Abstract
Objective
To investigate social inequalities underlying low birthweight (LBW) outcomes in Sri Lanka.
Design
Cross-sectional study.
Setting
This study used the Sri Lanka Demographic and Health Survey 2016, the first such survey to cover the entire country since the Civil War ended in 2001.
Participants
Birthweight data extracted from the child health development records available for 7713 babies born between January 2011 and the date of interview in 2016.
Outcome measures
The main outcome variable was birth weight, classified as LBW (≤2500 g) and normal.
Methods
We applied random intercept three-level logistic regression to examine the association between LBW and maternal, socioeconomic and geographic variables. Concentration indices were estimated for different population subgroups.
Results
The population-level prevalence of LBW was 16.9% but was significantly higher in the estate sector (28.4%) compared with rural (16.6%) and urban (13.6%) areas. Negative concentration indices suggest a relatively higher concentration of LBW in poor households in rural areas and the estate sector. Results from fixed effects logistic regression models confirmed our hypothesis of significantly higher risk of LBW outcomes across poorer households and Indian Tamil communities (AOR 1.70, 95% CI 1.02 to 2.83, p<0.05). Results from random intercept models confirmed there was substantial unobserved variation in LBW outcomes at the mother level. The effect of maternal biological variables was larger than that of socioeconomic factors.
Conclusion
LBW rates are significantly higher among babies born in poorer households and Indian Tamil communities. The findings highlight the need for nutrition interventions targeting pregnant women of Indian Tamil ethnicity and those living in economically deprived households.
Keywords: nutrition & dietetics, community child health, public health, social medicine
Strengths and limitations of this study.
The survey covered the entire island for the first time after the Civil War ended in 2001.
Birthweight data were obtained from child health records, and most of the births are institutional deliveries.
Birthweight data can be biased due to rounding errors or other errors related to weighing instruments.
Due to data constraints, data on genetic factors and prepregnancy weight that could have affected the low birth weight were not included in the analysis.
Introduction
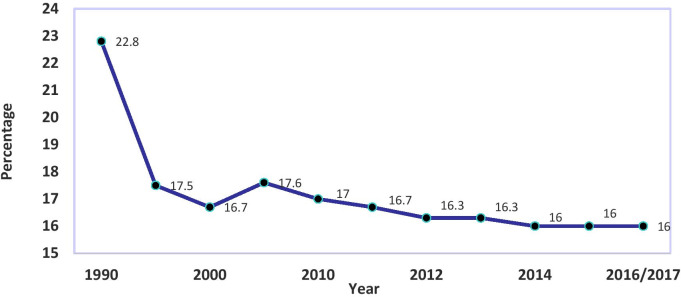
Over the last few decades, Sri Lanka has experienced a marked reduction in infant, child and maternal mortality rates,1 2 when compared with other South Asian countries. However, there has been little or no progress in child health indicators in Sri Lanka particularly low birthweight (LBW) outcomes, which have hindered the achievement of health-related United Nations Millennium Development Goals.3 For example, despite the reduction of LBW rates from 22.8% to 16.7% between 1990 and 2000, the percentage of children born with LBW has remained at around 17% since 2000 (figure 1).1–4
Figure 1.
Percentage of babies with low birth weight in Sri Lanka: 1990–2017. Data source: Department of Census and Statistics.1
LBW is a critical factor associated with neonatal and infant deaths, and nutritional and health outcomes at later stages of child development.4–9 LBW babies are more vulnerable to contracting infections, malnutrition and disability during childhood than those born with normal weight, particularly cognitive disorders related to behaviour and learning.6 LBW babies who survive infancy are also vulnerable to increased risks of non-communicable and chronic diseases in adulthood.9 10
Global and regional variations in LBW rates are pronounced, with the highest burden in low-income and middle-income countries, which account for more than 95% of all LBW babies. South Asia has the largest share of LBW babies, constituting 48% of all LBW babies globally4 11 with the highest rates recorded in Bangladesh, India and Pakistan.12 Maternal biobehavioural risk factors such as age, nutritional status, poor diet during pregnancy, body mass index (BMI), gestational age, interpregnancy interval, parity and lack of antenatal care as well as social, economic and environmental factors such as poverty and low socioeconomic status are associated with LBW outcomes globally.4 11–15
High rates of LBW remain a critical public health problem in Sri Lanka, with a long-term impact on health outcomes, disease burden and economic productivity.16 Despite a plethora of national health programmes, including a programme promoting universal access to antenatal care, a multisectoral food and micronutrient supplementation programme aligned to the National Nutrition Policy (2009–2013) and poverty alleviation programmes, there has been little reduction in the incidence of LBW outcomes.17 Previous small-scale community studies in Sri Lanka have identified that the risk of LBW babies is particularly high among mothers in the estate sector.17–20 The estate sector comprises mostly Indian Tamil tea plantation workers who live in the centre and south of Sri Lanka.21
Existing studies on LBW have been focused on homogeneous and relatively small samples in specific settings, for example, rural or hospital-based studies. There is little population-level research on the extent of inequalities in LBW outcome in Sri Lanka. The present research addresses this gap by analysing the social inequalities underlying LBW outcomes and associated risk factors in Sri Lanka, based on recent data from a nationally representative cross-sectional survey. We hypothesise that children born in poor households and to the Indian Tamil tea plantation workers in the estate sector are more vulnerable to LBW outcomes than their counterparts living in richer households in other rural areas and in towns and cities.
