Abstract
Brominated flame retardants (BFRs), including polybrominated diphenyl ethers and hexabromocyclododecane, leach out from consumer products into the environment. Exposure to BFRs has been associated with effects on endocrine homeostasis. To test the hypothesis that in utero and lactational exposure to BFRs may affect the reproductive system of female offspring, adult female Sprague Dawley rats were fed diets formulated to deliver nominal doses (0, 0.06, 20, or 60 mg/kg/day) of a BFR dietary mixture mimicking the relative congener levels in house dust from prior to mating until weaning. Vaginal opening and the day of first estrus occurred at a significantly earlier age among offspring from the 20 mg/kg/day BFR group, indicating that the onset of puberty was advanced. Histological analysis of ovaries from postnatal day 46 offspring revealed an increase in the incidence of abnormal follicles. A toxicogenomic analysis of ovarian gene expression identified upstream regulators, including HIF1A, CREB1, EGF, the β-estradiol, and PPARA pathways, predicted to be downregulated in the 20 or 60 mg/kg/day group and to contribute to the gene expression patterns observed. Thus, perinatal exposure to BFRs dysregulated ovarian folliculogenesis and signaling pathways that are fundamental for ovarian function in the adult.
Keywords: brominated flame retardants, ovary, folliculogenesis, endocrine disruptors, estrogens
Ovarian follicles are produced from a pool of primordial germ cells in the developing gonad early in gestation (Grive and Freiman, 2015). These primordial germ cells first form clusters (or “cysts”) that later undergo a breakdown during which they become surrounded by layers of somatic granulosa cells, forming the follicles that represent the entire ovarian reserve (Hirshfield and Midgley, 1978). In the rat, follicular histogenesis is initiated in the hours following birth and spreads throughout the gonad in subsequent days (Hirshfield and DeSanti, 1995) under the control of signaling mechanisms between oocytes and their surroundings (Grive and Freiman, 2015). Hence, primordial follicle assembly during the fetal and neonatal periods determines the long-term reproductive capacity of female mammals and constitutes a sensitive window of exposure to chemical insults.
Brominated flame retardants (BFRs) are added to a wide variety of consumer products, such as furniture, textiles, plastics, and electrical circuits, to slow down the spread of flames in the event of a fire (Camino et al., 1991; Stapleton et al., 2012). Because BFRs are usually not covalently bound to these products (de Wit, 2002; Webster et al., 2009), they can leach out and are found ubiquitously in our home environments. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecanes (HBCDDs), among the most prominent BFRs, are present in high concentrations in domestic dust in the majority of houses sampled in North America (reviewed in Malliari and Kalantzi [2017]). As a consequence, humans are continuously exposed to BFRs by inhalation and by ingestion (reviewed in Malliari and Kalantzi [2017]). Exposure is estimated to be higher early in life, in newborns and children, compared with adults (reviewed in Dufour and Charlier [2017]), due to the ingestion of house dust from hand-to-mouth contact and the consumption of contaminated breast milk (Jones-Otazo et al., 2005; Schecter et al., 2003; Stapleton et al., 2008b). Because they are lipophilic, BFRs bioaccumulate (Akortia et al., 2016; Darnerud, 2003; Segev et al., 2009) and are found in a multitude of human tissues and related matrices (Alaee, 2003; Poon et al., 2014), including follicular fluid (Johnson et al., 2012), breast milk (Marchitti et al., 2013; Meironyté et al., 1999), hair (Albert et al., 2018; Goodyer et al., 2017; Poon et al., 2014), and serum (Shin et al., 2016; Zota et al., 2013).
Over the past few decades, BFRs have been established to act as endocrine disrupting compounds (EDCs), affecting the activities of androgenic, estrogenic, and thyroid hormones (Darnerud, 2008; Linares et al., 2015; Lyche et al., 2015). Despite this, relatively few studies have examined their association with effects on female reproduction and fertility. Women with high PBDE levels in serum or follicular fluid have longer times to conception (Harley et al., 2010), decreased fertilization rates, embryo implantation failure (Johnson et al., 2012), and an increased risk of preterm birth (Peltier et al., 2015). High serum PBDE concentrations have also been associated with a younger age at menarche in adolescent girls (Chen et al., 2011).
Several animal studies have reported effects of exposure to a single PBDE congener on the female reproductive system. The exposure of rats to BDE-99 during gestation decreased the numbers of follicles in the ovaries of female offspring and delayed the onset of puberty (Lilienthal et al., 2006). Gestational exposure to BDE-47 was also reported to decrease follicle counts in female offspring; this effect was accompanied by a decrease in serum estradiol concentrations (Talsness et al., 2008). Previously, we demonstrated that the exposure of adult female rats prior to and during pregnancy to an environmentally relevant mixture of BFRs, formulated to mimic the BFR congener levels found in domestic dust (Allen et al., 2008; Stapleton et al., 2008a), adversely affected ovarian steroidogenesis and folliculogenesis (Lefèvre et al., 2016). Our goal in this study was to investigate the consequences of in utero and lactational exposure to this environmentally relevant mixture of PBDEs and HBCDDs on the onset of puberty and ovarian development in the F1 generation, the exposed female offspring.
MATERIALS AND METHODS
BFR mixture formulation
The median levels of the PBDEs and HBCDDs observed in the Boston house dust studies (Allen et al., 2008; Stapleton et al., 2008a) were used to develop a BFR mixture that was subsequently incorporated into an isoflavone-free diet formulated to reduce phytoestrogens (Teklad Global 2019 diet; Harlan Laboratories, Madison, Wisconsin), as described previously (Berger et al., 2014; Ernest et al., 2012; Lefèvre et al., 2016). Diets were prepared using 3 technical PBDE mixtures, DE-71 (52.1%), DE-79 (0.4%), and BDE-209 (44.2%), and 1 HBCDD mixture (3.3%) to give 0 (control), 0.75, 250, or 750 mg of BFR mixture per kg of diet, with target nominal doses of 0, 0.06, 20, and 60 mg/kg of body weight per day based on a daily food consumption of 80 g/kg body weight per day. The lowest dose reflects maximum human exposure based on a 100 mg/day dust ingestion rate in a child (16.5 kg), taking into account the surface ratio of the human body compared with that of the rat (1:6.9).
Experimental animals and treatments
The details of animal treatment and breeding have been described previously (Tung et al., 2016). Briefly, virgin female Sprague Dawley rats aged 6–7 weeks were purchased from Charles River Laboratories (St-Constant, Quebec, Canada) and acclimated for 2 weeks on control diet. All animals were housed under controlled light (12L: 12D), humidity (40%–70%), and temperature (20°C–24°C). All studies were completed in accordance with the guidelines of the Canadian Council on Animal Care; experimental procedures were approved by the Health Canada Animal Care Committee.
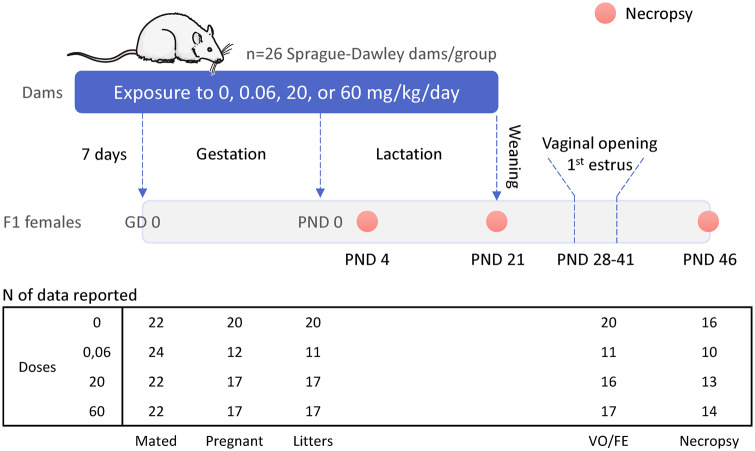
Females were randomized (n = 26 per treatment) to 1 of 4 BFR treatments (0, 0.06, 20, or 60 mg/kg body weight/day). Food and water were provided ad libitum. After a minimum of 1 week on these diets, estrous cycles were monitored daily by vaginal lavage; females observed to be in proestrus (n = 22–24 per treatment group) were housed overnight with proven fertile male Sprague Dawley rats. The presence of a sperm-positive vaginal swab the following morning indicated mating and this day was recorded as gestational day (GD) 0. All mated females were housed singly and allowed to deliver (this was denoted as postnatal day 0 or PND 0). The litters were reduced and balanced by sex to 8 pups each on PND 4. Dams were maintained on their respective diets throughout lactation until the pups were weaned onto the control diet on PND 21. Littermates of the same sex were cohoused and food consumption was monitored throughout development, as previously described (Tung et al., 2016, 2017).
