Background:
Management of acral lentiginous melanoma (ALM) remains controversial. Traditionally, ALM was managed with digit amputation (DA), resulting in significant morbidity, but recent evidence has advocated for digit sparing management. Furthermore, the significance of nodal metastasis for ALM is not well reported. The aims of this study were to determine if surgical approach for primary ALM impacts outcomes and to evaluate the predictive value of nodal status for ALM.
Methods:
Patients with localized ALM diagnosed from 1982 to 2017 were retrospectively identified. Clinicopathologic characteristics were correlated with surgical approach, nodal metastasis, overall survival, and recurrence-free survival.
Results:
There were 47 patients with ALM. Median age was 59 years, and median thickness was 3 mm. 51% of patients underwent wide local excision (WLE), 27.9% underwent DA, and 20.9% underwent partial digit amputation (PDA). ALM on the hand versus foot (OR: 12.7, 95%, confidence interval (CI), 2.0–80.1; P = 0.007) and subungual versus nonsubungual location (OR: 28.0, 95% confidence interval, 2.7–295.7; P = 0.006) were significantly associated with surgical approach (DA and PDA versus WLE). There were no significant differences in overall survival or recurrence-free survival between DA, PDA, or WLE cases (P = 0.481 and P = 0.778, respectively). There were no significant differences in overall survival or recurrence-free survival based on nodal status (P = 0.562 and P = 0.136, respectively).
Conclusions:
No significant differences in overall survival or recurrence-free survival were seen between ALM patients treated with DA, PDA, and WLE. Given these results, PDA or WLE may be options in select patients with digital ALM; however, careful consideration must be taken when deciding on the surgical approach.
INTRODUCTION
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma found on the acral skin of the hands and feet as well as the nail bed (subungual).1–4 ALM represents 1%–3% of all melanoma,5 yet it is the most common subtype of melanoma found in Fitzpatrick skin types III–VI, including people of African,5,6 Latin American,5,7–9 Chinese,10 Korean,11 and Singaporean12 descent. In addition, ALM has been associated with worse outcomes compared with other melanoma subtypes, which may in part be due to ALM often being diagnosed at more advanced clinical stages.1,13,14
Localized primary melanoma is treated with wide local excision (WLE). Recommended excision margins are well established and based on Breslow thickness.15–17 ALM located on the dorsal and volar surfaces of the hands and feet are often managed with WLE, but lesions on the nail fold, subungual region, or elsewhere on the digit have been traditionally managed with digit amputation (DA).1,8 It was initially believed that for digital and subungual ALM, amputation at the metacarpal–interphalangeal joint (MCPJ) was necessary to optimize survival.18 However, amputations can result in significant functional deficits. For instance, metacarpal–interphalangeal joint amputation results in a 40% reduction of hand function compared with a 10% reduction if an amputation is performed at the interphalangeal joint.19 Some reports have demonstrated that more distal amputation (proximal interphalangeal joint and distal interphalangeal joint) may not compromise survival,19–21 leaving patients with a much higher quality of life. However, there are limited data evaluating whether the extent of amputation or use of WLE instead of amputation on the digits affects outcomes in ALM patients.
In addition, the presence of nodal metastasis and sentinel lymph node biopsy (SLNB) status have been clearly shown to be prognostic for melanoma patients in general, with SLN metastasis seen in approximately 15%–20% of all melanoma cases.22–25 Reports suggest that SLN metastasis may be seen in up to 40% of ALM cases, but most studies evaluating SLNB for melanoma included relatively small numbers of patients with ALM.25,26 One recent study, by Bello et al., included 281 ALM patients and demonstrated that poor prognosis was correlated with a positive SLN.26 However, the prognostic significance of nodal disease and the value of SLNB specifically in ALM have not been well reported beyond this single study.26–28
Given the questions surrounding the management of ALM, we retrospectively reviewed a single institution experience in treating ALM. Our intent was to evaluate whether the type of surgical treatment for the primary ALM is associated with outcomes and to determine the prognostic significance of nodal metastasis in ALM patients.
METHODS
A retrospective review was performed, after obtaining Institutional Review Board approval, looking for patients diagnosed with invasive localized ALM who were treated at Yale-New Haven Hospital between 1982 and 2017. All of the patients were treated by plastic surgeons with the exception of a single patient who was treated by a surgical oncologist. All diagnoses of ALM were confirmed by Yale Dermatopathology. Patients with in situ lesions or patients who presented with clinically evident nodal or distant metastases were excluded.