Methods
Sample
We used data from the Sri Lanka Demographic and Health Survey (SLDHS) conducted during 2016–2017. This is the first nationally representative sample survey to be implemented since the Civil War ended in 2001. The SLDHS used a two-stage stratified sampling design. A total of 28 800 housing units were selected for the survey. Within the households, 18 302 married women aged 15–49 years were selected for interview. SLDHS collected detailed data on birth histories and mothers’ reproductive health behaviours, along with socioeconomic and demographic data.
The analysis considered 7072 mothers of reproductive age (15–49 years) who had at least one birth in the 5 years preceding the survey: 6069 had one birth, and 1003 had two or more births, of whom 27 had three children and 1 had four children. The total number of births to the 7072 mothers was 8104. Of these, 7964 were singleton (98.3%) and 140 (1.7%) were multiple births. For 251 singleton births, either the birthweight data were missing or the reported birth weight was extreme (over 6500 g (0.36% of births)).
For the remaining 7713 births, the mean birth weight was 2917 grams (95% CI 2906 to 2927), and the median was 2920 g. For 140 multiple births, the mean birth weight was 2135 g (95% CI 2050 to 2214) and the median was 2175 g. We excluded multiple births in the further analysis, since 81% of the multiple births had LBW. We found no statistical difference in the distribution of socioeconomic factors between singleton and multiple births. For 220 cases (2.6% of the total), birth weight was recorded at exactly 2500 g. Our final analysis sample includes 7713 singleton births with a recorded birth weight between January 2011 and November 2016 (survey date).
Outcome variable
We followed the standard definition of LBW (babies weighing less than 2500 g) and also considered those with a reported birth weight of exactly 2500 g22 to allow for potential rounding errors while entering LBW data on child health development records.
Explanatory variables
We grouped the explanatory variables into three categories: maternal depletion, socioeconomic and geographical. The classification of maternal depletion variables was on the basis of the theory of maternal depletion syndrome that states that women with closely spaced pregnancies are vulnerable to enter the reproductive cycle with reduced nutrition reserves.23 Maternal nutrition depletion may lead to negative outcomes such as LBW, infant mortality and reduced fecundity.23–25 SLDHS has limited variables to measure maternal depletion: maternal age, maternal BMI and height, preceding birth interval, micronutrient (iron and folic acid tablets) intake and food supplementation (Thriposha) received during pregnancy. Micronutrient supplementation and Thriposha are recommended by the government and are given free for pregnant and lactating mothers in Sri Lanka.17 We also have data on the frequency of antenatal care visits and the sex of the child. The survey asked mothers to report their gestational age in months. However, we did not use this information since the reported gestational data (in months) could be biased and grossly underestimated.
In addition, we considered the following socioeconomic variables: maternal education, a household wealth index as a proxy for measuring socioeconomic status and ethnicity.
Household wealth index quintile is a standard composite measure of household ownership of assets, materials and access to basic sanitation. The DHS estimates household wealth index using principal component analysis separately for urban, rural and sector areas. Finally, we considered two key geographic variables: (1) place of residence classified as urban, rural and estate sector (the urban sector is composed of areas administered by municipal and urban councils, the estate sector is predominantly concentrated in the tea plantation areas, while the rural sector comprises the areas not captured by the urban and estate sectors)1 and (2) nine administratively defined provinces.
Statistical analysis
We examined the binary association between birth weight and selected characteristics. The outcome variable is coded 0 (reference) for babies with a normal weight and 1 for those weighing 2500 g or less. Then we fit a series of binary logistic regression models. Model 1 includes maternal depletion variables, model 2 includes maternal depletion and socioeconomic variables and model 3 includes maternal depletion, socioeconomic variables and geographical variables. The variance inflation factor is used to check for collinearity and to ensure that the assumptions of multicollinearity are not violated. Due to the hierarchical nature of the data with some mothers having more than one child (903 mothers), and these mothers being grouped within communities (primary sampling units or clusters), we examine the variation in LBW at three levels: child, mother and community, using the same series of models, but taking account of the fact that some mothers have more than one child, and mothers are clustered within communities.
Additionally, we estimated concentration indices to measure the extent of wealth inequalities underlying LBW, which are illustrated graphically using concentration curves.
Patient and public involvement
Not applicable for this study
Results
Descriptive analysis
Table 1 shows the statistical association between birth weight and selected variables. About 17% of babies were born with a LBW, and the rate was significantly higher among babies born in the estate sector (28.4%) when compared with rural (16.6%) and urban (13.6) areas. LBW was concentrated among teenage and young mothers aged under 20 and 20–24 years. There is a positive association between maternal anthropometric measures (BMI and height) and LBW. The association between LBW and the number of antenatal visits is marginal (table 1). There was no significant association between LBW and receipt of Thriposha during pregnancy. However, LBW was relatively common among mothers who had not had iron and folic acid supplements. Female babies were more likely than male babies to be born with LBW. Among the socioeconomic characteristics, the prevalence of LBW was inversely related to educational attainment and household wealth. For example, 21.4% of mothers in the lowest wealth quintile had LBW babies, compared with only around half that proportion among the highest wealth quintile. Indian Tamils were more likely than the other ethnic groups to have LBW babies, and mothers living in the estate sector generally have a higher proportion of LBW babies (28.4%) compared with their counterparts living in rural and urban areas. LBW was common in Central and Sabaragamuwa regions and less common in the Northern region (table 1).
Table 1.
