Supplemental Digital Content is available in the text.
Background:
A calcium alginate dressing (ALGINATE) and negative pressure wound therapy (NPWT) are frequently used to treat wounds which heal by secondary intention. This trial compared the healing efficacy and safety of these 2 treatments.
Methods:
This randomized, non-inferiority trial enrolled patients who underwent skin excision (>30 cm2), which was left open to heal by secondary intention. They received ALGINATE or NPWT by a centralized randomization. Follow-up was performed weekly until optimal granulation tissue was obtained. The primary outcome was time to obtain optimal granulation tissue for a split thickness skin graft take (non-inferiority margin: 4 days). Secondary outcomes were occurrence of adverse events (AEs) and impact of the treatments on the patient’s daily life.
Results:
ALGINATE and NPWT were applied to 47 and 48 patients, respectively. The mean time to optimal granulation was 19.98 days (95% CI, 17.7–22.3) with ALGINATE and 20.54 (95% CI, 17.6–23.5) with NPWT. Between group difference was −0.56 days (95% CI −4.22 to 3.10). The non-inferiority of ALGINATE versus NPWT was demonstrated. No AE related to the treatment occurred with ALGINATE versus 14 AEs with NPWT. There was no difference in the impact of the treatments on the patient’s daily life.
Conclusion:
This trial demonstrates that ALGINATE has a similar healing efficacy to that of NPWT and that is markedly better with regard to patient safety.
Surgical excisions of the skin and the underlying soft tissue are carried out for various reasons including infections, trauma, and tumors. Such excisions are left open to heal by secondary intention to obtain optimal granulation tissue which allows a split-thickness skin graft (STSG) take.1–4 This clinical trial compared a calcium alginate wound dressing (ALGINATE) and negative pressure wound therapy (NPWT), medical devices widely used by French surgeons in the treatment of surgical excisions. ALGINATE5–8 and NPWT9,10 are known to eliminate excess exudate and promote granulation. Over the last 15 years, the use of NPWT increased exponentially in all kinds of wounds as a replacement for alginate, and other types of modern dressings. Despite numerous systematic reviews being performed, the Cochrane Collaboration still does not contain good-quality clinical evidence for informing health decision-making on the utility of NPWT in wound treatment versus dressings. The Cochrane Collaboration continuously asks for relevant and high-quality clinical trials to decide on this issue.11–13 The aim of thistial was to determine whether the healing efficacy, defined as the time to obtain optimal granulatin for STSG take, is non-inferior with ALGINATE versus NPWT and whether it is safer to use ALGINATE and whether the cost is inferior with ALGINATE. Results of the healing efficacy and safety of the 2 interventions are presented in this article. Cost results will be reported in another article.
PATIENTS AND METHODS
Trial Design
This was a multicenter, randomized, non-inferiority, parallel-group, and open-label clinical trial, with a blinded assessment of the primary outcome. Plastic and reconstructive surgery units of 17 French teaching hospitals participated in the trial. All patients signed an informed consent form before inclusion. The trial was initially designed to compare 2 wound treatments and was amended into a trial comparing 2 therapeutic strategies using ALGINATE or NPWT as the first-line treatment. The amendment, accepted by the ethics committee on 26 March 2015, allowed surgeons to replace the first-line treatment, if this failed, with another dressing until obtaining optimal granulation tissue.
Patients
Patients were included if they were 18 years or older and had at least 30 cm2 of their skin and soft tissue excised surgically for infectious, traumatic (except burns), or tumor-related reasons, with the wound left initially open to heal by secondary intention. Patients were not eligible if they had uncontrolled hyperglycaemia (HbA1c >10%), or had been treated during the 30 days before inclusion by immunosuppressant treatment, chemotherapy, or radiotherapy.
Treatments
ALGINATE (Algostéril, BROTHIER, France) is a wound dressing composed of pure calcium alginate obtained from brown seaweed. NPWT (3M, France; Smith & Nephew, UK; Hartmann, Germany; Mölnlycke, Sweden) is an electrical device comprising polyurethane synthetic foam, a tube, a canister, and an electrical generator. The 2 treatments were applied according to the manufacturer’s instructions: ALGINATE was changed once a day or every 2 days and the NWPT foam was changed every 2 to 3 days.
Evaluation
Primary Outcome
There is no definition of “optimal granulation tissue” in the literature; therefore, we defined it as “a homogeneous, pink, continuous, non-oozing, non-hemorrhagic, non-infected granulation tissue that is well-vascularized and uniformly covering the totality of the surgical excision area.” Its evaluation was performed by visual assessment.
The time to obtain an optimal granulation which allows STSG take was expressed in the number of days between the date of the surgical excision and the date on which optimal granulation tissue was achieved.
