Summary:
Collagen plays a fundamental role in wound healing and consequently defective collagen can impair normal wound healing processes. Kniest dysplasia (KD) is a collagenopathy that results from a pathogenic mutation in a gene that codes for type II collagen. Clinical manifestations of the dysplasia include short-trunk dwarfism, kyphoscoliosis, hand arthropathy, cleft palate, hearing loss, and ocular abnormalities. We present the case of a 21-year-old woman who desired reduction mammaplasty. A review of the literature was performed, and there were no published reports of any plastic surgery procedures in patients with KD. The patient proceeded with surgery and healed without any complications. Given that wound healing appears normal in this patient population, it is reasonable to consider elective plastic surgical procedures in patients with KD.
INTRODUCTION
Wound healing is a highly complex process that involves the interplay of a myriad of genes and molecular pathways. Collagen is central to healing and is the most abundant protein in mammalian proteins. Collagenopathies such as Ehlers–Danlos have a deleterious effect on healing.1 Kniest dysplasia (KD) is a type of collagenopathy that affects type II collagen through a pathogenic mutation in the COL2A1 gene.2 The majority of the mutations in KD occur de novo and are all autosomal dominant.3 The phenotype in KD varies in severity and typically includes short-trunk dwarfism, kyphoscoliosis, enlarged joints, hand arthropathy, cleft palate, hearing loss, and ocular abnormalities including retinal detachment.3 The classic x-ray finding is shortened vertebra.
There are no prior published reports of patients with KD undergoing major plastic surgery procedures. Concern about the ability of patient with KD to heal normally has been previously reported in the head and neck literature.4 We present a patient with KD who underwent bilateral reduction mammaplasty in whom routine healing was observed.
CASE REPORT
A 21-year-old woman with KD presented with symptomatic macromastia (Fig. 1). Her primary symptoms included neck pain and bra strap grooving. She had D cup breasts and her breast size had been stable for 3 years before presentation. Manifestations of KD included dwarfism, cervical arthritis, hip arthritis, knee arthritis, cleft palate, and hearing loss. She had undergone prior eye surgery, cleft palate repair, hip arthroscopy and replacement, knee arthroscopy, and elbow arthroscopy. She healed well after these prior procedures. On physical examination, her height was 4′6″, weight was 62 kg, and body mass index was 33.2 kg/m. There was an absence of point tenderness over her spine. She had grade 2 ptosis bilaterally with a suprasternal-notch-to-nipple distance of 25 cm bilaterally. Nipple-to-inframammary fold distance was 8 cm bilaterally. Insurance approved her procedure.
Fig. 1.

Preoperative photographs.
Preoperatively, 2 rheumatologists were asked to opine on her risk of postoperative wound healing problems. Although neither one had experience with KD, one noted that the Online Mendelian Inheritance in Man, a catalog of genetic disorders, made no mention of wound healing difficulties in patients with KD. Given all of the above, we felt comfortable offering her reduction mammaplasty.
She proceeded to surgery. Anesthesia noted a Mallampati grade I airway and had no difficulty with intubation. We used an inverted T pattern skin incision and an 11 cm wide inferior pedicle. To optimize perfusion, we designed a wide inferior pedicle (11 cm) relative to her nipple-to-IMF distance (8 cm) and maintained thick breast skin flaps. A total of 340 g were removed from the left breast and 310 g were removed from the right. Her postoperative course was uncomplicated, and all her incisions healed without wound breakdown (Fig. 2). After surgery, she reported a significant improvement in her neck pain and bra strap grooving.
Fig. 2.
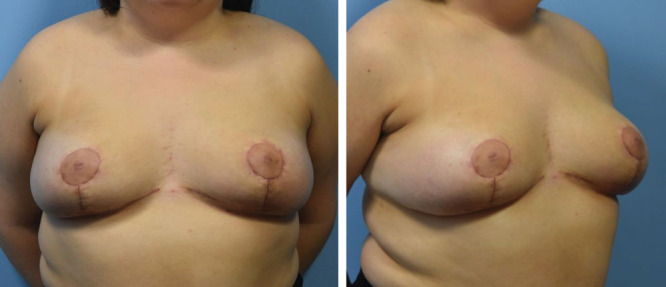
Postoperative photographs taken 4 weeks after surgery.
DISCUSSION
KD was first described in 1952 by a chief resident at the University of Jena, Dr. Wilhelm Kniest.8 A diagnosis of KD is usually made when clinical findings prompt radiographic evaluation which identifies platyspondyly, kyphoscoliosis, dumbbell-shaped long bones, and epiphyses with delayed ossification, as well as areas of high density dispersed within the epiphysis. Earlier diagnosis can be made antenatally with ultrasound, suggesting the diagnosis and magnetic resonance imaging with more characteristic findings.5 The diagnosis is confirmed by genetic analysis of the COL2A1 gene. Although types I and III collagen are the predominant variants involved in wound healing, other groups have raised concern that KD patients may have impaired wound healing.4 This concern is magnified with breast reduction surgery due to the large operative field and length of incisions required. To our knowledge, there are no reports of a plastic surgery procedure performed on a patient with this collagenopathy.
