Background:
Hyaluronic acid (HA), both crosslinked and uncrosslinked, is used clinically to treat fine lines and provides additional improvements in skin quality attributes. The purpose of this study was to assess potential early differences in the expression of biological markers of skin quality in living human skin explants injected with uncrosslinked and crosslinked HA gels.
Methods:
Living human skin explants injected with VYC-12L or noncrosslinked HA with mannitol (HYD) and noninjected controls were assessed via microscopy, histology, and immunohistochemistry on days 3 and/or 8 for biological markers of elasticity (collagen density, elastin, fibrillin-1) and hydration [aquaporin-3, acidic glycosaminoglycans (GAGs), HA]. Hydration was also assessed via a corneometer probe on days 0, 1, 2, and 8.
Results:
On day 3 versus controls, VYC-12L moderately increased collagen density in the upper reticular dermis and clearly increased fibrillin-1 expression, with slight increases persisting on day 8. Increases with HYD were smaller and did not persist on day 8. Both VYC-12L and HYD increased aquaporin-3 expression and GAG content on days 3 and 8, but VYC-12L produced greater GAG increases in the reticular dermis. Day 8 instrument-assessed hydration increased by 49% and 22% for VYC-12L and HYD, respectively. Elastin expression in oxytalan and elaunin fibers was unchanged. Upper-dermal HA reductions suggested HA injection-induced hyaluronidase expression.
Conclusion:
VYC-12L produced greater, more lasting improvements in biological markers of skin quality than HYD.
INTRODUCTION
Intrinsic processes, including aging and hormonal changes, and extrinsic factors, such as chronic sun exposure and smoking, contribute to structural and functional deficiencies in the skin.1–3 Structurally, the dermal extracellular matrix weakens, dermal collagen decreases, dermal elastic fibers become disorganized, the epidermal mitochondrial network becomes increasingly fragmented, and hyaluronic acid (HA) declines.1,3–5 Functionally, elasticity and hydration are reduced and barrier function is compromised.1,4,6,7 Over time, extrinsic and intrinsic forces can result in wrinkles, fine lines, dryness, and irregularities in tone and texture.1,2,6,8,9
Clinical dermatologic studies have examined elasticity and hydration to assess overall skin quality, particularly with regard to aging skin.10–12 HA, due to its natural abundance, stability, lack of toxicity and immunogenicity, and ability to attract and bind approximately 1,000 times its weight in water, has shown a wide range of benefits related to the quality of the skin, including wound healing, tissue regeneration, and skin repair.13,14 HA is involved in tissue hydration and preserving the integrity of the dermal extracellular matrix.15,16 Additionally, intradermal HA filler injections have demonstrated the ability to improve skin texture, luminance, hydration, and elasticity.12,17,18 HA fillers come in different forms: noncrosslinked, which has been shown to provide a moisturizing effect, and crosslinked, which improves durability, mechanical properties, and hydration.13 Juvéderm VOLITE [VYC-12L (Allergan, Annecy, France)] is a crosslinked HA (12.0 mg/mL) containing lidocaine developed using the Vycross technology platform (Allergan plc, Dublin, Ireland) and designed to treat superficial cutaneous depressions, such as fine lines, and provides additional improvements in skin quality attributes, such as elasticity and hydration.19 In a prospective, single-center, single-arm study in 131 subjects, VYC-12 (without lidocaine) was shown to be safe and effective for the treatment of superficial cutaneous depressions, such as fine lines, as measured by skin texture improvement, and for the improvement of skin quality in the face and neck, with improvements lasting up to 6 months and subject satisfaction with skin and improved hydration lasting up to 9 months (data on file; Allergan plc). Juvéderm HYDRATE [HYD (Allergan)],12,20 a noncrosslinked HA (13.5 mg/mL) with mannitol, is indicated for injection into the superficial dermis and dermal–epidermal junction to improve skin hydration and elasticity. A 2-month multicenter clinical trial showed that 27 subjects who received HYD by depot injection in the face and/or neck or décolletage area had significant improvements in skin hydration.12 VYC-12 and HYD are Conformité Européenne marked,19,20 but have not been approved by the US Food and Drug Administration.
Indicators of skin quality attributes may be evaluated in ex vivo models using biological markers. The density of dermal collagen, for example, is an indicator of skin elasticity because of collagen’s role in maintaining the strength of the skin, helping it to resist mechanical deformation.10,21,22 Other biological markers of elasticity are fibrillin-1, a glycoprotein that supports the integrity of the dermal elastic fiber network,6 and elastin, an essential protein of the elastic fibers of the skin, such as oxytalan and elaunin.6,23,24 Well-studied biological markers of skin hydration include aquaporin-3 (AQP3), water- and glycerol-transporting membrane proteins expressed in the epidermis,25,26 and glycosaminoglycans (GAGs), which are linear polysaccharide molecules, including HA, that bind water and help regulate the hydration of the dermis.3,7 The impact of these markers is often measured clinically using probes, such as the corneometer, which evaluates epidermal capacitance as a measure of hydration in the stratum corneum.27,28
The present study evaluated the early effects of VYC-12L injection compared with HYD or no injection on biological markers of hydration and elasticity in living human skin explants.
