Abstract.
Prevention of malaria in travelers to endemic countries is one of the complex challenges of travel medicine. Israel has a widespread culture of travel to developing countries, but information regarding malaria prevention is limited so far. Our study, conducted in Sheba Medical Center, Israel, during the years 2008–2018 examined malaria chemoprophylaxis usage and malaria cases in a large group of Israeli travelers returning from endemic countries with any medical complaint. Data were collected regarding travel destinations, conditions, duration of stay, and pretravel consultation. Altogether, 4,708 travelers were included in our study. Travel destinations included Asia (51%), Latin America (31%), and sub-Saharan Africa (SSA) (17%). Median travel duration was 26 days. Only 11.9% reported taking malaria chemoprophylaxis. Of the travelers to SSA, 41.3% took prophylaxis as opposed to only 6% outside of Africa. During the study years, 136 cases of malaria were diagnosed; among them, 82 (60%) were infected with Plasmodium falciparum, of whom all but two were from SSA and none adhered to prophylaxis. Malaria chemoprophylaxis usage was found to be negligible in travel to many countries still considered endemic. Higher prophylaxis usage was found among travelers to SSA, but numbers are still lower than recommended. The low number of malaria cases seen in destinations outside SSA, as documented in our cohort, is likely to represent travel to low risk areas and not high prophylaxis usage. We urge re-evaluation of current CDC and Israeli guidelines which still recommend using chemoprophylaxis in many low-risk countries, as focus on high-risk countries may increase adherence.
INTRODUCTION
Malaria is a potentially life-threatening disease and remains endemic in more than 90 tropical and subtropical countries. Every year, more than 125 million Western travelers visit malaria-endemic countries,1 including more than 160,000 Israelis.2 These nonimmune travelers are at a high risk of severe disease. Despite the declining rate of malaria globally,2 incident rates in travelers are increasing,3 with more than 10,000 cases of malaria reported annually in returning travelers.1 In Israel, where malaria is a notifiable disease, 50–100 cases of imported cases are diagnosed every year.4,5
Prevention of malaria in travelers to endemic countries is a complex challenge for travel medicine providers and numerous controversies on this subject among the professional community remain. The limited data regarding risk of malaria in travelers, which is different from the risk to local populations,6–8 as well as the inadequate and variable information about the prevalence of chemoprophylaxis side effects7,9–15 limit the ability to determine the risk/benefit ratio for travelers. The lack of optimal solutions for long-term travelers, who overall have a higher risk due to prolonged cumulative exposure time and low adherence to chemoprophylaxis,7,9,16–18 also presents a problem. Another challenge is the ineffectiveness of current chemoprophylaxis in prevention of late Plasmodium vivax and Plasmodium ovale infection.9,19–21
Lack of data and the previously discussed dilemmas lead to significant differences of recommendations between guidelines of various organizations regarding malaria chemoprophylaxis. The Israeli Ministry of Health, and the CDC have recommendations for malaria prevention calling for chemoprophylaxis in travel to many low-risk countries,3,22 recommendations which are no longer indorsed by many European authorities.23
Several studies found that adherence to malaria chemoprophylaxis among travelers is low. Most studies were conducted on small groups of travelers or specific populations and are not necessarily applicable to the general travel population. A series of international airport studies from 2004, which included 3,742 travelers to malaria-endemic countries,24–26 showed that between 40% and 84% of travelers to high-risk destinations carried chemoprophylaxis, compared with only 7–26% to travelers to low-risk destinations. These studies most likely overestimated adherence because they reported travelers carrying chemoprophylaxis on departure and not real compliance. A German study conducted during the same period, which included 1,001 returning travelers from Kenya, Senegal, and Thailand,27 showed 66% adherence to prophylaxis. Other small studies performed in pretravel clinics,15,28,29 a population that has a higher adherence in general, have shown 44–84% usage of chemoprophylaxis. Another study that examined long-term travelers reported only 25% adherence to chemoprophylaxis over time among British expatriates.17 To this date, only one Israeli study examined adherence in a group of 98 travelers who attended pretravel consultations in two travel clinics in Haifa.30 Only 38.6% reported they adhered to chemoprophylaxis after returning from travel.
Long-term travelers, business travelers, backpackers, immigrants visiting friends and family, and travelers to low-risk destinations were found to have lower adherence to malaria chemoprophylaxis.7,30–32 Reasons for nonadherence were addressed in several previous studies.15,17,24–28 Travelers stated that their decision not to take chemoprophylaxis was influenced by fear of potential side effects, previous side effects, personal perception of low risk of malaria or perception of the medication as ineffective in preventing the disease, conflicting recommendations from different medical sources, negative recommendation from other travelers and locals, perception of malaria as a relatively mild and easily treated disease, and high costs of the medications.
Israel has a widespread culture of travel to developing countries and a unique profile of travelers,2,33 including a large population of backpackers. Little is known about the use of malaria chemoprophylaxis or about the causes and risk factors for nonadherence among Israeli travelers. Our study examined the usage of malaria prophylaxis in a large and diverse group of travelers from all over Israel who returned from endemic countries.