Surgical resection of the primary was classified as DA, partial digit amputation (PDA), or WLE. In this report, PDA refers to distal digit amputation, where the amputation was limited to the closest joint proximal to the site of the primary lesion and standard margins (proximal interphalangeal joint or distal interphalangeal joint). WLE was planned using standard margins per the National Comprehensive Cancer Network guidelines,17 which were measured before excision; 1 cm for <1 mm thickness, 1–2 cm for 1–2 mm thickness, and 2 cm for >2 mm thickness. WLE included resection of a full thickness of skin and subcutaneous tissue using the above standard margins. For subungual cases, WLE consisted of resection of the nail unit along with peripheral and deep skin and soft tissue margins proximally, per the above standard margins, down to periosteum. Periosteal stripping was performed selectively if this was thought to be required to clear the deep margin. Reconstruction of all WLE defects consisted of full-thickness skin grafting versus local tissue flaps. DA consisted of amputation at or proximal to the metacarpal–interphalangeal joint or metatarsophalangeal joint. The choice of surgical therapy for the primary site was per the discretion of the treating surgeon. Of note, because WLE and amputation was used in both digit and nondigit cases and because of low numbers in certain comparison groups, digit and nondigit cases were combined. In 4 cases, the type of treatment was unable to be obtained due to incomplete medical records or treatments performed at satellite facilities.
For localized ALM cases, nodal staging was performed either as elective lymph node dissection (ELND), for patients diagnosed up to 1997, or as SLNB, for patients diagnosed from 1998 onwards. SLNB was performed according to techniques previously described.29,30 Specifically, SLNB was routinely offered to all patients with melanoma >1 mm in thickness, and selectively to patients with melanoma ≤1 mm in thickness, depending on the presence of additional risk factors such as thickness >0.75 mm, ulceration, and high mitotic rate (MR). Patients with a positive SLN were routinely offered completion lymph node dissection (CLND).
Primary tumor characteristics, such as thickness, Clark level, ulceration, and MR, were evaluated. Thickness was analyzed as a continuous variable. Clark level was categorized as V versus <V, while MR rate was recorded as mitoses per square millimeter and categorized as <3/mm2 versus ≥3/mm2. Assessment of lymph nodes consisted of serial sectioning, review of hematoxylin and eosin-stained sections, and evaluation with immunohistochemistry using any combination of S-100, HMB-45, Melan-A, and SOX-10. Patients were considered node-positive if metastatic disease was found in a node on SLNB or elective lymph node dissection.
Descriptive statistics including median with interquartile range (IQR) for continuous variables, and frequency with percentage for discrete variables, were reported for variables. Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square test (or Fisher’s exact test if the sample size was <5) for categorical variables were used to assess whether a variable had a different distribution between various comparison groups, including different surgical approaches to the primary ALM and nodal status. Logistic regression analysis was used to identify significant predictors for surgical approach to the primary ALM and for nodal positivity. Patient survival characteristics with 95% confidence interval (CI), including the median and 5-year overall survival (OS) and recurrence-free survival (RFS), were calculated based on the Kaplan–Meier product-limit method. The Kaplan–Meier curve plot was used to visualize survival distributions and log-rank test was used to compare groups. A P-value of ≤0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 (SAS, Cary, NC).
RESULTS
Patient Demographics, Primary Tumor Characteristics, and Treatment
Forty-seven patients were identified and included in the study (Table 1). The overall median age was 59 years (IQR: 53–74 years), and 24 patients (51.1%) were women. ALM was located most frequently on the foot (34 of 47 cases, 72.3%). For cases with data on subungual versus nonsubungual location (39 cases), the majority of lesions were subungual (21 of 39 cases, 53.8%) compared with nonsubungual sites (18 of 39 cases, 46.2%). For cases with available data, 10 of 11 (90.9%) hand ALM cases and 11 of 28 (39.3%) foot ALM cases were subungual, while the remaining cases were nonsubungual in these respective groups.
Table 1.
Clinicopathologic Characteristics
| N | 47 | |
|---|---|---|
| Age (median, years) | 59 [IQR: 53, 74] | |
| Gender | Male | 23 (49%) |
| Female | 24 (51%) | |
| Thickness (median, mm) | 3 [IQR: 1.1, 5] | |
| Hand or foot | Hand | 13 (28%) |
| Foot | 34 (72%) | |
| Subungual location | Subungual | 21 (54%) |
| Nonsubungual | 18 (46%) | |
| Surgical approach for primary | Digital amputation | 12 (28%) |
| Partial digital amputation | 9 (19%) | |
| Wide local excision | 22 (51%) | |
| Clark level | <V | 24 (71%) |
| V | 10 (29%) | |
| Ulceration | Yes | 17 (52%) |
| No | 16 (48%) | |
| Mitotic rate (per mm2) | >3 | 9 (43%) |
| ≤3 | 12 (57%) | |
IQR, interquartile range.