To minimize the subjectivity of the visual assessment of the optimal granulation, the date of optimal granulation was decided by a consensus between the investigator and a blind assessor, who was unaware of the treatment received by the patient. Subsequently, via anonymized photographs, an oversight committee validated this date or suggested another date. The date of optimal granulation chosen by the committee was used in the statistical analysis.
Secondary Outcomes
The safety evaluation was based on the frequency and nature of serious or non-serious adverse events (AEs) related to the tested treatments. Complications related to the tested treatments were evaluated using the number of AEs, withdrawals and treatment switches (post-hoc analysis). The quality of the wound dressing removal was assessed by nurses at each dressing change based on the degrees of the adherence to the wound, bleeding, and foul odor. The impact of the tested treatment on the pain and the discomfort during sleep and movement was self-evaluated by the patient: 0 (no impact) to 10 (maximum impact).
Assessments
At the inclusion and randomization visit that occurred between Day–15 and Day 0, day on which the surgical excision was performed, the investigator collected data on the patient. At the weekly follow-up visits, the investigator recorded the percentage of granulation tissue on the wound area and the impact of the treatment on the patient’s daily life. The end-of-trial visit took place on the day when the investigator and the blind assessor agreed that the optimal granulation tissue was obtained. At each visit, a photograph was taken according to a standardized protocol (see document, Supplemental Digital Content 1, http://links.lww.com/PRSGO/B320).
AEs related to the tested treatments were reported throughout the trial. At each wound dressing change, the quality of the dressing removal was recorded.
Patients were first followed up in the hospital (operating theater, intensive care unit, or hospitalization unit) and then in an after-care and rehabilitation facility and/or in home hospitalization, and/or at home under care provided by a district nurse.
Sample Size
The number of patients to be included was calculated by the RCTS company (France) using the SAS software (version 9.2). A non-inferiority margin of 4 days for the primary outcome was designated by the investigators in the absence of literature data. Using a SD of 7 days, a type I error rate of 0.025 (1-sided) and a type II error rate of 0.20, the number of patients required was estimated at 50 per group. Assuming that about 10% of patients would deviate markedly from the protocol or would be lost to follow-up, a sample size of 56 patients per group was decided upon.
Randomization
Patients were randomized to receive ALGINATE or NPWT in a 1:1 ratio. The allocation sequence, stratified according to the center and balanced using a block size of 4, was generated by ABPlus Company (France). The randomization was carried out via an interactive web-based or vocal response system.
Statistical Analysis
The RCTS Company performed the statistical analysis on the Intent-To-Treat (ITT) population, comprising all randomized patients who have been exposed at least once to 1 of the 2 treatments and on the Per Protocol (PP) population, comprising only patients of the ITT population who completed the trial without major protocol deviations. No interim analysis was planned nor performed.
Primary Outcome
The non-inferiority analysis was based on the mean time difference to obtain optimal granulation between the ALGINATE and NPWT groups. Non-inferiority will be shown if the upper limit of the 95% CI of the between group difference was less than the pre-specified non-inferiority margin of 4 days and will also include 0. If non-inferiority was met, it was planned to test the superiority of ALGINATE. Such superiority could be shown if the upper limit of the 95% CI was less than the non-inferiority margin and also less than 0.14
Secondary Outcomes
The secondary outcomes were analyzed for the ITT and PP populations, defined without a hierarchy and based on a search for a significant difference between the 2 groups defined using an α cutoff of 0.05 (2-sided). The safety analysis was descriptive and performed on the ITT population. The quality of the wound dressing removal and the impact of the treatment on the patient’s daily life were compared as follows: (a) continuous variables were compared using analysis of covariance with a mixed effect integrating the center effect as the random effect and the initial evaluation of the measure as the covariate, (b) binary variables were compared using logistic regression with mixed effects integrating the center effect as the random effect and their initial evaluation as a fixed factor.
Post-hoc Analyses
Complication rates were analyzed by the Axonal Company (France). The Fisher’s exact test was used to compare their incidence between the 2 groups in the ITT population. All of the collected variables were described using the usual statistical models. A 2-sided probability value of P < 0.05 was considered to be indicative of a statistically significant difference.
Reporting of the trial was conducted according to CONSORT rules published for non-inferiority trials.15
RESULTS
Participants
Between 11 July 2014 and 31 May 2016, 113 patients were included and randomized between D–15 and D0. The last patient completed the study on 13 July 2016. Baseline characteristics of patients and surgical excisions did not differ between the 2 treatment groups for the ITT (Table 1) as well as for the PP population (see tables a-e, Supplemental Digital Content 2, http://links.lww.com/PRSGO/B321). The ITT population consisted of 107 patients and the PP population of 95 patients (Fig. 1).
Table 1.