Percentage distribution of recorded birth weight by maternal depletion, socioeconomic and geographical factors: Sri Lanka, 2016
| Variable and category | Birth weight (in grams) | Number of births | P value | |||
| ≤2500 | 2501–3000 | 3001–3500 | 3501–6500 | |||
| All data | 16.9 | 38.0 | 34.9 | 10.2 | 7,713 | |
| Maternal age (years) | ||||||
| Under 20 | 25.6 | 39.1 | 31.0 | 4.0 | 74 | 0.001 |
| 20–24 | 19.7 | 41.9 | 31.2 | 7.1 | 1,012 | |
| 25–34 | 16.1 | 37.8 | 35.9 | 10.0 | 4,468 | |
| 35–39 | 16.2 | 36.1 | 34.2 | 13.2 | 1,622 | |
| 40 and over | 18.4 | 36.5 | 35.3 | 9.6 | 537 | |
| Maternal body mass index | ||||||
| Under 18.5 | 26.4 | 45.5 | 24.4 | 3.5 | 847 | 0.000 |
| 18.5–24.9 | 17.2 | 39.9 | 33.9 | 8.8 | 3,726 | |
| 25.0–29.9 | 14.1 | 33.9 | 38.4 | 13.5 | 2,171 | |
| 30.0 or more | 11.8 | 31.9 | 40.1 | 15.9 | 801 | |
| Maternal height | ||||||
| Short (up to 145.0 cm) | 28.8 | 41.2 | 24.5 | 5.3 | 545 | 0.000 |
| Average (145.1–155.0 cm) | 18.5 | 39.6 | 32.9 | 8.7 | 4,198 | |
| Tall (155.1 cm and over) | 12.0 | 34.8 | 39.5 | 13.5 | 2,821 | |
| Preceding birth interval | ||||||
| First birth | 19.5 | 40.6 | 32.0 | 7.7 | 3,011 | 0.000 |
| Under 24 months | 14.9 | 34.5 | 36.5 | 13.9 | 394 | |
| 24–47 months | 12.7 | 35.5 | 39.1 | 12.5 | 1,594 | |
| 48–59 months | 15.2 | 35.3 | 36.4 | 12.9 | 793 | |
| 60 months or more | 17.3 | 37.5 | 34.8 | 10.3 | 1,931 | |
| Received Thriposha | ||||||
| Received and consumed | 18.5 | 43.8 | 30.5 | 7.3 | 504 | 0.108 |
| Received and shared | 17.0 | 37.5 | 34.8 | 10.5 | 5,921 | |
| Not received | 9.7 | 40.7 | 37.8 | 11.6 | 103 | |
| Taken iron and folic acid supplements | ||||||
| Received and consumed | 16.5 | 38.1 | 35.0 | 10.3 | 6,503 | 0.000 |
| Not received and consumed | 25.7 | 36.0 | 26.6 | 11.5 | 1,210 | |
| Antenatal care visits | ||||||
| Fewer than three times | 16.9 | 38.2 | 35.7 | 9.0 | 1,378 | 0.041 |
| 3–5 times | 24.0 | 37.1 | 30.6 | 8.1 | 737 | |
| 6–10 times | 16.1 | 38.1 | 35.0 | 10.6 | 5,314 | |
| 11 or more times | 12.3 | 36.2 | 38.3 | 13.0 | 284 | |
| Sex of child | ||||||
| Male | 15.1 | 37.4 | 36.3 | 11.3 | 4,000 | 0.000 |
| Female | 18.7 | 38.8 | 33.5 | 9.0 | 3,794 | |
| Education level | ||||||
| No education and primary | 27.6 | 40.2 | 24.7 | 7.3 | 380 | 0.000 |
| Secondary and passed General Certificate of Education (GCE) O-level | 18.0 | 38.4 | 33.7 | 9.7 | 5,127 | |
| Passed GCE A-level | 11.6 | 39.0 | 38.0 | 11.2 | 1,761 | |
| Degree and above | 15.0 | 26.2 | 44.7 | 13.9 | 445 | |
| Wealth index quintile | ||||||
| Poorest | 21.4 | 40.6 | 29.8 | 8.0 | 1,900 | 0.000 |
| Poor | 17.8 | 38.0 | 35.3 | 8.7 | 1,571 | |
| Middle | 17.9 | 38.5 | 33.2 | 10.2 | 1,460 | |
| Rich | 14.1 | 36.5 | 37.8 | 11.4 | 1.514 | |
| Richest | 10.8 | 34.9 | 40.3 | 13.8 | 1,268 | |
| Ethnicity | ||||||
| Sinhala | 17.2 | 38.0 | 34.5 | 10.0 | 5,025 | 0.000 |
| Sri Lanka Tamil | 15.9 | 36.4 | 36.8 | 10.8 | 1,564 | |
| Indian Tamil | 32.6 | 42.5 | 23.5 | 1.2 | 242 | |
| Muslim | 12.1 | 38.6 | 36.7 | 12.4 | 857 | |
| Burgher and Malay | 12.0 | 48.0 | 28.0 | 12.0 | 25 | |
| Residential sector | ||||||
| Urban | 13.6 | 34.4 | 38.5 | 13.2 | 1,249 | 0.000 |
| Rural | 16.6 | 38.1 | 35.2 | 10.0 | 5,972 | |
| Estate | 28.4 | 45.1 | 21.9 | 4.4 | 492 | |
| Province | ||||||
| Western | 14.5 | 37.8 | 36.5 | 11.1 | 1,455 | 0.000 |
| Central | 20.2 | 38.8 | 32.7 | 8.3 | 996 | |
| Southern | 16.4 | 38.1 | 34.3 | 11.0 | 923 | |
| Northern | 12.0 | 34.4 | 40.3 | 13.1 | 905 | |
| Eastern | 17.0 | 37.5 | 35.0 | 10.3 | 857 | |
| North-Western | 17.1 | 34.9 | 35.7 | 12.1 | 832 | |
| North Central | 14.3 | 42.4 | 33.2 | 10.0 | 530 | |
| Uva | 18.7 | 41.0 | 35.1 | 4.9 | 543 | |
| Sabaragamuwa | 24.1 | 39.7 | 27.9 | 8.1 | 672 | |
Data source: Sri Lanka Demographic and Health Survey 2016.
