Abstract
Prenatal exposure to cypermethrin is a risk factor for adverse neurodevelopmental outcomes in children. In addition, maternal psychological stress during pregnancy has significant effects on fetal neurodevelopment and may influence end-stage toxicity to offspring by altering maternal xenobiotic metabolism. As such, this study examined effects of maternal exposure to alpha-cypermethrin and stress, alone and in combination, on offspring development, with a focus on fetal neurotoxicity. CD1 mouse dams were administered 10 mg/kg alpha-cypermethrin or corn oil vehicle via oral gavage from embryonic day 11 (E11) to E14. In addition, dams from each treatment were subjected to a standard model of restraint stress from E12 to E14. Cypermethrin treatment impaired fetal growth, reduced fetal forebrain volume, and increased ventral forebrain proliferative zone volume, the latter effects driven by combined exposure with stress. Cypermethrin also impaired migration of GABAergic progenitors, with different transcriptional changes alone and in combination with stress. Stress and cypermethrin also interacted in effects on embryonic microglia morphology. In addition, levels of cypermethrin were elevated in the serum of stressed dams, which was accompanied by interacting effects of cypermethrin and stress on hepatic expression of cytochrome P450 enzymes. Levels of cypermethrin in amniotic fluid were below the limit of quantification, suggesting minimal transfer to fetal circulation. Despite this, cypermethrin increased placental malondialdehyde levels and increased placental expression of genes responsive to oxidative stress, effects significantly modified by stress exposure. These findings suggest a role for interaction between maternal exposures to cypermethrin and stress on offspring neurodevelopment, involving indirect mechanisms in the placenta and maternal liver.
Keywords: cypermethrin, prenatal stress, pyrethroid, embryonic microglia, GABA, placenta
Pyrethroids are a class of insecticides with broad use in agriculture and household insect control. In the United States, the pyrethroid metabolite 3-PBA is detected in urine in 46%–79.8% of the general population, suggesting that exposure is widespread (Tang et al., 2018). Phasing out of organophosphate alternatives has led to significant increases in pyrethroid use, resulting in increased exposure, including to pregnant women (Barr et al., 2010; Williams et al., 2008). Pregnant women are of particular concern for exposure, as maternal pyrethroid exposure during pregnancy is negatively associated with adverse behavioral and motor outcomes in their children (Furlong et al., 2017; Xue et al., 2013). In addition, maternal pyrethroid exposure is linked to lower cognitive abilities and social-emotional measures (Coker et al., 2018; Watkins et al., 2016). Furthermore, increased proximity of pyrethroid application sites to prenatal maternal residence is associated with increased risk of developmental delay and autism spectrum disorders in their children (Shelton et al., 2014).
Cypermethrin, a type II pyrethroid, is among the most frequently detected pyrethroids in food—the main route of pesticide exposure in urban areas is through dietary intake of fruits and vegetables (Tang et al., 2018). In mammals, cypermethrin and other pyrethroids significantly affect a number of molecular targets including voltage-gated sodium channels, voltage-gated calcium channels, chloride channels, and ATPases (Field et al., 2017; Kakko et al., 2003). These molecular targets are critical in regulating neurodevelopmental processes such as neuronal differentiation and migration (Chen et al., 2014; Kamijo et al., 2018; Schwab et al., 2012). Although cypermethrin and other pyrethroids may alter neurodevelopment through direct actions on the embryonic brain, toxicity to the developing brain may also occur through indirect mechanisms, involving alterations in maternal and placental physiology. Epidemiological correlations between maternal pyrethroid exposure and low birth weight (Coker et al., 2018; Ding et al., 2015; Hanke et al., 2003) suggest that placental mechanisms, which support fetal growth through nutrient transport and growth factor synthesis, are likely targets for pyrethroids. Possible placental targets for pyrethroids include voltage-gated calcium channels, maxi chloride channels, and plasma membrane ATPases (Bernucci et al., 2006; Vallejos and Riquelme, 2007).
In addition to environmental toxicants, maternal stress during pregnancy is also a risk factor for altered neurodevelopment and has wide ranging effects on physiology. Prenatal stress (PS) is associated with increased risk for neuropsychiatric disorders in offspring, including increased rates of attention deficit hyperactivity disorder, schizophrenia, and childhood behavioral problems (Fine et al., 2014). PS affects several neurodevelopmental processes implicated in schizophrenia and autism (Benes and Berretta, 2001; Hashimoto et al., 2008; Yip et al., 2008), including microglia development and maturation of the GABAergic system (Bittle et al., 2019; Kawamura et al., 2006; Lussier and Stevens, 2016; Stevens et al., 2013). Previous work in our lab has demonstrated that PS alters the development of these cells through mechanisms involving increased inflammation and oxidative stress in the embryonic mouse brain (Bittle et al., 2019; Gumusoglu et al., 2017). In addition, placental dysfunction plays a role in mediating the effects of maternal stress on offspring neurodevelopment (Bronson and Bale, 2014; Gur et al., 2017; O’Donnell et al., 2009; Pehme et al., 2018).
Given the effects of both prenatal exposure to pyrethroids and maternal stress on neurodevelopment, these factors may have potentially additive or synergistic effects on neurodevelopmental processes (Antonelli et al., 2017). In this respect, pyrethroids interfere with signaling mechanisms necessary for neuronal migration (Kumar et al., 2013; Maurya et al., 2014) and induce oxidative stress (Afolabi et al., 2019; Nasuti et al., 2007), suggesting overlapping mechanisms with PS (Bittle et al., 2019; Stevens et al., 2013). In addition, combined maternal exposure to psychological stress and pyrethroids may have increased neurodevelopmental impacts through altered pyrethroid pharmacokinetics. For example, psychosocial stress significantly affects xenobiotic metabolism through transcriptional regulation of drug metabolizing enzymes in the liver (Konstandi et al., 2014). In the case of pyrethroids like cypermethrin, toxicity in adult animals is largely limited by rapid hepatic metabolism through oxidation reactions catalyzed by Cytochrome P450s or through hydrolysis by carboxylesterases (Crawford et al., 1981). In the event that maternal metabolism is not sufficient and pyrethroid levels persist in circulation, lipophilic xenobiotics such as cypermethrin could cross the placenta via passive diffusion and exert direct effects on the embryonic brain. Fetal levels of pyrethroids following maternal exposure are typically limited (Kaneko et al., 1984; Shiba et al., 1990) but may be increased due to these changes.
As such, we hypothesized that combined maternal exposure to stress and cypermethrin interact, resulting in enhanced effects on offspring neurodevelopment. This is a central hypothesis for understanding effect modifiers for epidemiological links which in turn may identify vulnerable populations to toxicological impacts. To test this hypothesis, experiments were performed using a well-validated mouse model of PS during mid-gestation with the addition of cypermethrin exposure overlapping with the timing of stress exposure. Several neurodevelopmental outcomes were significantly affected by cypermethrin and its combination with maternal stress, despite the short period of exposure. Furthermore, stress altered hepatic xenobiotic metabolism and maternal cypermethrin levels, suggesting that pharmacokinetic changes may play a role in effects of stress and chemical exposure on fetal neurodevelopment. Interestingly, the absence of detectable cypermethrin in amniotic fluid suggested that indirect impacts of stress and cypermethrin on placental functioning as demonstrated here may be responsible for fetal impacts.
MATERIALS AND METHODS
Chemicals
Neat α-cypermethrin (98%, Toronto Research Chemicals, North York, Canada) for the animal studies was further purified by recrystallization in isopropanol to 99% purity (based on relative peak area). The following analytical standards were used: α-cypermethrin (Catalog No. C2237; purity > 99%, Sigma-Aldrich, Saint Louis, Missouri); cis-permethrin (recovery standard, Catalog No. P288560; purity > 99%) and trans-permethrin (recovery standard, Catalog No. P288550; purity > 99%) both from Toronto Research Chemical; internal standard, deltamethrin (Catalog No. C1212000; purity > 99%, Crescent Chemical, Islandia, New York); untreated mouse plasma (Innovative Research, Novi, Michigan); pesticide grade solvents (Fisher Scientific, Pittsburg, Pennsylvania).
Animals and treatments
All experiments were conducted with the approval of the Institutional Animal Care and Use Committee of the University of Iowa. CD1 females (7–9 weeks old, Charles River) were mated with GAD67-GFP+/− knock-in males bred on a CD1 background. All mice were housed in cages on a 12-h light/dark cycle with free access to food and water. Gestational day 0 (E0) was determined upon detection of a vaginal plug. Pregnant dams were singly housed from E0 to E14.5.
A total of 24 pregnant CD1 dams were treated with 10 mg/kg alpha-cypermethrin (cyp) or corn oil vehicle (veh) via oral gavage each morning from gestational days 11 to 14. The dose of 10 mg/kg was determined from a pilot study in our lab to reduce fetal body weight and was tolerated throughout the treatment period by the pregnant dams. In comparison to human exposure limits set by health organizations, the dose of 10 mg/kg is relatively high. Use of a high dose was justified due to the goal of identifying mechanisms by which stress and toxicant exposure overlap and is not a translation into common human exposures. This short, 3-day treatment aligned with an established 3-day stress intervention protocol. Gestational days 11 through 14 encompass a concentrated period of neurogenesis and tangential migration of GABAergic progenitors from the ventral to the dorsal forebrain-endpoints central to this paper. Mice receiving either treatment (cypermethrin or vehicle) were split into nonstressed (NS) or PS group (NS veh: n = 5, PS veh: n = 5, NS cyp: n = 7, PS cyp: n = 7). On days 12 and 13, mice in the PS treatment groups were subjected to 3, 45-min sessions of restraint stress under bright light as previously described, the first episode 30 min after gavage. On gestational day 14, all dams received a final dose of cypermethrin or vehicle 90 min prior to euthanasia, followed by a final round of restraint stress in the stressed groups, ending 15 min prior to euthanasia. Pregnant dams were euthanized by ketamine/xylazine anesthesia followed by rapid decapitation.