METHODS
We conducted a prospective observational study in Sheba Medical Center, Israel, during the years 2008–2018. Patients who were examined in the Institute of Tropical Medicine for any complaint, on return from travel to developing countries, were eligible for the study.
We included patients returning from malaria-endemic countries, as defined by the CDC at the time of travel.3 We excluded patients who traveled to non-endemic countries, those who did not return from travel (and were examined because of locally contracted diseases), as well as foreign tourists or recent immigrants from endemic countries and patients who did not offer information regarding travel destination or usage of malaria prophylaxis.
Travelers included in the study were from all over Israel and attended different pretravel clinics before their trip.
For each patient, during the first visit on return from endemic countries, data were collected by the examining physician, eliciting the following independent variables: age, gender, travel destinations (all countries visited), trip duration, type of trip (business/expatriates, organized trip, high-standard non-organized trip, backpacking, and visiting friends and family), pretravel consultation, including location, and pretravel recommendation for malaria chemoprophylaxis (to note—data regarding recommendation for malaria prophylaxis were only recorded from 2012 to 2018). Dependent variables collected were use of malaria chemoprophylaxis during the trip, choice of medication, reported side effects, premature cessation of medication, and reasons for noncompliance. Malaria cases among the patients were documented, and all the aforementioned information about each malaria case was recorded. All data were computerized during the visit.
The study was approved by Sheba Medical Center Ethical Committee.
Statistical analysis.
The primary end point was self-reported usage of malaria chemoprophylaxis during travel. Secondary end points were malaria diagnosis, reasons for nonadherence to prophylaxis, and preference of specific malaria treatment when taken.
Categorical factors were summarized by frequency tables and continuous variables by their mean, median, and SD. Percentage of chemoprophylaxis use and the aforementioned secondary end points were compared between independent variables using chi-squared tests or Fisher’s exact tests (when the assumption of the parametric chi-squared test was not met) and continuous variables using Student’s t test (or Wilcoxon test). Malaria cases were analyzed accordingly between the aforementioned independent variables. We computed two-tailed P-values, where values of P < 0.05 were considered statistically significant. Statistical analyses were performed using the SAS software package, version 9.4 (Statistical Analysis System, Cary, NC).
RESULTS
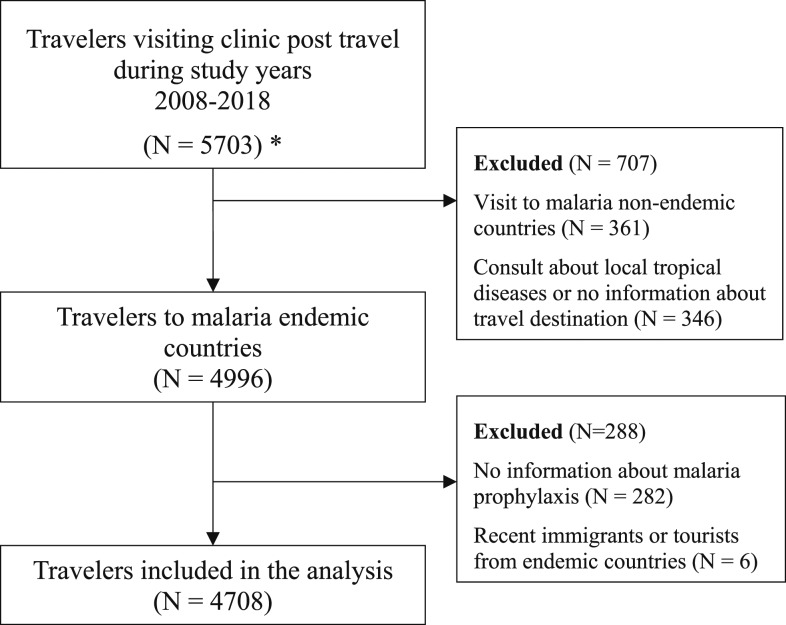
A total of 4,708 travelers were included in our study. Figure 1 shows the enrollment flow chart for the study. The general characteristics of our study population are shown in Table 1.
Figure 1.
Enrollment flow chart. * Patients who visited the clinic multiple times—only the first visit was counted.
Table 1.
General characteristics of study population
| Gender (N = 4,708) | |
| Male: female (%) | 53.1: 46.9 |
| Age (years) (N = 4,705) | |
| Mean | 36.8 ± 12.7 |
| Median | 33 |
| Range | 1–90 |
| Travel duration (days) (N = 4,687) | |
| Mean | 81 ± 175 |
| Median | 26 |
| Range | 1–3,356 |
| Purpose of travel (N = 4,643) | |
| High-standard trips | 1,955 (42.1%) |
| Backpackers | 1,816 (39.1%) |
| Business/expatriates | 619 (13.3%) |
| Organized trips | 161 (3.5%) |
| Visiting friends and relatives | 35 (0.8%) |
| Other | 57 (1.2%) |
| Travel destinations* (N = 4,708) | |
| Asia | 2,394 (50.9%) |
| Central America | 978 (20.8%) |
| South America | 712 (15.1%) |
| Africa | 845 (18%) |
| Pacific Islands† | 9 (0.2%) |
* Two hundred twenty-four patients traveled to multiple continents.