METHODS
Human Skin Explant Collection and Preparation
Two studies were performed at Laboratoire BIO-EC, Longjumeau, France. The first study, performed on explants from 1 donor, included all measurements described below. The second study was performed on explants from 3 different donors to confirm the hydration (corneometry) results obtained in the first study. Explants of living human skin were obtained during abdominal surgery from a total of 4 donors. Eighteen 11-mm round explants in study 1 (donor 1) were prepared for the analysis of biological markers of skin elasticity (collagen density, elastin, and fibrillin-1 expression) and skin hydration (AQP3 expression, GAG content, and HA). Eleven 1.5- by 2-cm rectangular explants from donors 1, 2, 3, and 4 were prepared for the analysis of epidermal capacitance via corneometry (n = 2 in study 1; n = 9 from study 2). Explants were stored in the BIO-EC survival culture medium at 37°C in 5% CO2-humidified air (Fig. 1). Half of the culture medium (1 mL) was refreshed on days 1, 2, 5, and 7.
Fig. 1.

Eleven-millimeter round human skin explants in BIO-EC survival culture medium. Image courtesy of Laboratoire BIO-EC, Longjumeau, France.
This study was conducted on explants derived from discarded abdominoplasty tissue; all subjects provided written informed consent before the use of these tissues. The only subject data obtained included age, race, and sex.
Product Application
In both studies, the product was injected via a needle into the dermis of the round 11-mm explants on day 0. In study 1, explants were injected with either 50 μL of VYC-12L (VYC-12 with lidocaine), 50 μL of noncrosslinked 13.5 mg/mL HYD, or no injection (control). (Three of the 21 original explants in study 1 were designated as untreated controls for testing after day 0.)
In parallel, 2 explants from study 1 consisting of 1.5- by 2-cm rectangular samples were injected on day 0 with either 3 × 50 μL of VYC-12L or of HYD, or left untreated (control). Nine 1.5- by 2-cm rectangular explants from study 2 were also injected on day 0 with either 3 × 50 μL of VYC-12L or of HYD, or left untreated (control). Both studies utilized the same batches of VYC-12L and HYD products. All image analyses were conducted on 9 to 12 analyses for each batch of staining, with the histopathologist blinded to treatment.
Histologic Evaluations
In study 1, on days 3 and 8 after injection, 3 round samples from each treatment group were collected and cut into 2 parts, with 1 part frozen, cryofixed, and cryosectioned, and the other part formalin fixed, paraffin embedded, and sectioned. The samples were observed under a Leica DMLB microscope (Leica Microsystems GmbH, Wetzlar, Germany) or a BX43 Olympus microscope (Olympus Corporation, Tokyo, Japan) and stained to assess general morphology and to evaluate biological markers of elasticity and hydration (Table 1)3,6,7,10,15,16,21–28 on days 3 and 8 versus noninjected controls, except for elastin expression, which was evaluated on day 8 only. The staining intensity of the biological markers was scored by a histopathologist as very weak, weak, moderate, quite clear, clear, very clear, or strong.
Table 1.
Biological Markers of Skin Quality
| Attribute | Marker | Definition | Rationale for Study |
|---|---|---|---|
| Elasticity | Collagen density10,21,22 | Concentration of collagen, a structural protein in the skin and other connective tissues | Collagen strengthens the skin, helping it to resist mechanical deformation |
| Elastin6,23,24 | Protein in the skin and connective tissue that maintains elasticity and flexibility | Elastin constitutes about 90% of the elastic fibers in the skin | |
| Fibrillin-16 | Glycoprotein that supports the integrity of the dermal elastic fiber network and is expressed in dermal–epidermal junctions | Fibrillin-1 is an essential component of the network of fibers that imparts elasticity to the skin | |
| Hydration | AQP325,26 | Water- and glycerol-transporting membrane proteins expressed in the epidermis | AQP3 is a major protein with a critical role in maintaining hydration in the skin |
| GAGs3,7 | Linear polysaccharide molecules, including hyaluronic acid, that bind water and help regulate the hydration of the dermis | GAGs hold and maintain water, and skin aging is associated with reduced GAG expression | |
| Hyaluronic acid (HA)7,15,16 | Linear polysaccharide molecule that maintains the hydration and structural integrity of skin and other connective tissues | HA has an immense capacity to retain water; a primary function in the skin is to regulate moisture homeostasis | |
| Epidermal capacitance27,28 | A measure of the humidity level of the outermost cutaneous layers of the stratum corneum | Epidermal capacitance is a well-established measure of skin hydration in dermatological studies |
AQP3, aquaporin-3; GAGs, glycosaminoglicans.
Assessment of General Morphology and Markers of Elasticity
To assess general morphology, including collagen density, paraffinized sections were stained according to Masson’s trichrome (Goldner variant) and examined at ×20 magnification. To evaluate elastin expression, frozen sections were stained with diluted rabbit antielastin polyclonal antibody and nuclei were post-stained with propidium iodide. Sections were examined at ×40 magnification. To evaluate the fibrillin-1 expression, frozen sections were stained with diluted mouse antifibrillin-1 monoclonal antibody, and nuclei were post-stained with propidium iodide. Sections were examined at ×40 magnification. Evaluation of elastin and fibrillin-1 expression was based on the qualitative microscopic observation of the stained sections.
