Abstract
Background
Avelumab, a human anti–programmed death-ligand 1 immunoglobulin G1 monoclonal antibody, showed favorable efficacy and safety in patients with metastatic Merkel cell carcinoma (mMCC) in the phase II JAVELIN Merkel 200 trial, leading to approval in multiple countries. We describe real-world experience with avelumab in patients with mMCC from an expanded access program.
Methods
Eligible patients had mMCC and progressive disease during or after chemotherapy or were ineligible for chemotherapy or clinical trial participation. Patients received an initial 3-month supply of avelumab (administered as 10 mg/kg intravenously every 2 weeks until progressive disease or unacceptable toxicity); resupply was allowed following complete response, partial response, stable disease, or clinical benefit per physician assessment.
Results
Between December 15, 2015, and March 4, 2019, 558 of 620 requests from 38 countries were medically approved, and 494 patients received avelumab. Among 240 evaluable patients, the objective response rate was 46.7% (complete response in 22.9%, including 3 of 16 potentially immunocompromised patients), and the disease control rate was 71.2%. The median duration of treatment in evaluable patients with response was 7.9 months (range, 1.0–41.7) overall and 5.2 months (range, 3.0–13.9) in immunocompromised patients. No new safety signals were identified. The expanded access program closed for new requests on December 31, 2018, as required after regulatory approval; benefitting patients continued to receive avelumab.
Conclusions
The avelumab expanded access program for patients with mMCC demonstrated efficacy and safety in a real-world setting, consistent with the results from JAVELIN Merkel 200, and provided a treatment for patients with limited options.
Keywords: tumors, dermatology
Background
Merkel cell carcinoma (MCC) is an aggressive skin cancer associated with a high risk of local and distant metastasis and poor survival outcomes in patients with metastatic disease (5-year survival rate with distant metastases of ≈20%).1–3 MCC is associated with clonal integration of the Merkel cell polyomavirus (MCPyV), ultraviolet radiation exposure, immunosuppression, and advanced age.4 5 MCC is considered a chemosensitive tumor, but responses to chemotherapy in patients with metastatic MCC (mMCC) are seldom durable.6–10 In addition, commonly used platinum-based chemotherapy regimens incur significant toxicity, particularly in a population that largely comprise older patients. There is evidence that impairment of the immune system can contribute to the pathogenesis of MCC, providing a rationale for therapies that promote antitumor immune responses.9
Avelumab is a human anti–programmed cell death protein-ligand 1 (PD-L1) immunoglobulin G1 monoclonal antibody that inhibits the interaction between PD-L1 and programmed cell death protein 1 (PD-1),11 12 which may induce T-cell reinvigoration and effector cell functions against tumor cells, as shown in preclinical models.13 14 Avelumab has shown antitumor activity and a tolerable safety profile in patients with various tumors,12 15–17 including patients who received first-line (1L) or second-line or later (2L+) therapy for mMCC.18–20 In mMCC, avelumab was initially assessed in part A of the JAVELIN Merkel 200 trial (NCT02155647), which enrolled 88 patients with mMCC and progressive disease (PD) while receiving or after receiving chemotherapy.18 After ≥2 years of follow-up in all patients, the objective response rate (ORR) was 33.0%, including a complete response (CR) in 11.4%, and the response duration was ≥2 years in 67.0% of responders based on Kaplan-Meier estimates.21 Grade ≥3 treatment-related adverse events (TRAEs) were observed in 11.4% of patients, and no treatment-related deaths occurred.18 21 Part B of JAVELIN Merkel 200 enrolled patients with mMCC who had not received prior systemic treatment for metastatic disease. In the primary analysis (n=116; ≥15 months of follow-up), the ORR was 39.7%, including CR in 16.4%, 30.2% had a durable response (lasting ≥6 months), and the median overall survival was 20.3 months. Grade ≥3 TRAEs were observed in 18.1% of patients, and no treatment-related deaths occurred.20 Based on the findings from JAVELIN Merkel 200, avelumab became the first treatment approved for mMCC in several locations, including the USA, European Union, Switzerland, Japan, Australia, Israel, Brazil, and Canada.11 18 22
An expanded access program (EAP; sometimes called a compassionate use program) provides an investigational treatment to patients who have a serious or terminal disease outside of a clinical trial, although definitions vary by country.23 After data became available from the avelumab registrational clinical trial in mMCC, the EAP was initiated to provide avelumab for compassionate use to patients with mMCC who had limited treatment options. The EAP was an ad hoc program that incorporated treatment guidelines but was not protocol-driven. Participation was permitted on a patient-by-patient basis; therefore, the EAP did not require clinical trial registration. However, to enable French patients to participate via the Temporary Authorization for Use designation, the global MCC EAP was registered with ClinicalTrials.gov (NCT03089658). Accordingly, available safety and efficacy data were not prioritized and were limited in scope. Here we describe real-world experience with avelumab for patients with mMCC enrolled in the global EAP.
