Supplemental Digital Content is available in the text.
Background:
Female-to-male mastectomy often renders the chest skin and nipple–areolar complex (NAC) insensate. We propose a new technique of preserving the intercostal nerves and using them to reinnervate the NAC after mastectomy.
Methods:
We performed a prospective analysis of transmasculine patients who underwent female-to-male mastectomy. The technique involves dissecting out the lateral intercostal nerves to length and performing a neurorrhaphy to nerve stumps at the base of the NAC. Sensory outcomes, as assessed with Semmes–Weinstein monofilaments, were compared to a cohort of patients who underwent mastectomy without neurotization.
Results:
Ten patients with a mean age of 17.5 years (range: 16–19 years) underwent mastectomy. The final follow-up was a mean of 15.4 ± 4.3 months for the treated group and 40.7 ± 12.9 months for the control group. Compared to control patients, treated patients had significant improvement in sensation at the nipple (P ≤ 0.0002), areola (P = 0.0001), and peripheral breast skin (P = 0.0001). For treated patients, there was no statistically significant difference in sensation between preoperative and postoperative sensation in all tested areas at final follow-up.
Conclusion:
This proof of concept study suggests that immediate reinnervation of the NAC after mastectomy enhances recovery of NAC sensation in patients undergoing female-to-male mastectomy and may be further generalized to women undergoing postmastectomy breast reconstruction.
INTRODUCTION
Female-to-male mastectomy is the most common procedure among transmasculine patients1 and is often the first surgical step of the transition process.2,3 Skin excision for gender-affirming mastectomy may be performed via a variety of techniques—semicircular, transareolar, concentric circular, extended concentric circular, and breast amputation—which may be nipple sparing, or require excision and reattachment of the nipple–areolar complex (NAC) with free nipple grafts.4 The NAC is innervated by the anterior and lateral cutaneous branches of the fourth intercostal nerve, with variable contributions from cutaneous branches of the third and fifth intercostal nerves.5 The NAC is thus anatomically denervated as these fine nerve branches are inevitably sacrificed, as the breast tissue is separated from the overlying skin in the subcutaneous plane. In cases of free nipple grafts, the NAC is insensate by definition since it is a skin graft that is completely detached from all surrounding tissues and transplanted to a different location on the chest wall.
Though female-to-male mastectomy alone has demonstrated psychological benefits among patients with gender dysphoria,6 loss of NAC sensation can lead to a lack of sexual arousal, a negative impact on self-esteem, and physical harm such as burns and mechanical trauma due to the absence of sensory awareness.7 Though much attention has been paid to the esthetics of female-to-male chest contouring,2–4,8,9 sensory outcomes have often been overlooked. In this study, we propose a novel technique for targeted reinnervation of the NAC during female-to-male mastectomy.
METHODS
This prospective study assessed consecutive patients who underwent female-to-male mastectomy by the lead author from 2016 to 2019. Approval for this study was provided by the Stanford Institutional Review Board. All patients met the World Professional Association for Transgender Health Standards of Care for chest surgery.10 Mastectomies were performed as nipple-sparing mastectomy (NSM) with a periareolar incision, or via a 2-incision approach with free nipple grafts. The extent of dissection and degree of undermining was similar to that of mastectomy as it is typically performed in women for breast pathology. During the dissection of the lateral outer quadrant, the third, fourth, and/or fifth lateral intercostal nerves were identified coming off the lateral border of the pectoralis major muscle and dissected free from the breast parenchyma while tracing the nerves toward the NAC (Figs. 1, 2). Neurorrhaphy was performed to the base of the NAC with 2 simple interrupted 7-0 prolene sutures, anchoring the perineurium to the dermis and biopsy-proven nerve stump, when available (Figs. 3, 4). In the case of free nipple grafts, the graft was affixed to a deepithelialized oval segment of skin; neurorrhaphy was performed to the underlying dermal surface of the deepithelialized area. No allograft was used.