One female pup from each litter was euthanized on PNDs 4, 21, and 46 (Figure 1). The ovaries were removed and weighed. One ovary from each female was fixed with 4% paraformaldehyde solution and the second was stored at −80°C for gene expression analysis. Whole blood was recovered, allowed to clot, and centrifuged, and serum was aliquoted and stored at −80°C. Beginning on PND 32, all remaining female pups (1 or 2 pups per litter; 36 pups in 20 litters for Control; 21 in 11 litters in 0.06; 28 in 17 in 20 and 32 in 16 litters in 60 mg/kg) were examined daily (between 9:00 and 10:30 Am) for vaginal patency; once this was observed, vaginal cytology was sampled by lavage to determine the age at first estrus (FE).
Figure 1.
Experimental design. Sprague Dawley virgin females, divided into 4 treatment groups, received a brominated flame retardant (BFR) mixture in the diet, with target nominal doses of 0, 0.06, 20, and 60 mg/kg of body weight per day, from at least 1 week prior to mating until weaning at postnatal day (PND) 21. Female offspring were necropsied on PNDs 4, 21, and 46, and were monitored from PND 32 to PND 37 for vaginal opening (VO) and first estrus (FE). Values are means ± SEM; n = 11–20 females from independent litters.
Hormone measurements
Serum from 53 PND 46 females (Control n = 16; 0.06 mg/kg BFR = 10; 20 mg/kg = 13; 60 mg/kg = 14) was sent to OpAns (Durham, North Carolina) for a comprehensive steroid panel analysis (cortisol, corticosterone, 11-deoxycortisol, androstenedione, deoxycorticosterone, testosterone, OH-progesterone, progesterone, pregnenolone, estrone, and estradiol) using HPLC/MS/MS. With the exception of corticosterone and progesterone, hormonal levels in the vast majority of samples were below the lower limit of quantitation (Supplementary Figure 1A).
Follicle counts
Fixed ovaries were paraffin-embedded, serially sectioned (section thickness, 5 μm), and stained with hematoxylin-eosin. Sections were examined with a Leica microscope using ×200 or ×400 magnification. Ovarian follicles were counted and classified based on the morphology and thickness of the granulosa cell layer, using previously defined criteria (Hirshfield and Midgley, 1978; Lefèvre et al., 2016). A primordial follicle was defined by a single layer of flattened granulosa cells whereas primary follicles were characterized by a row of cuboid granulosa cells. The entire ovary was sectioned and screened for follicle counts on PND 4. To avoid counting the same follicle more than once on PND 46 primordial and primary follicles were counted only in every 4th section; the peripheral parts of the ovary were not screened. The total numbers of primordial and primary follicles were calculated and standardized to the whole ovary as N = f × NN × 0.25, where N is the total number of follicles, f is the fraction of ovarian sections sampled, NN is the total number of follicles at each stage counted in the sections sampled, and 0.25 is a correction factor for the 1 in 4 sections that were counted.
Secondary follicles were defined by the presence of 2 or more layers of granulosa cells and antral follicles by the formation of an antrum. All sections were examined at ×200 and secondary follicles scored as present only in sections where the nucleus was fully visible. The formula used here was N = f × NN, where N is the total number of secondary or antral follicles, f is the fraction of ovarian sections sampled, and NN is the total number of follicles counted at each stage in the sampled sections.
Microarray analysis of RNA expression
RNA was extracted from the right ovaries from 1 female from separate litters (6 from control, 5 from 0.06, 5 from 20, and 6 from 60 mg/kg BFR treatment groups) using the Qiagen RNEasy Plus Mini Kit (Qiagen, Toronto, Ontario, Canada), as per the manufacturer’s instructions. RNA quality was measured using a NanoDrop 2000 Spectrophotometer (Thermo Fisher, Ottawa, Ontario, Canada), with 260/280 and 260/230 absorbance ratios comprised between 1.9–2.1 and 2.0–2.2, respectively. RNA was labeled with Cy3 using the Agilent One Color Low Input Quick Amp kit and hybridized on arrays using the SurePrint G3 Rat Gene Expression 8 × 60K microarray kit (Agilent Technologies, Mississauga, Ontario, Canada), as per the manufacturer’s instructions. Microarray analyses were done for RNA from the ovaries of 5–6 individual animals from independent litters for each treatment group and read with an Agilent SureScan Microarray Scanner G2600D. The resulting data were analyzed using GeneSpring version 14.9 (Agilent Technologies). Data were normalized to the 75th percentile using percentile shift normalization. Following quality control using principal component scores on all arrays, 1 sample was removed in the 0.06 mg/kg/day group. Transcripts affected by more than 2-fold were determined using a moderated t test and Benjamini-Hochberg FDR correction for each treatment. The microarray data have been uploaded to Gene Expression Omnibus (accession number GSE113000). The biological relationships between transcripts were analyzed using Ingenuity Pathway Analysis (IPA) version 01.12.
RT-qPCR
Seven genes of interest for validation were identified based on the microarray data (Supplementary Table 1). RNA from whole ovaries was converted to cDNA using the QuantiTect Reverse Transcription Kit (Qiagen), as per the manufacturer’s instructions. Gene expression was quantified using the QuantiTect SYBR Green PCR Kit (Qiagen) with the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, California) under the following thermal conditions: initial heat activation for 15 min at 95°C, followed by 50 cycles of denaturation for 15 s at 94°C, annealing for 30 s at 55°C, and extension for 30 s at 72°C. Serial dilutions of whole ovary RNA pooled from animals from all treatment groups were used as an internal reference and to create standard curves for primer efficiency and template concentration optimization. Each gene was amplified in triplicate from 6 independent samples for each treatment group. Relative levels of gene amplification compared with the control group and housekeeping gene, Ppia, were calculated using StepOnePlus Software (version 2.1). A melt curve was systematically generated to ensure the specificity of the PCR.
Statistical analyses
Statistical analyses were done using the Prism software (version 6.07, GraphPad Software, Inc, La Jolla, California). Progeny outcome data were systematically expressed as average ± SEM per litter, and histological observations were done on females from independent litters. Ovary weights, vaginal opening (VO), FE, and RT-qPCR data were analyzed using 1-way ANOVA followed by a post hoc Dunnett’s multiple comparison test. Follicle count data were analyzed with a Kruskal-Wallis nonparametric test followed by a post hoc Dunnett’s multiple comparison test. The level of significance for all statistical tests was set to p < .05.
RESULTS
Ovarian Development and Puberty
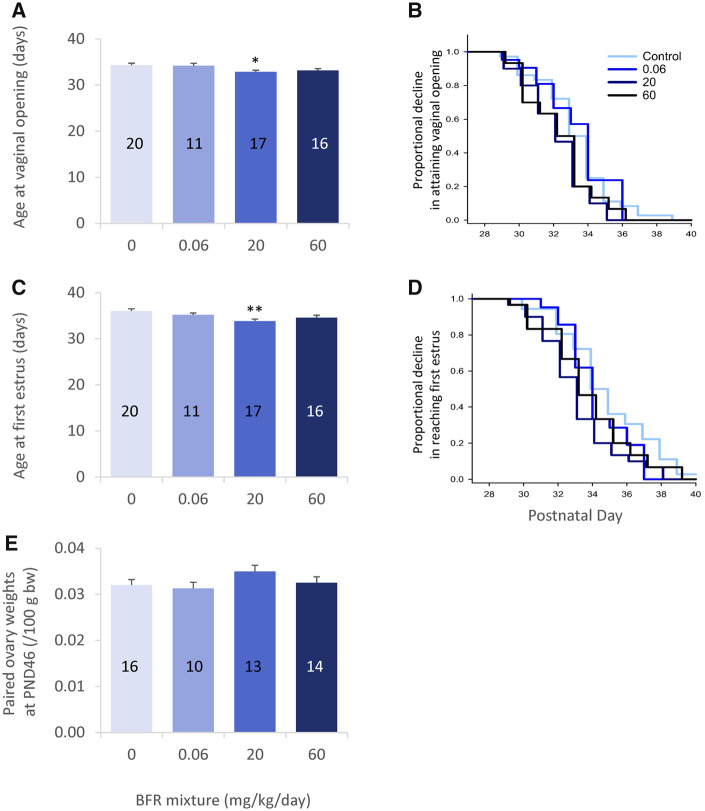
The effects of BFR exposure on mating, pregnancy, litter sizes, and pup growth rates have been reported elsewhere (Tung et al., 2016). Female offspring were weighed and necropsied on PNDs 4, 21, and 46 to assess the impact of exposure to BFRs on a variety of developmental landmarks (most described previously: Tung et al., 2016, 2017) and ovarian development (Figure 1). Previously published data showed no significant differences in body weight in female offspring on PND 4 or 21 (Tung et al., 2016), whereas there was a significant reduction in weight on PND 46 in the 60 mg/kg/day BFR treatment group. VO and FE were monitored to assess the effects of exposure to BFRs on pubertal development. Both VO and the day of FE occurred at a significantly earlier age in progeny from the 20 mg/kg/day BFR treatment group, whereas the apparent delay in these parameters observed in the 60 mg/kg/day treatment group did not reach statistical significance (p = .14 for VO and 0.052 for FE) (Figs. 2A, B and 2C, D, respectively). There were no significant differences in paired ovary weights between control and treated animals on PND 21 (data not shown) or PND 46 (Figure 2E).