Immunohistochemistry
GAD67-GFP+ offspring brains were fixed in 4% paraformaldehyde, cryoprotected (Optimal Cutting Temperature Compound, Fisher Scientific) and cryosectioned (Leica, 1510-3, Bannockburn, Illinois) at 25 μm. Slide mounted sections were immunostained using blocking (10% goat serum in PBS with 0.025% TritonX-100, 0.0125% Tween20) overnight incubation at 4°C in primary antibodies (anti-GFP, 1:1000, Abcam, AB13970, No. 660556; anti-Iba-1, 1:500, WAKO, rabbit polyclonal), followed by incubation with Alexa dye-conjugated secondary antibodies (1:500; Molecular Probes). Slides were coverslipped using DAPI (4′,6-diamidino-2-phenylindole, Vector Laboratories, No. H-1200).
Immunohistochemical measurements
Tangential migration of GAD67-GFP+ cells in E14 coronal tissue sections was measured using fluorescent microscopy with a Zeiss (Jena, Germany) Axiolmager M2 microscope with StereoInvestigator (MBF Biosciences, Vermont) software as previously described (Bittle et al., 2019). Migration was determined by the circumferential length of the superficial stream of GAD67GFP+ cells in dorsal forebrain as a percentage of the entire circumference of the cortical plate. Migration measurements were averaged across 3 anterior to posterior sections for each brain, using 1 male and 1 female (sex determined by genotyping) per litter when available (n = 5–7 per group).
Volume measurements of fetal forebrain and the ganglionic eminence proliferative ventricular zone were performed using 1 male and 1 female per litter when available (n = 5–7 per group). Every 20th section (6–7 sections per brain) was analyzed, defining the ganglionic eminence proliferative ventricular zone by an absence of GAD67-GFP expression.
Microglia assessment
Stereological cell counting was performed using the optical fractionator approach and unbiased counting rules. Counts were performed using 3-dimensional 200 × 150 × 15 μm counting frames, on a 500 × 300 μm grid with a ×40 objective lens (StereoInvestigator; MBF Biosciences). Stereological counting to determine cell density was assessed using 3–4 serial coronal sections (every 20th section) of the embryonic cortical plate as previously described (Bittle and Stevens, 2018; Gumusoglu et al., 2017). Brains were analyzed from 1 male and 1 female per litter when available (n = 5–7 per group).
MDA measurement
Lipid peroxidation marker malondialdehyde (MDA) was quantified in offspring placenta and brain homogenates using the thiobarbituric acid reactive substance assay and was normalized to total protein determined by BCA assay (Janero, 1990). Briefly, 1 male and 1 female placenta or brain from each litter (n = 4–7 per group) was homogenized separately in RIPA buffer, followed by centrifugation (3000 × g, 10 min, 4°C). MDA standards (0–50 μM) were prepared using 1,1,3,3-tetramethoxypropane. Sample supernatant or standard and acid solution (15% TCA, 0.37% TBA, 0.25 M HCl) were incubated (95°C, 45 min) and centrifuged (14 000 rpm, 5 min, 4°C). Supernatants were vortexed with n-butanol and saturated NaCl (30 s) and centrifuged (14 000 rpm, 2 min, 4°C). Supernatant absorbance was measured in duplicate at 535 and 572 nm (correcting for blank levels). Corrected absorbance values (Abs 535–572) were used to calculate concentrations of MDA in each sample using the standard curve.
Reduced thiol content
Total reduced thiol content in offspring placenta and brain was quantified using Ellman’s assay and normalized to total protein determined by BCA assay (Ellman, 1959). One male and 1 female placenta or brain from each litter (n = 4–7 per group) was homogenized and centrifuged as above. For determination of tissue reduced thiol concentration, 50 µl sample homogenate or standard (0–1000 µM glutathione) was mixed with 200 µl Tris HCl pH (8.9) and 20 µl DTNB solution (29.7 mg DTNB in 25 ml methanol). Absorbance was measured at 412 nm and sample reduced thiol concentrations were determined using the standard curve.
Gene expression analysis
RNA was isolated from maternal liver as well as placenta and ventral forebrain from 1 male and 1 female per litter (n = 5–7 per group) with the TRIzol-Chloroform method as previously described (Rio et al., 2010). Genomic DNA contamination was removed from RNA using the TURBO DNA-free kit (Thermo Scientific, AM1907). RNA concentrations were determined using a NanoDrop Spectrophotometer (Thermo Scientific). cDNA was synthesized using the AMV Reverse Transcriptase cDNA Synthesis Kit (M0277S). qPCR was performed with Power SYBR Green Master Mix (Thermo Fisher Scientific, Warrington, UK) and primers (see Supplementary Table 1) using an Applied Biosystems Model 7900HT instrument. The relative expression of each gene as compared with housekeeping gene expression was calculated using the ΔΔCt method. Hprt1, Ubc, and Gapdh were used as housekeeping genes for maternal liver, placenta, and embryonic brain samples, respectively.
RNAseq analysis of migrating GABAergic progenitors
Laser capture microdissection was utilized to isolate migrating GAD67GFP+ cells from the cortical plate of fixed embryonic brain 10-µm tissue slices on metal frame PET foil slides (Leica) from 1 male embryo per litter (n = 4 per group). Approximately 100 individual GFP+ cells were collected at the leading edge of migration from each of 10 tissue sections, giving approximately 1000 cells per sample. RNA was isolated from the tissue using the RecoverAll Total Nucleic Acid Isolation kit (Thermo Fisher Scientific, AM1975) and libraries were prepared using the Trio RNAseq kit (NuGen). Sixteen samples were run on a single lane using HiSeq4000 with a 75-bp Paired End chemistry. Reads were mapped with STAR 2.5 to mouse mm10 and a Refseq transcriptome. One sample in the PS veh group was removed prior to analysis due to sex-genotyping error. RNA sequencing (RNAseq) data were made publicly available at GEO (GSE144511).
Differential expression analysis was performed with edgeR (Version 3.26.3) (McCarthy et al., 2012; Robinson et al., 2010) to assess main effects of cypermethrin (NS veh + PS veh vs NS cyp + PS cyp) and stress (NS veh + NS cyp vs PS veh + PS cyp), along with the interaction between cypermethrin and stress (Table 1 and Supplementary Table 2). In addition, we assessed for effects of each individual treatment group in comparison with NS control (ie, NS veh vs PS veh, NS veh vs NS cyp, NS veh vs PS veh) (Table 2 and Figure 3). A total of 13 926 transcripts were included in the analyses after removing low count genes (mean counts < 5). Counts were normalized using the default TMM method. Genewise negative binomial generalized linear models were fit using the function glmFit followed by the function glmLRT. Differentially expressed (DE) genes were identified based on an FDR < .05. Due to a low number of DE genes at this cut-off, lower-confidence changes in genes with an uncorrected p value of less than .05 were included in pathway analysis conducted using Ingenuity Pathway Analysis (IPA) software. Canonical pathways enriched in genes altered by cypermethrin, stress, and the combination of both treatments were compared using IPA’s “Comparison Analysis” function.
Table 1.
Top DE Genes Induced by Main Effects and Interaction of Cypermethrin and Stressa
| Gene | logFC | FDR |
|---|---|---|
|
A. Altered genes by stress | ||
| Ky | −6.93 | .029 |
| Stbd1 | −5.93 | .029 |
| Ogg1 | 4.56 | .029 |
| Marveld1 | −2.55 | .029 |
| Gm10371 | 6.7 | .029 |
| Gdpd3 | 3.39 | .031 |
|
| ||
|
B. Altered genes by cypermethrin | ||
| C130046K22Rik | −4.4 | .009 |
| Cpeb1 | 4.08 | .028 |
| Map7D3 | 5.19 | .032 |
|
| ||
|
C. Top 10 unique genes with significant interaction | ||
| Gene | FDR | |
| A730020M07Rik | 1.12E−05 | |
| Prtg | 3.89E−05 | |
| Vmn2r6 | 4.41E−04 | |
| Nrxn2 | 4.41E−04 | |
| Mir669d-2 | 1.88E−03 | |
| Auh | 2.30E−03 | |
| Med25 | 2.71E−03 | |
| Kcnk18 | 2.71E−03 | |
| Grem1 | 4.19E−03 | |
| Plscr2 | 5.39E−03 | |
Differential expression analysis was performed using edgeR. Genes with FDR < .05 were considered DE.
Abbreviation: FC, fold change.
Table 2.
Pathway Enrichment Analysis of Genes Altered by Cypermethrin, Stress, and Combinationa
| A. Top pathways enriched by maternal stress (PS veh) | p Value |
|---|---|
| DNA double-strand break repair by nonhomologous end joining | 1.97E−03 |
| Thyroid hormone biosynthesis | 4.83E−03 |
| VEGF signaling | 5.68E−03 |
| 2-Ketoglutarate dehydrogenase complex | 9.41E−03 |
| Acute myeloid leukemia signaling | 9.53E−03 |
|
| |
|
B. Top pathways enriched by maternal cypermethrin exposure (NS cyp) |
p Value |
| Serine biosynthesis | 3.74E−04 |
| Superpathway of serine and glycine biosynthesis I | 1.24E−03 |
| LXR/RXR activation | 1.33E−03 |
| Type II diabetes mellitus signaling | 3.06E−03 |
| Angiopoietin signaling | 3.63E−03 |
|
| |
|
C. Top pathways enriched by combination of stress and cypermethrin (PS cyp) |
p Value |
| PRPP biosynthesis I | 6.61E−03 |
| DNA double-strand break repair by homologous recombination | 1.07E−02 |
| Serine biosynthesis | 1.08E−02 |
| Arginine degradation VI (arginase 2 pathway) | 1.58E−02 |
| Aryl hydrocarbon receptor signaling | 1.78E−02 |
Pathway analysis was performed using IPA’s “Core Analysis” function with the “Mouse” Species filter. Genes with uncorrected p values < .05 were included in the analysis.