The surgical approach for the primary ALM was known in 43 of 47 patients. Twelve of 43 patients (27.9%) underwent DA, whereas 9 of 43 patients (20.9%) were treated with PDA, and 22 of 43 patients (51.2%) underwent WLE. Of the 22 patients who underwent WLE, 3 (13.6%) patients had a positive deep margin, 17 (77.3%) patients had a negative deep margin, and the deep margin was unknown for 2 (9.1%) patients.
Overall median thickness was 3 mm (IQR: 1.1–5 mm). For cases that had Clark level and ulceration data, a greater number of patients had Clark level <V versus V tumors (71% versus 29%, respectively), while ulceration was seen in 17 of 33 cases (52%). In addition, a higher proportion of tumors had an MR ≥3/mm2 versus <3/mm2 in cases with MR data (57% versus 43%, respectively).
Overall, 31 patients had nodal staging with SLNB performed in 27 cases and elective lymph node dissection done in 4 cases. SLNB was positive in 11 of 27 cases (40.7%), while elective lymph node dissection was negative in all 4 cases, resulting in a 35.5% overall detection rate for nodal metastasis (11 positive nodal cases of 31 patients who underwent nodal staging). A completion lymph node dissection was performed in 9 of 11 (81.8%) positive SLN patients, and additional nodal disease was found in 3 of 9 completion lymph node dissection cases (33.3%). The reasons why a completion lymph node dissection was not performed in the remaining 2 positive SLN patients are unknown.
Two patients were treated with adjuvant therapy after surgery. Both patients had nodal metastases found on SLNB and received adjuvant interferon. Both patients later developed distant metastatic disease and subsequently died.
Factors Associated with Surgical Approach for Treating ALM Primary Site
As shown in Table 2, age and gender were not significantly different between the DA, PDA, and WLE groups (P = 0.28 and P = 0.74, respectively). Median thickness in the WLE group was the lowest at 2.4 mm (IQR: 1.1–4.3 mm), while the median thickness was the greatest in the PDA group at 4.5 mm (IQR: 2.5–5.9 mm); however, this failed to reach statistical significance (P = 0.62). In addition, Clark level, ulceration status, and MR were not significantly different between the DA, PDA, and WLE groups (P = 0.32, P = 0.87, and P = 0.1, respectively).
Table 2.
Univariable Factors Associated with Surgical Approach for Treating the Primary Site of Acral Lentiginous Melanoma
| Digital Amputation | Partial Digital Amputation | Wide Local Excision | P | ||
|---|---|---|---|---|---|
| n | 12 | 9 | 22 | ||
| Age (median, years) | 60 [IQR: 56, 76.5] | 61 [IQR: 55, 78] | 58 [IQR: 49, 67] | 0.28 | |
| Gender | Male | 7 (58%) | 4 (44%) | 10 (45%) | 0.74 |
| Female | 5 (42%) | 5 (56%) | 12 (55%) | ||
| Thickness (median, mm) | 3.6 [IQR: 1.1, 5] | 4.5 [IQR: 2.5, 5] | 2.4 [IQR: 1.1, 4.3] | 0.62 | |
| Hand or foot | Hand | 3 (25%) | 6 (67%) | 3 (14%) | 0.01 |
| Foot | 9 (75%) | 3 (33%) | 19 (86%) | ||
| Subungual location | Subungual | 7 (78%) | 8 (89%) | 4 (22%) | <0.001 |
| Nonsubungual | 2 (22%) | 1 (11%) | 14 (78%) | ||
| Clark level | <V | 6 (60%) | 4 (57%) | 14 (82%) | 0.32 |
| V | 4 (40%) | 3 (43%) | 8 (53%) | ||
| Ulceration | Yes | 6 (55%) | 4 (57%) | 7 (47%) | 0.87 |
| No | 5 (45%) | 3 (43%) | 8 (53%) | ||
| Mitotic rate (per mm2) |
>3 | 5 (71%) | 2 (50%) | 2 (20%) | 0.1 |
| ≤3 | 2 (29%) | 2 (50%) | 8 (80%) |
IQR, interquartile range.
DA was used in 3 of 12 (25%) hand cases and for 9 of 31 (29.0%) foot ALM, while PDA was used for 6 of 12 (50%) hand ALM and in only 3 of 31 foot cases (9.7%). WLE was used for 3 of 12 (25%) hand lesions and for 19 of 31 (61.3%) foot ALM (P = 0.01 comparing WLE rate in hand versus foot cases).
Patients treated with DA or PDA had significantly higher percentages of subungual versus nonsubungual ALM (DA: 78% versus 22% and PDA: 89% versus 11%) compared with WLE patients who had a significantly higher rate of nonsubungual versus subungual tumors (78% versus 22%; P < 0.001). In addition, DA or PDA was used in 15 of 19 (78.9%) subungual lesions but only in 3 of 17 (17.6%) nonsubungual cases, while WLE was used in 4 of 19 (21.1%) subungual ALM and in 14 of 17 (82.4%) nonsubungual tumors (P < 0.001).