*P<0.05; **p<0.01; ***p<0.001.
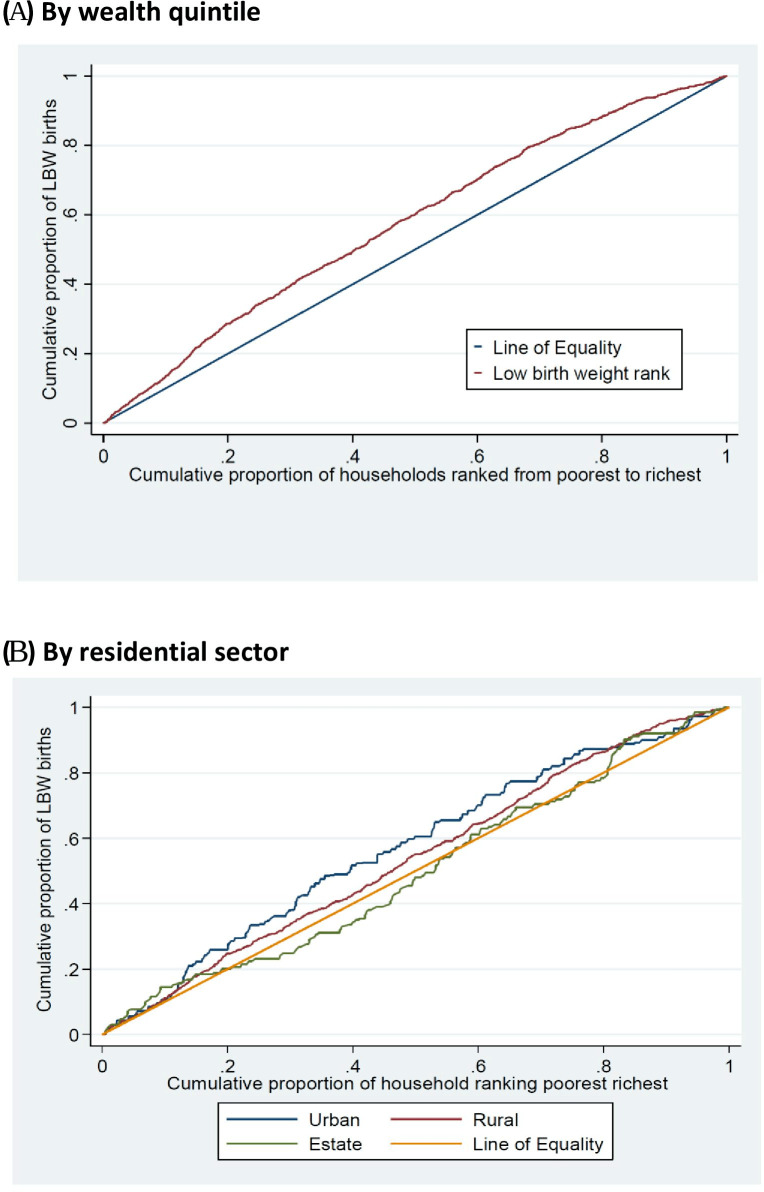
The socioeconomic differentials are further illustrated in the concentration curves (figure 2A, B). A concentration index ranges in value between −1 and +1. Negative values indicate that the variable is concentrated in poor households, a value of zero indicates there is no inequality and positive values indicate that the variable is concentrated in the richest households. The concentration curve is a graphical exploration of the concentration index. If the concentration curve lies on the diagonal 45° line, it shows perfect equality; when it lies below the line, the outcome is more concentrated among the higher SES (socioeconomic status) individuals of the population; if it lies above the 45° line, the outcome is more concentrated among the poor SES individuals in the population.26
Figure 2.
(A) Concentration curve showing the cumulative proportion of low birth weight (LBW) by wealth quintiles. (B) Concentration curves showing the cumulative proportion of LBW by residential sector.
The results for LBW show a concentration index of −0.13 (95% CI −0.15 to 0.10), suggesting that LBW is concentrated among the poorer households (figure 2A). The curve shows that, for example, the poorest 20% of households have about 30% of LBW babies, whereas the richest 20% of households have only about 10% of LBW babies. We graphed concentration curves by residential sector (figure 2B). The concentration curves for all sectors lie above the equality line, which suggests that LBW outcomes were higher among children in poorer households. The results show that that inequality within each sector is less than overall inequality and that, in particular, there is equality of LBW outcomes within the estate sector. This may be because the estate sector consists very largely of poor households.
Regression analysis
Table 2 shows the results of fixed effects logistic regression models with LBW as the outcome. In model 1, we included only maternal depletion variables. Mothers with a low BMI were more likely to have an LBW baby than those with normal BMI levels (adjusted OR (AOR) 1.76, 95% CI 1.41 to 2.20). There is a strong inverse association between maternal height and LBW outcome. Mothers who did not consume iron or folic acid (AOR 1.48, 95% CI 1.02 to 2.14) and those with a female birth (AOR 1.39, 95% CI 1.19 to 1.63) were more likely to have an LBW baby than those who did not consume iron or folic acid or who has a male baby, respectively. Babies born 24–47 months after their immediately elder sibling were at lower risk of having LBW compared with the first-born child (AOR 0.58, 95% CI 0.46 to 0.73).
Table 2.