Methods
Study design and participants
Eligible patients had measurable MCC by Response Evaluation Criteria in Solid Tumors 1.1 and PD following ≥1 prior line of chemotherapy in the metastatic setting; patients were also eligible if they were ineligible for chemotherapy (evaluated on a case-by-case basis). Patients were also not eligible for participation in any ongoing trials pertaining to advanced MCC. The population included patients who had an Eastern Cooperative Oncology Group performance score (ECOG PS) of 0–3, who had treated brain metastases (without steroid use) that were not progressing, or who were potentially immunocompromised (evaluated on a case-by-case basis by the sponsor based on medical history provided by the treating physician, including patients with medical conditions that can suppress the immune system, patients receiving immunosuppressive medications, and patients with HIV infection). Patients were not selected based on tumor PD-L1 expression or MCPyV status. Most patients received avelumab as 2L+ treatment, but a minority of patients received 1L avelumab after individual evaluation (including those unable to tolerate chemotherapy or who were ineligible for chemotherapy for various reasons, including age, renal disorder, and risk of pancytopenia).
Procedures
Patients received avelumab 10 mg/kg by 1-hour intravenous infusion every 2 weeks until confirmed PD, unacceptable toxicity, or occurrence of any other criterion for withdrawal. Continuation of avelumab beyond radiological PD was permitted in the absence of significant clinical deterioration and based on physician assessment of potential benefit on a case-by-case basis, with criteria including no new or worsening of existing symptoms, tolerance to avelumab, stable ECOG PS, and no delay to any imminent intervention to prevent serious complications of PD. A 3-month supply of avelumab was provided to treating physicians, and resupply was allowed for patients who had CR, partial response (PR), stable disease, or other clinical benefit according to radiography or treating physician assessment. Data were provided by the treating physician during the application for avelumab resupply. Antitumor activity was assessed by the treating physician per treatment guidelines and according to Response Evaluation Criteria in Solid Tumors 1.1 where applicable to determine best overall response. Other assessments included duration of treatment for patients with response, safety, and tolerability. Adverse events (AEs) were reported to Merck KGaA Global Patient Safety. An online portal to process EAP requests and collate responses was implemented in May 2017.
Results
Between December 15, 2015, and March 4, 2019 (data cut-off date), 620 requests for avelumab were received from 38 countries (online supplementary additional file 1, additional file 2). Of these requests, 558 were approved, 11 were withdrawn, and 51 were medically rejected for various reasons, including incorrect diagnosis, lack of appropriate prior therapy, and incomplete information. Countries providing the most requests were Italy (n=109), France (n=96), and Australia (n=83). The median age was 73 years (range, 23–95) and 66.8% of patients were male. Most patients (90.9%) had an ECOG PS of 0 or 1. Other available baseline characteristics are shown in table 1. Among approved patients, 522 (93.6%) were approved for 2L+ avelumab therapy and 36 (6.5%) for 1L therapy (patients judged ineligible for chemotherapy by the treating physician). The EAP was closed to enrollment on December 31, 2018, after regulatory approval of avelumab in multiple countries.