† These travelers were not included in the by-continent data analysis because of their negligible number.
For 29% of travelers, this was their first trip to a developing country. Fifty-seven percent of travelers spent 1 month or less abroad, whereas 12% traveled for more than 6 months.
Business travelers comprised a distinct group of our cohort. They were mostly male (83%), significantly older than the general population (median 42 versus 33 years, P < 0.001), and 62% of them traveled to sub-Saharan Africa (SSA), as opposed to only 11% of others. In fact, 50% of travelers to SSA were business travelers or expatriates. Travelers on organized trips were much older (median 64 years) and were also found to visit Africa more than other groups (38%). Backpackers traveled for a much longer duration than others (median 91 days).
Thirty-three thousand one hundred and fifty-two (67%) travelers attended pretravel consultation before their trip. Higher numbers were seen among younger travelers (76% of travelers under 30 years old), travelers to Africa (84%), backpackers (86%, accounting for the higher numbers among young travelers), and those who traveled for longer than 2 months (91%).
Usage of malaria chemoprophylaxis.
Of all travelers to countries considered endemic by the CDC, only 11.9% (N = 558) took chemoprophylaxis for malaria. This refers to reported usage, regardless of whether the traveler visited pretravel medical consultation. Included in this number are 112 patients who took prophylaxis only in high-risk areas during their trip. We did not include another 107 patients who reported stopping prophylaxis prematurely. Table 2 shows the characteristics of travelers who took malaria chemoprophylaxis.
Table 2.
Travelers taking malaria chemoprophylaxis (out of all travelers)
| Prophylaxis | No prophylaxis | P-value | |
|---|---|---|---|
| Total | 558 (11.9%) | 4,150 (88.1%) | |
| Gender (N = 4,708) | 558 | 4,150 | NS |
| Male: Female (%) | 12.1: 11.6 | 88.4: 87.9 | |
| Age (years) (N = 4,705) | 558 | 4,147 | |
| Mean (years) | 39 ± 15 | 36.5 ± 12 | 0.019 |
| Median (years) | 33 | 33 | |
| Travel duration* (N = 4,550) | 539 | 4,011 | |
| Mean (years) | 66 ± 79 | 58 ± 72 | NS |
| Median (years) | 30 | 24 | |
| Type of travel (N = 4,643) | 540 | 4,103 | |
| High-standard trips | 129 (7%) | 1,826 (93%) | |
| Backpackers | 259 (14%) | 1,557 (86%) | < 0.001 |
| Business/expatriates | 85 (14%) | 534 (86%) | |
| Organized trips | 50 (31%) | 111 (69%) | |
| Visiting friends and relatives | 6 (17%) | 29 (83%) |
NS = not significant.
* Not including 137 patients who spent more than 365 days abroad.
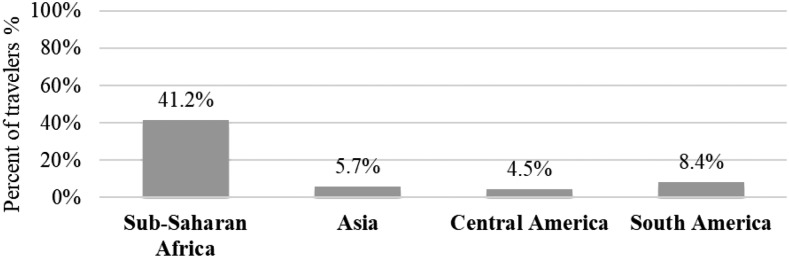
When analyzing data by travel destination (Figure 2), we found that 41.2% of travelers to SSA took chemoprophylaxis, when outside of Africa only 6% reported taking prophylaxis.
Figure 2.
Malaria chemoprophylaxis usage by travel destination.
Despite high attendance in pretravel clinics, backpackers did not report higher usage of chemoprophylaxis outside of SSA (8.2% only). In travel to SSA, the reported usage was 72%, which was significantly higher than other groups.
Fourteen percent of all business travelers and expatriates reported taking chemoprophylaxis. As mentioned earlier, most of this group traveled to Africa, and as such, their adherence is actually very low compared with other travelers to the same destination. Of those who traveled to SSA, we found a significant difference in the usage of chemoprophylaxis between different types of travel. Only 19% of business travelers to Africa took prophylaxis, compared with 72% of backpackers and 48% of high standard travelers. Only 7% of expatriates staying for a year or longer in endemic countries reported taking chemoprophylaxis, with those living in SSA reporting only 9% adherence.
Most patients who took chemoprophylaxis preferred atovaquone–proguanil (62%). Only 23% reported taking mefloquine. After 2013, the use of mefloquine was rare, and 83% of travelers used atovaquone–proguanil. Two events in 2013 probably influenced this prominent trend—the FDA black box warning issued for mefloquine, regarding risk of serious neurological and psychiatric side effects and the expiration of GlaxoSmithKline patent for atovaquone–proguanil, resulting in the reduction of its price.