Figure 2.
Pubertal onset in female offspring after in utero and lactational exposure to 0, 0.06, 20, or 60 mg/kg/day of an environmentally relevant brominated flame retardant (BFR) mixture. Female offspring were monitored for vaginal opening (VO; A, B) and first estrus (FE; C, D) from postnatal day (PND) 27 to PND 41 for 2 pups per litter, and their ovaries were weighed on PND 46 (E). Mean ± SEM of litter average age at VO and FE are depicted (A and C, respectively) with the number of representative litters indicated on each bar. Data for all pups are depicted for VO and FE as survival plots in B and D, respectively. *p < .05 and **p < .01.
Steroid Hormone Measurements
Serum from PND 46 females underwent a comprehensive steroid panel analysis. The concentrations of progesterone and corticosterone (Supplementary Figs. 1B and 1C) were not significantly affected by exposure to BFR mixtures in PND 46 females in diestrus.
Ovarian Folliculogenesis
To assess the effects of in utero and lactational exposure to BFRs on follicular histogenesis and progression, we counted ovarian follicles at PNDs 4 (Supplementary Figure 2) and 46 (Figure 3). No significant differences in the numbers of primordial and primary follicles were observed at PND 4 among any of the treatment groups (Supplementary Figs. 2A and 2B). However, we did observe an apparent increase in secondary follicles in the treated groups (Supplementary Figure 2C); this suggests a precocity in ovarian development in association with BFR treatment.
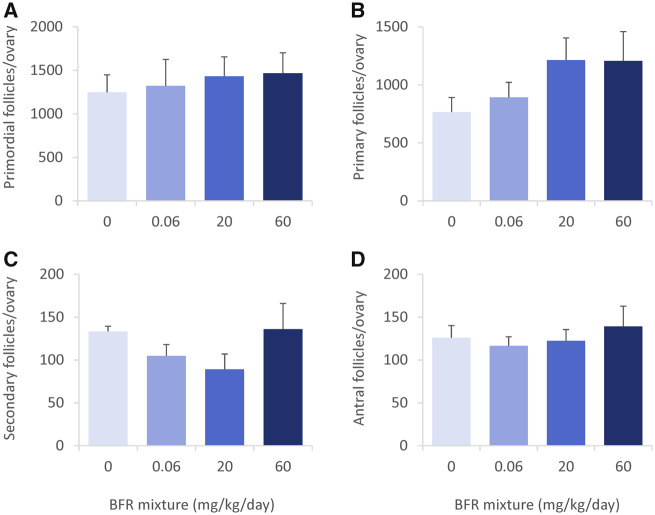
Figure 3.
Ovarian follicular counts in postnatal day 46 female offspring after in utero and lactational exposure to 0, 0.06, 20, or 60 mg/kg/day of an environmentally relevant brominated flame retardant (BFR) mixture. Primordial (A), primary (B), secondary (C), and antral (D) follicles were counted in all available sections and the total numbers calculated for the whole ovary. Values are expressed as means ± SEM; based on assessment of 1 female (left ovary) per litter for 6 litters from control, 5 from 0.06, 5 from 20, and 6 from 60 mg/kg treatment groups; there were no significant differences following a Kruskal-Wallis test and post hoc Dunnett’s multiple comparison test.
On PND 46 we observed a tendency toward an increase in primordial and primary follicles (Figs. 3A and 3B) that appeared dose-dependent and toward a decrease in the number of secondary follicles in the 20 mg/kg/day BFR treatment group (p = .19) (Figure 3C). The number of antral follicles did not differ from the control in treated animals (Figure 3D). Exposure to BFRs did not affect the total number of follicles on either PND.
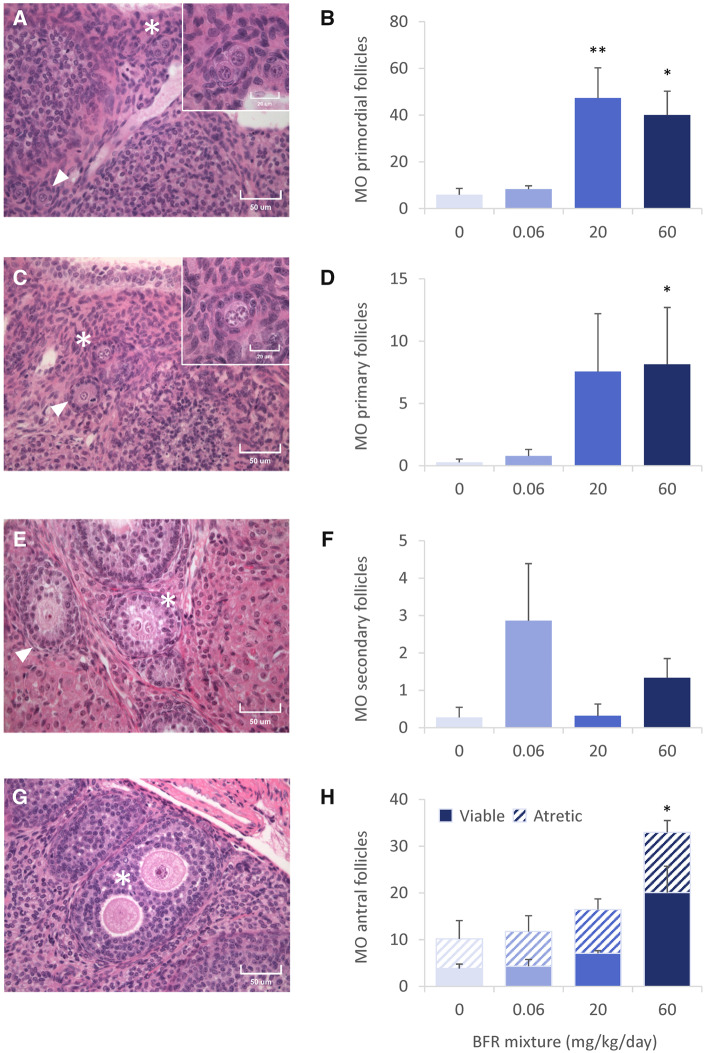
Interestingly, the histological analysis of follicles on PND 46 revealed the presence of 2 abnormal phenotypes: multioocyte follicles (MOFs) (Figure 4) and follicles with disorganized granulosa cell layers (Figure 5). We observed multioocyte primordial (Figure 4A), primary (Figure 4C), secondary (Figure 4E), and antral (Figure 4G) follicles in PND 46 ovaries. Although most MOFs contained 2 oocytes, follicles with 3 oocytes were also observed (Supplementary Figure 3A). Some follicles contained what appeared to be a dividing oocyte (Supplementary Figure 3B) whereas others appeared to contain follicles that were fusing (Supplementary Figure 3C). The numbers of primordial MOFs increased 8- and 7-fold, respectively, among offspring from 20 to 60 mg/kg/day BFRs mixture treatment groups (Figure 4B). Similarly, exposure to the highest dose of the BFR mixture significantly increased the number of primary MOFs by 15-fold (Figure 4D). Very few secondary MOFs were observed and there were no significant differences among the control and BFR exposed animals (Figure 4F). However, there was a dose-dependent increase in the numbers of viable and atretic antral MOFs that reached significance in the 60 mg/kg/day BFR treatment group (Figure 4H). In this study, atretic follicles were defined by the presence of a degenerating oocyte. MOFs were also counted on PND 4 to investigate their origin. Occasional MOFs (Supplementary Figure 2E) were observed in the 0.06 and 60 mg/kg/day BFR treatment groups on PND 4, but no treatment group differed significantly from control.
Figure 4.
Multioocyte (MO) follicles in ovaries from postnatal day 46 females after in utero and lactational exposure to 0, 0.06, 20, or 60 mg/kg/day of an environmentally relevant brominated flame retardant (BFR) mixture. High-quality photomicrographs and associated counts of multioocyte primordial (A, B), primary (C, D), secondary (E, F), and antral (G, H) follicles were calculated for the whole ovary. Follicles presenting with normal morphology are indicated with white arrowheads; abnormal follicles are indicated with an asterisk. Values are expressed as means ± SEM; based on the assessment of 1 female (left ovary) per litter from 6 litters for control, 5 from 0.06, 5 from 20, and 6 from 60 mg/kg treatment groups; *p < .05 and **p < .01 following a Kruskal-Wallis test and post hoc Dunnett’s multiple comparison test.
Figure 5.