Results of the fixed effects multiple logistic regression
| Variable and category | Model 1 | Model 2 | Model 3 |
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Maternal body mass index | |||
| Under 18.5 | 1.76 (1.41 to 2.20)*** | 1.62 (1.29 to 2.03)*** | 1.63 (1.31 to 2.03)*** |
| 18.5–24.9 | Ref | Ref | Ref |
| 25.0–29.9 | 0.78 (0.65 to 0.95)* | 0.83 (0.69 to 1.00) | 0.85 (0.71 to 1.03) |
| 30.0 or more | 0.73 (0.55 to 0.96)* | 0.80 (0.60 to 1.06) | 0.74 (0.56 to 0.98)* |
| Maternal height | |||
| Short (up to 145.0 cm) | 1.91 (1.47 to 2.74)*** | 1.76 (1.36 to 2.29)*** | 1.74 (1.35 to 2.24)*** |
| Average (145.1–155.0 cm) | Ref | Ref | Ref |
| Tall (155.1 cm and over) | 0.55 (0.46 to 0.66)*** | 0.58 (0.49 to 0.70) | 0.58 (0.49 to 0.69)*** |
| Preceding birth interval | |||
| First birth | Ref | Ref | Ref |
| Under 24 months | 0.68 (0.47 to 0.98)* | 0.67 (0.46 to 0.96)* | 0.73 (0.52 to 1.04) |
| 24–47 months | 0.58 (0.46 to 0.73)*** | 0.56 (0.44 to 0.70)*** | 0.59 (0.48 to 0.73)*** |
| 48–59 months | 0.77 (0.59 to 1.08) | 0.73 (0.56 to 0.96)* | 0.77 (0.59 to 0.99)* |
| 60 months or more | 0.92 (0.76 to 1.18) | 0.85 (0.70 to 1.04) | 0.87 (0.72 to 1.05) |
| Taken iron and folic acid supplements | |||
| Received and consumed | Ref | Ref | |
| Not received and consumed | 1.48 (1.02 to 2.14)* | 1.43 (0.98 to 2.08) | |
| Antenatal care visits | |||
| Fewer than three times | 1.30 (0.79 to 2.15) | 1.43 (0.86 to 2.37) | 1.25 (0.81 to 1.93) |
| 3–5 times | 1.73 (1.09 to 2.73)* | 1.78 (1.11 to 2.85)* | 1.75 (1.09 to 2.81)* |
| 6–10 times | 1.13 (0.75 to 1.70) | 1.14 (0.75 to 1.72) | 1.15 (0.76 to 1.74) |
| 11 or more times | Ref | Ref | Ref |
| Sex of child | |||
| Male | Ref | Ref | Ref |
| Female | 1.39 (1.19 to 1.63)*** | 1.40 (1.20 to 1.64)*** | 1.45 (0.16 to 1.67)*** |
| Education level | |||
| No education and primary | Ref | Ref | |
| Secondary and passed General Certificate of Education (GCE) O-level | 0.75 (0.55 to 1.03) | 0.80 (0.58 to 1.10) | |
| Passed GCE A-level | 0.58 (0.40 to 0.84)** | 0.63 (0.44 to 0.90)* | |
| Degree and above | 0.90 (0.57 to 1.44) | 0.92 (0.58 to 1.46) | |
| Wealth index quintile | |||
| Poorest | Ref | Ref | |
| Poor | 0.82 (0.65 to 1.04) | 0.82 (0.65 to 1.03) | |
| Middle | 0.81 (0.64 to 1.02) | 0.84 (0.66 to 1.07) | |
| Rich | 0.73 (0.56 to 0.94)* | 0.74 (0.58 to 0.96)* | |
| Richest | 0.50 (0.36 to 0.69)*** | 0.54 (0.40 to 0.73)*** | |
| Ethnicity | |||
| Sinhala | Ref | Ref | |
| Sri Lankan Tamil | 0.85 (0.68 to 1.05) | 1.03 (0.74 to 1.43) | |
| Indian Tamil | 1.48 (1.03 to 2.13)* | 1.70 (1.02 to 2.83)* | |
| Muslims | 0.82 (0.61 to 1.11) | 0.86 (0.63 to 1.18) | |
| Burgher and Malay | 0.54 (0.16 to 1.77) | 0.43 (0.13 to 1.45) | |
| Residential sector | |||
| Urban | Ref | ||
| Rural | 0.97 (0.77 to 1.23) | ||
| Estate | 1.06 (0.66 to 1.68) | ||
| Province | |||
| Western | Ref | ||
| Central | 0.99 (0.74 to 1.32) | ||
| Southern | 1.05 (0.78 to 1.41) | ||
| Northern | 0.60 (0.38 to 0.94)* | ||
| Eastern | 1.06 (0.76 to 1.47) | ||
| North-Western | 1.16 (0.89 to 1.51) | ||
| North Central | 0.93 (0.64 to 1.24) | ||
| Uva | 0.89 (0.63 to 1.24) | ||
| Sabaragamuwa | 1.42 (1.07 to 1.87)* |
***P<0.001; **p<0.01; *p<0.05; Ref: reference category.
Model 2 added socioeconomic variables. Although the ORs for the maternal depletion variables in models 1 and 2 cannot properly be compared because it is problematic to compare ORs across models with different independent variables in the sample as it reflects the degree of unobserved heterogeneity in the model, there was little or no change in the effect of the maternal depletion variables (table 2). Household wealth was a strong predictor of LBW outcome: babies born in the highest household wealth quintile had half the odds of LBW compared with those in the lowest quintile (AOR 0.50, 95% CI 0.36 to 0.69). Maternal education level was less important, although mothers with higher levels of education tended to have reduced odds of a LBW baby. There were some differences by ethnicity: Burgher and Malay mothers were less likely to have LBW babies, whereas the Indian Tamils were more likely to have LBW outcomes compared with Sinhala mothers (AOR 1.48, 95% CI 1.03 to 2.13).