Univariable logistic regression analysis showed that both tumor location on the hand versus foot (OR: 12.7, 95%, CI 2.0–80.1; P = 0.007) and subungual versus nonsubungual location (OR: 28.0, 95%, CI 2.7–295.7; P = 0.006) were significantly associated with surgical approach (DA and PDA versus WLE) for primary ALM.
Factors Associated with Nodal Metastasis
Age and gender were not significantly different between node-positive and node-negative patients (P = 0.79 and P = 0.77, respectively, Table 3). Node-positive patients had a greater median thickness compared with node-negative patients (4.7 mm versus 3 mm, respectively); however, this failed to reach statistical significance (P = 0.15). Similarly, a higher percentage of node-positive patients had Clark level V tumors (50%) compared with node-negative patients (24%), but this was not a significant difference (P = 0.16). Interestingly, patients without nodal metastasis demonstrated a trend of having ulceration compared with node-negative patients (76% versus 38%, respectively), but this was also not a significant difference (P = 0.06). Ultimately, no covariates were found to be significantly correlated with nodal metastasis.
Table 3.
Nodal Status in Acral Lentiginous Melanoma
| Node-Negative | Node-Positive | P | ||
|---|---|---|---|---|
| n | 20 | 11 | ||
| Age (median, years) | 60 [IQR: 56, 69] | 61 [IQR: 54, 75] | 0.79 | |
| Gender | Male | 12 (60%) | 6 (55%) | 0.77 |
| Female | 8 (40%) | 5 (45%) | ||
| Thickness (median, mm) | 3 [IQR: 2, 5.2] | 4.7 [3.5, 6.9] | 0.15 | |
| Hand or foot | Hand | 5 (25%) | 4 (36%) | 0.5 |
| Foot | 15 (75%) | 7 (64%) | ||
| Subungual location | Subungual | 9 (50%) | 4 (67%) | 0.48 |
| Nonsubungual | 9 (50%) | 2 (33%) | ||
| Surgical approach to the primary site | Digital amputation | 8 (40%) | 3 (30%) | 0.85 |
| Partial digital amputation | 4 (20%) | 2 (20%) | ||
| Wide local excision | 8 (40%) | 5 (50%) | ||
| Clark level | <V | 13 (76%) | 5 (50%) | 0.16 |
| V | 4 (24%) | 5 (50%) | ||
| Ulceration | Yes | 13 (76%) | 3 (38%) | 0.06 |
| No | 4 (24%) | 5 (63%) | ||
| Mitotic rate (per mm2) | >3 | 5 (45%) | 2 (25%) | 0.36 |
| ≤3 | 6 (55%) | 6 (75%) |
IQR, interquartile range.
Recurrence and Survival
Overall median follow-up was 42.5 months (IQR: 18.4–77.3 months), and a total of 19 of 47 patients (40.4%) died (Table 4). A recurrence developed in 17 of 47 patients (36.2%), with 11 (50%) occurring in the WLE group, 4 (33.3%) developing in the DA group, and 2 (22.2%) occurring in the PDA group. There were no statistically significant differences in recurrence rates between the WLE, DA, and PDA groups. Of note, a local recurrence developed in 2 patients, with 1 occurring after a DA for a 0.45 mm thickness melanoma and the other developing after a WLE for a 1.1 mm thickness melanoma. Both patients were treated with further surgical resection, and both patients are alive at last follow-up. The overall median OS was 126.6 months, while the overall median RFS was 14.1 months. The overall 5-year OS was 58.4%, and the overall 5-year RFS was 11.8%. On multivariable analyses, thickness was the only factor significantly prognostic of OS (continuous variable, HR: 1.14, 95%, CI: 1.01–1.30; P = 0.038), while gender was the only variable predictive of RFS (women versus men, HR: 6.80, 95%, CI: 1.65–29.49; P = 0.01).
Table 4.
Recurrence and Survival
| N | Deaths (%) | Median OS (95% CI) | Median RFS (95% CI) | 5-Year OS (95% CI) | 5-Year RFS (95% CI) | |
|---|---|---|---|---|---|---|
| All patients | 43 | 19 (40.4) | 126.6 months (44.2, 174.1) | 14.1 months (11.6, 16.6) | 58.4% (40.2, 76.6) | 11.8% (0, 27.1) |
| Digital amputation | 12 | 2 (16.7) | NR | 21.4 (0.46, 40.2) | 68.6% (32.1, 100) | 0% |
| Partial digital amputation | 9 | 5 (55.6) | 49.8 months (34.4, 140.3) | 13.8 months (13.5, 14.0) | 33.3% (0, 71.1) | 0% |
| Wide local excision | 22 | 10 (45.5) | 126.6 months (25.7, NR) | 12.7 months (10.7, 16.6) | 64.2% (41.1, 87.3) | 18.2% (0, 41) |
| Node-positive | 11 | 8 (72.7) | 55.4 months (21.6, 126.6) | 13.8 months (7.6, 15.5) | 39% (7.1, 70.9) | 0% |
| Node-negative | 20 | 8 (40) | 45.3 months (25.7, 140.3) | 16.2 months (10.7, 79.4) | 43.5% (12.5, 74.5) | 16.7% (0, 46.5) |
OS, overall survival; RFS, recurrence-free survival; CI, confidence interval; NR, not reached.