Assessment of Markers of Hydration
To assess AQP3 expression, paraffinized sections were stained with a rabbit anti-AQP3 polyclonal antibody and examined at ×40 magnification. To measure GAG content, paraffinized sections were stained by Alcian blue/periodic acid–Schiff for acidic GAGs and examined at ×10 magnification. To visualize HA, paraffinized sections were stained using a diluted, biotinylated antibody against HA binding protein and examined at ×10 magnification. Evaluation of HA staining, AQP3 expression, and GAG content was based on the qualitative microscopic observation of the stained sections. Evaluation of HA was also made by an analysis of images of the stained sections. The staining intensity was measured in these images using CellD data scoring software (CellD, Roquemaure, France) and expressed as a percentage of the preselected skin layer of interest.
Instrument Assessment of Hydration
In study 2, to evaluate hydration, the epidermal capacitance was measured in explants at day 0 and at days 1, 2, and 8 after injection using corneometry, a method that detects the hydration level of the outermost cutaneous layers of the stratum corneum. The Corneometer CM 825 (Courage+Khazaka electronic GmbH, Cologne, Germany) was used for this analysis, and results were expressed as the average value of 3 measurements.
Statistics
Quantitative data are expressed as mean (SD). For the analysis of staining for HA, comparisons between injected explants and noninjected controls were assessed at each time point using the Student t test, and significance was determined at P <0.05. All other markers were assessed by a histopathologist via visual evaluation and/or microscopic observation without statistical analyses. For corneometry measurements, a 2-way analysis of variance was used to assess the impact of the treatment group and time on the hydration measurements. Pairwise comparisons were made using Tukey’s multiple comparison test. Significance was determined at P <0.05.
RESULTS
Subject Data
The abdominoplasty tissue was obtained from 4 White female donors, aged 43 years old (study 1) and 42, 53, and 59 years old (study 2).
Elasticity Markers
Microscopic examination revealed elevated collagen density and associated extracellular matrix improvement in explants after injection of VYC-12L and HYD, but increases in collagen density with VYC-12L on day 3 were generally larger in magnitude than those observed with HYD (Fig. 2). Explants injected with VYC-12L displayed moderate increases in collagen density in the upper reticular dermis compared with controls on day 3, whereas the increases with HYD were slight. Improvements in the relief of the dermal–epidermal junction were also detected with both VYC-12L and HYD. Improvements in collagen density and associated extracellular matrix persisted on day 8 for VYC-12L but not for HYD.
Fig. 2.
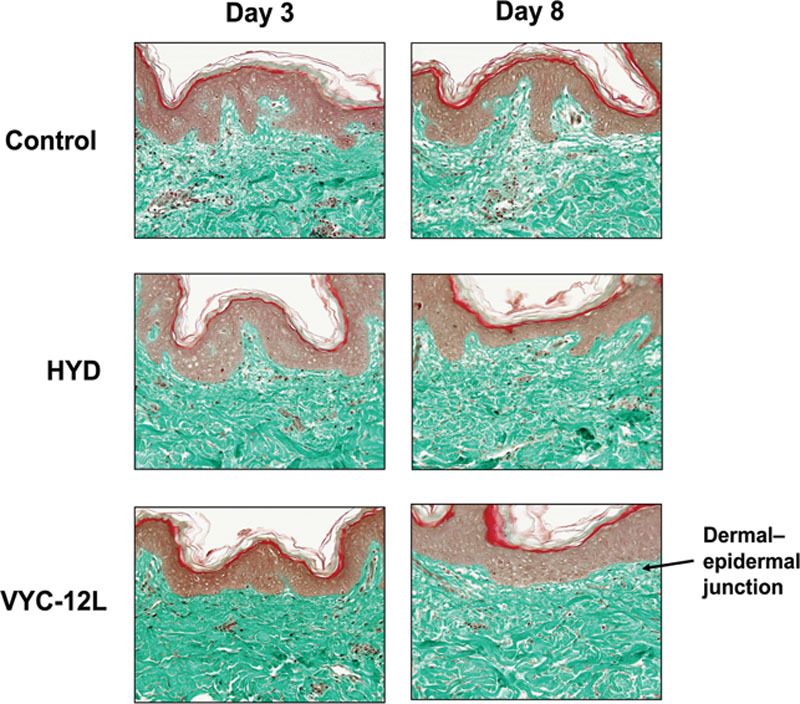
Microscopic observation of changes in collagen density on days 3 and 8 after injection. Increases in the density of the collagen network are observed following VYC-12L and HYD treatment in the papillary and upper reticular dermis by the heightened intensity of green staining as compared with control. Improvements in the relief of the dermal–epidermal junction may be seen with VYC-12L (arrow). HYD, noncrosslinked hyaluronic acid with mannitol; VYC-12L, crosslinked hyaluronic acid injectable gel with lidocaine.
As qualitatively assessed, explants injected with VYC-12L exhibited clear increases in fibrillin-1 expression compared with controls on day 3 (Fig. 3). These increases corresponded to the observed improvement in the dermal–epidermal junction on day 3, consistent with the presence of fibrillin-rich elastic fibers at the dermal–epidermal junction.6 The fibrillin-1 expression on day 3 was slightly higher in magnitude for VYC-12L than for HYD, and slight increases in fibrillin-1 expression with VYC-12L persisted on day 8. In contrast, there was no modification in expression with HYD on day 8.