Table 1.
Baseline characteristics of approved patients in avelumab MCC EAP
| Characteristics | All approved patients (n=558) | Approved immunocompromised patients (n=37) |
Approved 1L patients (n=36) |
| Age (years), n (%) | |||
| <65 | 116 (20.8) | 6 (16.2) | 4 (11.1) |
| ≥65 | 442 (79.2) | 31 (83.8) | 32 (88.9) |
| Median (range) | 73 (23–95) | 74 (50–86) | 79 (46–95) |
| Sex, n (%) | |||
| Male | 372 (66.8) | 21 (56.8) | 24 (66.7) |
| Female | 185 (33.2) | 16 (43.2) | 12 (33.3) |
| Weight (kg), n (%)* | |||
| <80 | 229 (50.9) | 24 (64.9) | 16 (44.4) |
| ≥80 | 221 (49.1) | 13 (35.1) | 20 (55.6) |
| Median (range) | 78.8 (40–150) | 75 (41–124) | 80 (49–138) |
| ECOG PS, n (%)* | |||
| 0 | 175 (39.0) | 14 (37.8) | 15 (41.7) |
| 1 | 233 (51.9) | 18 (48.7) | 18 (50.0) |
| 2 | 33 (7.4) | 3 (8.1) | 3 (8.3) |
| 3 | 8 (1.8) | 2 (5.4) | 0 |
| Line of therapy, n (%) | |||
| 1 | 36 (6.5) | 4 (10.8) | 36 (100) |
| ≥2 | 522 (93.6) | 33 (89.2) | 0 |
*Weight and ECOG PS at baseline were available only for the 450 patients enrolled via the EAP portal.
EAP, expanded access program; ECOG PS, Eastern Cooperative Oncology Group performance status; 1L, first-line; MCC, Merkel cell carcinoma.
jitc-2019-000313supp001.pdf (230KB, pdf)
Of 494 patients supplied with avelumab, starting from April 6, 2016, response data were not available in 254 patients for several reasons, which included patients whose entry predated the implementation of the EAP portal and who did not have outcome data, regardless of response or resupply, and patients who had no evaluable data beyond the 90-day period of initial drug supply (including those with rapid PD). Outcomes were therefore provided for 240 patients. Among patients who discontinued treatment, the most common reasons were radiographic or clinical progression. Of the 240 evaluable patients, 16 were classified as potentially immunocompromised based on available medical history (examples included patients who had chronic lymphocytic leukemia, were HIV-positive, had received a prior organ transplant, or were receiving high-dose steroids), and 15 received 1L avelumab (patients ineligible for chemotherapy).
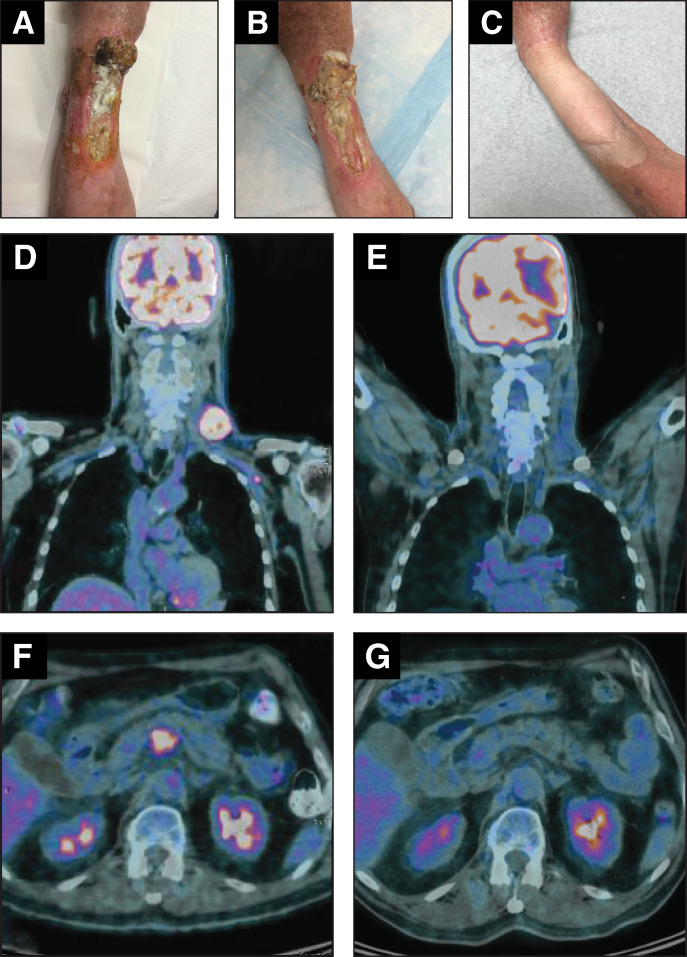
Duration of avelumab treatment (or duration that the drug was supplied) was considered a surrogate for duration of response or clinical benefit because resupply was dependent on these criteria. At data cut-off, the median duration of avelumab treatment was 7.9 months (range, 1.0–41.7) among all evaluable patients (n=240), 5.2 months (range, 3.0–13.9) in immunocompromised patients (n=16), and 4.5 months (range, 3.0–19.8) in 1L (chemotherapy-ineligible) patients (n=15). In total, 55 patients