Abbreviations: NS, nonstressed; PS, prenatal stress.
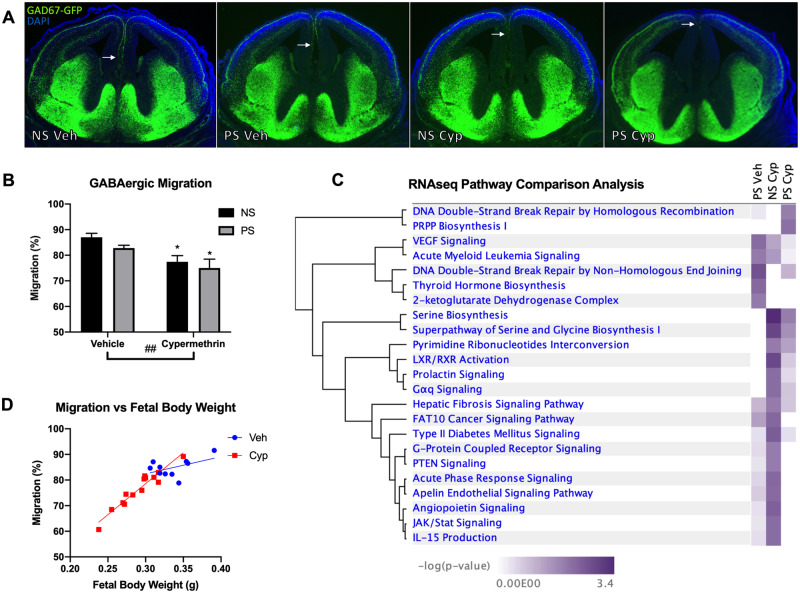
Figure 3.
Maternal cypermethrin exposure impaired tangential migration of GABAergic progenitors. A, Representative pictures highlighting the furthest extent of GABAergic progenitor tangential migration (arrows). B, Cypermethrin impaired GABAergic progenitor tangential migration. C, Ingenuity Pathway Analysis Pathway Comparison Analysis assessing canonical pathways enriched by cypermethrin (NS cyp), stress (PS veh), and the combination of the 2 treatments (PS cyp) in isolated GABAergic progenitors. D, Cypermethrin-induced delays in GABAergic migration were correlated with fetal growth restriction (r = .9598), despite no significant correlation in vehicle-treated embryos (r = .4936). Means ± SEMs are shown. n = 5–7 litters per treatment group (*p < .05 by post hoc tests) (##p < .01 main effect by 2-way ANOVA). Abbreviations: NS, nonstressed; PS, prenatal stress.
Measurement of cypermethrin in maternal serum and amniotic fluid
A standard operating procedure outlining the methods used to measure cypermethrin in biological samples was made available online at dx.doi.org/10.17504/protocols.io.y8ifzue. All cypermethrin analyses were performed with the analyst blinded to exposure group. Briefly, acetonitrile (900 µl), serum or amniotic fluid samples (200 µl), cis- and trans-permethrin (recovery standard, 100 ng each in acetonitrile [100 µl]), and water (800 µl) were added to quick, easy, cheap, effective, rugged, and safe (QuEChERS) tubes (15 ml, with 800 mg of magnesium sulfate and 200 mg of sodium chloride; United Chemical, Bristol, Pennsylvania) to give a final volume of 3 ml. After vortexing (1 min) and centrifugation (1791 × g, 5 min), aliquots of the acetonitrile phase (1 ml) were transferred into dispersive solid phase extraction (dSPE) tubes (2 ml, 150 mg magnesium sulfate and 50 mg CEC18; United Chemical) and vortexed (1 min) and centrifuged (1479 × g, 5 min). Aliquots (500 µl) from each dSPE tubes were stored overnight (RT) in glass vials, evaporated to dryness under a gentle stream of nitrogen, dissolved in ethyl acetate (800 µl), and a deltamethrin solution (200 ng in ethyl acetate [200 µl]) was added as the internal standard. Water blanks, method blanks, and ongoing precision and recovery (OPR) standards (200 µl of untreated serum or plasma spiked with 100 ng cypermethrin in acetonitrile [100 µl]) were extracted in parallel with each set of samples. All extracts were analyzed on an Agilent technologies 7890A gas chromatograph equipped with a 63Ni-micro electron capture detector (µECD) and an Agilent 7693 autosampler using a column (60-m length, 250-µm inner diameter, 0.25-µm film thickness; Supelco, St. Louis, Missouri) (Lentza-Rizos et al., 2001). The temperature program was as follows: 50°C, hold for 1 min, 15°/min to 220°C, 1°/min to 240°C, hold for 12 min, 15°/min to 280°C, and hold for 10 min. The helium (carrier gas) and makeup flow rates were 1.0 and 60 ml/min, respectively. The inlet and detector temperature were 240°C and 300°C, respectively. Levels of cypermethrin were determined using the internal standard method and are not adjusted for the recoveries of cis-/trans-permethrin.
The response of the μECD was linear (R2 ≥ .995) for all analytes (cypermethrin, cis- and trans-permethrin, and deltamethrin) within the concentration range encountered in the study (1–1000 ng/ml). Limits of detection (LOD) and limits of quantification (LOQ) were calculated based on water and serum blanks using the formula: LOD = average blanks + k * standard deviation blanks (k is Student’s t value for n−1 degrees of freedom at 99% confidence level and n is number of blanks) (Kania-Korwel et al., 2007). The limits of quantification (LOQ) were calculated as LOQ = LOD * 10 LOD. The LOD and LOQ for cypermethrin were 0.5 and 5 ng, respectively. Background levels of cypermethrin in blank plasma were 1 ± 0.27 ng/ml. The background levels of cypermethrin in serum and amniotic fluid from all control animals were 0.95 ng/ml (0.97 ng/g; n = 10) and 0.54 ng/ml (0.56 ng/g; n = 10), respectively. Recoveries of cypermethrin from the OPR samples were 81% (range: 70%–95%; n = 11). Cis- and trans-permethrin were used to assess the precision of the extractions. Recoveries of cis- and trans-permethrin were 72% (range: 52%–95%; n = 75) and 73% (range: 53%–95%; n = 75), respectively.
Statistical analysis
The litter was treated as the statistical unit for all measurements, using 1 male and 1 female per litter when available. On average, tissues from over half of all embryos from each litter were used for analysis, dispersed across multiple experiments. Sex differences were first evaluated for main effect and interaction and if not found, male and female samples within litters were averaged. Parametric data from treated groups were analyzed for main and interaction effects of stress and cypermethrin exposure by 2-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. With small sample sizes, ANOVA and post hoc tests may differ in their findings; this was considered in the interpretation to avoid type II statistical error. Nonparametric results were analyzed with a Mann-Whitney U test. Pearson correlation was used to evaluate inter-relationship between outcomes, using a Bonferroni correction for multiple comparisons with determine significance. Outliers identified using Grubbs’ test were removed prior to statistical analysis. All analyses were performed with GraphPad Prism (San Diego, California).
RESULTS
Maternal Cypermethrin and Stress Reduced Maternal and Fetal Body Weights
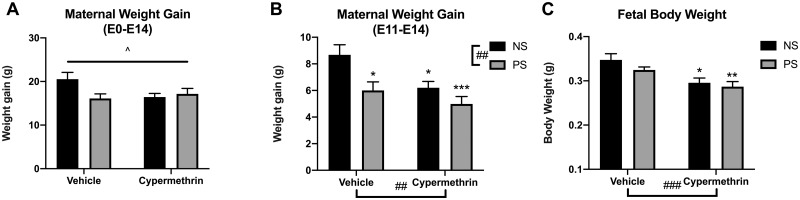
Maternal weight gain throughout pregnancy (E0–E14) was significantly affected by treatments (Figure 1A; ANOVA interaction, p = .042). Weight gain specifically during the treatment period (E11–E14) was significantly reduced by cypermethrin, stress, or combined exposure (Figure 1B; cyp main effect, F = 10.35, p = .0091; stress main effect, F = 8.33, p = .0043; post hoc tests: PS veh, p = .023; NS cyp, p = .023; PS cyp, p = .0009). Fetal body weight at E14 was reduced from control by cypermethrin treatment (Figure 1C; cyp main effect, F = 15.29, p = .0009; post hoc tests: NS cyp, p = .012; PS cyp, p = .0035). Cypermethrin-induced reductions in fetal weight may account for reduced maternal weight gain in these groups. There were no differences in total litter size.
Figure 1.
Maternal stress and cypermethrin exposure reduced maternal and fetal weight gain. A, Treatment changed maternal weight gain throughout pregnancy (E0–E14). B, Maternal weight gain specifically during the treatment period (E11–E14) was decreased by stress, cypermethrin, or combined exposure. C, Fetal body weight was reduced by cypermethrin exposure alone or in combination with stress. Means ± SEMs are shown. n = 5–7 litters per treatment group (*p < .05, **p < .01, ***p < .001 by post hoc tests) (##p < .01, ###p < .001 main effect by 2-way ANOVA). Abbreviations: NS, nonstressed; PS, prenatal stress.