Baseline Characteristics of the Patients and Surgical Excisions in the ITT Population
| ALGINATE | NPWT | |
|---|---|---|
| n = 52 | n = 55 | |
| Patients | ||
| Mean age, years (SD) | 50.8 (21.0) | 54.9 (22.4) |
| Male, n (%) | 26 (50.0) | 25 (45.5) |
| Diabetes mellitus, n (%) | 5 (9.6) | 9 (16.4) |
| Current smokers, n (%) | 16 (30.8) | 21 (38.2) |
| Obesity, n (%) | 11 (21.2) | 18 (32.7) |
| Surgical excisions | ||
| Etiology, n (%) | ||
| Infectious | 29 (55.8) | 27 (49.1) |
| Traumatic | 16 (30.8) | 19 (34.5) |
| Tumor-related | 7 (13.4) | 9 (16.4) |
| Localization, n (%) | ||
| Lower limb | 21 (40.4) | 26 (47.3) |
| Upper limb | 18 (34.6) | 17 (30.9) |
| Thoracic | 2 (3.8) | 3 (5.5) |
| Back | 4 (7.7) | 4 (7.3) |
| Abdominal | 2 (3.8) | 1 (1.8) |
| Lumbar/sacral | 0 | 2 (3.6) |
| Buttock | 1 (1.9) | 1 (1.8) |
| Anal/perineal/genital | 4 (7.7) | 1 (1.8) |
| Median surface area, cm2 (IQR) | 100 (60–174) | 80 (49–150) |
| Median volume, cm3 (IQR)* | 145 (75–300) | 150 (70–300) |
*Missing data for 1 patient in the ALGINATE group.
IQR, interquartile range.
Fig. 1.
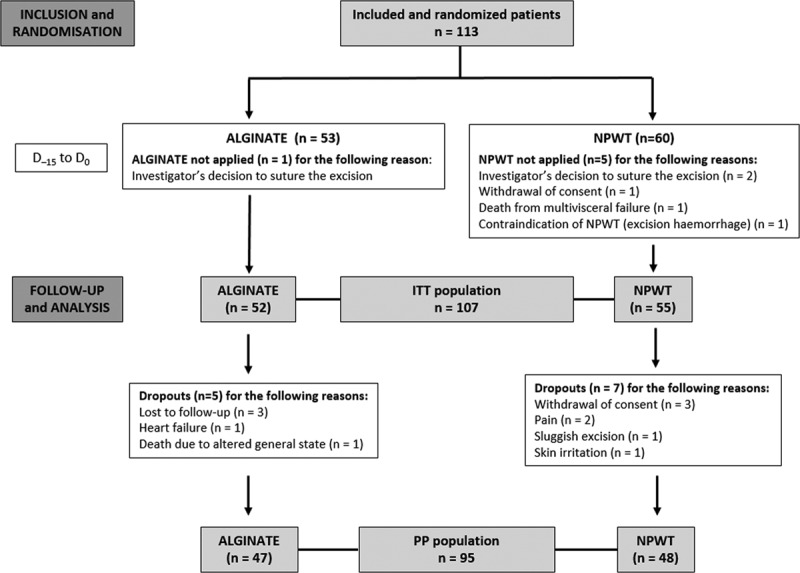
CONSORT participant flow diagram.
Primary Outcome
The mean time to optimal granulation was 19.98 days with ALGINATE and 20.54 with NPWT (Fig. 2). The non-inferiority of ALGINATE versus NPWT was met in the PP and ITT populations as the upper limit of the 95% CI of the difference, 3.10 days, was less than the pre-specified non-inferiority margin of 4 days and also included 0. The results for the primary outcome were identical for the PP (Table 2) and ITT populations (see tables a-e, Supplemental Digital Content 2, http://links.lww.com/PRSGO/B321). No conclusion could be drawn regarding the superiority of ALGINATE since the upper limit of the 95% CI exceeded 0. The time course of granulation development was similar for both treatments, with a rapid development during the first 2 weeks that slowed thereafter (Fig. 3).
Fig. 2.
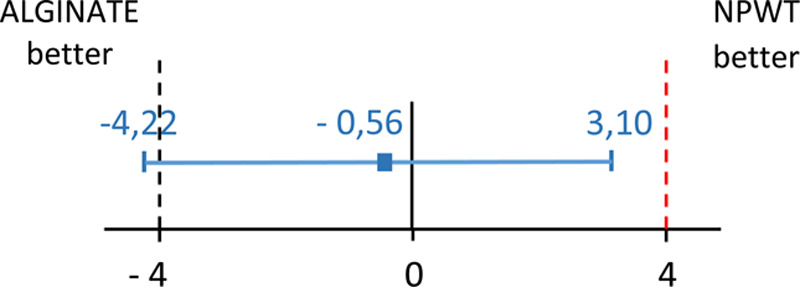
Presentation and interpretation of primary outcome “time to obtain an optimal granulation tissue,” using CI in relation to non-inferiority margin. Dashed lines indicate non-inferiority margin; the region between dashed lines is zone of non-inferiority. Error bar indicates 2-sided 95% CI. Given that CI for the difference in mean times (ALGINATE minus NPWT) lies to the left of the non-inferiority margin [Δ, (+ 4 days)] and also include 0, the interpretation is ALGINATE is non-inferior to the NPWT but not shown to be superior.
