrath S, A Radial Flow Microfluidic Device for Ultra-High-Throughput Affinity-Based Isolation of Circulating Tumor Cells, Small. 10 (2014) 4895–4904. doi: 10.1002/smll.201400719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Stott SL, Hsu C-H, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, Floyd FP, Gilman AJ, Lord JB, Winokur D, Springer S, Irimia D, Nagrath S, Sequist LV, Lee RJ, Isselbacher KJ, Maheswaran S, Haber DA, Toner M, Isolation of circulating tumor cells using a microvortex-generating herringbone-chip, Proc. Natl. Acad. Sci 107 (2010) 18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Li P, Stratton ZS, Dao M, Ritz J, Huang TJ, Probing circulating tumor cells in microfluidics, Lab Chip. 13 (2013) 602. doi: 10.1039/c2lc90148j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wu M, Huang P-H, Zhang R, Mao Z, Chen C, Kemeny G, Li P, Lee AV, Gyanchandani R, Armstrong AJ, Dao M, Suresh S, Huang TJ, Circulating Tumor Cell Phenotyping via High-Throughput Acoustic Separation, Small. 14 (2018) 1801131. doi: 10.1002/smll.201801131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Antfolk M, Magnusson C, Augustsson P, Lilja H, Laurell T, Acoustofluidic, Label-Free Separation and Simultaneous Concentration of Rare Tumor Cells from White Blood Cells, Anal. Chem 87 (2015) 9322–9328. doi: 10.1021/acs.analchem.5b02023. [DOI] [PubMed] [Google Scholar]
- [103].Antfolk M, Antfolk C, Lilja H, Laurell T, Augustsson P, A single inlet two-stage acoustophoresis chip enabling tumor cell enrichment from white blood cells, Lab Chip. 15 (2015) 2102–2109. doi: 10.1039/C5LC00078E. [DOI] [PubMed] [Google Scholar]
- [104].Augustsson P, Magnusson C, Nordin M, Lilja H, Laurell T, Microfluidic, Label-Free Enrichment of Prostate Cancer Cells in Blood Based on Acoustophoresis, Anal. Chem 84 (2012) 7954–7962. doi: 10.1021/ac301723s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Karthick S, Pradeep PN, Kanchana P, Sen AK, Acoustic impedance-based size-independent isolation of circulating tumour cells from blood using acoustophoresis, Lab Chip. 18 (2018) 3802–3813. doi: 10.1039/C8LC00921J. [DOI] [PubMed] [Google Scholar]
- [106].Austin RG, Huang TJ, Wu M, Armstrong AJ, Zhang T, Clinical utility of non-EpCAM based circulating tumor cell assays, Adv. Drug Deliv. Rev 125 (2018) 132–142. doi: 10.1016/j.addr.2018.01.013. [DOI] [PubMed] [Google Scholar]
- [107].Kumar NM, Gilula NB, The Gap Junction Communication Channel, Cell. 84 (1996) 381–388. doi: 10.1016/S0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- [108].Pawson T, Protein modules and signalling networks, Nature. 373 (1995) 573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- [109].Kurashina Y, Takemura K, Friend J, Cell agglomeration in the wells of a 24-well plate using acoustic streaming, Lab Chip. 17 (2017) 876–886. doi: 10.1039/C6LC01310D. [DOI] [PubMed] [Google Scholar]
- [110].Kurashina Y, Takemura K, Friend J, Miyata S, Komotori J, Efficient subculture process for adherent cells by selective collection using cultivation substrate vibration, IEEE Trans. Biomed. Eng 64 (2016) 1–1. doi: 10.1109/TBME.2016.2567647. [DOI] [PubMed] [Google Scholar]