Adherence to malaria chemoprophylaxis.
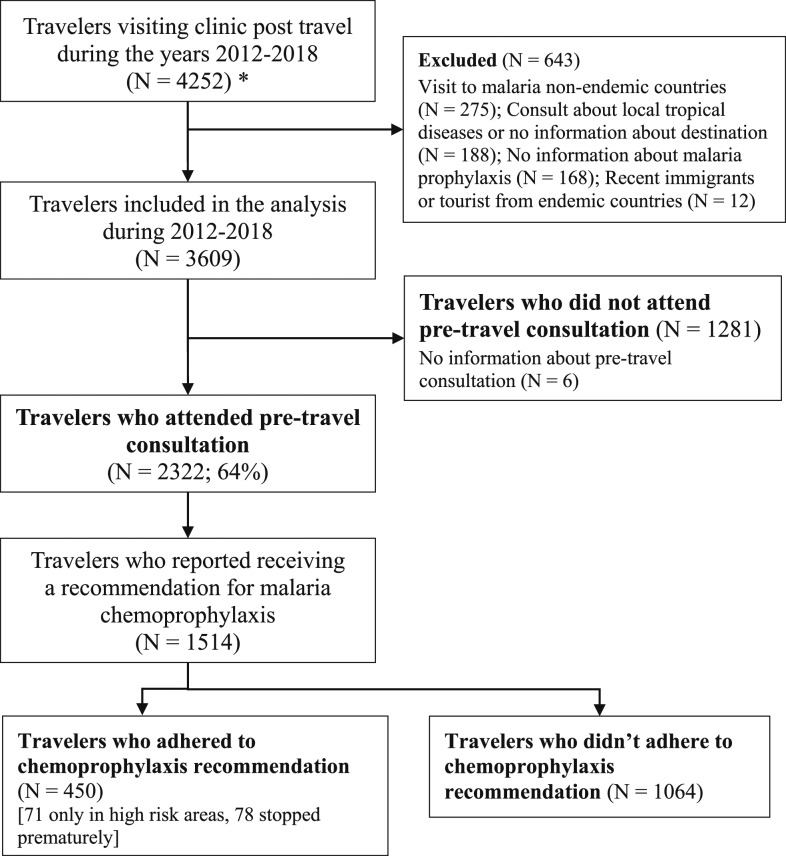
Data regarding pretravel clinic recommendations for chemoprophylaxis was only collected during the years 2012–2018 (see Figure 3). During this period, only 29.7% of travelers who visited a pretravel medical consultation and reported receiving a recommendation for malaria chemoprophylaxis adhered to the recommendation. Adherence was 61.2% in travel to SSA and only 16.7% outside of SSA.
Figure 3.
Adherence flow chart of years 2012–2018. * Patients who visited the clinic multiple times—only the first visit was counted.
Table 3 shows reasons for nonadherence as reported by those travelers who did not adhere to pretravel recommendations. Only 25% of travelers to SSA who did not take chemoprophylaxis reported they believed there was no need for the medication, whereas the majority, 51% feared potential side effects and 14% reported previous side effects.
Table 3.
Reasons for nonadherence to chemoprophylaxis
| (N = 1,064) (%) | |
|---|---|
| Believes there is no need to take malaria prophylaxis | 803 (75) |
| Concern of potential side effects | 244 (23) |
| Suffered side effects in the past | 40 (4) |
| The medicine is too expensive | 12 (1.1) |
| Believes medicine is not effective | 7 (0.7) |
Data on recommendation to take chemoprophylaxis was collected only from 2012 to 2018. During these years, 1,514 patients reported receiving a recommendation for malaria chemoprophylaxis. Of them, 1,064 (70.3%) did not adhere to the recommendation.
Malaria cases.