Fig. 3.
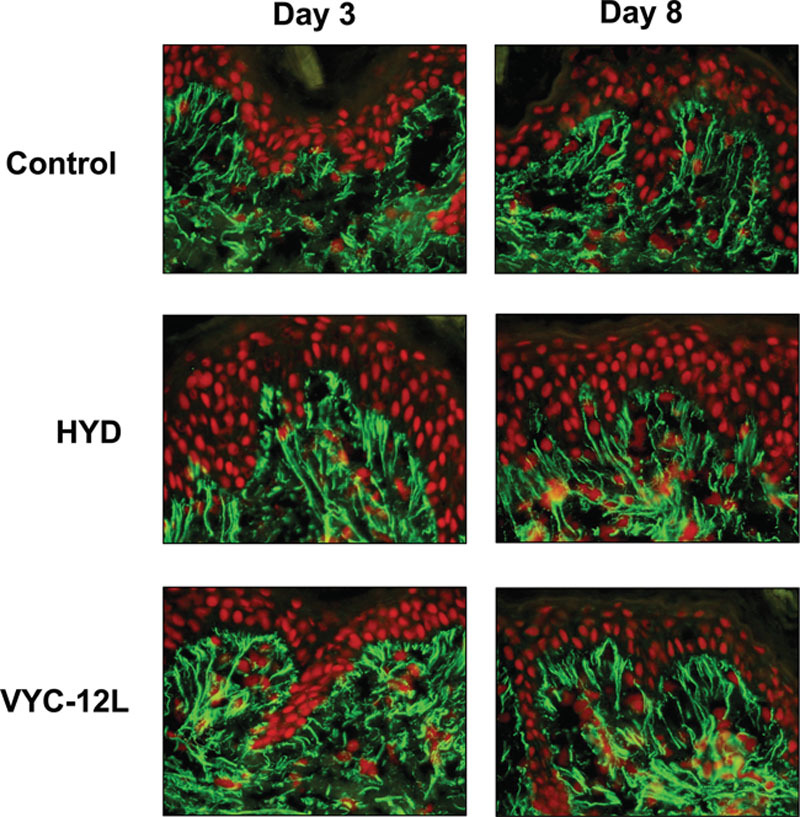
Microscopic observation of changes in fibrillin-1 expression on days 3 and 8 after injection. The increase in fibrillin-1 expression in the papillary dermis is shown following VYC-12L and HYD treatment as enhanced green immunofluorescence as compared with control. Nuclei are counterstained in red. HYD, noncrosslinked hyaluronic acid with mannitol; VYC-12L, crosslinked hyaluronic acid injectable gel with lidocaine.
Neither VYC-12L nor HYD was observed to affect elastin expression within oxytalan or elaunin fibers at day 3 or 8 relative to noninjected controls.
Hydration and Hydration Markers
Both VYC-12L and HYD slightly increased AQP3 expression on day 3. On day 8, the increase was greater (Fig. 4). The increases on both days, however, were clearly visible with VYC-12L. VYC-12L produced a strong increase in acidic GAG content in the upper and lower reticular dermis on day 3, which was sustained on day 8. The effect was less pronounced with HYD (Fig. 5).
Fig. 4.
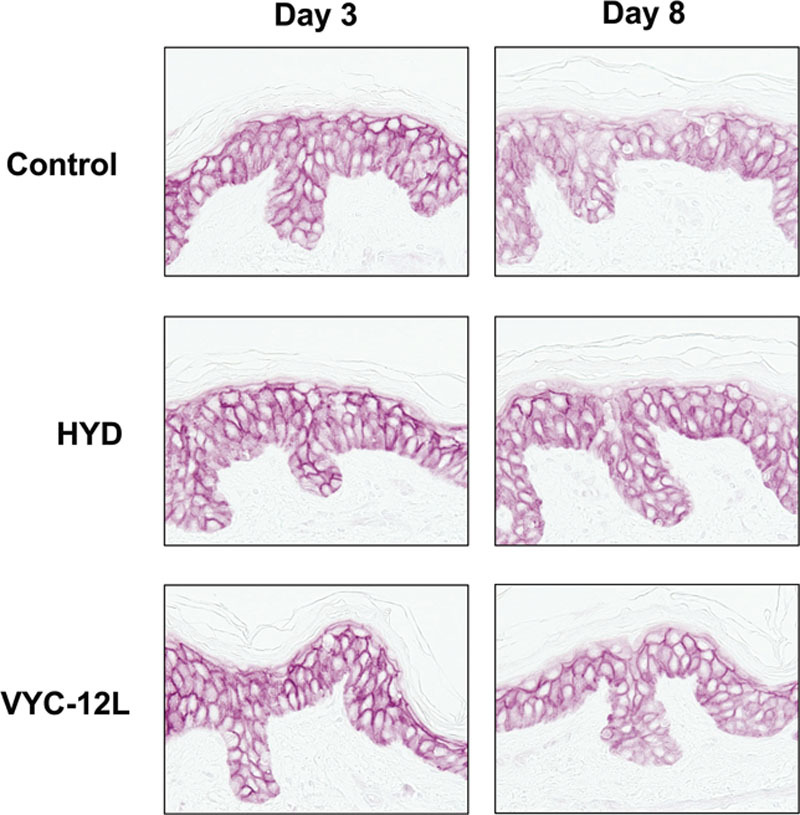
Microscopic observation of changes in AQP3 expression on days 3 and 8 after injection. The increase in AQP3 expression in the epidermis (basal layer of keratinocyte) is shown as an enhanced intensity of immunostaining (pink). AQP3, aquaporin-3; HYD, noncrosslinked hyaluronic acid with mannitol; VYC-12L, crosslinked hyaluronic acid injectable gel with lidocaine.