- [111].Li S, Guo F, Chen Y, Ding X, Li P, Wang L, Cameron CE, Huang TJ, Standing Surface Acoustic Wave Based Cell Coculture, Anal. Chem 86 (2014) 9853–9859. doi: 10.1021/ac502453z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kang B, Shin J, Park H-J, Rhyou C, Kang D, Lee S-J, Yoon Y, Cho S-W, Lee H, High-resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy, Nat. Commun 9 (2018) 5402. doi: 10.1038/s41467-018-07823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Collins DJ, Morahan B, Garcia-Bustos J, Doerig C, Plebanski M, Neild A, Two-dimensional single-cell patterning with one cell per well driven by surface acoustic waves, Nat. Commun 6 (2015) 8686. doi: 10.1038/ncomms9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chen K, Wu M, Guo F, Li P, Chan CY, Mao Z, Li S, Ren L, Zhang R, Huang TJ, Rapid formation of size-controllable multicellular spheroids via 3D acoustic tweezers, Lab Chip. 16 (2016) 2636–2643. doi: 10.1039/C6LC00444J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chen B, Wu Y, Ao Z, Cai H, Nunez A, Liu Y, Foley J, Nephew K, Lu X, Guo F, High-throughput acoustofluidic fabrication of tumor spheroids, Lab Chip. 19 (2019) 1755–1763. doi: 10.1039/C9LC00135B. [DOI] [PubMed] [Google Scholar]
- [116].Wu Y, Ao Z, Chen Bin, Muhsen M, Bondesson M, Lu X, Guo F, Acoustic assembly of cell spheroids in disposable capillaries, Nanotechnology. 29 (2018) 504006. doi: 10.1088/1361-6528/aae4f1. [DOI] [PubMed] [Google Scholar]
- [117].Fillafer C, Ratzinger G, Neumann J, Guttenberg Z, Dissauer S, Lichtscheidl IK, Wirth M, Gabor F, Schneider MF, An acoustically-driven biochip – impact of flow on the cell-association of targeted drug carriers, Lab Chip. 9 (2009) 2782. doi: 10.1039/b906006e. [DOI] [PubMed] [Google Scholar]
- [118].Zhang S, Cheng J, Qin Y-X, Mechanobiological Modulation of Cytoskeleton and Calcium Influx in Osteoblastic Cells by Short-Term Focused Acoustic Radiation Force, PLoS One. 7 (2012) e38343. doi: 10.1371/journal.pone.0038343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Greco G, Agostini M, Tonazzini I, Sallemi D, Barone S, Cecchini M, Surface-Acoustic-Wave (SAW)-Driven Device for Dynamic Cell Cultures, Anal. Chem 90 (2018) 7450–7457. doi: 10.1021/acs.analchem.8b00972. [DOI] [PubMed] [Google Scholar]
- [120].Stamp MEM, Brugger MS, Wixforth A, Westerhausen C, Acoustotaxis – in vitro stimulation in a wound healing assay employing surface acoustic waves, Biomater. Sci 4 (2016) 1092–1099. doi: 10.1039/C6BM00125D. [DOI] [PubMed] [Google Scholar]
- [121].Li S, Glynne-Jones P, Andriotis OG, Ching KY, Jonnalagadda US, Oreffo ROC, Hill M, Tare RS, Application of an acoustofluidic perfusion bioreactor for cartilage tissue engineering, Lab Chip. 14 (2014) 4475–4485. doi: 10.1039/C4LC00956H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Jonnalagadda US, Hill M, Messaoudi W, Cook RB, Oreffo ROC, Glynne-Jones P, Tare RS, Acoustically modulated biomechanical stimulation for human cartilage tissue engineering, Lab Chip. 18 (2018) 473–485. doi: 10.1039/C7LC01195D. [DOI] [PubMed] [Google Scholar]
- [123].Khedr MMS, Messaoudi W, Jonnalagadda US, Abdelmotelb AM, Glynne-Jones P, Hill M, Khakoo SI, Hilal MA, Generation of functional hepatocyte 3D discoids in an acoustofluidic bioreactor, Biomicrofluidics. 13 (2019) 014112. doi: 10.1063/1.5082603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Qi A, Friend JR, Yeo LY, Morton DAV, McIntosh MP, Spiccia L, Miniature inhalation therapy platform using surface acoustic wave microfluidic atomization, Lab Chip. 9 (2009) 2184. doi: 10.1039/b903575c. [DOI] [PubMed] [Google Scholar]
- [125].Ang KM, Yeo LY, Friend JR, Hung YM, Tan MK, Nozzleless spray cooling using surface acoustic waves, J. Aerosol Sci. 79 (2015) 48–60. doi: 10.1016/j.jaerosci.2014.10.004. [DOI] [Google Scholar]