had CR and 57 had PR, representing an ORR of 22.7% using the denominator of 494 patients in the total population. Considering only the 240 response-evaluable patients, the ORR was 46.7%, including CR in 22.9% and PR in 23.8%. In immunocompromised patients, the ORR was 37.5% (n=6), including CR in 18.8% (n=3; these patients had a history of acute myeloid leukemia, had undergone splenectomy, or had Sjögren’s syndrome). In the 1L subgroup, the ORR was 46.7% (n=7) (table 2). Images of responses in individual patients are shown in figure 1.
Table 2.
Physician-reported responses in all evaluable patients participating in avelumab MCC EAP
| Response parameter* | All patients (n=240) | Immunocompromised patients (n=16) | 1L (n=15) | 2L+ (n=225) |
| ORR, % | 46.7 | 37.5 | 46.7 | 46.7 |
| DCR, %† | 71.2 | 68.8 | 66.7 | 71.6 |
| Confirmed BOR, n (%) | ||||
| CR | 55 (22.9) | 3 (18.8) | 2 (13.3) | 53 (23.6) |
| PR | 57 (23.8) | 3 (18.8) | 5 (33.3) | 52 (23.1) |
| SD | 59 (24.6) | 5 (31.3) | 3 (20.0) | 56 (24.9) |
| PD‡ | 69 (28.8) | 5 (31.3) | 5 (33.3) | 64 (28.4) |
| Duration of treatment in patients with response§ | ||||
| Median (range), months | 7.9 (1.0–41.7) | 5.2 (3.0–13.9) | 4.5 (3.0–19.8) | 7.9 (1.0–41.7) |
Immunocompromised, 1L, and 2L+ are subsets of the total number of evaluable patients.
*Response was reported according to treating physician assessment of follow-up scans at the time of resupply.
†Among patients treated for a minimum of 3 months with available data.
‡Patients with PD or AEs who required treatment discontinuation within the first 90 days were never resupplied and did not have a follow-up response evaluation; thus, these values may be under-reported.
§Duration of avelumab treatment/drug supply is reported as a surrogate for duration of response or clinical benefit.
AE, adverse event; BOR, best overall response; CR, complete response; DCR, disease control rate; EAP, expanded access program; 1L, first-line; 2L+, second-line or later; MCC, Merkel cell carcinoma; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
Images of lesions in two patients treated with avelumab in the expanded access program. Patient 1: patient with large, ulcerated, intransit lesions at baseline (A), which regressed after two avelumab infusions (B); the patient subsequently received a skin graft (C). Patient 2: baseline (D and F) and post-treatment (E and G) FDG-PET CT images of a patient with chemotherapy-refractory metastatic Merkel cell carcinoma who achieved a complete response following four cycles of avelumab. Images A–C were kindly provided by JWW, while images D–G were kindly provided by RM. FDG, fluorodeoxyglucose; PET, positron emission tomography.