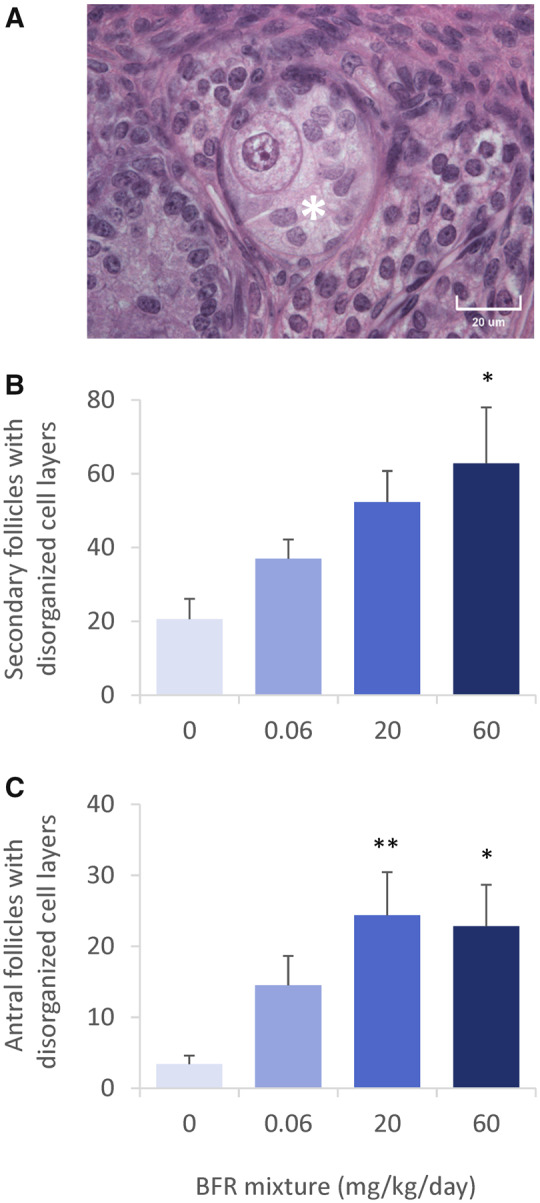
Follicles presenting with disorganized granulosa cell layers in ovaries from postnatal day 46 females after in utero and lactational exposure to 0, 0.06, 20, or 60 mg/kg/day of an environmentally relevant brominated flame retardant (BFR) mixture. High-quality photomicrograph of a secondary follicle with disorganized granulosa cell layers (A, indicated with an asterisk). Counts for secondary (B) and antral (C) follicles with disorganized granulosa cell layers, as calculated for the whole ovary. Values are expressed as means ± SEM based on the assessment of 1 female (left ovary) per litter of 6 litters from control, 5 from 0.06, 5 from 20, and 6 from 60 mg/kg treatment groups; *p < .05 and **p < .01 following a Kruskal-Wallis test and post hoc Dunnett’s multiple comparison test.
The second abnormal phenotype that was observed was the presence of follicles with absent or disorganized granulosa cell layers (Figure 5A). A dose-dependent increase in the numbers of disorganized secondary and antral follicles was observed (Figs. 5B and 5C).
Ovarian Gene Expression
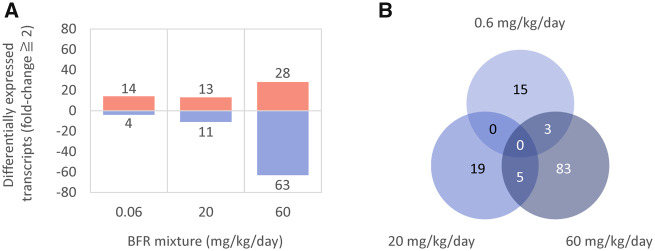
The impact of perinatal exposure to BFRs on ovarian gene expression was assessed to provide additional insights into their possible biological effects and to investigate mechanisms of action that may contribute to the impairment of folliculogenesis that was observed. Ovarian gene expression was analyzed in 4–6 independent PND 46 females per treatment group using single-color microarrays. The numbers of significantly differentially expressed transcripts (with a fold-change threshold ≥ 2) between the control and individual treatment groups are depicted in Figure 6A. In the low-dose BFR mixture exposure group (0.06 mg/kg/day), 14 transcripts were significantly upregulated and 4 were downregulated. Thirteen transcripts were significantly upregulated and 11 were downregulated in the 20 mg/kg/day BFR treatment group. Finally, exposure to 60 mg/kg/day altered the expression of a total of 91 genes: 28 were upregulated and 63 downregulated. The list of these transcripts is provided in Supplementary Table 2.
Figure 6.
Ovarian gene expression in postnatal day 46 females following in utero and lactational exposure to 0, 0.06, 20, or 60 mg/kg/day of an environmentally relevant brominated flame retardant (BFR) mixture. A, Number of uniquely mapped transcripts significantly up or downregulated by more than 2-fold determined by moderated t test and Benjamini-Hochberg FDR correction (p > .05). B, Venn diagram representing commonalities in differential gene expression between treatment groups. All data were generated using n = 4–6 female rats from independent litters.
The extent to which the significantly altered transcript sets for each of the treatment doses overlap is depicted in the Venn diagram in Figure 6B. The transcripts affected in the 3 BFR treatment groups had minimal overlap, with only the epidermal growth factor (EGF)-like repeats and discoidin domain 3 (Edil3), the olfactory receptor Orad4 and the patched domain Ptchd1 being commonly affected by exposure to 0.06 and 60 mg/kg/day, and 5 transcripts (C-X-C motif chemokine ligand 14, Cxc14; protocadherin, Pcdh8; secretogranin, Scg2; sortilin-related VPS10 domain containing receptor 2, Sorcs2; and TNF receptor superfamily member 11 b, Tnfrsf11b) commonly affected in the 20 and 60 mg/kg/day treatment groups.
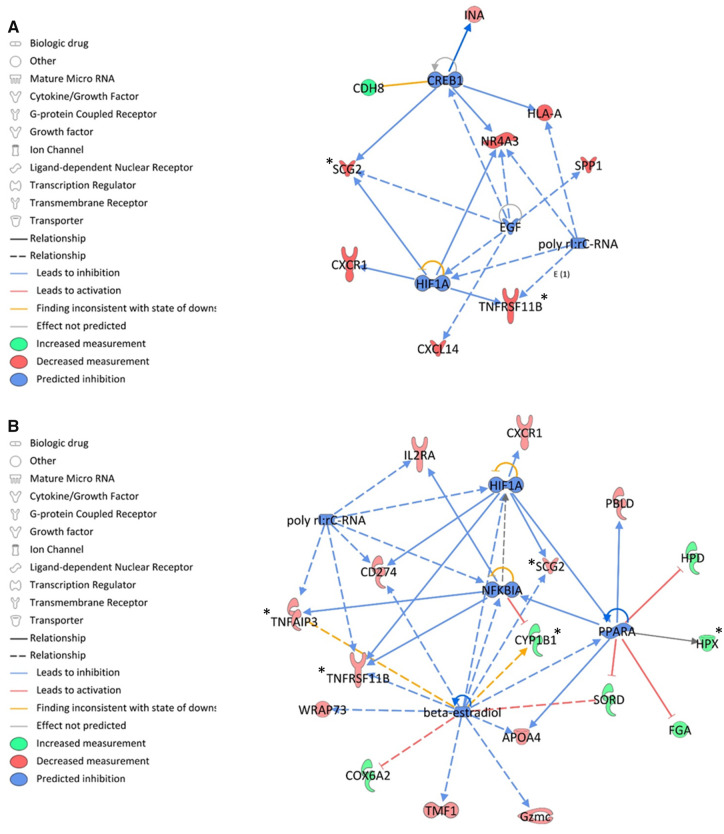
All transcripts that were differentially expressed with a fold-change ≥ 2 were imported into IPA software to investigate the biological relationships underlying these gene expression changes. Although canonical pathways were not specifically predicted to be activated or inhibited (null z-scores), we identified potential upstream regulators that may contribute to the gene expression patterns that were observed in BFR-treated groups (Table 1; Figure 7). No upstream regulator was identified in the lowest dose group. Hypoxia inducible factor 1 alpha (HIF1A) was predicted to be downregulated in both the medium- and high-dose groups (z-scores of −1.98 and −1.99, respectively), as was poly rI:rC RNA (z-scores of −1.97 and −2.00, respectively) (Table 1; Figs. 7A and 7B). Cyclic AMP responsive element binding protein 1 (CREB1; z-score = −2.20) and EGF (z-score = −2.00) were predicted to be downregulated in the 20 mg/kg/day BFR treatment group (Table 1; Figure 7A). Peroxisome proliferator-activated receptor alpha (PPARA; z-score = −2.20), β-estradiol (z-score = −2.20), and the Nuclear Factor of Kappa Light Polypeptide Gene Enhancer in B-Cells Inhibitor Alpha (NFKBIA; z-score = −2.0) were predicted to be downregulated in the 60 mg/kg/day treatment group (Table 1; Figure 7B).
Table 1.