- [126].Cortez-Jugo C, Qi A, Rajapaksa A, Friend JR, Yeo LY, Pulmonary monoclonal antibody delivery via a portable microfluidic nebulization platform, Biomicrofluidics. 9 (2015) 1–10. doi: 10.1063/1.4917181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Rajapaksa AE, Ho JJ, Qi A, Bischof R, Nguyen T-H, Tate M, Piedrafita D, McIntosh MP, Yeo LY, Meeusen E, Coppel RL, Friend JR, Effective pulmonary delivery of an aerosolized plasmid DNA vaccine via surface acoustic wave nebulization, Respir. Res 15 (2014) 60. doi: 10.1186/1465-9921-15-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ramesan S, Rezk AR, Yeo LY, High frequency acoustic permeabilisation of drugs through tissue for localised mucosal delivery, Lab Chip. 18 (2018) 3272–3284. doi: 10.1039/C8LC00355F. [DOI] [PubMed] [Google Scholar]
- [129].Ramesan S, Rezk AR, Dekiwadia C, Cortez-Jugo C, Yeo LY, Acoustically-mediated intracellular delivery, Nanoscale. 10 (2018) 13165–13178. doi: 10.1039/C8NR02898B. [DOI] [PubMed] [Google Scholar]
- [130].Bonnevier J, Hammerbeck C, Goetz C, Flow Cytometry: Definition, History, and Uses in Biological Research, in: 2018: pp. 1–11. doi: 10.1007/978-3-319-98071-3_1. [DOI] [Google Scholar]
- [131].Goddard GR, Sanders CK, Martin JC, Kaduchak G, Graves SW, Analytical Performance of an Ultrasonic Particle Focusing Flow Cytometer, Anal. Chem 79 (2007) 8740–8746. doi: 10.1021/ac071402t. [DOI] [PubMed] [Google Scholar]
- [132].Goddard G, Kaduchak G, Ultrasonic particle concentration in a line driven cylindrical tube, J. Acoust. Soc. Am 114 (2003) 2387–2387. doi: 10.1121/1.4777789. [DOI] [PubMed] [Google Scholar]
- [133].Ward MD, Kaduchak G, Fundamentals of Acoustic Cytometry, Curr. Protoc. Cytom 84 (2018) e36. doi: 10.1002/cpcy.36. [DOI] [PubMed] [Google Scholar]
- [134].Huang P-H, Ren L, Nama N, Li S, Li P, Yao X, Cuento RA, Wei C-H, Chen Y, Xie Y, Nawaz AA, Alevy YG, Holtzman MJ, McCoy JP, Levine SJ, Huang TJ, An acoustofluidic sputum liquefier, Lab Chip. 15 (2015) 3125–3131. doi: 10.1039/C5LC00539F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Li S, Ren L, Huang P-H, Yao X, Cuento RA, McCoy JP, Cameron CE, Levine SJ, Huang TJ, Acoustofluidic Transfer of Inflammatory Cells from Human Sputum Samples, Anal. Chem 88 (2016) 5655–5661. doi: 10.1021/acs.analchem.5b03383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhao S, He W, Ma Z, Liu P, Huang P-H, Bachman H, Wang L, Yang S, Tian Z, Wang Z, Gu Y, Xie Z, Huang TJ, On-chip stool liquefaction via acoustofluidics, Lab Chip. (2019) 42–45. doi: 10.1039/C8LC01310A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Friend J, Yeo LY, Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics, Rev. Mod. Phys 83 (2011) 647–704. doi: 10.1103/RevModPhys.83.647. [DOI] [Google Scholar]
- [138].Karthick S, Sen AK, Improved Understanding of Acoustophoresis and Development of an Acoustofluidic Device for Blood Plasma Separation, Phys. Rev. Appl 10 (2018) 034037. doi: 10.1103/PhysRevApplied.10.034037. [DOI] [Google Scholar]
- [139].Olsen H, Wergeland H, Westervelt PJ, Acoustic Radiation Force J Acoust. Soc. Am 30 (1958) 633–634. doi: 10.1121/1.1909718. [DOI] [Google Scholar]
- [140].Nyborg WL, Acoustic Streaming near a Boundary, J. Acoust. Soc. Am 30 (1958) 329–339. doi: 10.1121/1.1909587. [DOI] [Google Scholar]
- [141].Schwarz T, Petit-Pierre G, Dual J, Rotation of non-spherical micro-particles by amplitude modulation of superimposed orthogonal ultrasonic modes, J. Acoust. Soc. Am 133 (2013) 1260–1268. doi: 10.1121/1.4776209. [DOI] [PubMed] [Google Scholar]
- [142].Läubli N, Shamsudhin N, Ahmed D, Nelson BJ, Controlled Three-dimensional Rotation of Single Cells Using Acoustic Waves, Procedia CIRP. 65 (2017) 93–98. doi: 10.1016/j.procir.2017.04.028. [DOI] [Google Scholar]