Fig. 1.
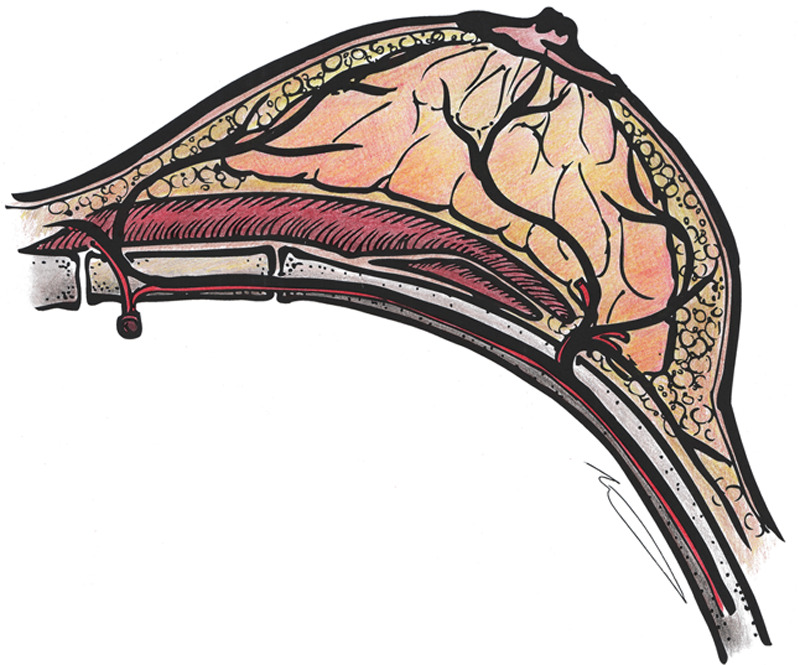
Schematic innervation of the nipple–areolar complex by branches of the intercostal nerve (black), showing the emergence of the lateral intercostal nerve branch at the lateral border of the pectoralis major muscle, along with branches of the intercostal artery (red). Illustration by Phil Brazio, MD.
Fig. 2.

Intraoperative photograph of lateral intercostal nerve dissected out to a length of 8 cm.
Fig. 3.

Postmastectomy coaptation of lateral intercostal nerve branch (black) to nerve stump of the nipple–areolar complex with a second lateral intercostal branch dissected to length, viewed through an extended periareolar incision. Illustration by Phil Brazio, MD.
Fig. 4.

Intraoperative photograph of neurotization to base of nipple–areolar complex, corresponding to illustration in Figure 3.
Patient demographics and sensory outcomes were recorded. The areola and breast skin were divided into 4 quadrants similar to prior methods of breast sensory assessment (See figure, Supplemental Digital Content 1, which displays a diagram of breast for sensory testing. Areola and the surrounding breast skin are divided into 4 quadrants. N, nipple; AS, areola superior; AM, areola medial; AI, areola inferior; AL, areola lateral; BS, breast superior; BM, breast medial; BI, breast inferior; BL, breast lateral. http://links.lww.com/PRSGO/B343).11,12 Sensation was assessed pre- and postoperatively for right (R) and left (L) sides using Semmes–Weinstein monofilaments (6.65, 4.56, 4.31, 3.61, and 2.83 g) at the nipple, and each quadrant of the areola and breast skin 2 cm from the areola. The temperature was assessed using a metal probe. Outcomes were compared to a cohort of patients who underwent prophylactic NSM without neurotization. Unpaired t tests were employed to compare the cohorts based on the mean lowest monofilament weight at which sensation was detected.