Maternal Cypermethrin and Stress Impaired Development of the Embryonic Brain
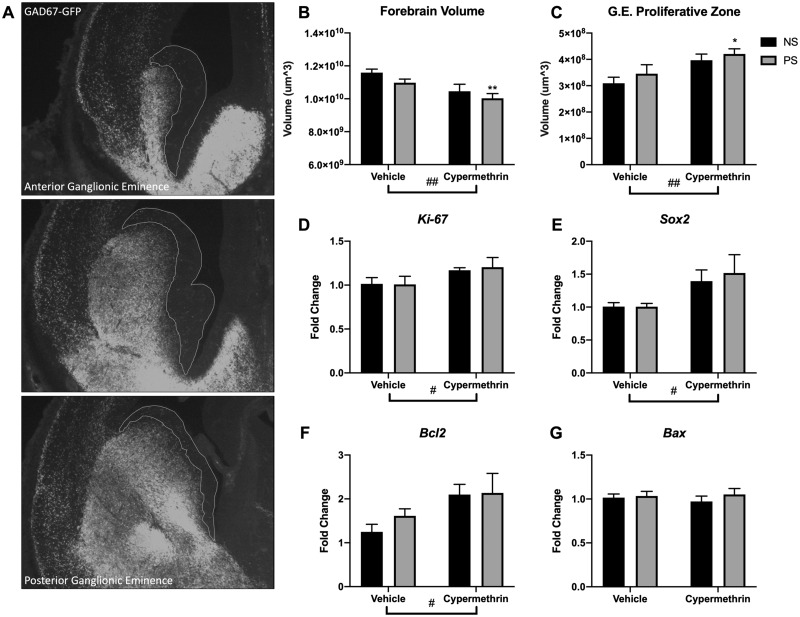
Cypermethrin reduced total E14 forebrain volume (Figure 2B; cyp main effect, F = 9.73, p = .0054) and increased E14 volume of the ganglionic eminence proliferative zone of forebrain GABAergic neurons (Figs. 2A and 2C; cyp main effect, F = 10.60, p = .0040). In both cases, these effects were driven by the stress and cypermethrin combined exposure group (PS cyp post hoc tests: forebrain, p = .0092; ganglionic eminence, p = .0131). Consistent with a larger proliferative zone, cypermethrin treatment increased E14 gene expression of the cell proliferation marker Ki-67 (Figure 2D; cyp main effect, F = 4.41, p = .049) and stem cell marker Sox2 (Figure 2E; cyp main effect, F = 5.13, p = .035) in ganglionic eminence. As brain growth may also reflect changes in cell death, gene expression at E14 of Bax and Bcl2 in ganglionic eminence was assessed; there was no change in the proapoptotic regulator, Bax (Figure 2G), but there was an increase in expression of the antiapoptotic regulator, Bcl2, with cypermethrin treatment (Figure 2H; cyp main effect, F = 4.68, p = .043), suggesting induction of this system to maintain cell survival.
Figure 2.
Maternal cypermethrin and stress exposure impaired forebrain growth and GABAergic system development. A, Representative pictures highlighting ganglionic eminence proliferative zone (white outline). B, Cypermethrin reduced forebrain volume. C, Cypermethrin increased ganglionic eminence proliferative zone volume. (D) Cypermethrin increased ventral forebrain gene expression of cell proliferation marker Ki-67 and (E) stem cell marker Sox2. (F) Cypermethrin increased gene expression of apoptotic inhibitor Bcl2 in the ventral forebrain (G) but had no effect on gene expression of proapoptotic regulator Bax. Means ± SEMs are shown. n = 5–7 litters per treatment group (*p < .05, **p < .01 by post hoc tests) (#p < .05, ##p < .01 main effect by 2-way ANOVA). Abbreviations: G.E., ganglionic eminence; NS, nonstressed; PS, prenatal stress.
Maternal Cypermethrin Exposure Impaired GABAergic Progenitor Migration
With the ganglionic eminence changes shown above suggesting increased GABAergic progenitor cell generation, further development of cortical GABAergic interneurons was assessed. Tangential migration of GABAergic cortical interneuron progenitors in embryonic brain is susceptible to maternal stress as well as chemical exposures including cocaine, alcohol, and caffeine (Crandall et al., 2004; Cuzon et al., 2008; Silva et al., 2013). Here, treatment E11–E14 with 10 mg/kg cypermethrin delayed tangential migration of cortical interneuron progenitors as measured at E14 (Figure 3B; cyp main effect, F = 10.62, p = .0043; post hoc tests: NS cyp, p = .036; PS cyp, p = .017). Although GABAergic migration was not correlated with fetal body weight in vehicle-treated animals (Figure 3D, r = .49, p = .15, corrected α .0022), there was a significant correlation between the degree of growth restriction and the degree of migration delay in cypermethrin-treated embryos at E14 (Figure 3D, r = .96, p < .0001, corrected α .0022). These results suggest that cypermethrin-induced impairments in GABAergic migration may be a component of fetal growth restriction or that these endpoints arise from common mechanisms.
Embryonic Brain Oxidative Stress Was Not Increased by Maternal Exposures
Previous work in our lab has demonstrated that alterations in GABAergic progenitor migration induced by maternal restraint stress may result from oxidative stress in the embryonic brain. We assessed here whether oxidative stress in the embryonic brain occurred with cypermethrin, stress, or combined exposures. Interestingly, there were no differences between groups in tissue malondialdehyde or reduced thiol content in offspring brain at E14 (Supplementary Figs. 1A and 1B). We also found no changes in ganglionic eminence gene expression of the endogenous antioxidant Sod1, the antioxidant response gene Nrf2, or the hypoxia-responsive gene Hif-1alpha (Supplementary Figs. 1C–E). These results prompted assessment of other potential mechanisms.
Altered Transcriptional Profile of Migrating GABAergic Progenitors
To specifically examine cortical interneuron progenitors and understand the mechanisms by which their migration was affected, E14 GABAergic cells in the outer migratory stream were isolated from all 4 groups via laser capture for RNA isolation and sequencing. By the conservative FDR cut-off, each exposure had distinct impacts by assessment of main effects of maternal stress and cypermethrin, as well as the interaction between the 2 exposures (Table 1). Three sequences were DE due to cypermethrin exposure: sequences for a long noncoding RNA (C130046K22Rik) near the proliferation enhancing gene, Wntb9; the translation regulator, Cpeb1; and a microtubule-interacting protein, Map7D3 (Table 1B). In this same analysis, stress exposure resulted in 6 DE genes with varied functions: Ky (regulates developmental autophagy), Stbd1 (has glycogen transport activity), Ogg1 (repairs oxidative DNA damage), Marveld1 (regulates neuronal migration), Gm10371 (unknown function), and Gdpd3 (a phospholipid metabolism protein) (Table 1A). The interaction of cypermethrin and stress exposures resulted in 60 unique DE transcripts including these top 10: A730020M07Rik (unknown), Prtg (a gene regulating neural progenitor migration), Vmn2r6 (a receptor implicated in pheromone detection), Nrxn2 (involved in synaptogenesis), Mir669d-2 (a microRNA with unknown function), Auh (RNA degradation), Med25 (RNA polymerase regulation), Kcnk18 (potassium channel domain), Grem1 (stem cell maintenance), and Plscr2 (regulator of phospholipids) (Table 1C).
We next performed pathway analysis of lower-confidence altered genes (p < .05) in interneuron progenitors to identify enriched canonical pathways of effect for each individual group in comparison with control. This revealed some distinctly different and some overlapping transcriptional changes due to stress, cypermethrin, and combined exposure (Figure 3C and Table 2). Cypermethrin exposure alone altered transcription in these interneuron progenitors with enrichment of biosynthetic, nuclear receptor-mediated, and angiogenic pathways (Table 2B). The stress exposure transcriptional profile was enriched for pathways that implicated DNA double-stranded breaks, alterations in thyroid hormone synthesis, and angiogenesis (Table 2A). Combined treatment of cypermethrin and stress altered transcription of biosynthetic, nuclear receptor-mediated, and DNA damage pathways (Table 2C). Overall, significant overlap between the cypermethrin-exposed groups was observed, in particular highlighting pathways involved in serine biosynthesis. In addition, both stressed groups showed enrichment of DNA damage repair pathways. These results suggest overlapping and unique mechanisms by which these 2 exposures impact neuronal migration. Pathway analyses for main effects and interaction showed similar findings (Supplementary Table 2).
Maternal Cypermethrin and Stress Alter Microglia Morphology in the Embryonic Brain
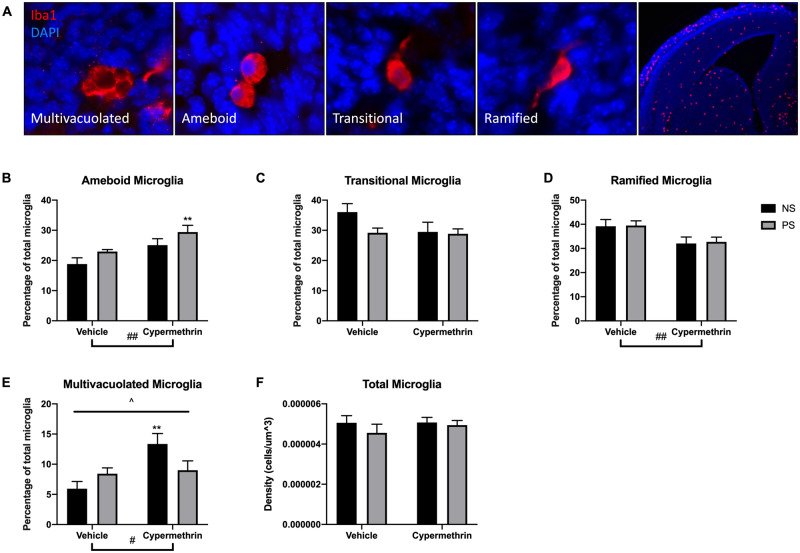
Previous work has shown that cypermethrin and other pyrethroids alter adult microglia morphology in vitro and in vivo (Hossain et al., 2017; Singh et al., 2011, 2016). In addition, we have previously shown that maternal stress alters the morphology of microglia in the embryonic brain through inflammatory mechanisms (Gumusoglu et al., 2017). Microglia morphology in E14 brain may indicate developmental stage or activation by indicating the degree of ramification (ameboid, transitional, or ramified) or the presence of multiple vacuoles indicating phagocytic state (multivacuolated) (Figure 4A). Cypermethrin exposure increased ameboid microglia at E14 (Figure 4B, cyp main effect, F = 9.62, p = .0056), driven by the combined exposure of cypermethrin with maternal stress (PS cyp post hoc test, p = .0043). Along with the increase in ameboid morphology, cypermethrin treatment decreased ramified microglia, suggesting a general delay in development of these cells (Figure 4D, cyp main effect, F = 8.17, p = .0097). Interestingly, maternal cypermethrin exposure and stress interacted in their effects on multivacuolated microglia, with only the cypermethrin alone group increasing this morphology (Figure 4E, ANOVA interaction, F = 4.92, p = .038; cyp main effect, F = 6.73, p = .017; NS cyp post hoc test, p = .0075). Neither exposure in this study altered total microglia density in the embryonic brain (Figure 4F). Overall, these results suggest a delay in development of microglia in response to combined cypermethrin and stress exposure, with cypermethrin exposure alone inducing an activated microglia state.