Upstream Regulators in PND 46 Female Offspring Ovaries That Are Predicted Using Ingenuity Pathway Analysis to be Significantly Affected by Exposure to 20 or 60 mg/kg/day of an Environmentally Relevant BFR Mixture
| Upstream Regulator | Predicted State | z-Score | p Value of Overlap | Target Molecules in Dataset | |
|---|---|---|---|---|---|
| 20 mg/kg/day | CREB1 | Inhibited | −2.20 | 6.84E−02 | Cdh8, Hla-a, Ina, Nr4a3, Scg2 |
| miR-423-5p | Inhibited | −2.00 | 2.11E−01 | Angpt4, C11orf87, Cacfd1, Slc22a12 | |
| EGF | Inhibited | −2.00 | 1.17E−01 | Cxcl14, Nr4a3, Scg2, Spp1 | |
| HIF1A | Inhibited | −1.98 | 6.22E−02 | Cxcr1, Nr4a3, Scg2, Tnfrsf11b | |
| poly rI:rC RNA | Inhibited | −1.97 | 6.48E−02 | Hla-a, NR4a3, Spp1, Tnfrsf11b | |
| 60 mg/kg/day | PPARA | Inhibited | −2.20 | 2.73E−02 | Apoa4, Fga, Hpd, Hpx, Pbld, Sord |
| ß-estradiol | Inhibited | −2.20 | 3.04E−01 | Apoa4, Cd274, Cox6a2, Cyp1b1, Gzmc, Scg2, Sord, Tmf1, Tnfaip3, Tnfrsf11b, Wrap73 | |
| NFKBIA | Inhibited | −2.00 | 1.95E−01 | Cyp1b1, Il2rA, Tnfaip3, Tnfrsf11b | |
| poly rI:rC RNA | Inhibited | −2.00 | 1.52E−01 | Cd274, Il2rA, Tnfaip3, Tnfrsf11b | |
| HIF1A | Inhibited | −1.99 | 1.46E−01 | Cd274, Cxcr1, Scg2, Tnfrsf11b |
Figure 7.
Ingenuity Pathway Analysis predictions of upstream regulators significantly affected by exposure to an environmentally relevant brominated flame retardant mixture. A, Upstream regulators predicted to be affected by exposure to 20 mg/kg/day of the BFR mixture. B, Upstream regulators predicted to be affected by exposure to 60 mg/kg/day of the BFR mixture. An asterisk indicates the transcripts associated with these pathways that were validated using qRT-PCR.
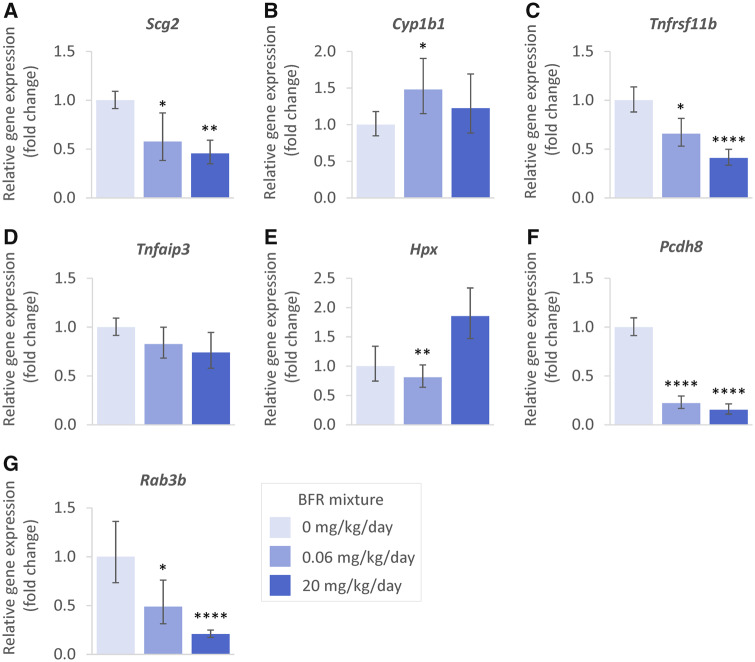
Seven target genes of interest were selected for validation by RT-qPCR based on pathways analysis of the gene expression data (Figure 8). Because we did not find a housekeeping gene with stable expression in the high-dose group RT-qPCR, data are provided only for the 0, 0.06, and 20 mg/kg/day groups. Within the CREB1 and HIF1A pathways, we confirmed Scg2 to be significantly downregulated in both 0.06 and 20 mg/kg/day BFR treatment groups (Figure 8A). Within the β-estradiol pathway, we confirmed the significant upregulation of Cyp1b1 at the lowest dose (Figure 8B), and the significant downregulation of Tnfrsf11b and Scg2 in both the 0.06 and 20 mg/kg/day treatment groups (Figs. 8A and 8C). This effect on Tnfrsf11b was also consistent with the predicted inactivation of NFKBIA; however, qRT-PCR did not confirm the significant downregulation of Tnfaip3 (Figure 8D). Finally, within the PPARA pathway, we found Hpx to be significantly downregulated at 0.06 mg/kg/day and upregulated, although not significantly, in the 20 mg/kg/day group (Figure 8E). Other transcripts of interest included the protocadherin Pcdh8 (Figure 8F) as well as the Ras-related protein Rab3b (Figure 8G), which were both confirmed to be significantly downregulated in the 0.06 and 20 mg/kg/day BFR treatment groups.
Figure 8.
RT-qPCR analyses of the expression of selected transcripts following in utero and lactational exposure to 0, 0.06, 20, or 60 mg/kg/day of an environmentally relevant brominated flame retardant mixture. Relative levels of gene amplification compared with the 0 mg/kg/day group and housekeeping gene Ppia were calculated following the ΔΔCt method. Significance was determined by 1-way ANOVA corrected by Dunnett’s multiple comparison test; n = 6 biological replicates plated in triplicate; *p < .05; **p < .01; ****p < .0001.
DISCUSSION
To the best of our knowledge, this study is the first to demonstrate that early life exposure to an environmentally relevant BFR mixture leads to disruption of ovarian development in postpubertal female rats that is accompanied by accelerated puberty and abnormal follicular structure. Ovarian histological analysis revealed a significant elevation in the incidence of MOFs and a dose-dependent increase in the numbers of follicles with disorganized granulosa cell layers. Using a toxicogenomic approach, we found that exposure to BFRs significantly altered the expression of a number of genes in the ovary and identified potential upstream regulators that may be targeted; these included HIF1A, poly rI:rC RNA, β-estradiol, PPARA, NFKB1A, CREB1 and EGF.
A limited number of studies have investigated the effects of exposure to individual PBDE congeners on the onset of puberty. Previously, we reported that exposure to the environmentally relevant mixture of BFRs during gestation and lactation altered developmental programing and induced thyroid hormone dysfunction (Tung et al., 2016, 2017) in the offspring. Here, we observed the premature onset of puberty in female offspring after perinatal exposure to 20 mg/kg/day of this BFR mixture; the apparent delay observed in females exposed to a higher dose failed to reach statistical significance. These data corroborate epidemiological studies showing associations between exposure to PBDEs and lower age at menarche (Chen et al., 2011) or premature thelarche (Deodati et al., 2016). However, other studies have reported associations between PBDE exposures and a higher age at pubertal transition (Windham et al., 2015) or menarche (Harley et al., 2017) in girls. In an animal study, the exposure of pregnant Long-Evans dams to a single PBDE congener, BDE-99, from GD 10 to 18 produced a significant delay in the onset of puberty in female offspring (Lilienthal et al., 2006); a trend toward a delay was also observed in a second study (Ceccatelli et al., 2006). These discrepancies in human and animal studies highlight the need for a better understanding of the mechanisms of action that are at play and intricacies involved in interpreting the consequences of exposure to mixtures of endocrine disrupting chemicals.
Previous studies have reported decreases in the numbers of follicles in Long-Evans rats after treatment with a single BDE congener, BPE-99 (Lilienthal et al., 2006) or BDE-47 (Talsness et al., 2008). Here, we report that the overall numbers of ovarian follicles at PND 46 remained unaffected by exposure to BFRs, although there was a trend toward a dose-dependent increase in the number of primordial and primary follicles and a decrease in the number of secondary follicles in the 0.06 and 20 mg/kg/day groups.
In utero and lactational exposure to our environmentally relevant BFR mixture induced a dose-dependent increase in the number of follicles displaying 2 abnormal phenotypes: the presence of more than 1 oocyte in a single follicle (MOFs) and a disorganization of granulosa cell layers. MOFs are often found in rats during the prepubertal period but are uncommon in adulthood (Davis and Hall, 1950). However, MOFs have been reported in different species (rodents, sheep, caiman crocodiles, and primates) after exposure to endocrine-disrupting chemicals. Their presence may be due to the division of an oocyte within a follicle, perhaps because of mitotic slippage or dysregulation of the separation of germ cell clusters, commonly referred to as germinal cysts. Alternatively, there is speculation that MOFs can arise in juvenile rat ovaries through fusion of adjacent follicles (Gaytán et al., 2014). Our data do not allow us to distinguish among these possibilities because some follicles contained what appeared to be a dividing oocyte (Supplementary Figure 3B) whereas others appeared to contain follicles that were fusing (Supplementary Figure 3C). We also found no statistically significant differences in the numbers of follicles at any stage at PND 4, suggesting that the nondifferentiation of cysts is a possibility.