RESULTS
Ten female-to-male transgender patients underwent bilateral mastectomy with NAC reinnervation; 3 patients underwent bilateral NSM, and 7 underwent 2-incision mastectomy with free nipple grafts. The average number of grafted nerves per side was 1.7 (range: 1–3) with a mean length of 3.8 ± 0.8 cm. The diameter of the nerves was 1–3 mm. Average age was 17.5 years (range: 16–19 years) and the mean body mass index was 27.1 ± 9.7 kg/m2. The control group consisted of 10 female BRCA1 or BRCA2 carriers with a mean age of 36.6 years (range: 18–59 years) and the mean body mass index of 24.2 ± 4.1 kg/m2. Mean Regnault classification of ptosis was 1.6 for the reinnervated group and 1.0 for the control group (range: 0–3 for both).
Compared to control patients, treated patients had significant improvement in sensation at the nipple (control: R, 5.58 ± 1.13, L, 4.95 ± 0.90; treated: R, 3.41 ± 0.53, L, 3.21 ± 0.57; P ≤ 0.0002), areola (control: R, 5.09 ± 0.60, L, 5.13 ± 0.57; treated: R, 3.34 ± 0.39, L, 3.41 ± 0.44; P = 0.0001), and peripheral breast skin (control: R, 4.70 ± 0.91, L, 4.47 ± 0.78; treated: R, 3.00 ± 0.35, L, 2.98 ± 0.35; P = 0.0001) at average final follow-up time of 15.4 ± 4.3 months for the treated group and 40.7 ± 12.9 months for controls (see figure, Supplemental Digital Content 2, which demonstrates that compared to control patients, treated patients had significant improvement in sensation at the (A) nipple, (B) areola, and (C) peripheral breast skin. http://links.lww.com/PRSGO/B344).
For treated patients, there was no statistically significant difference in sensation between preoperative baseline and postoperative sensation at the nipple (before: R, 3.20 ± 0.61, L, 2.93 ± 0.28; after: R, 3.51 ± 0.58, L, 3.21 ± 0.57), areola (before: R, 3.02 ± 0.23, L, 3.00 ± 0.23; after: R, 3.34 ± 0.39, L, 3.41 ± 0.44), and peripheral breast skin (before: R, 2.95 ± 0.20, L, 2.93 ± 0.15; after: R, 3.00 ± 0.35, L, 2.98 ± 0.35) at a final follow-up (see figure, Supplemental Digital Content 3, which displays that for treated patients, there was no significant difference in sensation between preoperative baseline and postoperative sensation at the (A) nipple, (B) areola, and (C) peripheral breast skin at final follow up. http://links.lww.com/PRSGO/B345). For control patients, there was a statistically significant decrease in sensation between preoperative baseline (nipple: R, 2.83 ± 0, L, 2.83 ± 0; areola: R, 2.87 ± 0.12, L, 2.89 ± 0.18; peripheral breast skin: R, 2.83 ± 0, L, 2.85 ± 0.06) and postoperative sensation (nipple: R, 5.58 ± 1.13, L, 4.95 ± 0.90; areola: R, 5.09 ± 0.60, L, 5.12 ± 0.57; peripheral breast skin: R, 4.70 ± 0.91, L, 4.47 ± 0.78) in all areas (P < 0.001). Three treated patients in the treatment group additionally reported recovery of ticklish sensation with light touch. All treated patients had intact temperature sensation. No patients experienced adverse effects of reinnervation.
DISCUSSION
Among the transgender population, there are limited data surrounding the extent of NAC sensory loss and the rate of spontaneous return of sensation following female-to-male mastectomy. From studies in women undergoing mastectomy for oncologic indications, we know that sensation to the NAC can return spontaneously without neurotization13–16; however, the extent, quality, and timing of reinnervation are highly variable.17 In a survey of 68 transmasculine postsurgical youth, Olson-Kennedy et al18 found the main complaint to be an NAC sensory loss, with 59% sustaining temporary loss and 41% reporting permanent loss. Also based on the survey data, Knox et al19 reported that 43 out of 92 breasts (46.7%) managed with nipple-sparing techniques had full or partial sensation, whereas Wolter et al2 reported “very good” or “good” sensation in 80.3% of breasts (212 out of 264). For patients with free nipple grafts, Nelson et al20 showed that 7 out of 16 patients (43.8%) self-reported some postoperative NAC sensation, whereas Knox et al19 found that all 110 breasts treated with free nipple grafts lost sensation. Without the use of objective measures, it is difficult to determine the true postoperative pattern of NAC sensation. However, from the aforementioned studies, it is clear that sensory recovery is highly variable and loss of sensation is a major risk in female-to-male mastectomy.