Figure 4.
Maternal cypermethrin and stress altered microglia morphology in the E14 dorsal forebrain. A, Representative pictures of E14 microglia morphologies: (1) Multivacuolated, with multiple vacuoles and/or pyknotic nuclei, (2) Ameboid, with no processes and normal nucleus, (3) Transitional, with 1 process and normal nucleus, (4) Ramified, with 2 or more processes and normal nuclei (high magnification). Representative picture of the forebrain stained for Iba1 to the right (low magnification). B, Cypermethrin increased percentage of ameboid microglia. C, No effect for either treatment on transitional microglia. D, Cypermethrin decreased the percentage of ramified microglia. E, Cypermethrin alone, but not in combination with stress, increased the percentage of multivacuolated microglia. F, No effect for either treatment on total microglia density. Means ± SEMs are shown. n = 5–7 litters per treatment group (**p < .01 by post hoc tests) (#p < .05, ##p < .01 main effect by 2-way ANOVA) (^p < .05 interaction by 2-way ANOVA). Abbreviations: NS, nonstressed; PS, prenatal stress.
Maternal Stress Increased Cypermethrin Concentration in Maternal Serum
Levels of cypermethrin were assessed in maternal and embryonic compartments to determine the possibility of direct toxicity. Cypermethrin level was increased in the serum of stressed versus NS dams (Figure 5A; Mann-Whitney test, p = .014). Previous studies have suggested that restraint stress may alter xenobiotic metabolism, so hepatic expression of enzymes responsible for cypermethrin metabolism were evaluated. Consistent with higher serum cypermethrin resulting from reduced metabolism after stress, restraint stress suppressed cypermethrin-induced upregulation of Cyp1a1 in maternal liver (Figure 5C; ANOVA interaction, F = 5.06, p = .036; NS cyp post hoc test, p = .011). In addition, restraint stress decreased hepatic expression of Cyp1a2 (Figure 5D, stress main effect, F = 5.09, p = .036; PS cyp post hoc test, p = .034) and Cyp2b10 (Figure 5E, stress main effect, F = 12.47, p = .0022; NS cyp vs PS cyp post hoc test, p = .013). However, neither stress nor cypermethrin affected hepatic expression of Cyp2e1, Cyp3a11, or Cyp3a41b (Figs. 5F–H). In addition, neither stress nor cypermethrin affected hepatic expression of carboxylesterase 1 or 2, enzymes that catalyse the hydrolysis of pyrethroids (Supplementary Figure 2). Interestingly, levels of cypermethrin in amniotic fluid were below LOQ for all cypermethrin-treated samples, suggesting that there was minimal transfer of cypermethrin from maternal to fetal circulation (Figure 5B).
Figure 5.
Maternal stress increased concentration of cypermethrin in maternal serum and altered maternal hepatic expression of cytochrome P450 enzymes after cypermethrin exposure. A, Restraint stress increased the concentration of cypermethrin in maternal serum. B, Cypermethrin levels in amniotic fluid were below the limit of quantification. C, Stress suppressed cypermethrin-dependent upregulation of hepatic Cyp1a1 expression. D, Stress reduced hepatic expression of Cyp1a2. E, Stress reduced hepatic expression of Cyp2b10. F–H, Stress or cypermethrin did not affect hepatic expression of Cyp2e1, Cyp3a11, or Cyp3a41b. Means ± SEMs are shown. n = 5–7 litters per treatment group (*p < .05 by Mann-Whitney or post hoc tests) (#p < .05, ##p < .01 main effect by 2-way ANOVA) (^p < .05 interaction by 2-way ANOVA). Abbreviations: NS, nonstressed; PS, prenatal stress.
Maternal Cypermethrin and Stress-induced Oxidative Stress and Endothelial Changes in Placental Tissue
Given the low level of cypermethrin in the embryonic compartment, neurodevelopmental changes observed in this study may be mediated through indirect mechanisms. Placental dysfunction is a possible mechanism for these effects, as shown for maternal stress (Dahlerup et al., 2018; Mairesse et al., 2007; Monk et al., 2016; Reynolds et al., 2013). The placenta is a prime target for the effects of toxicants as it comes into contact with large amounts of maternal blood, concentrates lipophilic toxicants via protein binding, and has the potential to bioactivate toxicants due to the presence of cytochrome P450 enzymes (Murayama et al., 2017).
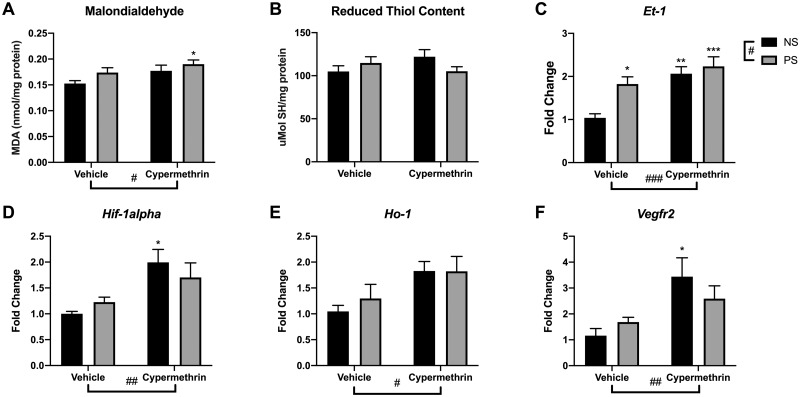
Given that previous studies have implicated oxidative stress as a common mechanism by which pyrethroids and stress impact physiology, we investigated whether oxidative stress occurred in placental tissue here. Analysis of malondialdehyde, a byproduct of lipid peroxidation, revealed an increase in placental tissue due to cypermethrin exposure (Figure 6A; main effect, F = 4.99, p = .041), driven by the combined impact of cypermethrin and stress (Figure 6A; PS cyp post hoc test, p = .032). However, placental reduced thiol content was not affected by either exposure (Figure 6B). Consistent with increased tissue malondialdehyde, analysis of placental gene expression revealed that cypermethrin exposure increased the expression of several genes relevant to hypoxia/oxidative stress processes, including Hif-1alpha, Vegfr2, Et-1, and Ho-1 (Figs. 6C–F; cyp main effects, F = 10.24, p = .0045; F = 8.49, p = .0086; F = 15.11, p = .0009; F = 7.44, p = .013, respectively). Gene expression of endothelin-1 (Et-1), a potent vasoconstrictor, was also increased by stress exposure (Figure 6C; stress main effect, F = 6.72, p = .017). Interestingly, the effects of cypermethrin on Hif-1alpha and Vegfr2 gene expression, critical factors in the angiogenic response to oxidative stress, were driven by exposure to cypermethrin alone (Figs. 6D and 6F; NS cyp post hoc test, p = .016 and p = .021, respectively). Examination of the relationship of developmental measures to these placental responses revealed negative correlations of GABAergic migration and fetal weight with heme oxygenase 1 (Ho-1) expression (r = −.63, p = .0009; r = −.66, p = .0005, respectively, corrected α .0022) and a negative correlation of forebrain volume with Et-1 expression (r = −.60, p = .0022, corrected α .0022).
Figure 6.
Maternal cypermethrin and stress altered placental oxidative stress and gene expression. A, Cypermethrin increased lipid peroxidation marker malondialdehyde in placental tissue. B, Neither treatment significantly affected placental reduced thiol content. C, Placental endothelin-1 expression increased in response to both cypermethrin and stress. (D) Cypermethrin treatment increased placental gene expression of several angiogenic genes responsive to oxidative stress, including Hif-1alpha, (E) heme oxygenase 1, and (F) Vegfr2. Means ± SEMs are shown. n = 4–7 litters per treatment group (*p < .05, **p < .01, ***p < .001 by post hoc tests) (#p < .05, ##p < .01, ###p < .001 main effect by 2-way ANOVA). Abbreviations: NS, nonstressed; PS, prenatal stress.