Fig. 5.
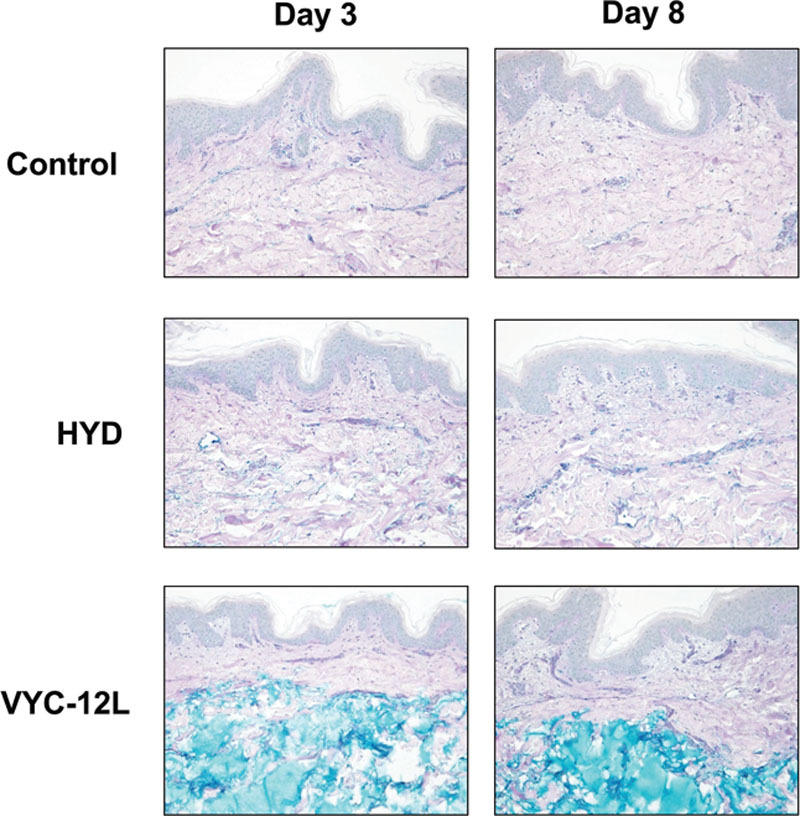
Microscopic observation of changes in GAG content on days 3 and 8 after injection. The increase in acidic GAG expression in the papillary dermis and upper reticular dermis is shown as an enhanced intensity of blue staining. GAG, glycosaminoglican; HYD, noncrosslinked hyaluronic acid with mannitol; VYC-12L, crosslinked hyaluronic acid injectable gel with lidocaine.
On day 3, explants injected with HYD and VYC-12L showed significantly reduced HA in the epidermis above the injection site and in the papillary and upper reticular dermis versus controls, with a concurrent HA increase in the lower reticular dermis. For example, on day 3, the average area of the epidermis that was positive for the presence of HA in controls was 68.6% versus 30% and 6.6% (P < 0.01) in explants injected with HYD and VYC-12L, respectively. On day 3, the average area of the lower reticular dermis positive for HA in controls was 6.4% versus 8.5% (P < 0.1) and 12.0% (P < 0.01) with HYD and VYC-12L, respectively. After 8 days, HA levels recovered to approximately the same level as in controls with VYC-12L for all skin layers; for example, on day 8, the average epidermal area positive for HA was 69.9% for controls and 72.4% for VYC-12L; for HYD, it was 35.8%.
Corneometry readings for hydration in study 2 revealed significant increases on days 1, 2, and 8 for VYC-12L-injected explants relative to control and HYD-injected explants. The corneometry readings for HYD were significantly increased at day 8 compared with control. Relative to control, the average corneometry-measured skin hydration increase in explants from the 3 donors was 49% in the VYC-12L-injected explants on day 8, compared with 22% in the HYD-injected explants, a significant increase (Fig. 6).
Fig. 6.