In the WLE group, 10 of 22 patients (45.5%) died, and the median OS was 126.6 months, while the median RFS was 14.1 months. Five-year OS for the WLE group was 64.2%, and the 5-year RFS was 18.2%. In the PDA group, 5 of 9 patients (55.6%) died. The PDA group demonstrated a lower median OS at 49.8 months, although RFS was similar at 13.8 month. The 5-year OS for the PDA group was also lower compared with the WLE group at 33%, and the 5-year RFS was 0%. A total of 2 of 12 patients (16.7%) died in the DA group. The median OS in the DA group was not reached, while median RFS was 21.4 months. For the DA group, the 5-year OS was 68.6%, and the 5-year RFS was 0%. As demonstrated in Figure 1A, the surgical approach for the primary ALM (DA, PDA, and WLE) had no significant impact on OS (P = 0.481) or on RFS (P = 0.778, Fig. 1B).
Fig. 1.

Survival in acral lentiginous melanoma stratified by surgical approach for the primary site. (A) Overall survival. (B) Recurrence-free survival.
For the 2 patients with digit ALM on the hand and the 4 patients with digit ALM on the foot treated with WLE, 2 deaths were observed (33.3%). For the 6 patients with digit ALM on the hand and the 2 patients with digit ALM on the foot treated with PDA, 4 patients died (50%). For the 3 patients with digit ALM on the hand and the 7 patients with digit ALM on the foot treated with DA, there was 1 death (10%). For patients with subungual tumors, there was 1 death for the 4 patients (25%) treated with WLE, 0 deaths for the 7 patients treated with DA, and 4 deaths for the 8 patients (50%) treated with PDA.
Node-negative patients had a median OS of 45.3 months and a median RFS of 16.2 months. The 5-year OS and 5-year RFS were 43.5% and 16.7%, respectively, in the node-negative group. Patients with nodal metastasis had a median OS of 55.4 months and a median RFS of 13.8 months. The 5-year OS for node-positive patients was 39%, and the 5-year RFS was 0%, which were lower compared with node-negative patients, but these differences were not significantly different. Figure 2A demonstrates that nodal status had no significant impact on OS (P = 0.562) or RFS (P = 0.136, Fig. 2B).
Fig. 2.
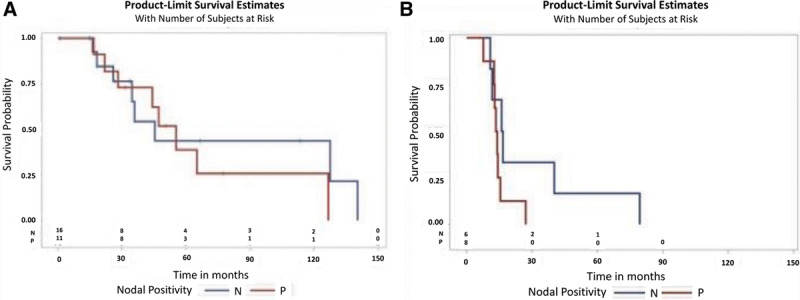
Survival in acral lentiginous melanoma stratified by nodal status. (A) Overall survival. (B) Recurrence-free survival.
DISCUSSION
Contemporary data regarding the management of ALM are relatively limited. Historically, DA was the accepted treatment of digital and subungual ALM, while ALM located on the dorsal and volar surfaces of the hands and feet could be managed with WLE.1,8 The acceptance of DA as the treatment of choice originates from Hutchinson’s31 descriptions in 1886, which was corroborated by the case series published by Das Gupta and Bradfield in 1965.6 Initial studies suggested that for digital and subungual ALM, amputation at the metacarpal–interphalangeal joint was necessary to optimize survival.18 However, patients can have significant functional deficits after DA.
Recently, the belief that a proximal amputation was necessary for digital or subungual melanomas has been called into question by some studies supporting more limited excisions to help preserve hand function. Heaton et al.20 concluded that proximal amputation versus distal amputation did not significantly impact survival or disease recurrence. Similarly, Park et al.3 demonstrated that prognosis and survival were independent of the level of the digital amputation. Our data support these studies as the majority of partial amputations (PDA) were performed for ALM located in subungual locations (P < 0.001) and on the hand (P = 0.01), whereas complete amputations (DA) were most likely to be performed for foot lesions (P = 0.01), although DA was also used relatively frequently for subungual ALM. Regardless, no differences in survival were seen between ALM patients who were treated with WLE, PDA, or DA.