Table 2.
Time to Optimal Granulation Tissue in the PP Population
| ALGINATE, n = 47 | NPWT, n = 48 | |
|---|---|---|
| Mean time, days (SD) | 19.98 (7.76) | 20.54 (10.03) |
| 95% CI | 17.7–22.3 | 17.6–23.5 |
| Median (IQR) | 19.0 (14.0–24.0) | 19.5 (14.0–27.0) |
| Range | 8.0–37.0 | 7.0–47.0 |
| Mean time ALGINATE minus NPWT, days (SD) | −0.56 (1.84) | |
| 95% CI | −4.22 to 3.10 | |
Fig. 3.
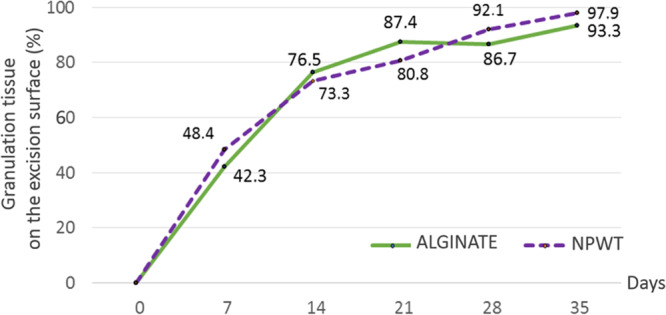
Time course of granulation development.
Secondary Outcomes
No AE was reported in the ALGINATE group. The 14 AEs reported in the NPWT group, related to the treatment, occurred in 13 (23.6%) patients. One patient experienced 2 AEs and 12 patients experienced a single AE (with 1 of these patients experiencing a serious AE) (Table 3).
Table 3.
AEs Related to Tested Products in the ITT Population
| ALGINATE, n = 52 | NPWT, n = 55 | |||
|---|---|---|---|---|
| Adverse Event | Aes, n | Patients, n (%) | Aes, n | Patients, n (%) |
| At least one AE | 0 | 0 (0) | 14 | 13 (23.6) |
| Severe pain | 0 | 0 (0) | 5 | 5 (9.1) |
| Hemorrhage (one serious*) | 0 | 0 (0) | 4 | 3(5.5) |
| Skin irritation | 0 | 0 (0) | 2 | 2(3.6) |
| Infection | 0 | 0 (0) | 2 | 2(3.6) |
| Joint ankylosis | 0 | 0 (0) | 1 | 1(1.8) |
*Blood loss of 300 mL.
The quality of the wound dressing removal was significantly better for ALGINATE than for NPWT, with less adherence (52.2% versus 73.2%, P < 0.0001), less bleeding (38.9% versus 55.4%, P < 0.0001), and less foul odor (22.7% versus 47.4%, P < 0.0001). There was no intergroup difference concerning the impact of the tested treatments on the patients’ daily life (noise nuisance, background pain, or discomfort during sleep and movement).
Excisions with healthy perilesional skin (without maceration) are more numerous in the ALGINATE group: 78% versus 58% for NPWT (see tables a-e, Supplemental Digital Content 2, http://links.lww.com/PRSGO/B321).
Post-hoc Analyses
The proportion of patients who had at least 1 complication related to the tested treatments was significantly higher in the NPWT group (34.5%) than in the ALGINATE group (0%) (see tables a-e, Supplemental Digital Content 2, http://links.lww.com/PRSGO/B321). Easiness of handling was evaluated: 88% of ALGINATE applications were considered easy versus 55% of NPWT and 83% of ALGINATE removals were considered easy versus 56% of NPWT.