As a rare condition, situations in which a patient with KD requires surgical intervention beyond cleft palate repair or vitrectomy are equally rare. Husain and colleagues performed multiple head and neck operations on a single patient without any difficulty in wound healing.3,4 We report a similar situation, albeit in a surgical region without as robust collateral blood supply as the head and neck. The rate of complication in reduction mammaplasty using an inferior pedicle wise technique has been published as high as 31.5%. Infection and wound dehiscence are the most common complications, affecting 8.9% of patients after breast reduction.6 As such, it is a procedure that stresses the wound healing capability of the recipient. Our case demonstrates a normal wound healing outcome despite a formal diagnosis of KD.
There are other concerns that a surgeon should be aware of before offering surgical therapy to patients with KD. Cervical atlantoaxial instability has been described with neurological sequelae and patients requiring occipitocervical arthrodesis.7 Before any anesthetic event, the cervical spine should be evaluated. The airway represents the most challenging aspect of operating on patients with KD, and anesthesia practitioners may encounter a variety of airway morphology such as tracheomalacia as described in a neonate with KD.8 Additionally, dysmorphic growth may lead to a difficult airway as a KD patient ages,9 and a specific endotracheal tube may be required to secure the airway.10
CONCLUSIONS
KD is a rare genetic condition resulting in a type II collagenopathy. Concern regarding wound healing in these patients should be tempered by this report of a successful reduction mammaplasty in a 21-year-old woman. Further studies are warranted to assess surgical risk with this and other collagenopathies. We recommend careful history taking to elucidate the symptomatology of macromastia in contradistinction to symptomatic kyphoscoliosis. Even with rare conditions, the relationship between the genotype and phenotype can be elucidated with clinical relevance for practitioners,11 and Online Mendelian Inheritance in Man may prove useful in this regard. Our case adds to the literature of KD and we recommend that operative intervention should not be withheld from this group of patients provided appropriate cervical evaluation and airway management can be ensured.
Footnotes
Published online 24 March 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Harrison B, Sanniec K, Janis JE. Collagenopathies-implications for abdominal wall reconstruction: a systematic review. Plast Reconstr Surg Glob Open. 2016;4:e1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobling R, D’Souza R, Baker N, et al. The collagenopathies: review of clinical phenotypes and molecular correlations. Curr Rheumatol Rep. 2014;16:394. [DOI] [PubMed] [Google Scholar]
- 3.Sergouniotis PI, Fincham GS, McNinch AM, et al. Ophthalmic and molecular genetic findings in Kniest dysplasia. Eye (Lond). 2015;29:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husain Q, Cho J, Neugarten J, et al. Surgery of the head and neck in patient with Kniest dysplasia: is wound healing an issue? Int J Pediatr Otorhinolaryngol. 2017;93:97–99. [DOI] [PubMed] [Google Scholar]
- 5.Yazici Z, Kline-Fath BM, Laor T, et al. Fetal MR imaging of Kniest dysplasia. Pediatr Radiol. 2010;40:348–352. [DOI] [PubMed] [Google Scholar]
- 6.Ogunleye AA, Leroux O, Morrison N, et al. Complications after reduction mammaplasty: a comparison of Wise pattern/inferior pedicle and vertical scar/superomedial pedicle. Ann Plast Surg. 2017;79:13–16. [DOI] [PubMed] [Google Scholar]
- 7.McKay SD, Al-Omari A, Tomlinson LA, et al. Review of cervical spine anomalies in genetic syndromes. Spine (Phila Pa 1976). 2012;37:E269–E277. [DOI] [PubMed] [Google Scholar]
- 8.Hicks J, De Jong A, Barrish J, et al. Tracheomalacia in a neonate with Kniest dysplasia: histopathologic and ultrastructural features. Ultrastruct Pathol. 2001;25:79–83. [DOI] [PubMed] [Google Scholar]
- 9.Segawa Y, Enomoto H, Nakagawa T, et al. Growth causes difficult tracheal intubation in a patient with Kniest dysplasia. J Anesth. 2001;15:104–105. [DOI] [PubMed] [Google Scholar]
- 10.Niwa Y, Hirabayashi Y, Seo N, et al. Use of Parker flex-tip tracheal tube in a patient with Kniest dysplasia. Masui. 2011;60:631–634. [PubMed] [Google Scholar]
- 11.Spranger J. Changes in clinical practice with the unravelling of diseases: connective-tissue disorders. J Inherit Metab Dis. 2001;24:117–126. [DOI] [PubMed] [Google Scholar]