- [143].Xie Y, Ahmed D, Lapsley MI, Lin S-CS, Nawaz AA, Wang L, Huang TJ, Single-Shot Characterization of Enzymatic Reaction Constants K m and k cat by an Acoustic-Driven, Bubble-Based Fast Micromixer, Anal. Chem 84 (2012) 7495–7501. doi: 10.1021/ac301590y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Schwed Lustgarten DE, Thompson J, Yu G, Vachani A, Vaidya B, Rao C, Connelly M, Udine M, Tan KS, Heitjan DF, Albelda S, Use of Circulating Tumor Cell Technology (CELLSEARCH) for the Diagnosis of Malignant Pleural Effusions, Ann. Am. Thorac. Soc 10 (2013) 582–589. doi: 10.1513/AnnalsATS.201303-068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Farace F, Massard C, Vimond N, Drusch F, Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L, Planchard D, Le Moulec S, André F, Fizazi K, Soria JC, Vielh P, A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas, Br. J. Cancer 105 (2011) 847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Di Carlo D, A Mechanical Biomarker of Cell State in Medicine, J. Lab. Autom 17 (2012) 32–42. doi: 10.1177/2211068211431630. [DOI] [PubMed] [Google Scholar]
- [147].Guo F, French JB, Li P, Zhao H, Chan CY, Fick JR, Benkovic SJ, Huang TJ, Probing cell–cell communication with microfluidic devices, Lab Chip. 13 (2013) 3152. doi: 10.1039/c3lc90067c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee H-H, Alves SE, Salmon M, Ferrante TC, Weaver JC, Bahinski A, Hamilton GA, Ingber DE, Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro, Nat. Methods 13 (2016) 151–157. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- [149].Huh D, Hamilton GA, Ingber DE, From 3D cell culture to organs-on-chips, Trends Cell Biol. 21 (2011) 745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Guo F, Xie Y, Li S, Lata J, Ren L, Mao Z, Ren B, Wu M, Ozcelik A, Huang TJ, Reusable acoustic tweezers for disposable devices, Lab Chip. 15 (2015) 4517–4523. doi: 10.1039/C5LC01049G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ma Z, Collins DJ, Ai Y, Detachable Acoustofluidic System for Particle Separation via a Traveling Surface Acoustic Wave, Anal. Chem 88 (2016) 5316–5323. doi: 10.1021/acs.analchem.6b00605. [DOI] [PubMed] [Google Scholar]
- [152].Mao Z, Li P, Wu M, Bachman H, Mesyngier N, Guo X, Liu S, Costanzo F, Huang TJ, Enriching Nanoparticles via Acoustofluidics, ACS Nano. 11 (2017) 603–612. doi: 10.1021/acsnano.6b06784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Jin H, Zhou J, He X, Wang W, Guo H, Dong S, Wang D, Xu Y, Geng J, Luo JK, Milne WI, Flexible surface acoustic wave resonators built on disposable plastic film for electronics and lab-on-a-chip applications, Sci. Rep 3 (2013) 2140. doi: 10.1038/srep02140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Huang PH, Nama N, Mao Z, Li P, Rufo J, Chen Y, Xie Y, Wei CH, Wang L, Huang TJ, A reliable and programmable acoustofluidic pump powered by oscillating sharp-edge structures, Lab Chip. 14 (2014) 4319–4323. doi: 10.1039/c4lc00806e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bachman H, Huang P-H, Zhao S, Yang S, Zhang P, Fu H, Huang TJ, Acoustofluidic devices controlled by cell phones, Lab Chip. 18 (2018) 433–441. doi: 10.1039/C7LC01222E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Bachman H, Fu H, Huang P-H, Tian Z, Embry-Seckler J, Rufo J, Xie Z, Hartman JH, Zhao S, Yang S, Meyer JN, Huang TJ, Open source acoustofluidics, Lab Chip. (2019). doi: 10.1039/C9LC00340A. [DOI] [PMC free article] [PubMed] [Google Scholar]