Physician-reported safety data from the EAP are summarized in table 3. Infusion-related reaction, fever, fatigue, and rash were the most frequently occurring TRAEs. Safety events were likely under-reported because data were reported at the treating physician’s discretion at the time of resupply, and many patients had no evaluable data beyond the 3-month supply.
Table 3.
Physician-reported TRAEs with avelumab in the MCC EAP
| TRAEs* | n=494 | ||
| Non-serious events, n | Serious events, n | Total events, n | |
| Infusion-related reaction | 7 | 2 | 9 |
| Fever | 5 | 2 | 7 |
| Fatigue | 5 | 1 | 6 |
| Rash | 4 | 0 | 4 |
| Asthenia | 3 | 1 | 4 |
| Abdominal pain | 3 | 0 | 3 |
| Chills | 1 | 2 | 3 |
| Dyspnea | 0 | 3 | 3 |
*Data shown are preferred terms of all TRAEs observed in ≥3 patients in the EAP extracted from the safety database, including unsolicited cumulative events provided by treating physicians; overall safety events may have been under-reported in this ad hoc program.
EAP, expanded access program; MCC, Merkel cell carcinoma; TRAE, treatment-related adverse event.
Discussion
The global MCC EAP for avelumab answered an urgent, unmet medical need for patients with mMCC, a rare cancer with limited treatment options; to our knowledge, this is the largest EAP performed for this patient population. Before 2017, no treatment was approved for mMCC by the US Food and Drug Administration, European Commission, or any other regulatory authority. In this large EAP population, avelumab provided a clinical benefit in both immune-competent and immunocompromised patients (based on available medical history). The physician-reported ORR was 46.7% among all evaluable patients, including three immunocompromised patients who achieved CR and three who achieved PR, and the ORR was consistent in 1L and 2L+ subgroups. Durable responses were observed in both immune-competent and immunocompromised patients. Importantly, no new safety signals were identified in the EAP population. In both the EAP and JAVELIN Merkel 200 study,21 infusion-related reaction, fatigue, and rash were among the most frequently occurring TRAEs. Infusion-related reactions were managed per established guidance for avelumab.11
The data summarized in this manuscript have some limitations. Safety and efficacy data for the EAP were reported at the treating physician’s discretion and are therefore potentially under-reported in this program. Furthermore, because resupply of avelumab was only provided to patients following an assessment of clinical benefit by the treating physician, this may have affected reporting. Additionally, patients with PD or an AE who required treatment discontinuation within the first 90 days did not receive a resupply of avelumab and subsequently were not provided with a follow-up response evaluation. Limited information was provided by clinicians about each patient’s medical history, and the classification of patients as immunocompromised was made without specific assessments or knowledge of ongoing medications. Nevertheless, these findings highlight that in a real-world setting, avelumab showed efficacy and safety consistent with results from the JAVELIN Merkel 200 trial, despite including patients who would have been ineligible for the trial (eg, those with ECOG PS of 2 or 3, treated brain metastases, or immunosuppressive conditions including HIV infection).21 Although the results presented are limited by the nature of data reporting within the MCC EAP, these results provide valuable insight into responses to anti-PD-1/PD-L1 antibodies in vulnerable or immunocompromised patients with this disease.
EAPs enable patients with limited treatment options to access therapies that they otherwise would not receive. To the best of our knowledge, this MCC EAP is the largest and only EAP for an immune checkpoint inhibitor in this rare disease. In addition to evaluating the efficacy and safety profile of avelumab in a real-world setting, this EAP has revealed the need for additional treatment options for this rare aggressive cancer.
In conclusion, avelumab provided benefit in a real-world population of patients with mMCC who had various factors that excluded participation in clinical trials. Efficacy and safety data were consistent with pivotal trial data.