Our study demonstrated no significant differences in OS (P = 0.48) or RFS (P = 0.778) between WLE, PDA, and DA. Of note, each treatment group consisted of both digit and nondigit cases. This was because both WLE and amputation were used in both anatomic locations, and there were low numbers in certain comparison groups. For instance, there were only 5 cases of nonsubungual digit ALM, of which 3 cases were treated with DA and 2 cases were treated with WLE. Furthermore, the number of deaths and recurrences specifically for digit ALM or subungual ALM were too small to statistically analyze. By combining digit with nondigit cases, both of which were treated with WLE and amputation, statistical analyses could be performed regarding overall treatment of ALM. In looking at WLE for both digit and subungual ALM combined, we were able to perform a baseline comparison to PDA and DA, in which there did not appear to be significant differences in survival.
These results suggest that DA may not be necessary in all cases of digital or subungual ALM and that PDA and WLE may be options in select ALM cases since oncologic outcomes may not be compromised while function may be improved. This lack of statistically significant differences in survival was observed despite PDA patients having the greatest median thickness (4.5 mm) compared with WLE patients (lowest median thickness: 2.4 mm) and DA patients (median thickness: 3.6 mm). This may account for the higher number of deaths observed in the PDA group; however, again these differences were not significantly different.
Other studies have also suggested using WLE for ALM in select cases. In 2002, Clarkson et al.32 made a formal recommendation for WLE for in situ melanomas occurring anywhere on the hand and reported no cases of recurrence; however, our study did not include in situ lesions. Most recently, Moehrle et al. published a study of 62 patients presenting with stage I and II subungual melanomas and found no difference in survival between functional surgery (WLE and/or resection of the distal portion of the distal phalanx) versus amputation. However, this study was weakened by the relatively few patients (n = 3) who underwent WLE.33 In contrast, our study included 22 patients who underwent WLE and showed no significant differences in OS or RFS as compared with patients who underwent PDA or DA.
While our data demonstrate no significant differences in survival based on surgical approach to primary ALM, the number of recurrences was highest in the WLE group (n = 11, 50%), followed by the DA group (n = 4, 33.3%), and was lowest in the PDA group (n = 2, 22.2%). Our recurrence rate for ALM following WLE is consistent with other reports by Cohen et al.,34 Gumaste et al.,35 Moerhle et al.,33 and Phan et al.,36 as seen in Table 5, which displays recurrence rates for all ALM and subungual ALM. These recurrence rates are in stark contrast to published recurrence rates reported by Urist et al.37,38 for primary cutaneous melanoma overall. One report by Lee et al. demonstrated a lower recurrence rate for ALM at 10.8%, although this study included thin and thick lesions at a variety of primary locations.39 Despite the varying rates of recurrences, OS in the current study was not significantly different between the surgical approaches, and it is likely that patients in the WLE group who recurred were salvaged with further surgical treatment such as PDA or DA. Of note, these findings are similar to those of Cohen et al. who published a series of 49 patients with melanoma in situ managed with WLE and found that these patients were at increased risk of recurrence.34 All of these data highlight the importance of close follow-up for patients who undergo WLE for digital and subungual ALM.
Table 5.
Recurrence Rates Reported for Acral Lentiginous Melanoma
| Publication | n | Surgical Approach | Diagnosis | Recurrence Rate (%) |
|---|---|---|---|---|
| Gumase et al. | 61 | WLE | ALM | 49.0 |
| Moehrle et al. | 31 | Amputation | Subungual specifically | 48.4 |
| Cohen et al. | 49 | Mixed | Subungual specifically | 27 |
| Moehrle et al. | 31 | WLE | Subungual specifically | 35.5 |
| Phan et al. | 126 | WLE | ALM | 30.0 |
| Lee et al. | 129 | Mixed | ALM | 10.8 |
| Heaton et al. | 46 | Amputation | Subungual specifically | 65 |
| Urist et al. (1985) | 3,445 | WLE | Primary cutaneous | 2.7 |
| Urist et al. (1996) | 3,147 | WLE | Primary cutaneous | 3.2 |
WLE, wide local excision; ALM, acral lentiginous melanoma.