DISCUSSION
This trial compared the healing efficacy and safety of ALGINATE versus NPWT in treating surgical excisions left open to heal by secondary intention. The primary outcome was the time (days) to obtain optimal granulation tissue, which allows a STSG take. This time was similar in the ALGINATE and NPWT groups, at an average of 20 days. This similarity in mean time may be explained by their similar mechanisms of action: the 2 treatments drain the wound exudate and accelerate granulation. ALGINATE drains the exudate thanks to its highly hydrophilic nature and to the capillarity of its multilobal fibers. ALGINATE accelerates the formation of granulation tissue by releasing calcium ions, which activate endothelial cells and fibroblasts.16 By applying negative pressure to the wound, NPWT drains the exudate and creates a tissue hypoxia gradient, which generates new blood vessels and hence accelerates granulation.9 In contrast to these results, a single-center trial by Monsen et al17 that compared NPWT with 2 alginate-based wound dressings, found that the healing of surgical wounds was 2-fold faster with NPWT. The Cochrane review revealed the presence of selection, detection, and analysis biases in Monsen trial and concluded that “the benefits of NPWT versus wound dressings in surgical wounds remain largely uncertain, due to the absence of a rigorous randomized controlled trial.”12 Beyond the methodology biases, the difference in results between the Monsen et al trial and our trial is understandable given that the 2 alginate dressings used by Monsen et al were different from ALGINATE tested in our trial. The clinical efficacy of alginate wound dressings rests on the release of their calcium ions into a wound,18,19 which is influenced by the ratio between 2 constituents of alginate dressings, mannuronic and guluronic acids.20,21 This ratio varies between manufacturers.21–23 Different compounds added by manufacturers to alginate dressings, such as carboxymethyl cellulose and sodium ions21,23–25 alter further the release of calcium ions and subsequently the healing efficacy of alginate. The characteristics of alginate fibers, such as their form, resistance, and textiling, impact their drainage capacity and vary considerably between manufacturers.21,23 The nonequivalence of alginates, well described in the literature,26–28 is why clinical results are not transposable from one alginate wound dressing to another. During the course of our trial, no complications nor AE occurred in the ALGINATE group, whereas 20 complications were reported in the NPWT group. These complications involved 19 patients (34.5%) and 14 were reported as AEs related to NPWT. The causes of these complications are well explained in the literature and reported frequently: (a) bleeding and pain occur due to the granulation tissue infiltrating the foam and being torn apart when removing the NPWT foam,10,29–31 (b) infection is frequently caused by fragments of foam remaining trapped in the granulation tissue,30,32–34 (c) irritation of the perilesional skin is due to an allergic reaction to the polyurethane film used to ensure the airtightness of the wound,35–37 and (d) ankylosis results from immobilization of the joint in proximity to the wound treated with NPWT. Our results demonstrate that, upon removal, there is significantly less wound adherence and less bleeding for ALGINATE than for NPWT. These results were expected: the granulation tissue often infiltrates the NPWT foam which removal causes bleeding, whereas ALGINATE dressing does not adhere to the wound bed because it jellifies when in contact with exudate.8,38 These features result in the non-traumatic removal of the ALGINATE wound dressing, with little bleeding.38 The drawbacks of NPWT are described in the literature: pain during the functioning of the device and when removing the foam, noise generated by the device, reduced mobility, increased stress, and anxiety of the patient.39,40 Yet, our trial found that there was no difference between the treatments in their impact on patient’s daily life. This may be explained by each patient evaluating the impact of only 1 treatment, without knowing the effect of the other.
In this trial, some limits can be underlined. Three patients in the ALGINATE group were lost to follow-up: almost all ALGINATE patients were discharged from the hospital to home care with a prescription, which enabled them to have their wound dressing renewed by a nurse of their choice without needing to return to the hospital. No patient was lost to follow-up for NPWT because their treatment depended on this device, which was provided and monitored by a hospital. Several brands of NPWT were used in this usual care trial, which could be considered as its limit, but, according to health authorities there is no difference in efficacy between NPWT brands. The NNT (number needed to treat) was calculated to be 50 per group. Fifty-three (ALGINATE) and 60 (NPWT) patients were included in the trial. Due to premature dropouts, the PP population consisted of 47 (ALGINATE) and 48 (NPWT) patients. This could have been a limit of the trial, but the PP population was sufficient to demonstrate the non-inferiority of ALGINATE. In the field of medical devices, the literature is often poor in data needed to calculate the NNT and therefore this calculation is often under- or overestimated.
To avoid the limitation of an open-label design,41 the primary outcome of our trial was reevaluated by a blind assessor and then by an oversight committee on the basis of anonymized excision photographs. One strength of our trial was its strict methodology, centralized randomization, inclusion of only 1 wound type (surgical excision), and comparison of NPWT versus only 1 type of alginate dressing. The use of NPWT in wound treatment has increased over the past 15 years at the expense of wound dressings. Is this change in practice justified by clinical evidence of NPWT benefits versus wound dressings? Several systematic Cochrane reviews were performed since 2007 on the utility of NPWT versus wound dressings in burns,42 skin grafts and sutured wounds,13 pressure sores,11 leg ulcers,43 surgical excisions,12 diabetic feet,44 traumatic open wounds,45 and sutured wounds.46 For all these types of wounds other than traumatic wounds, the authors could not draw conclusions about the utility of NPWT versus wound dressings due to the poor quality of evidence of randomized controlled trials. Only the systematic review on traumatic open wounds obtained a conclusion, thanks to the moderate level of evidence,45 with the authors concluding that there was no difference between NPWT and wound dressings regarding the percentage of healed wounds.