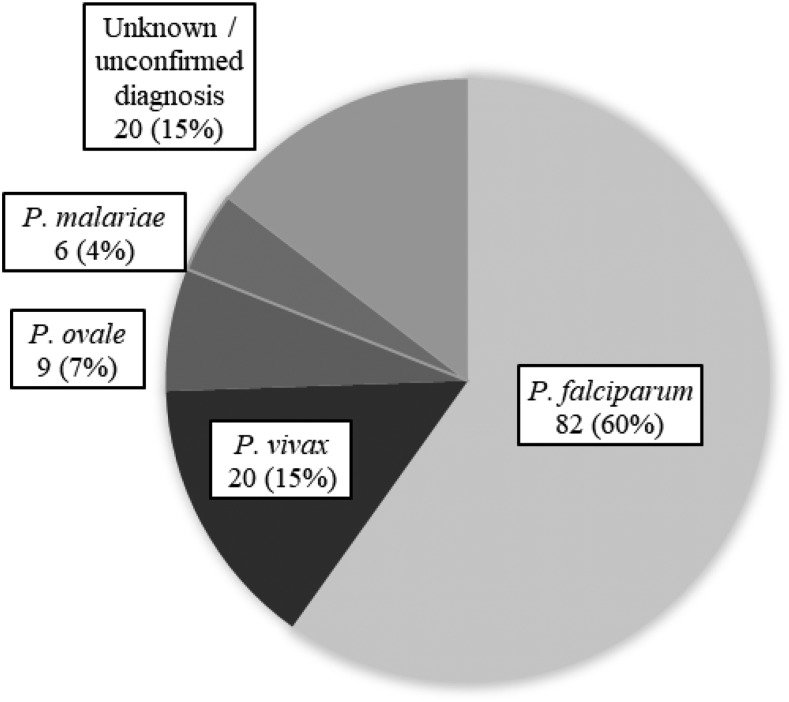
During the years 2008–2018,136 cases of malaria were diagnosed in the study population. One hundred and twenty-three (90%) of them were diagnosed in travelers returning from SSA, although only 18% of travelers in our cohort visited this continent. Another six (4%) were returning travelers from Asia, five (4%) from Latin America, and two other patients visited multiple continents. Figure 4 shows the prevalence of different malaria species diagnosed in our study population.
Figure 4.
Malaria cases by species.
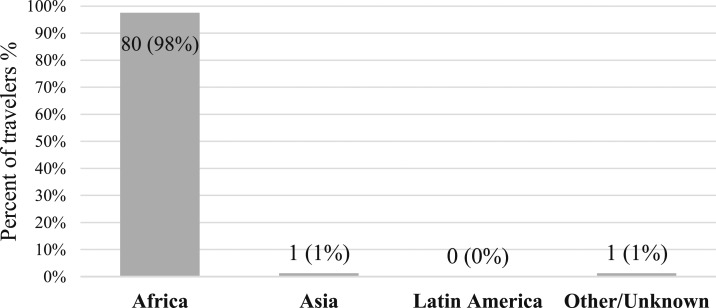
Of proven Plasmodium falciparum cases, 80 of 82 (98%) were diagnosed in travelers to SSA, as can be seen in Figure 5.
Figure 5.
Plasmodium falciparum cases by travel destination.
Of proven P. vivax cases, 11 (55%) were contracted in SSA, five (25%) in Asia, and four (20%) in Latin America. All Plasmodium malariae cases were diagnosed in travelers returning from West Africa.
Of all malaria cases in our study population, 104 (77%) were diagnosed in business travelers or expatriates, and another 22 (16%) in backpackers. Forty-four (32%) spent 1 month or less traveling, and only 23 (16%) spent more than 6 months abroad. This does not accurately show exposure time because for business travelers, we recorded only the length of the last trip, when many had multiple trips to risk destinations, and as such a much higher cumulative exposure time.
Only 14 (10%) of malaria cases in our clinic took malaria chemoprophylaxis as prescribed during their trip. None of the 82 P. falciparum cases in our cohort adhered to chemoprophylaxis, as opposed to P. vivax patients, of which eight (40%) reported taking chemoprophylaxis. Two (22%) of the P. ovale cases reported adherence to prophylaxis and only one P. malariae case used atovaquone–proguanil.
DISCUSSION
Our study was conducted on a large and diverse cohort of 4,708 returning travelers from all over Israel and demonstrates that most Israelis do not take malaria chemoprophylaxis while traveling to countries considered endemic. In travel to destinations outside of Africa, where both the CDC and the Israeli Ministry of Health still recommend malaria chemoprophylaxis,3,22 only 6% of Israeli travelers take medications. These numbers represent the actual chemoprophylaxis coverage in travelers, regardless of whether they received pretravel consultation and prophylaxis recommendation.
Previous studies show that the rate of malaria cases in travelers returning from South East Asia, the Indian subcontinent and Latin America6,8,34 is very low. The U.S. malaria surveillance35 also reports that 86% of all malaria cases and 96.3% of P. falciparum cases, with known region of acquisition, were contracted in Africa. Data collected from the Israeli Ministry of Health over a period of 17 years and compared the number of Israeli travelers with those of developing countries established by using the World Tourism Organization database showed an attack rate range of 51–6,683/100,000 travelers to SSA, compared with an attack rate of 2–15/100,000 in travel to Asia and Latin America.36
Accordingly, we found in our cohort, which includes approximately 20% of all malaria cases reported in Israel during the study years, a very small number of malaria cases contracted outside of SSA. This is despite the much larger volume of travel to Asia and Latin America, as reported in previous Israeli studies2,33,36 and reflected in our study. Because malaria chemoprophylaxis usage is shown here to be negligible in travel to destinations outside of Africa, it is reasonable to conclude that the low rate of malaria is due to actual low risk to travelers and not high prophylaxis coverage. The low risk of malaria raises the question whether we should continue recommending chemoprophylaxis to travelers outside of SSA (excluding some of the Pacific Islands who still show a high risk for travelers and were not included in our analysis because of small number of travelers).