Acknowledgments
The authors thank all the patients and their families as well as the investigators who participated in the global avelumab EAP for MCC. The authors would also like to thank Yushun Lin, PhD, for support with statistical analysis.
Footnotes
Correction notice: Since the online publication of this article, the authors have noticed an errors in Table 1 and 2. In Table 1, the header 'Approved immunocompromised patients (n=33)' should read 'Approved immunocompromised patients (n=37)' as the total number of patients is 37. Additionally in Table 2 row ‘CR’, column ‘1L (n=15)’, the data value is incorrectly stated as ‘5 (33.3)2 (13.3)’. The correct data value is ‘2 (13.3)’.
Contributors: Provision of study materials or patients: JWW, CL, GG, PN, LD, EF, PAA, SS, RM. Collection and assembly of data: all authors. Data analysis and interpretation: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
Funding: The EAP was sponsored by Merck KGaA, Darmstadt, Germany, as part of an alliance between Merck KGaA and Pfizer. Medical writing support was provided by ClinicalThinking and funded by Merck KGaA, Darmstadt, Germany, and Pfizer.
Competing interests: CL reports a consulting or advisory role for Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche; speaker services for Amgen, Bristol-Myers Squibb, Novartis, and Roche; research funding from Bristol-Myers Squibb and Roche; and travel expenses from Amgen, Bristol-Myers Squibb, and Roche. GG reports a consulting or advisory role for Bayer, Eisai, Eli Lilly, Novartis, Pfizer, and PharmaMar; research funding from Bayer, Novartis, and PharmaMar; and travel expenses from PharmaMar. PN reports a consulting or advisory role for AstraZeneca, Bristol-Myers Squibb, Novartis, Immunocure, Roche, and Pfizer. RM reports a consulting or advisory role for Eli Lilly, Merck KGaA, Merck Sharp & Dohme, Pierre Fabre, Roche, and Sanofi; speaker services for Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche; research funding from Bristol-Myers Squibb, Eli Lilly, Merck KGaA, Merck Sharp & Dohme, Novartis, and Roche; and travel expenses from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, and Sanofi. PAA reports a consulting or advisory role for 4SC, Array, AstraZeneca, Bristol-Myers Squibb, Genmab, Idera, Immunocore, Incyte, MedImmune, Merck KGaA, Merck Sharp & Dohme, NewLink Genetics, Novartis, Pierre Fabre, Roche/Genentech, Sandoz, Sanofi, Sun Pharma, Syndax, and Ultimovacs; research funding from Array, Bristol-Myers Squibb, and Roche/Genentech; and travel expenses from Merck Sharp & Dohme. SS reports a consulting or advisory role for Amgen, AstraZeneca, Bristol-Myers Squibb, Janssen, Merck Sharp & Dohme, Novartis, and Roche; and research funding from Amgen, AstraZeneca, Bristol-Myers Squibb, and Merck Sharp & Dohme. EB is an employee of Merck KGaA, and SF, JR, and VK are employees of EMD Serono, a business of Merck KGaA. AE is an employee of Pfizer Pharma. SH is an employee of and has ownership of equity in Pfizer. All other authors declare that they have no competing interests.
Patient consent for publication: Not required.
Ethics approval: The trial was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation Guideline for Good Clinical Practice. The protocol was approved by the institutional review board or independent ethics committee of each center, and all patients provided written informed consent before enrollment.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. For all new products or new indications approved in both the European Union and the USA after January 1, 2014, Merck KGaA, Darmstadt, Germany will share patient-level and study-level data after deidentification, as well as redacted study protocols and clinical study reports from clinical trials in patients. These data will be shared with qualified scientific and medical researchers, upon researchers' request, as necessary for conducting legitimate research. Such requests must be submitted in writing to the company’s data sharing portal. More information can be found at https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. Where Merck KGaA has a coresearch, codevelopment or comarketing/copromotion agreement or where the product has been out-licensed, it is recognized that the responsibility for disclosure may be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavor to gain agreement to share data in response to requests.