CONCLUSION
The results of this trial demonstrate that the healing efficacy of ALGINATE is similar to that of NPWT. ALGINATE is markedly superior in terms of patient safety, with AEs occurring in 23.6% of patients in the NPWT group versus none in the ALGINATE group. These findings indicate that ALGINATE is preferable for the treatment of surgical excisions. The results of this trial can be extrapolated to all non-infected acute wounds left open to heal by secondary intention. They cannot be extrapolated to chronic wounds because they involve a different healing process.47
ACKNOWLEDGMENTS
The authors thank all the investigators for their time and commitment to the trial: Antoine Alliez, Claire Baptista, Baptiste Bertrand, and Julien Serri (La Conception Hospital, Marseille); Nawaf Aljudaibi, Yasmine Bennis, and Louise Pasquesoone (Roger Salengro Hospital, Lille); Nizar Assaf and Gautier Michel (Hôpital Sud, Amiens); Eric Watier and Sylvie Aillet (Hôpital Sud, Rennes); Pierre Moullot (Hôpital Nord, Marseille); Rémi Foissac (Pasteur II Hospital, Nice); Pauline Ridel (Hôtel Dieu Hospital, Nantes); Nathaniel Stroumza (Tenon Hospital, Paris); Thibault Vimont Doguet (Angers Hospital, Angers); Fabienne Braye and Mehdi Ismail (La Croix Rousse Hospital, Lyon); Isabelle Barthélémy and Charles Irthum (Estaing Hospital, Clermont-Ferrand); Vincent Casoli and Camille Poujardieu (Pellegrin Hospital, Bordeaux); Damien Feuvrier (Jean-Minjoz Hospital, Besançon); Catherine Bruant-Rodier and Frédéric Bodin (Hautepierre Hospital, Strasbourg); Carine Blanc, Charlotte Monnerie, and Alexandra Trimaille (La Cavale Blanche Hospital, Brest); Mihai Gorj, Yassin Guenane, and Vincent NGuyen (Saint-Louis Hospital). We also thank Cédric Portugues, Frédéric Mistretta (RCTs, Lyon), and Nicolas Lemaire (Axonal, Nanterre) for the statistical analysis and data interpretation, as well as Hervé Maisonneuve (H2MW, Paris) and Marko Vidak (Lingua franca, Puteaux) for their contribution to the article (drafting, reviewing, and editing), and the BROTHIER company for funding.
Supplementary Material
Footnotes
Published online 27 March 2020.
Presented at the 61th Annual Congress of the French Plastic Surgery Reconstructive and Esthetic Society in Paris, France, November 2016; at the 37th Annual Congress of French Burn Society, La Rochelle, France, June 2017; at the 3rd Congress Le Pied Diabétique in Montpellier, France, February 2017; at the 13th Conference of the European Council of Enterostomal Therapists, in Berlin, Germany, June 2017; at the 27th conference of the European Wound Management Association, Amsterdam, Netherlands, May 2017; at the 70th Congress of Thoracic and Cardiovascular Surgery, Marseille, France, June 2017; at the 53th Congress of the French Society of Stomatology, Maxillofacial Surgery and Oral Surgery, Marseille, France, October 2017; at the 8th edition of the Midi-Pyrenees Days Healing, Toulouse, France, March 2018; and at the 38th Overseas Othopedy Days, Tahiti, March 2019.
This trial was funded by a grant from BROTHIER, resources directly paid to each investigator’s institutions. Trial treatments were not provided by the funder given that the trial was conducted as a usual care clinical trial. The funding sources contributed to the study design, monitoring, data analysis, data interpretation, and to the writing of the study report.
This trial was sponsored by BROTHIER Company, Nanterre, France. All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/conflicts-of-interest/. All authors (except G.C.) reports grants from BROTHIER during the conduct of the study. B.C.-S., P.G., C.P., N.B., A.C.-B., R.S., I.P., F.D., and W.H. report personal fees and P.R. reports non-financial support from BROTHIER during the conduct of the study. D.C., B.C.-S., N.B., F.D., M.A., F.B., C.P., I.P., and W.H. report non-financial support and F.D., N.B., F.B., and C.P. report personal fees from BROTHIER outside the submitted work. D.C. reports personal fees and non-financial support from URGO outside the submitted work. R.S. reports non-financial support from URGO and MOLNLYCKE outside the submitted work. B.C.-S. reports non-financial support from SMITH & NEPHEW and CONVATEC outside the submitted work. F.D. reports personal fees and non-financial support from URGO, SMITH & NEPHEW, and HARTMANN and non-financial support from CONVATEC, and personal fees from GENEVRIER outside the submitted work. P.R. reports non-financial support from KCI outside the submitted work. N.P.D. reports non-financial support from CONVATEC outside the submitted work.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the regional ethics committee of Ile-de-France IV on 15 July 2013.