In fact, the currently recommended prophylaxis mainly prevents P. falciparum malaria, which is the primary cause of severe disease and mortality and is not effective in preventing late P. vivax and P. ovale infection.6,18,19 Because 98% of all P. falciparum cases in our cohort were contracted in Africa (similar to numbers reported in previous studies19,31,35,36) and none of these travelers fully adhered to chemoprophylaxis, the question regarding the yield of chemoprophylaxis outside of SSA is further stressed.
Many European countries, including Germany, Italy, Austria, England, Switzerland, and others, have already adopted this view and issued guidelines23 limiting the recommendation for chemoprophylaxis only to high-risk areas, including SSA, some of the Pacific Islands, Surinam, Guyana, and French Guyana in South America. Moreover, only a few of those countries recommend chemoprophylaxis for travel to parts of India, Thailand, Cambodia, and Laos. In travel to other destinations with limited malaria risk, recommendations include personal protection measures only, and at times of emergency stand-by treatment. This differs significantly from the current CDC and Israeli Ministry of Health recommendations, which still recommend chemoprophylaxis to many low-risk Asian countries and most of South and Central America. We believe that this wide recommendation is unjustified by the low risk of malaria to travelers to those destinations as shown in our cohort and previous studies.
Adherence to chemoprophylaxis recommendations.
When looking at travelers who visited pretravel medical consultation and received a recommendation for malaria chemoprophylaxis, adherence is still very low.
Principal reasons for nonadherence to malaria chemoprophylaxis in our study were belief that there was no need for prophylaxis and concern about potential side effects. In high-risk destinations, fear of potential side effects and report of side effects when using chemoprophylaxis in the past were the leading causes, although still a considerable number stated medications were not needed. These concerns should be addressed during pretravel consultations to raise the still low adherence of chemoprophylaxis in high malaria-risk destinations.
Special risk groups.
A specific high-risk group identified in our study is business travelers and expatriates. Only 19% of business travelers to Africa used chemoprophylaxis and numbers were as low as 9% in expatriates living for a year or longer is SSA. Although comprising only 13.3% of our cohort and 50% of travelers to Africa, 77% of all malaria cases were diagnosed in this group. Only 5% of businessmen diagnosed with malaria reported adherence to prophylaxis, and none among the P. falciparum cases. The characteristics of this population and the profile of malaria morbidity in business travelers reported in our study were similar to those shown in a GeoSentinel analysis of returning business travelers from 29 countries, published in January 2018.37 As previously reported,7,24,31 we found that business and long-term travelers tend to disregard recommendations for malaria chemoprophylaxis and probably rely on treatment in case of disease. Being nonimmune with high risk of exposure, staying in locations with limited access to quality medical care and the problem of counterfeit drugs put them at risk for severe malaria. Rethinking is needed to address the problem of this specific high-risk group of travelers who account for the vast majority of imported malaria cases to Israel.
Backpackers also merit special consideration. Israel has a common culture of backpacking to developing countries, as can be seen by the large number of backpackers in our study, making up 39% of our cohort. Israeli backpackers are in general young travelers (many just after their mandatory army service), traveling for long durations of time, and usually in rudimentary conditions. Most Israeli backpackers tend to visit pretravel clinics before their trip, as shown also in previous Israeli studies.2,33 Despite this cultural phenomenon, this group does not show higher adherence to malaria prophylaxis outside of SSA.
Backpackers were the second most likely group to acquire malaria during their trips (N = 22, 16%), but out of a much larger numbers of travelers. As opposed to business travelers, most backpackers (64%) had non-falciparum malaria, and 36% of all cases reported adhering to chemoprophylaxis. However, here as well, all P. falciparum cases except one were acquired in Africa, and none of those reported full adherence to chemoprophylaxis. As current prophylaxis does not prevent late P. vivax and P. ovale malaria, it is reasonable to question whether it is justified to recommend chemoprophylaxis to backpackers outside of Africa, in spite of the long trip durations and higher risk conditions.
LIMITATIONS
Our study had a few limitations. First, data regarding the usage of malaria chemoprophylaxis and pretravel recommendation for prophylaxis were recorded according to the patients’ self-report only. As data were collected shortly after return from travel, the chance for recall bias is low.
Second, our cohort included travelers who turned to medical consultation because of travel-related illnesses on return from their trip. This population does not necessarily represent the general population of returning travelers. These travelers were examined in our clinic because of various medical conditions randomly acquired during their trip, such as acute gastrointestinal or skin conditions. Their demographic profile and the distribution of travel destinations were similar to a national survey of Israeli travelers to endemic countries.2 As such, there is no reason to assume that this group greatly differs from the general travelers’ population.
Most previous studies who examined adherence enrolled travelers attending pretravel clinics or outbound travelers at airports and did not assess their actual adherence post travel. The advantage of this cohort selection is the ability to report actual adherence in a large population of returning travelers, from all over Israel, including those who did not consult a travel clinic before their travel.