The second abnormal phenotype that we observed was an increase in the numbers of follicles with disorganized granulosa cells. The connections between oocytes and granulosa cells are established by cytoplasmic extensions bearing communicating junctions formed by connexins. A deficiency in connexin-43 in granulosa cells at the preantral stage has been reported to induce incompetent oocytes (Kidder and Mhawi, 2002). Although microarray analyses revealed connexin-43 to be present in all samples, there was no difference in mRNA levels between any BFR treatment groups. Moreover, in rats irradiated in utero an imbalance between the number of oocytes and somatic cells prevented follicular growth, leading to a reduction in follicular stock (Mazaud Guittot et al., 2006). Such a phenomenon may be involved in the appearance of disorganized follicles after exposure to BFRs.
The consequences of either MOFs or follicles with disorganized structure for health and fertility are not clear. The lack of observed effects on hormone levels in this study suggests that these are not associated with major endocrine disruption. It is not clear if MOFs can produce fully viable oocytes but oocytes collected from MOFs of ovaries from mice perinatally exposed to diethylstilbestrol (Iguchi et al., 1991) or from patients in fertility clinics (Dandekar et al., 1988) can be fertilized; however, in both cases the resulting embryos develop more slowly than those from single ovular follicles. These observations demonstrate that MOFs produce mature oocytes via hormonal manipulation but do not necessarily reveal their fate under normal physiological conditions.
We identified several potential upstream regulators to be targeted by BFRs; these include HIF1A and poly rI:rC RNA (in ovaries from both the middle and high BFR treatment groups). HIF1A plays an important role in mammalian ovarian follicular development and ovulation (Wang et al., 2012; Zhang et al., 2012) and is expressed in both granulosa cells and mature oocytes (Takahashi et al., 2016; Wang et al., 2012). HIF1A has been implicated in the regulation of progesterone synthesis in luteinizing granulosa cells and is downregulated in atretic follicles (Fadhillah et al., 2017). Double-stranded RNA such as poly rI:rC RNA plays a key role in activating protein kinase R, a key innate immune sensor (Hull and Bevilacqua, 2016). Double-stranded RNA is an upstream regulator linking damage sensing to multiple responses, including the insulin signaling pathway (Hage Hassan et al., 2016) and wound healing (Zhu et al., 2017). In the ovary, the administration of poly rI:rC RNA inhibited estradiol synthesis and induced ovarian granulosa cell apoptosis (Yan et al., 2015).
In the middle-dose group, CREB1 and EGF were also identified as potential upstream regulators. The CREB1 pathway plays a critical role in mediating the gonadotropin hormone responsiveness of granulosa cells (Mukherjee et al., 1996). The knockdown of CREB attenuates the induction of StAR expression and progesterone synthesis by proinflammatory cytokines in human granulosa-lutein cells (Dang et al., 2017). EGF signaling also plays a crucial role in the ovary in regulating oocyte maturations and ovulation (Richani and Gilchrist, 2018). Pharmacological downregulation of the EGF pathway has been reported to suppress the induction of cumulus expansion and oocyte maturation (Shimada et al., 2016).
Finally, in the high-dose group, NFKB1A, PPARA, and β-estradiol were identified as potential upstream regulators targeted by BFR exposure. NFKBIA encodes a protein that negatively regulates the NF-kappa-B pathways, and thus would be expected to affect expression of cytokines involved in inflammatory responses. To our knowledge, this pathway has not been specifically implicated in folliculogenesis in the ovary, but gene association studies have linked NFKBIA variants with some inflammatory diseases (Hong et al., 2018).
The β-estradiol signaling pathway was predicted to be downregulated in ovaries from the high BFR dose group. Although serum estradiol concentrations were below the level of detection in our study (Supplementary Figure 1A), previous studies have reported a decrease in estradiol concentration after exposure to BDE-10 (Lilienthal et al., 2006) and BDE-47 (Talsness et al., 2008). However, developmental exposure to certain BDEs was reported to upregulate the expression of estrogen target genes in the uterus (Ceccatelli et al., 2006; Dang et al., 2017). It is consistent with this finding that several PBDE congeners, and their hydroxylated metabolites, have been reported to act as NR3A1 (ER alpha) and NR3A2 (ER beta) receptor agonists (Meerts et al., 2001; Mercado-Feliciano and Bigsby, 2008). Hydroxylated BDE-47 metabolites have also been reported to stimulate ovarian aromatase activity (Karpeta et al., 2013). Thus, the mechanism by which exposure to the mixture of BFRs we have studied affects β-estradiol pathway endpoints is likely to be complex. Previously, our lab reported that exposure to a mixture of PBDEs similar to that in human follicular fluid reduced estradiol secretion in KGN cells, an immortalized human granulosa cell line (Lefevre et al., 2016). Dysregulation of estradiol signaling may play an important role in dysregulating ovarian folliculogenesis. Treatment with diethylstilbestrol or estradiol has been reported to decrease the number of small antral follicles and induce MOF formation in the neonatal rodent ovary (Iguchi et al., 1990; Kipp et al., 2007). Increased follicular recruitment and MOFs have also been described after exposure to estrogenic compounds such as bisphenol A (Hunt et al., 2012; Rodríguez et al., 2010).
The PPARA pathway was also predicted to be significantly inhibited in the 60 mg/kg/day BFR exposure group; this pathway is involved in estradiol biosynthesis in granulosa cells (reviewed in Komar [2005]). Numerous studies have shown that PPARs are expressed throughout the female reproductive system (Vitti et al., 2016) and are critical for normal ovarian function (Huang, 2008; Komar, 2005; Vélez et al., 2013). Certain compounds, such as herbicides (eg, 2,4-dichlorophenoxyacetic acid, 2,4-D), plasticizers (eg, di-(2-ethylhexyl) phthalate, DEHP; di-(2-ethylhexyl) adipate, DEHA; and dibutyl phthalate, DBP), and perfluorooctanoic acid, as well as other hygiene components (hair spray and solvent for perfumes) activate PPARs in the ovary and testis (Nakajima et al., 2002), reviewed in Huang (2008) and Vitti et al. (2016), resulting in effects such as prolonged sexual cycle, suppressed ovulation, contracted preovulatory follicles, decreased estradiol levels, elevated FSH, and alterations in steroidogenesis. The effects of reduced activation of PPARA on ovarian function are not clear as female mice that lack a functional PPARA gene are fertile (Lee et al., 1995; Yessoufou et al., 2006) with a slightly increased rate of spontaneous abortion (Yessoufou et al., 2006). Consequently, it is not clear what reduced PPARA activation might predict for ovarian health and fertility.
Using a rodent model, we have shown that in utero and lactational exposure to an environmentally relevant mixture of BFR congeners representative of those present in house dust produces several effects on the ovaries of female offspring. These include an early onset of puberty and an increase in the incidence of MOFs. Our data suggest that such effects may be a consequence of the downregulation of pathways that are fundamental for ovarian function; these include the HIF1A, CREB1, EGF, β-estradiol, and PPAR pathways, in addition to pathways that regulate inflammatory responses.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank Emily Tung for coordinating animal breeding and early life monitoring, Kevin Kittle for mixing and pelleting of animal diets, Lydia Goff for ovary sectioning and Marie-Ève Ruest for RNA extractions.