This trial is registered under the name “Comparison of the efficacy, tolerance and cost of Algostéril vs Negative Pressure Therapy in preparation for skin grafting following surgical excision,” ISRCTN Clinical Trial Registry number ISRCTN60292377 (http://www.isrctn.com/ISRCTN60292377).
REFERENCES
- 1.Saaiq M, Hameed-Ud-Din, Khan MI, et al. Vacuum-assisted closure therapy as a pretreatment for split thickness skin grafts. J Coll Physicians Surg Pak. 2010;20:675–679. [PubMed] [Google Scholar]
- 2.Revol M, Servant JM. Cicatrisation dirigée. Techniques Chirurgicales - Chirurgie plastique reconstructrice et esthétique. 201045–50. [Google Scholar]
- 3.Revol M, Servant JM. Greffes cutanées. Techniques Chirurgicales - Chirurgie plastique reconstructrice et esthétique. 201045–70. [Google Scholar]
- 4.Penn JG. Skin graft and skin flaps. In: Patient Care in Plastic Surgery. 1996Mosby: St. Louis; 552–563. [Google Scholar]
- 5.Lalau JD, Bresson R, Charpentier P, et al. Efficacy and tolerance of calcium alginate versus vaseline gauze dressings in the treatment of diabetic foot lesions. Diabetes Metab. 2002;28:223–229. [PubMed] [Google Scholar]
- 6.André J, Guillotreau J, Rouffi J, et al. Intérêt de la mèche d’alginate de calcium et de la mèche imprégnée de polyvidone iodée dans le traitement local du sinus pilonidal abcédé. Revue de l’ADPHSO. 1997;22:69–74. [Google Scholar]
- 7.Sayag J, Lieaume S, Bohbot S. Healing properties of calcium alginate dressings. J Wound Care. 1996;5:357–362. [DOI] [PubMed] [Google Scholar]
- 8.Qin Y. Alginate fibers: an overview of the production processes and applications in wound management. Polym Int. 2007;57:171–180. [Google Scholar]
- 9.Huang C, Leavitt T, Bayer LR, et al. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51:301–331. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Yu A. Complications of negative pressure wound therapy: a mini review. Wound Repair Regen. 2014;22:457–461. [DOI] [PubMed] [Google Scholar]
- 11.Dumville JC, Webster J, Evans D, et al. Negative pressure wound therapy for treating pressure ulcers. Cochrane Database Syst Rev. 2015;5:CD011334. [DOI] [PubMed] [Google Scholar]
- 12.Dumville JC, Owens GL, Crosbie EJ, et al. Negative pressure wound therapy for treating surgical wounds healing by secondary intention. Cochrane Database Syst Rev. 2015;6:CD011278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster J, Scuffham P, Stankiewicz M, et al. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev. 2014;10:CD009261. [DOI] [PubMed] [Google Scholar]
- 14.CPMP. Point to consider on switching between superiority and non-inferiority (CPMP/EWP/482/99). 2000. London: European Agency for the Evaluation of Medicinal Products; https://www.ema.europa.eu/en/switching-between-superiority-non-inferiority. [Google Scholar]
- 15.Piaggio G, Elbourne DR, Pocock SJ, et al. ; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–2604. [DOI] [PubMed] [Google Scholar]
- 16.Munaron L. Intracellular calcium, endothelial cells and angiogenesis. Recent Pat Anticancer Drug Discov. 2006;1:105–119. [DOI] [PubMed] [Google Scholar]
- 17.Monsen C, Wann-Hansson C, Wictorsson C, et al. Vacuum-assisted wound closure versus alginate for the treatment of deep perivascular wound infections in the groin after vascular surgery. J Vasc Surg. 2014;59:145–151. [DOI] [PubMed] [Google Scholar]
- 18.Tintle TE, Jeter KF. Early experience with a calcium alginate dressing. Ostomy Wound Manage. 1990;28:74–81. [PubMed] [Google Scholar]
- 19.Attwood AI. Calcium alginate dressing accelerates split skin graft donor site healing. Br J Plast Surg. 1989;42:373–379. [DOI] [PubMed] [Google Scholar]
- 20.Szekalska M, Pucilowska A, Szymankiewicz E, et al. Alginate: current use and future perspectives in pharmaceutical and biomedical applications. International Journal of Polymer Science. 2016;2016:17. [Google Scholar]
- 21.Qin Y. The gel swelling properties of alginate fibers and their applications in wound management. Polym Adv Technol. 2008;19:6–14. [Google Scholar]
- 22.Qin Y. Ion-exchange properties of alginate fibers. Textile Research Journal. 2005;75:165–168. [Google Scholar]
- 23.Qin Y. The characterization of alginate wound dressings wih different fiber and textile structures. Journal of Applied Polymer Science. 2006;100:2516–2520. [Google Scholar]