During the study, data were collected regarding countries visited during the trip and not areas within each country. Many countries have a non-uniform risk of malaria and travelers might have visited only lower risk areas within the country. Current CDC recommendations still advise using chemoprophylaxis even in many such lower risk areas (including urban areas in India and Cambodia). In addition, a large population out of our cohort (39%) were backpackers who spent a median of 91 days traveling and commonly travel to rural areas and not only to highly touristic destinations.
Israeli travelers have a unique profile, including characteristic travel itineraries and habits. There is no doubt that our conclusions are applicable mainly to Israeli travelers and not necessarily to other travelers.
The total number of imported malaria cases reported in non-endemic countries does not necessarily include all cases because long-term travelers are at times treated for febrile illness during their trip and continue traveling. As part of our study, returning travelers were questioned regarding febrile illness suffered abroad, and whether diagnosis of malaria was received from local health services. This method enabled us to receive a more accurate picture regarding malaria prevalence. On the other hand, 15% of malaria cases reported in our study were a presumed diagnosis, based on suggestive clinical presentation and a diagnosis made abroad. Because many endemic countries tend to over-diagnose malaria, some of these cases may not be truly malaria disease. Despite this limitation, even when looking only at malaria cases confirmed in Israel, we received the same results regarding the geographical distribution of the disease.
CONCLUSION
Our study demonstrates an overwhelming low adherence to chemoprophylaxis among Israeli travelers to destinations outside of SSA. Despite this, only a small number of malaria cases in our cohort were acquired in those areas. In addition, previous studies6,8,34,36 have shown that the risk of malaria in travelers outside of SSA is very low, including in many countries that are still considered endemic. Therefore, we urge the re-evaluation of current guidelines which continue to recommend using chemoprophylaxis in many low-risk countries and advise limiting the recommendation only to areas where malaria risk to travelers justifies it. Focusing on high-prevalence countries and specific risk groups (such as business travelers) may increase the adherence and result in fewer malaria cases.
REFERENCES
- 1.World Health Organization , 2017. International Travel and Health–Malaria Update 2017. Available at: http://www.who.int/ith/2017-ith-chapter7.pdf?ua=1. Accessed Septmber 19, 2019. [Google Scholar]
- 2.Berko-Ball J, 2007. A Profile of Israeli Travelers to Endemic Countries: A National Survey. Annual Meeting of the Israeli Society for Parasitology, Protozoology and Tropical Diseases, Ramat Gan, Israel, 10–12, 32. [Google Scholar]
- 3.Centers for Disease Control and Prevention , 2019. CDC Yellow Book 2020: Health Information for International Travel. New York, NY: Oxford University Press. [Google Scholar]
- 4.Israel Center for Disease Control (ICDC), Division of Epidemiology Public Health Services , 2012. Notifiable Infectious Diseases in Israel 1951–2010. Ramat Gan, Israel: Ministry of Health; Publication 342. [Google Scholar]
- 5.Berger S, 2019. Infectious Diseases of Israel. Los Angeles, CA: Global Infectious Diseases and Epidemiology ON-line, Gideon Informatics, Inc; Available at: http://www.gideononline.com. Accessed September 6, 2019. [Google Scholar]
- 6.Behrens RH, et al. 2007. TropNetEurop. The low and declining risk of malaria in travelers to Latin America: is there still an indication for chemoprophylaxis? Malar J 6: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlaqenfauf P, Petersen E, 2008. Malaria chemoprophylaxis: strategies for risk groups. Clin Microbiol Rev 21: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behrens RH, et al. 2010. The incidence of malaria in travelers to south-east Asia: is local malaria transmission a useful risk indicator? Malar J 9: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LH, Wilson ME, Schlagenhauf P, 2007. Controversies and misconceptions in malaria chemoprophylaxis for travelers. JAMA 297: 2251–2263. [DOI] [PubMed] [Google Scholar]
- 10.Lobel HO, Miani M, Eng T, Bernard KW, Hightower AW, Campbell CC, 1993. Long-term malaria prophylaxis with weekly mefloquine. Lancet 341: 848–851. [DOI] [PubMed] [Google Scholar]
- 11.Steffen R, Fuchs E, Schildknecht J, Naef U, Funk M, Schlagenhauf P, Philips-Howard P, Nevill C, Stürchler D, 1993. Mefloquine compared with other malaria chemoprophylactic regiments in tourists visiting east Africa. Lancet 341: 1299–1303. [DOI] [PubMed] [Google Scholar]
- 12.Croft AM, World MJ, 1996. Neuropsychiatric reactions with mefloquine chemoprophylaxis. Lancet 347: 326. [DOI] [PubMed] [Google Scholar]