References
- 1.Lebbe C, Becker JC, Grob J-J, et al. . Diagnosis and treatment of Merkel cell carcinoma. European consensus-based interdisciplinary guideline. Eur J Cancer 2015;51:2396–403. 10.1016/j.ejca.2015.06.131 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: Merkel cell carcinoma. V2, 2019. Available: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf [Accessed 26 June 2019].
- 3.Prieto Muñoz I, Pardo Masferrer J, Olivera Vegas J, et al. . Merkel cell carcinoma from 2008 to 2012: reaching a new level of understanding. Cancer Treat Rev 2013;39:421–9. 10.1016/j.ctrv.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 4.Schadendorf D, Lebbé C, Zur Hausen A, et al. . Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer 2017;71:53–69. 10.1016/j.ejca.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 5.Feng H, Shuda M, Chang Y, et al. . Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096–100. 10.1126/science.1152586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer JG, Blom A, Doumani R, et al. . Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med 2016;5:2294–301. 10.1002/cam4.815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker JC, Lorenz E, Ugurel S, et al. . Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget 2017;8:79731–41. 10.18632/oncotarget.19218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowey CL, Mahnke L, Espirito J, et al. . Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol 2017;13:1699–710. 10.2217/fon-2017-0187 [DOI] [PubMed] [Google Scholar]
- 9.Nghiem P, Kaufman HL, Bharmal M, et al. . Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol 2017;13:1263–79. 10.2217/fon-2017-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanetti I, Coati I, Alaibac M. Interaction between Merkel cell carcinoma and the immune system: pathogenetic and therapeutic implications. Mol Clin Oncol 2017;7:729–32. 10.3892/mco.2017.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bavencio (avelumab) [prescribing information]. Rockland, MA: EMD Serono, 2019. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761049s000lbl.pdf [Accessed 26 June 2019].
- 12.Heery CR, O'Sullivan-Coyne G, Madan RA, et al. . Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017;18:587–98. 10.1016/S1470-2045(17)30239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyerinas B, Jochems C, Fantini M, et al. . Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015;3:1148–57. 10.1158/2326-6066.CIR-15-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii R, Friedman ER, Richards J, et al. . Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget 2016;7:33498–511. 10.18632/oncotarget.9256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel MR, Ellerton J, Infante JR, et al. . Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64. 10.1016/S1470-2045(17)30900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulley JL, Rajan A, Spigel DR, et al. . Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18:599–610. 10.1016/S1470-2045(17)30240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly K, Infante JR, Taylor MH, et al. . Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN Solid Tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer 2018;124:2010–7. 10.1002/cncr.31293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman HL, Russell JS, Hamid O, et al. . Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018;6:7. 10.1186/s40425-017-0310-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Angelo SP, Russell JS, Bhatia S, et al. . 18-Month efficacy and safety update from JAVELIN Merkel 200 Part A: a phase II study of avelumab in metastatic Merkel cell carcinoma progressed on chemotherapy. J Clin Oncol 2018;36192. 10.1200/JCO.2018.36.5_suppl.192 [DOI] [Google Scholar]
- 20.D’Angelo S, Lebbé C, Mortier L, et al. . First-line avelumab treatment in patients with metastatic Merkel cell carcinoma: primary analysis after ≥15 months of follow-up from JAVELIN Merkel 200, a registrational phase 2 trial. J Immunother Cancer 2019;7:282. 10.1186/s40425-019-0763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nghiem P, Bhatia S, Brohl AS, et al. . Two-year efficacy and safety update from JAVELIN Merkel 200 part A: a registrational study of avelumab in metastatic Merkel cell carcinoma progressed on chemotherapy. J Clin Oncol 2018;36:9507 10.1200/JCO.2018.36.15_suppl.9507 [DOI] [Google Scholar]
- 22.Kaufman HL, Russell J, Hamid O, et al. . Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17:1374–85. 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iudicello A, Alberghini L, Benini G, et al. . Expanded access programme: looking for a common definition. Trials 2016;17:21. 10.1186/s13063-015-1108-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2019-000313supp001.pdf (230KB, pdf)