FUNDING
The Canadian Institutes of Health Research (CIHR) Institute of Human Development, Child and Youth Health (RHF100625, RHF100626); the Health Canada Chemicals Management Plan Research Initiative. O.A. and P.L.C.L. were the recipients of Fonds de Recherche en Santé du Québec postdoctoral fellowships. B.F.H and B.R. are James McGill Professors.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Akortia E., Okonkwo J. O., Lupankwa M., Osae S. D., Daso A. P., Olukunle O. I., Chaudhary A. (2016). A review of sources, levels, and toxicity of polybrominated diphenyl ethers (PBDEs) and their transformation and transport in various environmental compartments. Environ. Rev. 24, 253–273. [Google Scholar]
- Alaee M. (2003). Recommendations for monitoring of polybrominated diphenyl ethers in the Canadian environment. Environ. Mon. Assess. 88, 327–341. [DOI] [PubMed] [Google Scholar]
- Albert O., Huang J. Y., Aleksa K., Hales B. F., Goodyer C. G., Robaire B., Chevrier J., Chan P. (2018). Exposure to polybrominated diphenyl ethers and phthalates in healthy men living in the greater Montreal area: a study of hormonal balance and semen quality. Environ. Int. 116, 165–175. [DOI] [PubMed] [Google Scholar]
- Allen J. G., McClean M. D., Stapleton H. M., Webster T. F. (2008). Critical factors in assessing exposure to PBDEs via house dust. Environ. Int. 34, 1085–1091. [DOI] [PubMed] [Google Scholar]
- Berger R. G., Lefèvre P. L., Ernest S. R., Wade M. G., Ma Y. Q., Rawn D. F., Gaertner D. W., Robaire B., Hales B. F. (2014). Exposure to an environmentally relevant mixture of brominated flame retardants affects fetal development in Sprague-Dawley rats. Toxicology 320, 56–66. [DOI] [PubMed] [Google Scholar]
- Camino G., Costa L., Luda di Cortemiglia M. P. (1991). Overview of fire retardant mechanisms. Polym. Degrad. Stabil. 33, 131–154. [Google Scholar]
- Ceccatelli R., Faass O., Schlumpf M., Lichtensteiger W. (2006). Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology 220, 104–116. [DOI] [PubMed] [Google Scholar]
- Chen A., Chung E., DeFranco E. A., Pinney S. M., Dietrich K. N. (2011). Serum PBDEs and age at menarche in adolescent girls: Analysis of the National Health and Nutrition Examination Survey 2003–2004. Environ. Res. 111, 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar P. V., Martin M. C., and Glass R. H. (1988). Polyovular follicles associated with human in vitro fertilization. Fertil. Steril. 49, 483–486. [PubMed] [Google Scholar]
- Dang X., Zhu Q., He Y., Wang Y., Lu Y., Li X., Qi, J., Wu, H., and Sun, Y. (2017). IL-1β upregulates StAR and progesterone production through the ERK1/2- and p38-mediated CREB signaling pathways in human granulosa-lutein cells. Endocrinology 158, 3281–3291. [DOI] [PubMed] [Google Scholar]
- Darnerud P. O. (2003). Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 29, 841–853. [DOI] [PubMed] [Google Scholar]
- Darnerud P. O. (2008). Brominated flame retardants as possible endocrine disrupters. Int. J. Androl. 31, 152–160. [DOI] [PubMed] [Google Scholar]
- Davis D. E., Hall O. (1950). Polyovuly and anovular follicles in the wild Norway rat. Anat. Rec. 107, 187–192. [DOI] [PubMed] [Google Scholar]
- Deodati A., Sallemi A., Maranghi F., Germani D., Puglianiello A., Baldari F., Busani L., Mancini F. R., Tassinari R., Mantovani A., et al. (2016). Serum levels of polybrominated diphenyl ethers in girls with premature thelarche. Horm. Res. Paediatr. 86, 233–239. [DOI] [PubMed] [Google Scholar]
- de Wit C. A. (2002). An overview of brominated flame retardants in the environment. Chemosphere 46, 583–624. [DOI] [PubMed] [Google Scholar]
- Dufour P., Charlier C. (2017). Brominated flame retardant: Environmental and exposed individuals’ health impact. Ann. Biol. Clin. (Paris) 75, 146–157. [DOI] [PubMed] [Google Scholar]
- Ernest S. R., Wade M. G., Lalancette C., Ma Y. Q., Berger R. G., Robaire B., Hales B. F. (2012). Effects of chronic exposure to an environmentally relevant mixture of brominated flame retardants on the reproductive and thyroid system in adult male rats. Toxicol. Sci. 127, 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadhillah Y. S., Nishimura R., Yamamoto Y., Kimura K., Okuda K. (2017). Hypoxia-inducible factor 1 Mediates hypoxia-enhanced synthesis of progesterone during luteinization of granulosa cells. J. Reprod. Dev. 63, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytán F., Morales C., Manfredi-Lozano M., Tena-Sempere M. (2014). Generation of multi-oocyte follicles in the peripubertal rat ovary: Link to the invasive capacity of granulosa cells? Fertil. Steril. 101, 1467–1476. [DOI] [PubMed] [Google Scholar]
- Goodyer C. G., Poon S., Aleksa K., Hou L., Atehortua V., Carnevale A., Koren G., Jednak R., Emil S., Bagli D., et al. (2017). A case–control study of maternal polybrominated diphenyl ether (PBDE) exposure and cryptorchidism in Canadian populations. Environ. Health Perspect. 125, 057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grive K. J., Freiman R. N. (2015). The developmental origins of the mammalian ovarian reserve. Development 142, 2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage Hassan R., Pacheco de Sousa A. C., Mahfouz R., Hainault I., Blachnio-Zabielska A., Bourron O., Koskas F., Górski J., Ferré P., Foufelle F., et al. (2016). Sustained action of ceramide on the insulin signaling pathway in muscle cells. J. Biol. Chem. 291, 3019–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley K. G., Marks A. R., Chevrier J., Bradman A., Sjödin A., Eskenazi B. (2010). PBDE concentrations in women’s serum and fecundability. Environ. Health Perspect. 118, 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley K. G., Rauch S. A., Chevrier J., Kogut K., Parra K. L., Trujillo C., Lustig R. H., Greenspan L. C., Sjödin A., Bradman A., et al. (2017). Association of prenatal and childhood PBDE exposure with timing of puberty in boys and girls. Environ. Int. 100, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield A. N., DeSanti A. M. (1995). Patterns of ovarian cell proliferation in rats during the embryonic period and the first three weeks postpartum. Biol. Reprod. 53, 1208–1221. [DOI] [PubMed] [Google Scholar]
- Hirshfield A. N., Midgley A. R. Jr (1978). Morphometric analysis of follicular development in the rat. Biol. Reprod. 19, 597–605. [DOI] [PubMed] [Google Scholar]
- Hong M., Ye B. D., Yang S. K., Jung S., Lee H. S., Kim B. M., Lee S. B., Hong J., Baek J., Park S. H., et al. (2018). Immunochip meta-analysis of inflammatory bowel disease identifies three novel loci and four novel associations in previously reported loci. J. Crohns Colitis 12, 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. C. (2008). The role of peroxisome proliferator-activated receptors in the development and physiology of gametes and preimplantation embryos. PPAR Res. 2008, e732303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C. M., Bevilacqua P. C. (2016). Discriminating self and non-self by RNA: Roles for RNA structure, misfolding, and modification in regulating the innate immune sensor PKR. Acc. Chem. Res. 49, 1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P. A., Lawson C., Gieske M., Murdoch B., Smith H., Marre A., Hassold T., VandeVoort C. A. (2012). Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc. Natl. Acad. Sci. U.S.A. 109, 17525–17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi T., Fukazawa Y., Uesugi Y., Takasugi N. (1990). Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol. Reprod. 43, 478–484. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Kamiya K., Uesugi Y., Sayama K., Takasugi N. (1991). In vitro fertilization of oocytes from polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. In Vivo 5, 359–363. [PubMed] [Google Scholar]
- Johnson P. I., Altshul L., Cramer D. W., Missmer S. A., Hauser R., Meeker J. D. (2012). Serum and Follicular fluid concentrations of polybrominated diphenyl ethers and in-vitro fertilization outcome. Environ. Int. 45, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Otazo H. A., Clarke J. P., Diamond M. L., Archbold J. A., Ferguson G., Harner T., Richardson G. M., Ryan J. J., Wilford B. (2005). Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ. Sci. Technol. 39, 5121–5130. [DOI] [PubMed] [Google Scholar]
- Karpeta A., Barc J., Ptak A., Gregoraszczuk E. L. (2013). The 2,2′,4,4′-tetrabromodiphenyl ether hydroxylated metabolites 5-OH-BDE-47 and 6-OH-BDE-47 stimulate estradiol secretion in the ovary by activating aromatase expression. Toxicology 305, 65–70. [DOI] [PubMed] [Google Scholar]
- Kidder G. M., Mhawi A. A. (2002). Gap junctions and ovarian folliculogenesis. Reproduction 123, 613–620. [DOI] [PubMed] [Google Scholar]
- Kipp J. L., Kilen S. M., Bristol-Gould S., Woodruff T. K., Mayo K. E. (2007). Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology 148, 1968–1976. [DOI] [PubMed] [Google Scholar]
- Komar C. M. (2005). Peroxisome proliferator-activated receptors (PPARs) and ovarian function—Implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod. Biol. Endocrinol. 3, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S., Pineau T., Drago J., Lee E. J., Owens J. W., Kroetz D. L., Fernandez-Salguero P. M., Westphal H., Gonzalez F. P., (1995). Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 15, 3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre P. L., Berger R. G., Ernest S. R., Gaertner D. W., Rawn D. F., Wade M. G., Robaire B., Hales B. F. (2016). Exposure of Female rats to an environmentally relevant mixture of brominated flame retardants targets the ovary, affecting folliculogenesis and steroidogenesis. Biol. Reprod. 94, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre P. L., Wade M., Goodyer C., Hales B. F., Robaire B. (2016). A mixture reflecting polybrominated diphenyl ether (PBDE) profiles detected in human follicular fluid significantly affects steroidogenesis and induces oxidative stress in a female human granulosa cell line. Endocrinology 157, 2698–2711. [DOI] [PubMed] [Google Scholar]