The final model included the geographical variables residential sector and province in addition to maternal and socioeconomic factors (table 2). We removed the iron and folic acid variable from the model, as it was no longer significant in model 2 (though we note that mothers who had not received and consumed iron and folic acid had a higher risk of LBW babies than mothers who had received and consumed both these supplements). Both maternal and socioeconomic factors remain important predictors of LBW; however, residential sector was less important. The effect of Indian Tamil ethnicity remained significant with a higher odds (AOR 1.70, 95% CI 1.02 to 2.83). Similarly, mothers who lived in Sabaragamuwa province had higher odds of LBW than those from the Western province (AOR 1.42, 95% CI 1.07 to 1.87). LBW babies were more common among Indian Tamils than among other ethnic groups. The Indian Tamils lived and worked mostly at tea plantation estates in Sabaragamuwa province.
Random effects
Our data are hierarchical, in that some quantities are specific to children, whereas others are defined and measured at the mother level and yet others, such as provinces, are defined at a broader community level. It might be that characteristics of mothers and/or communities lead to the risk of LBW among children born to the same mother, or born within the same community, being correlated. Some of these characteristics can be observed (eg, mother’s BMI) but others (eg, genetic factors) cannot be observed. To assess the magnitude of these correlation effects, we estimated a model of LBW with no covariates but three variance parameters at the child level, the mother level and the community level. We found very little correlation between the risk of LBW for babies within the same community but substantial correlation between the risk of LBW for children of the same mother. More than 60% of the variance in LBW is the result of variation between mothers. This suggests that any community-level effects were those deriving from the characteristics of mothers living in the same community.
To take account of this mother-level variation, we re-estimated model 3 described previously adding a random effect at the mother level. The results are shown in table 3. The effect of the covariates is similar to that in the comparable fixed effects model, though in some cases (eg, maternal height) their impact is amplified.
Table 3.
Results of the two-level random intercept logistic regression model
| Variable and category | Adjusted OR (95% CI) |
| Maternal body mass index | |
| Under 18.5 | 2.14 (1.48 to 3.09)*** |
| 18.5–24.9 | Ref |
| 25.0–29.9 | 0.71 (0.54 to 0.94)* |
| 30.0 or more | 0.60 (0.39 to 0.91)* |
| Maternal height | |
| Short (up to 145.0 cm) | 2.48 (1.60 to 3.83)*** |
| Average (145.1–155.0 cm) | Ref |
| Tall (155.1 cm and over) | 0.44 (0.32 to 0.57)*** |
| Antenatal care visits | |
| Fewer than three times | 1.65 (0.84 to 3.24) |
| 3–5 times | 2.79 (1.35 to 5.30)** |
| 6–10 times | 1.41 (0.75 to 2.64) |
| 11 times or more | Ref |
| Sex of child | |
| Male | Ref |
| Female | 1.55 (1.24 to 1.95)*** |
| Preceding birth interval | |
| First birth | Ref |
| Under 24 months | 0.55 (0.32 to 0.92)* |
| 24–47 months | 0.46 (0.33 to 0.63)*** |
| 48–59 months | 0.61 (0.40 to 0.90)* |
| 60 months or more | 0.74 (0.55 to 0.98)* |
| Education level | |
| No education and primary | Ref |
| Secondary and passed General Certificate of Education (GCE) O-level | 0.59 (0.36 to 0.98)* |
| Passed GCE A-level | 0.38 (0.21 to 0.70)** |
| Degree and above | 0.76 (0.36 to 1.59) |
| Wealth index quintile | |
| Lowest | Ref |
| Second | 0.77 (0.54 to 1.08) |
| Middle | 0.81 (0.55 to 1.17) |
| Fourth | 0.63 (0.41 to 0.93)* |
| Highest | 0.43 (0.25 to 0.70)** |
| Ethnicity | |
| Sinhala | Ref |
| Sri Lankan Tamil | 0.91 (0.60 to 1.38) |
| Indian Tamil | 2.13 (1.12 to 4.06)* |
| Muslims | 0.71 (0.46 to 1.08) |
| Burgher and Malay | 0.72 (0.08 to 5.90) |
| Province | |
| Western | Ref |
| Central | 1.25 (0.81 to 1.91) |
| Southern | 1.02 (0.66 to 1.58) |
| Northern | 0.66 (0.37 to 1.17) |
| Eastern | 1.27 (0.78 to 2.06) |
| North-Western | 1.36 (0.88 to 2.11) |
| North Central | 0.90 (0.53 to 1.52) |
| Uva | 0.96 (0.55 to 1.63) |
| Sabaragamuwa | 1.82 (1.14 to 2.89)* |
| Mother-level variance (SE) | 2.40 (0.324)*** |
| Intracluster correlation coefficient | 0.63 |
| Log likelihood | −2,831.64 |
| Akaike information criterion | 5735.29 |
| Bayes information criterion | 5983.02 |
***P<0.001; **p<0.01; *p<0.05; Ref: reference category.
Discussion
Our findings confirm the research hypothesis of a clear socioeconomic gradient in the risk of LBW in Sri Lanka. Mothers from poor households, especially those from Indian Tamil communities living in the estate sector, have increased risk of LBW babies. The persistence of LBW among this group might be attributed to genetic factors deriving from the selected group of marginalised communities of Indian Tamils who were originally brought to Sri Lanka to work in the tea plantations in the 19th century.20 There is a lack of research on genetic causes of LBW in Sri Lanka, and a more thorough investigation of the genetic factors associated with LBW is needed.