- 24.Paddle-Ledinek JE, Nasa Z, Cleland HJ. Effect of different wound dressings on cell viability and proliferation. Plast Reconstr Surg. 2006;1177 Suppl110S–118S; discussion 119S. [DOI] [PubMed] [Google Scholar]
- 25.Clark M. Rediscovering alginate dressings. Wounds International. 2012;3:1. [Google Scholar]
- 26.Thomas S. Alginate dressings in surgery and wound management–part 1. J Wound Care. 2000;9:56–60. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt RJ, Fry JR. Alginate dressings. Pharm Journal. 1986;236:36–37. [Google Scholar]
- 28.Thomas S. Alginate dressings in surgery and wound management: part 3. J Wound Care. 2000;9:163–166. [DOI] [PubMed] [Google Scholar]
- 29.Wagstaff MJ, Driver S, Coghlan P, et al. A randomized, controlled trial of negative pressure wound therapy of pressure ulcers via a novel polyurethane foam. Wound Repair Regen. 2014;22:205–211. [DOI] [PubMed] [Google Scholar]
- 30.Mattox EA. Reducing risks associated with negative-pressure wound therapy: strategies for clinical practice. Crit Care Nurse. 2017;37:67–77. [DOI] [PubMed] [Google Scholar]
- 31.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–76; discussion 577. [PubMed] [Google Scholar]
- 32.Beral D, Adair R, Peckham-Cooper A, et al. Chronic wound sepsis due to retained vacuum assisted closure foam. BMJ. 2009;338:b2269. [DOI] [PubMed] [Google Scholar]
- 33.Rencuzogullari A. Retention of vacuum-assisted closure device sponge leading to a perianal abscess and fistula. Int Wound J. 2015;12:739–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazoch M, Montgomery C. Retained wound vacuum foam in non-healing wounds: a real possibility. J Wound Care. 2015;246 SupplS18–S20. [DOI] [PubMed] [Google Scholar]
- 35.Khashram M, Huggan P, Ikram R, et al. Effect of TNP on the microbiology of venous leg ulcers: a pilot study. J Wound Care. 2009;18:164–167. [DOI] [PubMed] [Google Scholar]
- 36.Daar DA, Wirth GA, Evans GR, et al. The bagautdinov dressing method: negative pressure wound therapy in a patient with an allergy to acrylate adhesive. Int Wound J. 2017;14:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manoharan V, Grant AL, Harris AC, et al. Closed incision negative pressure wound therapy vs conventional dry dressings after primary knee arthroplasty: A randomized controlled study. J Arthroplasty. 2016;31:2487–2494. [DOI] [PubMed] [Google Scholar]
- 38.Chevillard C, Rugina M, Bonfils P, et al. Evaluation of calcium alginate nasal packing (algostéril) versus polyvinyl acetal (merocel) for nasal packing after inferior turbinate resection. Rhinology. 2006;44:58–61. [PubMed] [Google Scholar]
- 39.Treating Complex Wounds - Negative Pressure Wound Therapy. Available at https://www.inesss.qc.ca/nc/en/publications/publications/publication/traitement-des-plaies-complexes-therapie-par-pression-negative-tpn.html. Accessed December 16, 2019.
- 40.Apelqvist J, Willy C, Fagerdahl AM, et al. EWMA document: negative pressure wound therapy. J Wound Care. 2017;26Sup3S1–S154. [DOI] [PubMed] [Google Scholar]
- 41.Boutron I, Tubach F, Giraudeau B, et al. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J Clin Epidemiol. 2004;57:543–550. [DOI] [PubMed] [Google Scholar]
- 42.Dumville JC, Munson C, Christie J. Negative pressure wound therapy for partial-thickness burns. Cochrane Database Syst Rev. 2014;12:CD006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumville JC, Land L, Evans D, Peinemann F. Negative pressure wound therapy for treating leg ulcers. Cochrane Database Syst Rev. 2015;7:CD011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Dumville JC, Hinchliffe RJ, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. 2018;10:CD010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iheozor-Ejiofor Z, Newton K, Dumville JC, et al. Negative pressure wound therapy for open traumatic wounds. Cochrane Database Syst Rev. 2018;7:CD012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webster J, Liu Z, Norman G, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2019;3:CD009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haalboom M. Chronic wounds: innovations in diagnostics and therapeutics. Curr Med Chem. 2018;25:5772–5781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