We demonstrate a novel technique of targeted NAC reinnervation that enhances NAC sensation following female-to-male mastectomy. In contrast to prior studies, we employ monofilament testing and a control group to objectively quantify the extent of sensory restoration in the reinnervated group. All patients had sensory recovery at the nipple, in contrast to fractions of patients in prior studies. The return of ticklish sensation furthermore signifies recovery of meaningful sensation. We hypothesize that the success of our technique is related to the fact that we are essentially performing a direct peripheral nerve repair, avoiding a delay in sensory recovery inherent with the use of autograft or allograft.21 In addition, we posit that sensory recovery in the peripheral breast skin is due to spontaneous cutaneous connections that form with branches of the intercostal nerve that are preserved during the dissection.
The limitations of our study include the small study size. We focus on objective sensory outcomes based on monofilament and temperature testing, though subjective outcomes, such as sexual arousal and self-esteem, are also important clinical endpoints. In addition, the placebo effect is a potential bias, as knowledge of neurotization may have impacted the treated group’s perception of sensation. To avoid this potential bias, future studies could employ a prospective, single-blind randomized controlled study design comparing transmasculine patients undergoing mastectomy with and without neurotization to more definitively evaluate sensory outcomes. Alternatively, future studies could evaluate differential sensory recovery of the chest in patients receiving unilateral nipple neurotization. Comparison of sensory recovery in patients who undergo NSM versus free nipple grafts is also an interesting line of inquiry that can be addressed in future investigations.
Neurotization in chest reconstruction is not a novel concept, as authors have been reporting this technique for autologous breast reconstruction for over 20 years. Blondeel et al11 demonstrated higher-quality sensory recovery following deep inferior epigastric perforator flap breast reconstruction among patients who underwent neurorrhaphy of the sensory branch of nerves to the rectus abdominis muscle to the fourth intercostal nerve, compared to those who underwent nonreinnervated deep inferior epigastric perforator flaps. However, targeted NAC reinnervation has neither been demonstrated in autologous or implant-based breast reconstruction nor in the transgender population. Immediate targeted NAC reinnervation thus has the potential to herald the “next frontier”7 in breast reconstruction, while also improving outcomes in transmasculine patients undergoing gender-affirming mastectomy.
Supplementary Material
Footnotes
Published online 24 March 2020.