The foregoing analyses of SLDHS data confirms the prominent role of maternal factors in determining LBW outcomes. Maternal depletion factors such as maternal BMI and height and preceding birth interval were more influential in determining LBW than socioeconomic and geographical factors. Multilevel analysis revealed that more than 60% of the variation in LBW occurred at the maternal level. Once this had been accounted for, there was very little additional variation (6% of the total) at the community level. Birth weights of children born to the same mother were highly correlated, partly reflecting the impact of unmeasured factors such as genetic and environmental factors that were not taken into account in the fixed effect model.
Our findings highlight the need for nutrition interventions targeting pregnant women from the Indian Tamil ethnicity and those living in economically deprived households. The government in Sri Lanka has taken several measures to improve the nutritional status of pregnant mothers, particularly the free distribution of Thriposha targeted at poor families. However, the effect of receiving and consuming Thriposha was not significant, consistent with findings from previous research.20 This might be due to the fact that Thriposha fulfils only 400 kcal of energy needs,27 which is not adequate for undernourished mothers28 or our inability to identify true recipients of it. The present study suggests revisiting the effectiveness of Thriposha programme in addressing the nutritional needs of mothers. The other existing poverty alleviation programme in Sri Lanka is Samurdhi (prosperity), which was launched in 1994. This also only provides a modest quantity of monetary support (only 500–1000 rupees) (around US$2.75–5.5) and does not always target the right beneficiaries.29 30
This study showed that increasing the frequency of antenatal care visits tends to reduce the risk of LBW outcome. Antenatal clinics provide comprehensive health promotion and pregnancy care services for mothers, such as dietary advice including micronutrient and Thriposha supplementation, methods of newborn care, monitoring of the fetus, examination of maternal biomarkers and haemoglobin.15–17 Therefore, it is vital to expand the services and coverage targeting vulnerable women settled in the estate sector.
LBW is concentrated among poor people, especially within the estate sector. Hence, to be more effective in reducing the prevalence of LBW, the Samurdhi programme should be expanded to target the poorest mothers in the estate sector. Since the maternal level is more influential in determining LBW in the context of Sri Lanka, policies should be more centred on improving maternal factors including nutritional level.
Strengths and limitations of this study
The present research is based on cross-sectional data at the national level, which has been collected for the first time after the war and civil conflict in Sri Lanka. The analysis is based on data from health records, which are fairly accurate in Sri Lanka where institutional birth is universal. However, previous studies show that birth weight data may be biased due to rounding errors or other errors related to weighing instruments even in hospital settings.31 32 SLDHS has several limitations. There are no data on genetic factors as well as on nutrition/dietary intake before, during and after pregnancy. However, maternal anthropometric data offer useful proxies to assess the relationship between maternal nutritional status and LBW outcomes. SLDHS has also no data on gestational weight gain and prepregnancy weight: the present study used height and weight data measures at the time of the survey to calculate BMI values. However, maternal weight before and after pregnancy may differ considerably. Therefore, it is recommended that future studies consider both anthropometric measures and pregestational BMI to examine if there is a relationship with birth weight.
Conclusion
Our study concludes that lower socioeconomic status mothers, particularly Indian Tamil mothers have higher LBW, and it differs substantially from other groups. Maternal factors such as maternal BMI and height and preceding birth interval along with antenatal care visits have more influence in determining LBW outcome. Socioeconomic and geographic factors such as maternal education, wealth and residential sector are also important determinants of LBW outcomes in Sri Lanka. Public health nutrition policies and programme interventions should address these key factors to reduce the overall burden of LBW, with a focus on the marginalised Indian Tamil mothers and those with lower socioeconomic status.
Supplementary Material
Acknowledgments
The authors wish to thank the Department of Census and Statistics in Sri Lanka for granting permission to access the DHS data.
Footnotes
Contributors: All authors have substantially contributed to this manuscript. GA designed, prepared the dataset and conducted the statistical analysis under the supervision of SP and AH. GA prepared the initial draft of the paper. SP and AH revised the paper for intellectual content and contributed to preparing the final draft of the paper for submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Ethical approval was granted from the Ethics Research and Governance unit of the University of Southampton (reference: 42179).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party but are not publicly available. The data are not publicly available but can be obtained through written request to the Department of Census and Statistics in Sri Lanka.
References
- 1.Department of Census and Statistics Demographic and Health Survey 2016. Colombo: Department of Census and Statistics, Sri Lanka, 2017. [Google Scholar]
- 2.World Bank Sri Lanka: attaining the millennium development goals in Sri Lanka. Washington DC: World Bank Human Development Unit, South Asia Region, 2005. https://openknowledge.worldbank.org/bitstream/handle/10986/8635/321340LK.pdf?sequence=1&isAllowed=y [Google Scholar]
- 3.World Health Organization Paradox of Healthcare in Sri Lanka: a Snapshot of the Last Decade from a Partnership of Sixty Years. Colombo: World Health Organization, 2014.. Available: https://apps.who.int/iris/bitstream/handle/10665/255195/ParadoxofhealthcareinSri%20Lanka.pdf?sequence=1&isAllowed=y [Accessed 10 Jun 2019].
- 4.World Health Organization Global Nutrition Targets 2025: Low Birth Weight Policy Brief. Geneva: World Health Organization, 2014. Available: https://www.who.int/nutrition/topics/globaltargets_lowbirthweight_policybrief.pdf[Accessed 10 Apr 2019].