Maternal Cypermethrin and Stress Altered Placental Gene Expression of Nutrient Transporters But Not Growth Factors
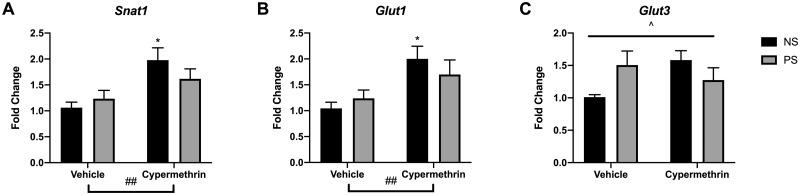
Due to reductions in fetal weight and forebrain volume, assessments were made of nutrient transporters and placental growth factors. Cypermethrin exposure upregulated placental expression of nutrient transporters Snat1 (Figure 7A, cyp main effect, F = 10.61, p = .0040) and Glut1 (Figure 7B, cyp main effect, F = 8.77, p = .0077). This effect on transporters was driven primarily by cypermethrin exposure alone (Figs. 7A and 7B; NS cyp post hoc tests: Snat1, p = .011; Glut1, p = .027). Interestingly, stress and cypermethrin interacted to influence Glut3 expression (Figure 7C, ANOVA Interaction, F = 5.64, p = .028) in a pattern somewhat similar to Snat1 and Glut1, suggesting that stress may impair the placenta’s ability to upregulate nutrient transporters in response to cypermethrin-induced fetal growth restriction.
Figure 7.
Maternal cypermethrin and stress altered placental gene expression of nutrient transporters. A, Cypermethrin increased gene expression of Snat1. B, Cypermethrin increased expression of Glut1. C, Cypermethrin and stress had interacting effects on placental Glut3 expression. Means ± SEMs are shown. n = 5–7 litters per treatment group (*p < .05 by post hoc tests) (#p < .05, ##p < .01 main effect by 2-way ANOVA) (^p < .05 interaction by 2-way ANOVA). Abbreviations: NS, nonstressed; PS, prenatal stress.
Examination of the relationship of developmental measures to these placental nutrient transporter changes revealed that vehicle-exposed groups had a negative correlation of fetal weight with Snat-1 expression (r = −.84, p = .0028, corrected α .0022), a relationship that was completely abolished with cypermethrin exposure (r = .05, p = .87, corrected α .0022). This further suggests an interaction of cypermethrin and stress on the relationship between growth impairment and placental nutrient transport. Together with other placental findings, these data suggest that stress may modify the placenta’s transcriptomic response to the effects of cypermethrin. No significant effects were observed on placental expression of Plgf, Igf-2, or Vegf-a (Supplementary Figure 3) suggesting that impaired placental production of growth factors was not a persistent feature of stress or cypermethrin impacts.
DISCUSSION
In this study, maternal restraint stress and cypermethrin exposure during a critical period of forebrain development produced signs of neurodevelopmental delay, fetal growth restriction, and placental toxicity. Maternal exposure to cypermethrin was the driving force behind many of the adverse effects observed, including delays in GABAergic progenitor migration, microglia response, fetal growth restriction, and placental compensation for oxidative stress. However maternal stress was a significant effect modifier on some outcomes, resulting in differences from control driven by combined exposure for fetal forebrain growth, microglia development, and GABAergic progenitor proliferative zone size. For other endpoints, maternal restraint stress suppressed effects induced by cypermethrin, such as increased embryonic multivacuolated microglia and upregulation of placental nutrient transporters.
To date, no previous study has examined the effect of cypermethrin on the tangential migration of GABAergic progenitors. Through this study, we found that maternal cypermethrin exposure delayed GABAergic cortical interneuron progenitor migration, alone and in combination with stress. Delays in the development of these neurons may significantly affect their function in the mature brain (Le Magueresse and Monyer, 2013) and play a role in childhood neurodevelopmental problems (Sgado et al., 2011). Cell-autonomous mechanisms were uncovered by transcriptomic assessment, suggesting distinct underpinnings of altered migration despite possible overlap between effects of maternal cypermethrin and stress on other mediators. Interestingly, migration measurements and fetal body weight were highly correlated in cypermethrin-exposed offspring. These results suggest that this neuronal developmental delay may be either a secondary consequence of fetal growth restriction, or simply the result of a common mechanism responsible for both outcomes which may involve biosynthetic and cellular development genes. Gene expression altered by maternal stress in migrating interneuron progenitors implicated pathways involved in cellular stress processes and immune function similar to previous work (Lussier and Stevens, 2016; Stevens et al., 2013).
Maternal restraint stress did not alter GABAergic progenitor migration to the same degree as previous studies (Bittle et al., 2019). Possible explanations for this difference include: housing of NS females individually instead of cohoused and corn oil vehicle administration; both of which may influence stress physiology (Kamakura et al., 2016; Nemeth et al., 2014; Tuli et al., 1995). The lack of antioxidant gene expression changes in embryonic brain due to maternal stress as shown previously (Bittle et al., 2019), despite some evidence for stress-induced oxidative DNA damage (indicated by altered Ogg1 expression in isolated GABAergic progenitors), may be due to different time points of assessment; with the current studying examining effects at E14, 24 h later than effects shown previously.
In addition to the GABAergic system assessment, we also investigated embryonic microglia morphology changes. Microglia play significant roles in neurodevelopment, including synaptic pruning, regulation of neurogenesis, and response to tissue injury (Paolicelli et al., 2011; Streit, 2000; Tong and Vidyadaran, 2016). Early in development, microglia maintain an ameboid morphology that facilitates migration (Monier et al., 2007). Once distributed in the CNS, microglia maturate to more ramified forms (Schilling et al., 2001). Although pyrethroids are known to activate adult microglia (Hossain et al., 2017; Singh et al., 2011, 2016), this is the first study to assess cypermethrin’s effects on embryonic microglia. Analysis here revealed a cypermethrin-mediated shift away from ramified morphology, suggesting delayed development. We also found that this effect of increasing ameboid microglia was driven by combined exposure with maternal stress. This is consistent with increased embryonic ameboid microglia after maternal corticosterone exposure (Bittle and Stevens, 2018). Cypermethrin and stress interacted to influence multivacuolated microglia-cypermethrin alone increased this activated state, but not when combined with stress. Multivacuolated microglia density increases in response to maternal immune activation via IL-6 injections, suggesting that maternal cypermethrin alone may induce inflammation (Gumusoglu et al., 2017). It is interesting that combined exposure did not increase multivacuolated microglia, as previous work in our lab had shown with maternal stress exposure (Gumusoglu et al., 2017). In this respect, it is possible that vehicle administration may modify the response to stress.
A particularly interesting finding of this study is that combined administration of cypermethrin and maternal restraint stress increased maternal serum concentrations of cypermethrin, suggesting stress-induced enhancement of chemical toxicity during pregnancy. Previous studies have shown that restraint stress influences chemical toxicity through altered regulation of hepatic enzymes, and this is the first study to demonstrate these effects in the pregnant mouse dam. We observed a decrease in Cyp1a1 induction in maternal liver when cypermethrin and stress exposure were combined, as well as stress-induced decreases in hepatic Cyp1a2 and Cyp2b10 expression (Figure 5).
Cypermethrin levels in the amniotic fluid below the limit of quantification, despite significant concentrations found in maternal serum, were an unexpected finding due to previous work showing cypermethrin in the amniotic fluid of rats (Bossi et al., 2013). To our knowledge, this is the first study to measure cypermethrin distribution in the pregnant mouse following a controlled exposure and suggests minimal transfer of cypermethrin by placenta from maternal to fetal circulation with or without stress. Effects on neurodevelopment may therefore occur through indirect mechanisms or low levels of cypermethrin inducing direct neurotoxicity. Distinguishing between direct and indirect mechanisms is limited by no measurement of pyrethroid metabolites in the fetal compartment. It is also possible that measurements in amniotic fluid may underestimate fetal brain exposure.
Placental dysfunction is a significant factor in offspring outcomes such as fetal growth restriction and neurodevelopmental delay in offspring (Cetin and Antonazzo, 2009; Longo et al., 2013). As such, altered placental function may be responsible for the cypermethrin-induced fetal growth restriction. Although few studies have assessed the effects of pyrethroid insecticides on placental function, there are relevant molecular targets for pyrethroids in the placenta that regulate nutrient transfer and growth factor synthesis, including voltage-gated calcium channels, maxi chloride channels, and plasma membrane ATPases (Bernucci et al., 2006; Vallejos and Riquelme, 2007). In the context of these placental mechanisms, it is possible that the increased expression of placental Glut1 and Snat1 observed in cypermethrin-treated placentas reflected a compensatory response to growth restriction driven by impairments in placental nutrient transfer (Langdown and Sugden, 2001). This reaction is likely complex, as the strong correlation between fetal weight and placental Snat1 shown here in vehicle-exposed samples was lacking in the presence of cypermethrin exposure.
Placental findings from this study suggest other possible mechanisms by which maternal exposure to cypermethrin could underlie effects on fetal growth and neurodevelopment. The data here suggest a general placental response to tissue hypoxia, attempted compensatory responses to increased fetal nutrient demand, or a response to maternal diffusible factors including immune mediators or growth factors. For example, increased levels of placental malondialdehyde accompanied by increased expression of genes involved in the angiogenic response to hypoxia/oxidative stress (Et-1, Hif1-alpha, Ho-1, and Vegfr2) implicate cypermethrin-induced oxidative stress as a plausible mechanism. It is well established that pyrethroids induce oxidative stress in a variety of cell types, with mitochondrial dysfunction as a proposed origin of this effect (Gassner et al., 1997). In the context of this study, upregulated endothelin-1 expression could induce placental oxidative stress, as previously shown through an NADPH oxidase-dependent mechanism (Fiore et al., 2005; Pollock and Pollock, 2005).
It is interesting that the pattern of expression for several of these genes responsive to oxidative stress (Hif-1alpha and Vegfr2) did not coincide with tissue malondialdehyde levels; cypermethrin alone induced expression of these genes whereas combined cypermethrin and stress exposure did not. Although the study may have been underpowered to detect these changes, it is also possible that stress may impair the placenta’s ability to respond to insults at a transcriptional level. This may be due to components of physiological stress with specific effects on cellular injury response (eg, glucocorticoids) (Carolina et al., 2018; Ito et al., 2006).