Presented at American Society of Reconstructive Microsurgery (ASRM) Annual Meeting, January 10-14, 2020, Ft. Lauderdale, Fla.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.James S, Herman J, Rankin S, et al. The Report of the 2015 U.S. Transgender Survey. 2016Washington, DC: National Center for Transgender Equality. [Google Scholar]
- 2.Wolter A, Diedrichson J, Scholz T, et al. Sexual reassignment surgery in female-to-male transsexuals: an algorithm for subcutaneous mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:184–191. [DOI] [PubMed] [Google Scholar]
- 3.Cohen WA, Shah NR, Iwanicki M, et al. Female-to-male transgender chest contouring: a systematic review of outcomes and knowledge gaps. Ann Plast Surg. 2019;83:589–593. [DOI] [PubMed] [Google Scholar]
- 4.Monstrey S, Selvaggi G, Ceulemans P, et al. Chest-wall contouring surgery in female-to-male transsexuals: a new algorithm. Plast Reconstr Surg. 2008;121:849–859. [DOI] [PubMed] [Google Scholar]
- 5.Jaspars JJ, Posma AN, van Immerseel AA, et al. The cutaneous innervation of the female breast and nipple-areola complex: implications for surgery. Br J Plast Surg. 1997;50:249–259. [DOI] [PubMed] [Google Scholar]
- 6.van de Grift TC, Elfering L, Greijdanus M, et al. Subcutaneous mastectomy improves satisfaction with body and psychosocial function in trans men: findings of a cross-sectional study using the BODY-Q chest module. Plast Reconstr Surg. 2018;142:1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabin RC. After mastectomies, an unexpected blow: numb new breasts - the new york times. The New York Times; https://www.nytimes.com/2017/01/29/well/live/after-mastectomies-an-unexpected-blow-numb-new-breasts.html. Published January 29, 2017. Accessed October 28, 2017. [Google Scholar]
- 8.Berry MG, Curtis R, Davies D. Female-to-male transgender chest reconstruction: a large consecutive, single-surgeon experience. J Plast Reconstr Aesthet Surg. 2012;65:711–719. [DOI] [PubMed] [Google Scholar]
- 9.Bluebond-Langner R, Berli JU, Sabino J, et al. Top surgery in transgender men: how far can you push the envelope? Plast Reconstr Surg. 2017;139:873e–882e. [DOI] [PubMed] [Google Scholar]
- 10.WPATH World Professional Association for Transgender Health. Standards of care. https://www.wpath.org/publications/soc. Accessed December 15, 2019. [PubMed]
- 11.Blondeel PN, Demuynck M, Mete D, et al. Sensory nerve repair in perforator flaps for autologous breast reconstruction: sensational or senseless? Br J Plast Surg. 1999;52:37–44. [DOI] [PubMed] [Google Scholar]
- 12.van Verschuer VM, Mureau MA, Gopie JP, et al. Patient satisfaction and nipple-areola sensitivity after bilateral prophylactic mastectomy and immediate implant breast reconstruction in a high breast cancer risk population: nipple-sparing mastectomy versus skin-sparing mastectomy. Ann Plast Surg. 2016;77:145–152. [DOI] [PubMed] [Google Scholar]
- 13.Dossett LA, Lowe J, Sun W, et al. Prospective evaluation of skin and nipple-areola sensation and patient satisfaction after nipple-sparing mastectomy. J Surg Oncol. 2016;114:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg. 2009;62:586–590. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Unda NA, Bello RJ, Clarke-Pearson EM, et al. Nipple-sparing mastectomy improves long-term nipple but not skin sensation after breast reconstruction: quantification of long-term sensation in nipple sparing versus non-nipple sparing mastectomy. Ann Plast Surg. 2017;78:697–703. [DOI] [PubMed] [Google Scholar]
- 16.Chirappapha P, Srichan P, Lertsithichai P, et al. Nipple-areola complex sensation after nipple-sparing mastectomy. Plast Reconstr Surg Glob Open. 2018;6:e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou A, Ducic I, Momeni A. Sensory restoration of breast reconstruction - the search for the ideal approach continues. J Surg Oncol. 2018;118:780–792. [DOI] [PubMed] [Google Scholar]
- 18.Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knox ADC, Ho AL, Leung L, et al. A review of 101 consecutive subcutaneous mastectomies and male chest contouring using the concentric circular and free nipple graft techniques in female-to-male transgender patients. Plast Reconstr Surg. 2017;139:1260e–1272e. [DOI] [PubMed] [Google Scholar]
- 20.Nelson L, Whallett EJ, McGregor JC. Transgender patient satisfaction following reduction mammaplasty. J Plast Reconstr Aesthet Surg. 2009;62:331–334. [DOI] [PubMed] [Google Scholar]
- 21.Boeckstyns MEH, Sørensen AI, Viñeta JF, et al. Collagen conduit versus microsurgical neurorrhaphy: 2-year follow-up of a prospective, blinded clinical and electrophysiological multicenter randomized, controlled trial. J Hand Surg. 2013;38:2405–2411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