- 5.Mothers BDJP. Babies and Health in Later Life. 2 edn Edinburgh: Churchill Livingstone, 1998. [Google Scholar]
- 6.Hack M, Klein NK, Taylor HG. Long-Term developmental outcomes of low birth weight infants. Future Child 1995;5:176–96. 10.2307/1602514 [DOI] [PubMed] [Google Scholar]
- 7.Khan A, Nasrullah FD, Jaleel R. Frequency and risk factors of low birth weight in term pregnancy. Pak J Med Sci 2016;32:138–42. 10.12669/pjms.321.8120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 9.Fall CHD. Fetal programming and the risk of noncommunicable disease. Indian J Pediatr 2013;80 Suppl 1:13–20. 10.1007/s12098-012-0834-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivasankaran S, Thankappan KR. Prevention of non-communicable diseases requires a life course approach: a case study from Kerala. Indian J Med Res 2013;137:874–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Mahumud RA, Sultana M, Sarker AR. Distribution and determinants of low birth weight in developing countries. J Prev Med Public Health 2017;50:18–28. 10.3961/jpmph.16.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leon DA, Moser KA. Low birth weight persists in South Asian babies born in England and Wales regardless of maternal country of birth. slow pace of acculturation, physiological constraint or both? analysis of routine data. J Epidemiol Community Health 2012;66:544–51. 10.1136/jech.2010.112516 [DOI] [PubMed] [Google Scholar]
- 13.Bian Y, Zhang Z, Liu Q, et al. Maternal risk factors for low birth weight for term births in a developed region in China: a hospital-based study of 55,633 pregnancies. J Biomed Res 2013;27:14–22. 10.7555/JBR.27.20120046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlui M, Azahar N, Oche OM, et al. Risk factors for low birth weight in Nigeria: evidence from the 2013 Nigeria demographic and health survey. Glob Health Action 2016;9:28822. 10.3402/gha.v9.28822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kader M, Perera NKPP. Socio-Economic and nutritional determinants of low birth weight in India. N Am J Med Sci 2014;6:302–8. 10.4103/1947-2714.136902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health Nutrition and Indigenous Medicine Annual Health Bulletin Sri Lanka. Colombo: Ministry of Health, Nutrition and Indigenous Medicine, 2015. [Google Scholar]
- 17.Rajapaksa LC, Arambepola C, Gunawardena N. Nutritional status in Sri Lanka, determinants and interventions: a desk review. Colombo: UNICEF, 2011. http://files.unicef.org/srilanka/2012_SL_Nutri_Desk_review.pdf [Google Scholar]
- 18.Perera PJ, Ranathunga N, Fernando MP, et al. Growth parameters at birth of babies born in Gampaha district, Sri Lanka and factors influencing them. WHO South East Asia J Public Health 2013;2:57–62. 10.4103/2224-3151.115845 [DOI] [PubMed] [Google Scholar]
- 19.Jayawardena P. Underlying causes of child and maternal nutrition in the estate sector of Sri Lanka. J South Asian Stud 2014;2:241–55. [Google Scholar]
- 20.Anuranga C, Wickramasinghe R, Rannan-Eliya RP, et al. Trends, inequalities and determinants of low birth weight in Sri Lanka. Ceylon Med J 2012;57:61–9. 10.4038/cmj.v57i2.4429 [DOI] [PubMed] [Google Scholar]
- 21.Department of Census and Statistics Population and housing census final report. Colombo: Department of Census and Statistics, 2012. http://www.statistics.gov.lk/PopHouSat/CPH2011/Pages/Activities/Reports/FinalReport/FinalReport.pdf [Google Scholar]
- 22.World Health Organization WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization, 2006. [Google Scholar]
- 23.Winkvist A, Rasmussen KM, Habicht JP. A new definition of maternal depletion syndrome. Am J Public Health 1992;82:691–4. 10.2105/AJPH.82.5.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King JC. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J Nutr 2003;133:1732S–6. 10.1093/jn/133.5.1732S [DOI] [PubMed] [Google Scholar]
- 25.Shell-Duncan B, Yung SA. The maternal depletion transition in northern Kenya: the effects of settlement, development and disparity. Soc Sci Med 2004;58:2485–98. 10.1016/j.socscimed.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell O, van Doorslaer E, Wagstaff A, et al. Analyzing Health Equity Using Household Survey Data. Washington, DC, 2008. [Google Scholar]
- 27.Sri Lanka Thriposha Limited Nutrient content, 2016. Available: http://www.thriposha.lk/thriposha-production/nutrient-content/ [Accessed 10 Oct 2019].
- 28.Rannan-Eliya R. Understanding the paradox of Undernutrtion in Sri Lanka. Paper presented at the International Conference on Maternal and Child Nutrition; 23-24 November, Colombo, 2015. [Google Scholar]
- 29.Damayanthi MKN, Champika PAJ. An Evaluation of Samurdhi Banks in Poverty Alleviation. Colombo: Hector Kobbekaduwa Agrarian Research and Training Institiute, 2014. http://www.harti.gov.lk/images/download/reasearch_report/new1/165.pdf [Google Scholar]
- 30.Center for Public Impact The Samurdhi programme in Sri Lanka, 2019. Available: https://www.centreforpublicimpact.org/case-study/samurdhi-programme-sri-lanka/ [Accessed 23 Oct 2019].
- 31.Channon AAR, Padmadas SS, McDonald JW. Measuring birth weight in developing countries: does the method of reporting in retrospective surveys matter? Matern Child Health J 2011;15:12–18. 10.1007/s10995-009-0553-3 [DOI] [PubMed] [Google Scholar]
- 32.Blencowe H, Krasevec J, de Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2019;7:e849–60. 10.1016/S2214-109X(18)30565-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.