Nodal status is prognostic for melanoma patients in general, and studies have validated the role for SLNB for nodal staging in melanoma.22,40,41 However, most studies that have evaluated nodal status for melanoma have only included a small number of patients with ALM and were unable to report on the significance of SLN status specifically for ALM. Recently, Bello et al. have shown that SLN status was a significant predictor of disease-specific survival on multivariable analysis.26 In the current study, the nodal metastasis rate of 35.5% is consistent with prior reports22,24,25,40,42; however, no significant differences in survival were seen between node-positive and node-negative patients. These results are likely due to the low number of patients who underwent nodal staging and the low number of patients with nodal metastasis. With larger numbers, significant differences may be seen, although differences in disease biology may also play a potential role.31–37
Our study has several limitations. It is retrospective in nature and reflects the referral bias and practice setting of a single institution with 4 surgeons treating melanomas. Furthermore, there may have been a selection bias for the surgical modality chosen to treat the primary site. In addition, the relatively low number of patients in each of the comparison groups may have also limited our ability to see significant differences between the WLE, PDA, and DA groups and in the node-positive versus node-negative groups. Given the limitations of any one center to gather enough of these rare cases, treatment of ALM will require a clinical trial approach with multicenter participation to develop a true and powered consensus on the surgical management of this disease.
CONCLUSIONS
Although studies have evaluated surgical management of ALM, there are no consensus recommendations in the literature as to the preferred and standard method to treat the primary site, particularly for digital and subungual lesions. In the current study, patients with foot or nonsubungual tumors were more likely to be treated with WLE, while patients with hand and subungual ALM were more likely to have PDA. Importantly, there appeared to be no significant differences in OS or RFS between ALM patients treated with DA, PDA, and WLE. Given these results, WLE may be considered for certain appropriately selected foot and nonsubungual ALM, while PDA may be considered in some appropriately selected hand and subungual ALM. PDA or WLE may be options in select patients with ALM on the digit to allow for preservation of function, minimization of morbidity, and maintaining oncologic outcomes. However, careful consideration of multiple clinicopathologic factors, including the consensus of the institution’s tumor board, must be taken into account when deciding the most appropriate treatment.
ACKNOWLEDGMENTS
We would like to acknowledge the time and effort of our research fellows, Dr. Alex Sun, MD, and Dr. Cyril Gary, MD, in building and maintaining the acral lentiginous melanoma database.
Footnotes
Published online 23 March 2020.
Presented at the New England Hand Society Annual Meeting (Sturbridge, Mass. – December 2018) and at the American Association of Hand Surgery Annual Meeting (Palm Desert, Calif. – January 2019)
Disclosures: Database was managed and supported by the Yale Cancer Center via NIH Research Grant CA-16359 from the National Cancer Institute.
REFERENCES
- 1.Goydos JS, Shoen SL. Acral lentiginous melanoma. Cancer Treat Res. 2016;167:321–329. [DOI] [PubMed] [Google Scholar]
- 2.Piliang MP. Acral lentiginous melanoma. Clin Lab Med 2011;31:281–288. [DOI] [PubMed] [Google Scholar]
- 3.Desai A, Ugorji R, Khachemoune A. Acral melanoma foot lesions. Part 1: epidemiology, aetiology, and molecular pathology. Clin Exp Dermatol. 2017;42:845–848. [DOI] [PubMed] [Google Scholar]
- 4.Desai A, Ugorji R, Khachemoune A. Acral melanoma foot lesions. Part 2: clinical presentation, diagnosis, and management. Clin Exp Dermatol. 2018;43:117–123. [DOI] [PubMed] [Google Scholar]
- 5.Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson DA, Krige JE. Melanoma in black South Africans. J Am Coll Surg. 1995;180:65–71. [PubMed] [Google Scholar]
- 7.Pollitt RA, Clarke CA, Swetter SM, et al. The expanding melanoma burden in California Hispanics: importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. 2011;117:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black WC, Goldhahn RT, Jr, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331–1334. [PubMed] [Google Scholar]
- 9.Lino-Silva LS, García-Gómez MA, Salcedo-Hernández RA. In response: can we rely on the adequate mesorectum excision and the complete pathological response in case of rectal signet-ring cell carcinoma. J Surg Oncol. 2016;114:650. [DOI] [PubMed] [Google Scholar]
- 10.Chi Z, Li S, Sheng X, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer. 2011;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roh MR, Kim J, Chung KY. Treatment and outcomes of melanoma in acral location in Korean patients. Yonsei Med J. 2010;51:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HY, Chay WY, Tang MB, et al. Melanoma: differences between Asian and Caucasian patients. Ann Acad Med Singapore. 2012;41:17–20. [PubMed] [Google Scholar]