- Lilienthal H., Hack A., Roth-Härer A., Grande S. W., Talsness C. E. (2006). Effects of developmental exposure to 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ. Health Perspect. 114, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares V., Bellés M., Domingo J. L. (2015). Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 89, 335–356. [DOI] [PubMed] [Google Scholar]
- Lyche J. L., Rosseland C., Berge G., Polder A. (2015). Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 74, 170–180. [DOI] [PubMed] [Google Scholar]
- Malliari E., Kalantzi O. I. (2017). Children’s exposure to brominated flame retardants in indoor environments—A review. Environ. Int. 108, 146–169. [DOI] [PubMed] [Google Scholar]
- Marchitti S. A., LaKind J. S., Naiman D. Q., Berlin C. M., Kenneke J. F. (2013). Improving infant exposure and health risk estimates: Using serum data to predict polybrominated diphenyl ether concentrations in breast milk. Environ. Sci. Technol. 47, 4787–4795. [DOI] [PubMed] [Google Scholar]
- Mazaud Guittot S., Guigon C. J., Coudouel N., Magre S. (2006). Consequences of fetal irradiation on follicle histogenesis and early follicle development in rat ovaries. Biol. Reprod. 75, 749–759. [DOI] [PubMed] [Google Scholar]
- Meerts I. A., Letcher R. J., Hoving S., Marsh G., Bergman A., Lemmen J. G., van der Burg B., Brouwer A. (2001). In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A Compounds. Environ. Health Perspect. 109, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meironyté D., Norén K., Bergman A. (1999). Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J. Toxicol. Environ. Health A 58, 329–341. [DOI] [PubMed] [Google Scholar]
- Mercado-Feliciano M., Bigsby R. M. (2008). Hydroxylated metabolites of the polybrominated diphenyl ether mixture DE-71 Are weak estrogen receptor-alpha ligands. Environ. Health Perspect. 116, 1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Park-Sarge O. K., Mayo K. E. (1996). Gonadotropins induce rapid phosphorylation of the 3′,5′-cyclic adenosine monophosphate response element binding protein in ovarian granulosa cells. Endocrinology 137, 3234–3245. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Ichihara G., Kamijima M., Itohara S., Aoyama T. (2002). Functional activation of peroxisome proliferator-activated receptor alpha (PPARalpha) by environmental chemicals in relation to their toxicities. Nagoya J. Med. Sci. 65, 85–94. [PubMed] [Google Scholar]
- Peltier M. R., Koo H. C., Getahun D., Menon R. (2015). Does exposure to flame retardants increase the risk for preterm birth? J. Reprod. Immunol. 107, 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon S., Wade M. G., Aleksa K., Rawn D. F., Carnevale A., Gaertner D. W., Sadler A., Breton F., Koren G., Ernest S. R., et al. (2014). Hair as a biomarker of systemic exposure to polybrominated diphenyl ethers. Environ. Sci. Technol. 48, 14650–14658. [DOI] [PubMed] [Google Scholar]
- Richani D., Gilchrist R. B. (2018). The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 24, 1–14. [DOI] [PubMed] [Google Scholar]
- Rodríguez H. A.1, Santambrosio N., Santamaría C. G., Muñoz-de-Toro M., Luque E. H. (2010). Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod. Toxicol. 30, 550–557. [DOI] [PubMed] [Google Scholar]
- Schecter A., Pavuk M., Päpke O., Ryan J. J., Birnbaum L., Rosen R. (2003). Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ. Health Perspect. 111, 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev O., Kushmaro A., Brenner A. (2009). Environmental impact of flame retardants (persistence and biodegradability). Int. J. Environ. Res. Public Health 6, 478–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M., Umehara T., Hoshino Y. (2016). Roles of epidermal growth factor (EGF)‐like factor in the ovulation process. Reprod. Med. Biol. 15, 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M. Y., Lee S., Kim H. J., Lee J. J., Choi G., Choi S., Kim S., Kim S. Y., Park J., Moon H. B., et al. (2016). Polybrominated diphenyl ethers in maternal serum, breast milk, umbilical cord serum, and house dust in a South Korean birth panel of mother-neonate pairs. Int. J. Environ. Res. Public Health 13, pii: E767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Allen J. G., Kelly S. M., Konstantinov A., Klosterhaus S., Watkins D., McClean M. D., Webster T. F. (2008. a). Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 42, 6910–6916. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Kelly S. M., Allen J. G., Mcclean M. D., Webster T. F. (2008. b). Measurement of polybrominated diphenyl ethers on hand wipes: Estimating exposure from hand-to-mouth contact. Environ. Sci. Technol. 42, 3329–3334. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Sharma S., Getzinger G., Ferguson P. L., Gabriel M., Webster T. F., Blum A. (2012). Novel and high volume use flame retardants in US couches reflective of the 2005 pentaBDE Phase out. Environ. Sci. Technol. 46, 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Davy P. M., Gardner L. H., Mathews J., Yamazaki Y., Allsopp R. C. (2016). Hypoxia inducible factor 1 alpha is expressed in germ cells throughout the murine life cycle. PLoS One 11, e0154309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsness C. E., Kuriyama S. N., Sterner-Kock A., Schnitker P., Grande S. W., Shakibaei M., Andrade A., Grote K., Chahoud I. (2008). In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ. Health Perspect. 116, 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung E. W., Yan H., Lefèvre P. L., Berger R. G., Rawn D. F., Gaertner D. W., Kawata A., Rigden M., Robaire B., Hales B. F., et al. (2016). Gestational and early postnatal exposure to an environmentally relevant mixture of brominated flame retardants: General toxicity and skeletal variations. Birth Defects Res. B Dev. Reprod. Toxicol. 107, 157–168. [DOI] [PubMed] [Google Scholar]
- Tung E. W. Y., Kawata A., Rigden M., Bowers W. J., Caldwell D., Holloway A. C., Robaire B., Hales B. F., Wade M. G. (2017). Gestational and lactational exposure to an environmentally-relevant mixture of brominated flame retardants: Effects on neurodevelopment and metabolism. Birth Defects Res. 109, 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez L. M., Abruzzese G. A., Motta A. B. (2013). The biology of the peroxisome proliferator-activated receptor system in the female reproductive tract. Curr. Pharm. Des. 19, 4641–4646. [DOI] [PubMed] [Google Scholar]
- Vitti M., Di Emidio G., Di Carlo M., Carta G., Antonosante A., Artini P. G., Cimini A., Tatone C., Benedetti E. (2016). Peroxisome proliferator-activated receptors in female reproduction and fertility. PPAR Res. 2016, e4612306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhang Z., Wu Y., Chen L., Luo Q., Zhang J., Chen J., Luo Z., Huang X., Cheng Y. (2012). Effects of echinomycin on endothelin-2 expression and ovulation in immature rats primed with gonadotropins. Exp. Mol. Med. 44, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster T. F., Harrad S., Millette J. R., Holbrook R. D., Davis J. M., Stapleton H. M., Allen J. G., McClean M. D., Ibarra C., Abdallah M. A., et al. (2009). Identifying transfer mechanisms and sources of decabromodiphenyl ether (BDE 209) in indoor environments using environmental forensic microscopy. Environ. Sci. Technol. 43, 3067–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham G. C., Pinney S. M., Voss R. W., Sjödin A., Biro F. M., Greenspan L. C., Stewart S., Hiatt R. A., Kushi L. H. (2015). Brominated flame retardants and other persistent organohalogenated compounds in relation to timing of puberty in a longitudinal study of girls. Environ. Health Perspect. 123, 1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K., Cheng L., Liu P., Liu Z., Zhao S., Zhu W., Wang Q., Wu H., Han D. (2015). Polyinosinic-polycytidylic acid perturbs ovarian functions through toll-like receptor 3-mediated tumor necrosis factor a production in female mice. Biol. Reprod. 93, 11. [DOI] [PubMed] [Google Scholar]
- Yessoufou A., Hichami A., Besnard P., Moutairou K., Khan N. A. (2006). Peroxisome proliferator-activated receptor alpha deficiency increases the risk of maternal abortion and neonatal mortality in murine pregnancy with or without diabetes mellitus: Modulation of T cell differentiation. Endocrinology 147, 4410–4418. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang Z., Wu Y., Chen L., Luo Q., Chen J., Huang X., Cheng Y., Wang Z. (2012). Regulatory effect of hypoxia-inducible factor-1α on hCG-stimulated endothelin-2 expression in granulosa cells from the PMSG-treated rat ovary. J. Reprod. Dev. 58, 678–684. [DOI] [PubMed] [Google Scholar]
- Zhu A. S., Li A., Ratliff T. S., Melsom M., Garza L. A. (2017). After skin wounding, noncoding dsRNA coordinates prostaglandins and Wnts to promote regeneration. J. Invest. Dermatol. 137, 1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota A. R., Linderholm L., Park J. S., Petreas M., Guo T., Privalsky M. L., Zoeller R. T., Woodruff T. J. (2013). A temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ. Sci. Technol. 47, 11776–11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.