There are several possible contributors to the observed effects of combined maternal stress and cypermethrin exposure on the placenta and fetal brain. The stress-induced increase in maternal serum cypermethrin levels could enhance toxicity to the feto-placental unit. In addition, maternal stress may induce a degree of maternal toxicity that enhances effects of cypermethrin on offspring. We did not assess here potential alterations in the maternal psychological response to stress due to the maternal neurological effects of cypermethrin (Michael Caudle, 2016), but despite the short-term exposure here, this may also play a role.
In conclusion, the results of this study show that combined maternal exposure to cypermethrin and restraint stress altered several neurodevelopmental outcomes, including embryonic forebrain volume, microglia morphology, and ventral forebrain proliferative zone size. Migration of cortical interneuron progenitors in offspring was delayed by cypermethrin alone and in combination with stress, however the transcriptional changes in these neurons demonstrated distinct patterns of effect with each exposure. Assessment of cypermethrin levels demonstrated that maternal restraint stress increased maternal serum levels of cypermethrin. However, stress did not alter the finding that cypermethrin was not present in significant levels in the amniotic fluid, suggesting minimal direct effects cypermethrin on the embryonic brain. Lastly, placental toxicity and transcriptional changes suggest altered nutrient transport with maternal cypermethrin exposure, which may be modified by maternal stress. These findings together implicate maternal stress as an effect modifier not only of neurodevelopmental endpoints in offspring, but of the intermediate mechanisms that likely underlie them. Overall, an important future direction suggested by our results is the investigation of placenta-mediated mechanisms responsible for the effects of environmental factors such as cypermethrin and maternal stress on neurodevelopment. Given that the dose of 10 mg/kg used in this study is relatively high in comparison with human exposures, more studies are needed to determine whether endpoints consistent with placental toxicity and alterations in GABAergic system development are relevant at lower doses. Understanding how maternal stress and xenobiotic exposures influence placental functioning is an important next step that will offer great insight into preventing effects of environmental exposures on fetal growth and neurodevelopment.
Supplementary Material
ACKNOWLEDGMENTS
RNA sequencing was performed with support of the Iowa Institute of Human Genetics Core. RNAseq reads were mapped to a reference genome with the support of Dr Diana Kolbe and the University of Iowa Bioinformatics Division. The University of Iowa EHSRC core facilities supported RNA sequencing analysis and chemical analysis. We would like to thank Dr Andrea Adamcakova-Dodd for her help on this project. We would also like to thank the Stevens lab for helpful discussion.
FUNDING
The American College of Toxicology (North American Graduate Fellowship to B.A.E.); University of Iowa Pappajohn Biomedical Institute (Microfinance Grant to B.A.E.); University of Iowa Central Microscopy Research Facility (Pilot Project Seed Grant to B.A.E.); University of Iowa Environmental Health Sciences Research Center (EHSRC) Pilot Grant and Career Development Award (NIH P30 ES005605 to H.E.S.). Any opinions, findings, or conclusions expressed in this material are those of the authors and do not necessarily reflect the views of the funding organizations.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Afolabi O. K., Aderibigbe F. A., Folarin D. T., Arinola A., Wusu A. D. (2019). Oxidative stress and inflammation following sub-lethal oral exposure of cypermethrin in rats: Mitigating potential of epicatechin. Heliyon 5, e02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli M. C., Pallares M. E., Ceccatelli S., Spulber S. (2017). Long-term consequences of prenatal stress and neurotoxicants exposure on neurodevelopment. Prog. Neurobiol. 155, 21–35. [DOI] [PubMed] [Google Scholar]
- Barr D. B., Olsson A. O., Wong L. Y., Udunka S., Baker S. E., Whitehead R. D., Magsumbol M. S., Williams B. L., Needham L. L. (2010). Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National health and nutrition examination survey 1999–2002. Environ. Health Perspect. 118, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes F. M., and Berretta, S. (2001). GABAergic interneurons: Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25, 1–27. [DOI] [PubMed]
- Bernucci L., Henriquez M., Diaz P., Riquelme G. (2006). Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane. Placenta 27, 1082–1095. [DOI] [PubMed] [Google Scholar]
- Bittle J., Menezes E. C., McCormick M. L., Spitz D. R., Dailey M., Stevens H. E. (2019). The role of redox dysregulation in the effects of prenatal stress on embryonic interneuron migration. Cereb. Cortex (New York, NY: 1991) 29, 5116–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittle J., Stevens H. E. (2018). The role of glucocorticoid, interleukin-1beta, and antioxidants in prenatal stress effects on embryonic microglia. J. Neuroinflamm. 15, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi R., Vinggaard A. M., Taxvig C., Boberg J., Bonefeld-Jorgensen E. C. (2013). Levels of pesticides and their metabolites in Wistar rat amniotic fluids and maternal urine upon gestational exposure. Int. J. Environ. Res. Public Health 10, 2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson S. L., Bale T. L. (2014). Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolina E., Kato T., Khanh V. C., Moriguchi K., Yamashita T., Takeuchi K., Hamada H., Ohneda O. (2018). Glucocorticoid impaired the wound healing ability of endothelial progenitor cells by reducing the expression of CXCR4 in the PGE2 pathway. Front. Med. (Lausanne) 5, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin I., Antonazzo P. (2009). The role of the placenta in intrauterine growth restriction (IUGR). Z. Geburtshilfe Neonatol. 213, 84–88. [DOI] [PubMed] [Google Scholar]
- Chen D., Yu S. P., Wei L. (2014). Ion channels in regulation of neuronal regenerative activities. Transl. Stroke Res. 5, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker E., Chevrier J., Rauch S., Bradman A., Obida M., Crause M., Bornman R., Eskenazi B. (2018). Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ. Int. 113, 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall J. E., Hackett H. E., Tobet S. A., Kosofsky B. E., Bhide P. G. (2004). Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb. Cortex (New York, NY: 1991) 14, 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford M. J., Croucher A., Hutson D. H. (1981). The metabolism of the pyrethroid insecticide cypermethrin in rats; excreted metabolites. Pestic. Sci. 12, 399–411. [Google Scholar]
- Cuzon V. C., Yeh P. W., Yanagawa Y., Obata K., Yeh H. H. (2008). Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J. Neurosci. 28, 1854–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlerup B. R., Egsmose E. L., Siersma V., Mortensen E. L., Hedegaard M., Knudsen L. E., Mathiesen L. (2018). Maternal stress and placental function, a study using questionnaires and biomarkers at birth. PLoS One 13, e0207184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G., Cui C., Chen L., Gao Y., Zhou Y., Shi R., Tian Y. (2015). Prenatal exposure to pyrethroid insecticides and birth outcomes in Rural Northern China. J. Expo. Sci. Environ. Epidemiol. 25, 264–270. [DOI] [PubMed] [Google Scholar]
- Ellman G. L. (1959). Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77. [DOI] [PubMed] [Google Scholar]
- Field L. M., Emyr Davies T. G., O’Reilly A. O., Williamson M. S., Wallace B. A. (2017). Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur. Biophys. J. 46, 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine R., Zhang J., Stevens H. E. (2014). Prenatal stress and inhibitory neuron systems: Implications for neuropsychiatric disorders. Mol. Psychiatry 19, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore G., Florio P., Micheli L., Nencini C., Rossi M., Cerretani D., Ambrosini G., Giorgi G., Petraglia F. (2005). Endothelin-1 triggers placental oxidative stress pathways: Putative role in preeclampsia. J. Clin. Endocrinol. Metab. 90, 4205–4210. [DOI] [PubMed] [Google Scholar]
- Furlong M. A., Barr D. B., Wolff M. S., Engel S. M. (2017). Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 62, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassner B., Wuthrich A., Scholtysik G., Solioz M. (1997). The pyrethroids permethrin and cyhalothrin are potent inhibitors of the mitochondrial complex I. J. Pharmacol. Exp. Ther. 281, 855–860. [PubMed] [Google Scholar]
- Gumusoglu S. B., Fine R. S., Murray S. J., Bittle J. L., Stevens H. E. (2017). The role of IL-6 in neurodevelopment after prenatal stress. Brain Behav. Immun. 65, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur T. L., Shay L., Palkar A. V., Fisher S., Varaljay V. A., Dowd S., Bailey M. T. (2017). Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun. 64, 50–58. [DOI] [PubMed] [Google Scholar]
- Hanke W., Romitti P., Fuortes L., Sobala W., Mikulski M. (2003). The use of pesticides in a polish rural population and its effect on birth weight. Int. Arch. Occup. Environ. Health 76, 614–620. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Arion D., Unger T., Maldonado-Aviles J. G., Morris H. M., Volk D. W., Mirnics K., Lewis D. A. (2008). Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 13, 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. M., Liu J., Richardson J. R. (2017). Pyrethroid insecticides directly activate microglia through interaction with voltage-gated sodium channels. Toxicol. Sci. 155, 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Getting S. J., Charron C. E. (2006). Mode of glucocorticoid actions in airway disease. Sci. World J. 6, 1750–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero D. R. (1990). Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 9, 515–540. [DOI] [PubMed] [Google Scholar]