- 13.Marchetti MA, Chung E, Halpern AC. Screening for acral lentiginous melanoma in dark-skinned individuals. JAMA Dermatol. 2015;151:1055–1056. [DOI] [PubMed] [Google Scholar]
- 14.Jackson CR, Fernelius C, Arora N. Ramifications of poor medical education and screening in minority populations: an extensive acral melanoma. BMJ Case Rep. 2015;2015bcr2014207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bichakjian CK, Halpern AC, Johnson TM, et al. ; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American academy of dermatology. J Am Acad Dermatol. 2011;65:1032–1047. [DOI] [PubMed] [Google Scholar]
- 16.Coit DG, Thompson JA, Algazi A, et al. NCCN guidelines insights: melanoma, version 3.2016. J Natl Compr Canc Netw. 2016;14:945–958. [DOI] [PubMed] [Google Scholar]
- 17.Coit DG, Andtbacka R, Anker CJ, et al. ; National Comprehensive Cancer Network (NCCN). Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:395–407. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta T, Brasfield R. Subungual melanoma: 25-year review of cases. Ann Surg. 1965;161:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635–638. [DOI] [PubMed] [Google Scholar]
- 20.Heaton KM, el-Naggar A, Ensign LG, et al. Surgical management and prognostic factors in patients with subungual melanoma. Ann Surg. 1994;219:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KG, Blessing K, Kernohan NM. Surgical aspects of subungual malignant melanomas. The Scottish melanoma group. Ann Surg. 1992;216:692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton DL, Thompson JF, Cochran AJ, et al. ; MSLT Group. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. [DOI] [PubMed] [Google Scholar]
- 23.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. [DOI] [PubMed] [Google Scholar]
- 24.Morton DL, Wanek L, Nizze JA, et al. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991;214:491–499; discussion 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavri SN, Han G, Khan S, et al. Does sentinel lymph node status have prognostic significance in patients with acral lentiginous melanoma? J Surg Oncol. 2019;119:1060–1069. [DOI] [PubMed] [Google Scholar]
- 26.Bello DM, Chou JF, Panageas KS, et al. Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol. 2013;20:3618–3625. [DOI] [PubMed] [Google Scholar]
- 27.Marek AJ, Ming ME, Bartlett EK, et al. Acral lentiginous histologic subtype and sentinel lymph node positivity in thin melanoma. JAMA Dermatol. 2016;152:836–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito T, Wada M, Nagae K, et al. Acral lentiginous melanoma: who benefits from sentinel lymph node biopsy? J Am Acad Dermatol. 2015;72:71–77. [DOI] [PubMed] [Google Scholar]
- 29.Ross MI, Reintgen D, Balch CM. Selective lymphadenectomy: emerging role for lymphatic mapping and sentinel node biopsy in the management of early stage melanoma. Semin Surg Oncol. 1993;9:219–223. [PubMed] [Google Scholar]
- 30.Morton DL, Wen DR, Foshag LJ, et al. Intraoperative lymphatic mapping and selective cervical lymphadenectomy for early-stage melanomas of the head and neck. J Clin Oncol. 1993;11:1751–1756. [DOI] [PubMed] [Google Scholar]
- 31.Hutchinson J. Melanosis often not black: melanotic whitlow. Br Med J. 1886;1:491. [Google Scholar]
- 32.Clarkson JH, McAllister RM, Cliff SH, et al. Subungual melanoma in situ: two independent streaks in one nail bed. Br J Plast Surg. 2002;55:165–167. [DOI] [PubMed] [Google Scholar]
- 33.Moehrle M, Metzger S, Schippert W, et al. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366–374. [DOI] [PubMed] [Google Scholar]
- 34.Cohen T, Busam KJ, Patel A, et al. Subungual melanoma: management considerations. Am J Surg. 2008;195:244–248. [DOI] [PubMed] [Google Scholar]
- 35.Gumaste PV, Fleming NH, Silva I, et al. Analysis of recurrence patterns in acral versus nonacral melanoma: should histologic subtype influence treatment guidelines? J Natl Compr Canc Netw. 2014;12:1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phan A, Touzet S, Dalle S, et al. Acral lentiginous melanoma: a clinicoprognostic study of 126 cases. Br J Dermatol. 2006;155:561–569. [DOI] [PubMed] [Google Scholar]
- 37.Urist MM, Balch CM, Soong S, et al. The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer. 1985;55:1398–1402. [DOI] [PubMed] [Google Scholar]
- 38.Urist MM. Surgical management of primary cutaneous melanoma. CA Cancer J Clin. 1996;46:217–224. [DOI] [PubMed] [Google Scholar]
- 39.Lee KT, Kim EJ, Lee DY, et al. Surgical excision margin for primary acral melanoma. J Surg Oncol. 2016;114:933–939. [DOI] [PubMed] [Google Scholar]
- 40.Morton DL, Thompson JF, Cochran AJ, et al. ; MSLT Group. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coit DG. NCCN guidelines and quality cancer care: where have we come from, and where should we be going? J Natl Compr Canc Netw. 2016;14:373–377. [DOI] [PubMed] [Google Scholar]
- 42.Balch CM, Morton DL, Gershenwald JE, et al. Sentinel node biopsy and standard of care for melanoma. J Am Acad Dermatol. 2009;60:872–875. [DOI] [PubMed] [Google Scholar]