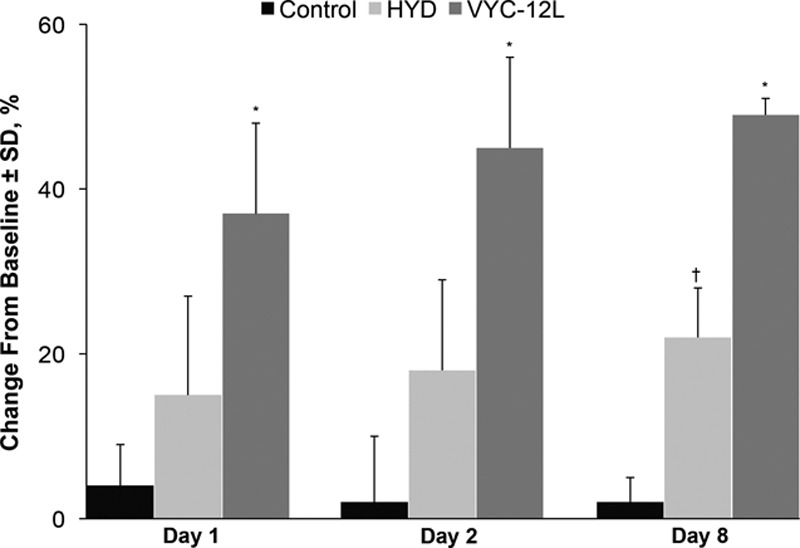
Change from baseline in corneometric hydration values on days 1, 2, and 8 after injection. HYD, noncrosslinked hyaluronic acid with mannitol; VYC-12L, crosslinked hyaluronic acid injectable gel with lidocaine. *P <0.05 vs control and HYD. †P <0.05 vs control.
DISCUSSION
An increasing array of injectable HA fillers are used in aesthetic medicine to restore facial volume loss and to smooth wrinkles. Numerous reports exist in the literature that describes HA fillers on the basis of their various biophysical characteristics, including cohesivity, elasticity, and viscosity.29–33 Most of these studies were conducted in vitro, and their results have helped to predict clinical outcomes and guide physicians to select the most relevant products to optimize the intended aesthetic objectives.29–33
In the present analyses, biological markers of skin hydration and elasticity in living human skin explants were studied ex vivo. This model has been used previously to evaluate early treatment effects on these markers,34 as the tissue maintains viability for 7 to 14 days (maximum) in culture.35–37 This model allows injection of the product directly into human skin, allowing for exposure of the cells and tissue to the hydrogel composition and hydrogel physical properties, both of which can impact tissue response. Improvements were seen following VYC-12L injection, supporting the benefits of VYC-12L with respect to specific skin quality attributes. Collagen density, fibrillin-1 and AQP3 expression, and acidic GAG content increased following VYC-12L injection. The observation of increases in these markers persisted from day 3 to day 8 after injection. Microscopic evaluation of collagen density revealed associated extracellular matrix and dermal–epidermal junction improvements with VYC-12L, demonstrating the potential of the product to improve microscopic structural properties of the skin in this model. Elastin expression did not increase in injected explants, whereas fibrillin-1 expression clearly improved, which may reflect a delayed synthesis of elastin or maintenance of a lower ratio of elastin to fibrillin in the oxytalan and elaunin elastic fibers studied.6,38,39 Furthermore, VYC-12L injection in the dermis produced strong increases in acidic GAG content in both the upper and lower reticular dermis, correcting hydration levels in layers of the skin deeper than the direct site of injection. Corneometry results also showed a significantly increased improvement in hydration with VYC-12L than with HYD, a noncrosslinked HA formulation that has a similar HA content and has been shown to increase hydration clinically.12 Injection with VYC-12L generally resulted in more pronounced and longer-lasting increases in skin quality biomarkers than HYD. These results with VYC-12L support the role of VYC-12L as a dermal filler with additional potential for improving skin elasticity and hydration.
This is the first evaluation in living human skin explants of the impact of an injectable crosslinked HA filler (VYC-12L) on markers of hydration and elasticity versus an injectable noncrosslinked HA formulation (HYD) or no injection. Quan et al40 examined fibroblast behavior and collagen production in cultured skin specimens from healthy elderly volunteers (mean age 81 years) biopsied 1, 2, 4, and 12 weeks after injection with crosslinked HA and vehicle and determined that the HA-injected skin samples exhibited heightened pro-collagen fibroblast function and extracellular matrix stability. Sundaram et al35 demonstrated that a topically applied crosslinked HA product was more effective than a noncrosslinked HA topical formulation in improving measures of hydration and skin barrier function in living human skin explants analyzed on day 9 after treatment. The results of our analyses are consistent with such findings, but they are also unique, deriving from studies conducted after direct HA injection into explants and showing improvements in various biological markers of skin quality that were sustained over 8 days. Although interpretations of our data are limited by the short study duration due to tissue degradation over time and by the inability to replicate the conditions of live human skin in vivo, such limitations are common to all studies using human skin explants.35–37 Moreover, the ability to inject an HA gel directly into a living skin explant allows physical interaction between HA and the tissue and cells. As the primary effect of HA gels is to provide a filling effect, and the physical interaction (ie, stretch effect) has been shown to impact collagen and elastin production,41 this model provides a good opportunity to evaluate HA fillers in an in situ tissue environment with similarities to clinical HA injection conditions. As a result, living human skin explants provide a realistic model for the effects that might be expected in human skin in vivo.35–37 Further evaluation of these results in longer-term clinical studies would help to confirm the longer-term time course of these effects. A recently published prospective clinical study by Niforos et al42 evaluated the same formulation and reported skin smoothness up to 6 months and hydration lasting 9 months, consistent with and expanding upon the results of the current study.