- 13.Schlagenhauf P, et al. 2003. Tolerability of malaria chemoprophylaxis in non-immune travelers to sub-Saharan Africa: multicenter, randomised, double blind, four arm study. BMJ 327: 1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overbosch D, et al. 2001. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in non-immune travelers: results from a randomized, double-blind study. Clin Infect Dis 33: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 15.Goodyer L, Rice L, Martin A, 2001. Choice of and adherence to prophylactic antimalarials. J Travel Med 18: 245–249. [DOI] [PubMed] [Google Scholar]
- 16.Chen LH, Wilson ME, Schlagenhauf P, 2006. Prevention of malaria in long-term travelers. JAMA 296: 2234–2244. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham J, Horsley J, Patel D, Tunbridge A, Lalloo DG, 2014. Compliance with long-term malaria prophylaxis in British expatriates. Travel Med Infect Dis 12: 341–348. [DOI] [PubMed] [Google Scholar]
- 18.Landman KZ, Tan KR, Arguin PM, 2015. Adherence to malaria prophylaxis among peace corps volunteers in the African region, 2013. Travel Med Infect Dis 12: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz E, Parise M, Kozarsky P, Cetron M, 2003. Delayed onset of malaria–implications for chemoprophylaxis in travelers. N Engl J Med 349: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz E, 2009. Tropical Diseases in Travelers. Oxford, United Kingdom: Wiley-Blackwell, 216–228. [Google Scholar]
- 21.Meltzer E, Rahav G, Schwartz E, 2018. Vivax malaria chemoprophylaxis: the role of atovaquone-proguanil compared to other options. Clin Infect Dis 66: 1751–1755. [DOI] [PubMed] [Google Scholar]
- 22.The Israeli Ministry of Health , 2017. Guidelines for Travel Clinics. Available at: https://www.health.gov.il/Subjects/vaccines/Vaccines_abroad/Pages/vaccination_abroad.aspx. Accessed June 19, 2018. [Google Scholar]
- 23.TropNet: European Network for Tropical Medicine and Travel Health , 2019. European Recommendations & Guidelines in Travel Medicine. Available at: http://www.tropnet.net/index.php?id=105. Accessed September 6, 2019. [Google Scholar]
- 24.Van Herck K, et al. 2004. Knowledge, attitudes and practices in travel-related infectious diseases: the European airport survey. J Travel Med 11: 3–8. [DOI] [PubMed] [Google Scholar]
- 25.Hamer DH, Connor BA, 2004. Travel health knowledge, attitudes and practices among Unites States travelers. J Travel Med 11: 23–26. [DOI] [PubMed] [Google Scholar]
- 26.Wilder-Smith A, Khairullah NS, Song JH, Chen CY, Torresi J, 2004. Travel health knowledge, attitudes and practices among Australasian travelers. J Travel Med 11: 9–15. [DOI] [PubMed] [Google Scholar]
- 27.Ropers G, Du Ry van Beest Holle M, Wichmann O, Kappelmayer L, Stüben U, Schönfeld C, Stark K, 2008. Determinants of malaria prophylaxis among German travelers to Kenya, Senegal and Thailand. J Travel Med 15: 162–171. [DOI] [PubMed] [Google Scholar]
- 28.Stoney RJ, et al. 2016. Malaria prevention strategies: adherence among Boston area travelers visiting malaria-endemic countries. Am J Trop Med Hyg 94: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belderok SM, van den Hoek A, Roeffen W, Sauerwein R, Sonder GJ, 2013. Adherence to chemoprophylaxis and Plasmodium falciparum anti-circumsporozoite seroconverstion in a prospective cohort study of Dutch short-term travelers. PLoS One 8: e56863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein I, Grefat R, Ephros M, Rishpon S, 2015. Intent-to-adhere and adherence to malaria prevention recommendations in two travel clinics. J Travel Med 22: 130–132. [DOI] [PubMed] [Google Scholar]
- 31.Vliegenthart-Jongbloed K, de Mendonca Melo M, van Wolfswinkel ME, Koelewijn R, van Hellemond JJ, van Genderen PJ, 2013. Severity of imported malaria: protective effect of taking malaria chemoprophylaxis. Malar J 12: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ, 2006. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg 75: 402–415. [PubMed] [Google Scholar]
- 33.Stienlauf S, Meltzer E, Leshem E, Rendi-Wagner P, Schwartz E, 2010. The profile of Israeli travelers to developing countries: perspectives of a travel clinic. Harefuah 149: 559–562. [PubMed] [Google Scholar]
- 34.Behrens RH, et al. 2006. Malaria prophylaxis policy for travelers from Europe to the Indian sub-continent. Malar J 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mace KE, Arguin PN, Lucchi NW, Tan KR, 2019. Malaria surveillance–United States, 2016. MMWR Surveill Summ 68: 1–35. [DOI] [PubMed] [Google Scholar]
- 36.Stienlauf S, Goldman D, Meltzer E, Anis E, Segal G, Schwartz E, 2014. Geographic Distribution of Malaria Incidence among Israeli Travelers–An Implication for Malaria Prophylaxis? 10th Asia Pacific Travel Health Conference, Caravelle Hotel, Ho Chi Minh City, Vietnam, 79–80. [Google Scholar]
- 37.Chen LH, et al. 2018. Business travel-associated illness: a GeoSentinel analysis. J Travel Med 25: tay030. [DOI] [PMC free article] [PubMed] [Google Scholar]