- Kakko I., Toimela T., Tahti H. (2003). The synaptosomal membrane bound ATPase as a target for the neurotoxic effects of pyrethroids, permethrin and cypermethrin. Chemosphere 51, 475–480. [DOI] [PubMed] [Google Scholar]
- Kamakura R., Kovalainen M., Leppaluoto J., Herzig K. H., Makela K. A. (2016). The effects of group and single housing and automated animal monitoring on urinary corticosterone levels in male C57BL/6 mice. Physiol. Rep. 4, e12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo S., Ishii Y., Horigane S. I., Suzuki K., Ohkura M., Nakai J., Fujii H., Takemoto-Kimura S., Bito H. (2018). A critical neurodevelopmental role for L-type voltage-gated calcium channels in neurite extension and radial migration. J. Neurosci. 38, 5551–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H., Izumi T., Ueda Y., Matsuo M., Miyamoto J. (1984). Metabolism and placental-transfer of stereoisomers of tetramethrin isomers in pregnant rats. J. Pestic. Sci. 9, 249–258. [Google Scholar]
- Kania-Korwel I., Shaikh N. S., Hornbuckle K. C., Robertson L. W., Lehmler H. J. (2007). Enantioselective disposition of PCB 136 (2,2′,3,3′,6,6′-hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality 19, 56–66. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Chen J., Takahashi T., Ichitani Y., Nakahara D. (2006). Prenatal stress suppresses cell proliferation in the early developing brain. Neuroreport 17, 1515–1518. [DOI] [PubMed] [Google Scholar]
- Konstandi M., Johnson E. O., Lang M. A. (2014). Consequences of psychophysiological stress on cytochrome P450-catalyzed drug metabolism. Neurosci. Biobehav. Rev. 45, 149–167. [DOI] [PubMed] [Google Scholar]
- Kumar K., Patro N., Patro I. (2013). Impaired structural and functional development of cerebellum following gestational exposure of deltamethrin in rats: Role of reelin. Cell. Mol. Neurobiol. 33, 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdown M. L., Sugden M. C. (2001). Enhanced placental GLUT1 and GLUT3 expression in dexamethasone-induced fetal growth retardation. Mol. Cell. Endocrinol. 185, 109–117. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C., Monyer H. (2013). GABAergic interneurons shape the functional maturation of the cortex. Neuron 77, 388–405. [DOI] [PubMed] [Google Scholar]
- Lentza-Rizos C., Avramides E. J., Visi E. (2001). Determination of residues of endosulfan and five pyrethroid insecticides in virgin olive oil using gas chromatography with electron-capture detection. J. Chromatogr. A 921, 297–304. [DOI] [PubMed] [Google Scholar]
- Longo S., Bollani L., Decembrino L., Di Comite A., Angelini M., Stronati M. (2013). Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J. Matern. Fetal Neonatal Med. 26, 222–225. [DOI] [PubMed] [Google Scholar]
- Lussier S. J., Stevens H. E. (2016). Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress. Dev. Neurobiol. 76, 1078–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairesse J., Lesage J., Breton C., Bréant B., Hahn T., Darnaudéry M., Dickson S. L., Seckl J., Blondeau B., Vieau D., et al. (2007). Maternal stress alters endocrine function of the feto-placental unit in rats. Am. J. Physiol. Endocrinol. Metab. 292, E1526–1533. [DOI] [PubMed] [Google Scholar]
- Maurya S. K., Mishra J., Tripathi V. K., Sharma R., Siddiqui M. H. (2014). Cypermethrin induces astrocyte damage: Role of aberrant Ca(2+), ROS, JNK, P38, matrix metalloproteinase 2 and migration related reelin protein. Pestic. Biochem. Physiol. 111, 51–59. [DOI] [PubMed] [Google Scholar]
- McCarthy D. J., Chen Y., Smyth G. K. (2012). Differential expression analysis of multifactor RNA-seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael Caudle W. (2016). This can’t be stressed enough: The contribution of select environmental toxicants to disruption of the stress circuitry and response. Physiol. Behav. 166, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier A., Adle-Biassette H., Delezoide A. L., Evrard P., Gressens P., Verney C. (2007). Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J. Neuropathol. Exp. Neurol. 66, 372–382. [DOI] [PubMed] [Google Scholar]
- Monk C., Feng T., Lee S., Krupska I., Champagne F. A., Tycko B. (2016). Distress during pregnancy: Epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am. J. Psychiatry 173, 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama N., Kazuki Y., Satoh D., Arata K., Harada T., Shibata N., Guengerich F. P., Yamazaki H. (2017). Induction of human cytochrome P450 3A enzymes in cultured placental cells by thalidomide and relevance to bioactivation and toxicity. J. Toxicol. Sci. 42, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuti C., Gabbianelli R., Falcioni M. L., Di Stefano A., Sozio P., Cantalamessa F. (2007). Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology 229, 194–205. [DOI] [PubMed] [Google Scholar]
- Nemeth M., Millesi E., Wagner K. H., Wallner B. (2014). Effects of diets high in unsaturated fatty acids on socially induced stress responses in guinea pigs. PLoS One 9, e116292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K., O’Connor T. G., Glover V. (2009). Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Dev. Neurosci. 31, 285–292. [DOI] [PubMed] [Google Scholar]
- Paolicelli R. C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T. A., Guiducci E., Dumas L., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Pehme P. M., Zhang W., Finik J., Pritchett A., Buthmann J., Dana K., Hao K., Nomura Y. (2018). Placental MAOA expression mediates prenatal stress effects on temperament in 12-month-olds. Infant Child Dev. 27, e2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock D. M., Pollock J. S. (2005). Endothelin and oxidative stress in the vascular system. Curr. Vasc. Pharmacol. 3, 365–367. [DOI] [PubMed] [Google Scholar]
- Reynolds L. P., Vonnahme K. A., Lemley C. O., Redmer D. A., Grazul-Bilska A. T., Borowicz P. P., Caton J. S. (2013). Maternal stress and placental vascular function and remodeling. Curr. Vasc. Pharmacol. 11, 564–593. [DOI] [PubMed] [Google Scholar]
- Rio D. C., Ares M. Jr, Hannon G. J., Nilsen T. W. (2010). Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K. (2010). edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T., Nitsch R., Heinemann U., Haas D., Eder C. (2001). Astrocyte-released cytokines induce ramification and outward K+ channel expression in microglia via distinct signalling pathways. Eur. J. Neurosci. 14, 463–473. [DOI] [PubMed] [Google Scholar]
- Schwab A., Fabian A., Hanley P. J., Stock C. (2012). Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913. [DOI] [PubMed] [Google Scholar]
- Sgado P., Dunleavy M., Genovesi S., Provenzano G., Bozzi Y. (2011). The role of GABAergic system in neurodevelopmental disorders: A focus on autism and epilepsy. Int. J. Physiol. Pathophysiol. Pharmacol. 3, 223–235. [PMC free article] [PubMed] [Google Scholar]
- Shelton J. F., Geraghty E. M., Tancredi D. J., Delwiche L. D., Schmidt R. J., Ritz B., Hansen R. L., Hertz-Picciotto I. (2014). Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The charge study. Environ. Health Perspect. 122, 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Kaneko H., Kakuta N., Yoshitake A., Miyamoto J. (1990). Placental-transfer of esfenvalerate and fenvalerate in pregnant rats. J. Pestic. Sci. 15, 169–174. [Google Scholar]
- Silva C. G., Metin C., Fazeli W., Machado N. J., Darmopil S., Launay P.-S., Ghestem A., Nesa M.-P., Bassot E., Szabo E., et al. (2013). Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci. Transl. Med. 5, 197ra104. [DOI] [PubMed] [Google Scholar]
- Singh A. K., Tiwari M. N., Dixit A., Upadhyay G., Patel D. K., Singh D., Prakash O., Singh M. P. (2011). Nigrostriatal proteomics of cypermethrin-induced dopaminergic neurodegeneration: Microglial activation-dependent and -independent regulations. Toxicol. Sci. 122, 526–538. [DOI] [PubMed] [Google Scholar]
- Singh A., Tripathi P., Prakash O., Singh M. P. (2016). Ibuprofen abates cypermethrin-induced expression of pro-inflammatory mediators and mitogen-activated protein kinases and averts the nigrostriatal dopaminergic neurodegeneration. Mol. Neurobiol. 53, 6849–6858. [DOI] [PubMed] [Google Scholar]
- Stevens H. E., Su T., Yanagawa Y., Vaccarino F. M. (2013). Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology 38, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J. (2000). Microglial response to brain injury: A brief synopsis. Toxicol. Pathol. 28, 28–30. [DOI] [PubMed] [Google Scholar]
- Tang W., Wang D., Wang J., Wu Z., Li L., Huang M., Xu S., Yan D. (2018). Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 191, 990–1007. [DOI] [PubMed] [Google Scholar]
- Tong C. K., Vidyadaran S. (2016). Role of microglia in embryonic neurogenesis. Exp. Biol. Med. (Maywood) 241, 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli J. S., Smith J. A., Morton D. B. (1995). Stress measurements in mice after transportation. Lab. Anim. 29, 132–138. [DOI] [PubMed] [Google Scholar]
- Vallejos C., Riquelme G. (2007). The maxi-chloride channel in human syncytiotrophoblast: A pathway for taurine efflux in placental volume regulation? Placenta 28, 1182–1191. [DOI] [PubMed] [Google Scholar]
- Watkins D. J., Fortenberry G. Z., Sanchez B. N., Barr D. B., Panuwet P., Schnaas L., Osorio-Valencia E., Solano-Gonzalez M., Ettinger A. S., Hernandez-Avila M., et al. (2016). Urinary 3-phenoxybenzoic acid (3-PBA) levels among pregnant women in Mexico City: Distribution and relationships with child neurodevelopment. Environ. Res. 147, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. K., Rundle A., Holmes D., Reyes M., Hoepner L. A., Barr D. B., Camann D. E., Perera F. P., Whyatt R. M. (2008). Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U. S. environmental protection agency restriction of organophosphates. Environ. Health Perspect. 116, 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z., Li X., Su Q., Xu L., Zhang P., Kong Z., Xu J., Teng J. (2013). Effect of synthetic pyrethroid pesticide exposure during pregnancy on the growth and development of infants. Asia Pac. J. Public Health 25, 72S–79. [DOI] [PubMed] [Google Scholar]
- Yip J., Soghomonian J. J., Blatt G. J. (2008). Increased GAD67 mRNA expression in cerebellar interneurons in autism: Implications for Purkinje cell dysfunction. J. Neurosci. Res. 86, 525–530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.