The reduction in HA content with HYD and VYC-12L at day 3 was consistent with effects previously observed by the laboratory with injected HA, suggesting increased expression of hyaluronidase in response to the influx of HA15; by day 8, HA levels had increased and normalized with VYC-12L, but not to the same extent with HYD, which may reflect a more durable cutaneous response to VYC-12L than HYD, consistent with effects observed for the biological markers of collagen density, fibrillin-1 and AQP3 expression, and acidic GAG content. The more sustained response to VYC-12L compared with HYD in this study may be related to the crosslinking of VYC-12L, which increases persistence in tissue relative to noncrosslinked HYD, as observed in the increased GAG staining in the lower reticular dermis.43–46
CONCLUSIONS
VYC-12L improved biological markers of skin quality in human living skin explants. Increases in biological markers of skin elasticity and hydration were generally greater and persisted longer with VYC-12L compared with HYD. The data suggest that VYC-12L could improve microscopic structural aspects of the skin, consistent with previous observations investigating the physical interaction41; however, as these analyses were conducted ex vivo over early time points, additional in vivo or clinical investigations, such as that of Niforos et al,42 will be valuable for offering further insight on additional effects following treatment.
Footnotes
Published online 25 March 2020.
Presented in poster form at the 13th Aesthetic and Anti-aging Medicine European Congress, September 15–17, 2017, Monte Carlo, Monaco.
Disclosure: L. Nakab, C.K. Hee, and O. Guetta are employees of Allergan plc and may own stock/stock options in the company. This study was funded by Allergan plc, Dublin, Ireland. Medical writing support for this article was provided at the request of the authors by Regina Kelly of Peloton Advantage, LLC, an OPEN Health company, Parsippany, New Jersey, and was funded by Allergan plc. The opinions expressed in this article are those of the authors. The authors received no honorarium/fee or other forms of financial support related to the development of this article.
REFERENCES
- 1.Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trojahn C, Dobos G, Lichterfeld A, et al. Characterizing facial skin ageing in humans: disentangling extrinsic from intrinsic biological phenomena. Biomed Res Int. 2015;2015:318586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DH, Oh JH, Chung JH. Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci. 2016;83:174–181. [DOI] [PubMed] [Google Scholar]
- 4.Lapière CM. The ageing dermis: the main cause for the appearance of ‘old’ skin. Br J Dermatol. 1990;122suppl 355–11. [DOI] [PubMed] [Google Scholar]
- 5.Mellem D, Sattler M, Pagel-Wolff S, et al. Fragmentation of the mitochondrial network in skin in vivo. PLoS One. 2017;12:e0174469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langton AK, Sherratt MJ, Griffiths CE, et al. A new wrinkle on old skin: the role of elastic fibres in skin ageing. Int J Cosmet Sci. 2010;32:330–339. [DOI] [PubMed] [Google Scholar]
- 7.Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol. 2006;12:145–154. [DOI] [PubMed] [Google Scholar]
- 8.Giacomoni PU, Rein G. A mechanistic model for the aging of human skin. Micron. 2004;35:179–184. [DOI] [PubMed] [Google Scholar]
- 9.Nkengne A, Bertin C. Aging and facial changes–documenting clinical signs, part 1: clinical changes of the aging face. Skinmed. 2013;11:281–286. [PubMed] [Google Scholar]
- 10.Choi JW, Kwon SH, Huh CH, et al. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19:e349–e355. [DOI] [PubMed] [Google Scholar]
- 11.Berardesca E, Cameli N, Primavera G, et al. Clinical and instrumental evaluation of skin improvement after treatment with a new 50% pyruvic acid peel. Dermatol Surg. 2006;32:526–531. [DOI] [PubMed] [Google Scholar]
- 12.Taieb M, Gay C, Sebban S, et al. Hyaluronic acid plus mannitol treatment for improved skin hydration and elasticity. J Cosmet Dermatol. 2012;11:87–92. [DOI] [PubMed] [Google Scholar]
- 13.Sundaram H, Cegielska A, Wojciechowska A, et al. Prospective, randomized, investigator-blinded, split-face evaluation of a topical crosslinked hyaluronic acid serum for post-procedural improvement of skin quality and biomechanical attributes. J Drugs Dermatol. 2018;17:442–450. [PubMed] [Google Scholar]
- 14.Bukhari SNA, Roswandi NL, Waqas M, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120pt B1682–1695. [DOI] [PubMed] [Google Scholar]
- 15.Kogan G, Soltes L, Stern R, et al. Pethrick RA, Ballada A, Zaikov GE. Hyaluronic acid: a biopolymer with versatile physico-chemical and biological properties. In: Handbook of Polymer Research: Monomers, Oligomers, Polymers and Composites. 2007Hauppauge, NY: Nova Science Publishers, Inc; 393–439. [Google Scholar]
- 16.Yoshida H, Komiya A, Ohtsuki R, et al. Relationship of hyaluronan and HYBID (KIAA1199) expression with roughness parameters of photoaged skin in caucasian women. Skin Res Technol. 2018;24:562–569. [DOI] [PubMed] [Google Scholar]
- 17.Niforos F, Acquilla R, Ogilvie P, et al. A prospective, open-label study of hyaluronic acid-based filler with lidocaine (VYC-15L) treatment for the correction of infraorbital skin depressions. Dermatol Surg. 2017;43:1271–1280. [DOI] [PubMed] [Google Scholar]
- 18.Landau M, Fagien S. Science of hyaluronic acid beyond filling: fibroblasts and their response to the extracellular matrix. Plast Reconstr Surg. 2015;1365 suppl188S–195S. [DOI] [PubMed] [Google Scholar]
- 19.Juvederm Volite B [direction for use]. 2017Pringy, France: Allergan. [Google Scholar]
- 20.Juvederm Hydrate [EU Direction for Use]. 2016Pringy, France: Allergan. [Google Scholar]
- 21.Abreu EL, Palmer MP, Murray MM. Collagen density significantly affects the functional properties of an engineered provisional scaffold. J Biomed Mater Res A. 2010;93:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wunsch A, Matuschka K. A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Photomed Laser Surg. 2014;32:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin AK, Simpson A, Steer R, et al. Elastic fibres in health and disease. Expert Rev Mol Med. 2013;15:e8. [DOI] [PubMed] [Google Scholar]
- 24.Bensouilah J, Buck P. Bensouilah J, Buck P. Skin structure and function. In: Aromadermatology: Aromatherapy in the Treatment and Care of Common Skin Conditions. 2006Abingdon, United Kingdom: Radcliffe Publishing; 1–11. [Google Scholar]
- 25.Xing F, Liao W, Jiang P, et al. Effect of retinoic acid on aquaporin 3 expression in keratinocytes. Genet Mol Res. 2016;15:15016951. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106–112. [DOI] [PubMed] [Google Scholar]
- 27.Le Fur I, Reinberg A, Lopez S, et al. Analysis of circadian and ultradian rhythms of skin surface properties of face and forearm of healthy women. J Invest Dermatol. 2001;117:718–724. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto T, Yuasa H, Kai R, et al. Skin capacitance in normal and atopic infants, and effects of moisturizers on atopic skin. J Dermatol. 2007;34:447–450. [DOI] [PubMed] [Google Scholar]
- 29.Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and drug administration-approved fillers. Plast Reconstr Surg. 2015;136:678–686. [DOI] [PubMed] [Google Scholar]
- 30.Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;1324 suppl 25S–21S. [DOI] [PubMed] [Google Scholar]
- 31.Gavard Molliard S, Albert S, Mondon K. Key importance of compression properties in the biophysical characteristics of hyaluronic acid soft-tissue fillers. J Mech Behav Biomed Mater. 2016;61:290–298. [DOI] [PubMed] [Google Scholar]
- 32.Gavard Molliard S, Bon Betemps J, Hadjab B, et al. Key rheological properties of hyaluronic acid fillers: from tissue integration to product degradation. Plast Aesthet Res. 2018;5:17. [Google Scholar]
- 33.La Gatta A, Salzillo R, Catalano C, et al. Hyaluronan-based hydrogels as dermal fillers: the biophysical properties that translate into a “volumetric” effect. PLoS One. 2019;14:e0218287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgher F, Mathieu L, Lati E, et al. Experimental 70% hydrofluoric acid burns: histological observations in an established human skin explants ex vivo model. Cutan Ocul Toxicol. 2011;30:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundaram H, Mackiewicz N, Burton E, et al. Pilot comparative study of the topical action of a novel, crosslinked resilient hyaluronic acid on skin hydration and barrier function in a dynamic, three-dimensional human explant model. J Drugs Dermatol. 2016;15:434–441. [PubMed] [Google Scholar]
- 36.Lebonvallet N, Jeanmaire C, Danoux L, et al. The evolution and use of skin explants: potential and limitations for dermatological research. Eur J Dermatol. 2010;20:671–684. [DOI] [PubMed] [Google Scholar]
- 37.Park GH, Chang SE, Bang S, et al. Usefulness of skin explants for histologic analysis after fractional photothermolysis. Ann Dermatol. 2015;27:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro SD, Endicott SK, Province MA, et al. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haydont V, Bernard BA, Fortunel NO. Age-related evolutions of the dermis: clinical signs, fibroblast and extracellular matrix dynamics. Mech Ageing Dev. 2019;177:150–156. [DOI] [PubMed] [Google Scholar]
- 40.Quan T, Wang F, Shao Y, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133:658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paliwal S, Fagien S, Sun X, et al. Skin extracellular matrix stimulation following injection of a hyaluronic acid-based dermal filler in a rat model. Plast Reconstr Surg. 2014;134:1224–1233. [DOI] [PubMed] [Google Scholar]
- 42.Niforos F, Ogilvie P, Cavallini M, et al. VYC-12 injectable gel is safe and effective for improvement of facial skin topography: a prospective study. Clin Cosmet Investig Dermatol. 2019;12:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther. 2011;13:21–27. [DOI] [PubMed] [Google Scholar]
- 44.Eccleston D, Murphy DK. Juvéderm Volbella in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sattler G, Philipp-Dormston WG, Van Den Elzen H, et al. A prospective, open-label, observational, postmarket study evaluating VYC-17.5L for the correction of moderate to severe nasolabial folds over 12 months. Dermatol Surg. 2017;43:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monheit G, Beer K, Hardas B, et al. Safety and effectiveness of the hyaluronic acid dermal filler VYC-17.5L for nasolabial folds: results of a randomized, controlled study. Dermatol Surg. 2018;44:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]


