Summary:
Carpal tunnel release (CTR) surgery continues to evolve. Carpal tunnel syndrome remains a primarily clinical diagnosis, although ultrasound has supplemented electrodiagnostic testing as a confirmatory tool. Magnetic resonance imaging of the carpal tunnel has also showed some promise as an alternative method for the examination of the median nerve. Open CTR surgery remains the traditional, and most popular, method of CTR. Wide-Awake, with Local Anesthesia only, and No Tourniquet CTR has emerged as a means to decrease cost and improve pain control and convenience for patients. Endoscopic CTR is increasing in popularity due to its more rapid recovery. The safety profile of endoscopic CTR has improved, and recent studies show similar rates of major complications between open and endoscopic techniques. Nonsurgeon operated ultrasound-guided techniques for release of the transverse carpal ligament have emerged. While promising in early studies, the current evidence in their favor is limited in terms of patient numbers and direct comparison with other techniques. The outcomes of CTR continue to be excellent. Recent research has demonstrated that nerve conduction continues to recover postoperatively over a longer period of time than previously believed. Patient psychological factors play a significant role in outcomes after surgery but do not appear to limit the improvement provided by intervention.
Carpal tunnel syndrome is the most common compressive neuropathy of the upper extremity, affecting an estimated 3.1% of the population aged 18–64 each year.1 Over 400,000 carpal tunnel releases (CTRs) are performed each year—representing approximately 0.1% of the US population annually—with direct costs of greater than 2 billion dollars per year.2,3 Risk factors for carpal tunnel syndrome requiring surgery include increasing age and body mass index (odds ratio [OR] = 1.04/year for both) and female sex (OR = 2.26 versus males).4
Surgical release of the carpal tunnel was first described by Galloway in 1924 (cited by Amadio)5 and later popularized by Phalen.6 Over time it has evolved to become the most common and among the safest hand surgeries. A 2015 meta-analysis found complication rates of only 3.2% for endoscopic CTR and 2.6% for open CTR.7 Despite the high degree of efficacy and safety already demonstrated in current CTR techniques, new technologies and data continue to change practice. These advances include new diagnostic techniques, new less-invasive surgical techniques, and new studies of outcome and complications.
DIAGNOSIS OF CARPAL TUNNEL SYNDROME
Traditionally, the diagnosis of carpal tunnel syndrome (CTS) has been a clinical one. However, electrodiagnostic studies (EDX) have become common place in the diagnosis of CTS as they can provide objective parameters that can better aide in prognostication, as well as evaluate other possible causes of nerve dysfunction. EDX is inclusive of the duo of tests most commonly performed by electromyographers consisting of a nerve conduction study and electromyogram. Multiple studies comparing clinical impression or validated scoring systems with EDX have demonstrated that EDX adds minimal to no sensitivity or specificity to the diagnosis.8–11 In a seminal 2008 study, Graham8 demonstrated that routine use of EDX provided only a 2%–6% increase in the probability of CTS versus from the use of a clinical checklist alone. Further research has demonstrated a false-positive rate of 16% in a general population of Japanese and American workers.12 Nonetheless, a 2016 study of 62,894 American patients found that 58% had a preoperative EDX performed before CTR surgery.13
In response to this mounting body of evidence, the American Academy of Orthopaedic Surgeons (AAOS) changed its Clinical Practice Guidelines (CPG) in 2016. The AAOS now states that “moderate evidence supports that…electrodiagnostic studies could be used to aid the diagnosis” of CTS14 in its 2016 CPG.
Ultrasound (US) has emerged as an alternative to EDX. A 2011 meta-analysis with a total sample size of 3,131 wrists calculated a sensitivity and specificity of 77.3% and 92.8%, respectively, versus clinical findings.15 Reliability improved with user experience.16
Cross-sectional area (CSA) of the median nerve is a highly reproducible US parameter indicative of CTS. A 2018 study found that CSA provides sensitivity and specificity of 75% and 87.5% respectively in diagnosing CTS18 (Fig. 1). The CSA of the median nerve correlates moderately with EDX findings.18 Short periods of training can result in acceptable levels of accuracy in imaging the carpal tunnel, even in novice US operators.19,20 Despite these promising studies, however, there remained some question as to the optimal CSA cutoff for CTS diagnosis, as well as the optimal site for measurement. As a result, the 2016 AAOS CPG for CTS states: “limited evidence supports not routinely using US for the diagnosis of carpal tunnel syndrome.”14
Fig. 1.
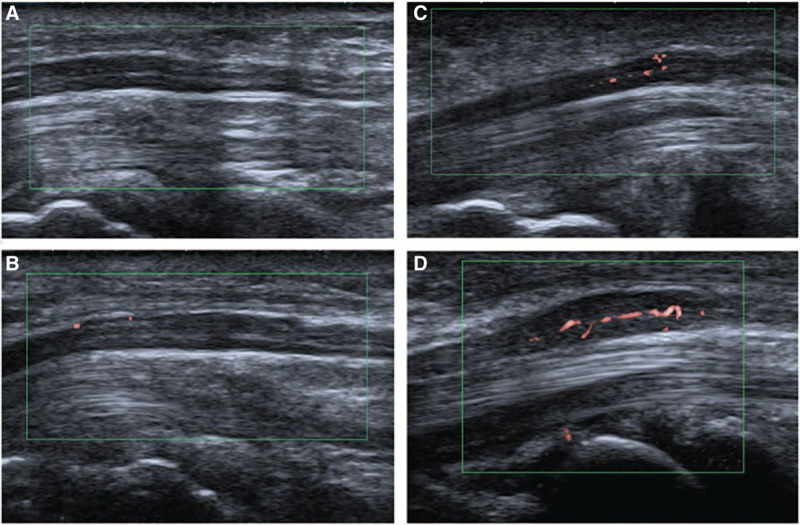
Other studies have also demonstrated improved diagnostic accuracy of US when nerve hypervascularity78 or morphology79 is factored into consideration. This image demonstrates different levels of vascularity as detected in the median nerve by color Doppler US, from grade 0 (A, no flow) to grade 3 (D, best flow). Adapted with permission from Med (United States). 2017:96;e6862 © 2013 Chen et al.70
Magnetic resonance imaging (MRI) is a diagnostic alternative to EDX for CTS. A 2002 study using nerve conduction studies (NCS) and a hand pain diagram as a gold standard demonstrated that MRI of the carpal tunnel has up to a 96% sensitivity to detect CTS, but only 33% specificity.21 A 2019 study directly comparing US and MRI in healthy volunteers and patients with a known diagnosis of CTS found that CSA of the median nerve is both sensitive and specific, with receiver operator curve area of 0.874–0.997 (statistically indistinguishable from US-measured CSA).22 The 2016 AAOS CPG on CTS states that “moderate evidence supports not routinely using MRI for the diagnosis of carpal tunnel syndrome.”14
There remains no perfect modality for the diagnosis of CTS. All modalities including clinical examination, questionnaire-based tests, EDX, US, and MRI have potential value in the diagnosis of CTS.
Clinical impression has been augmented with the use of diagnostic scoring systems for CTS. The CTS-6 diagnostic tool has been compared with NCS and US and demonstrated 95% sensitivity and 91% specificity for diagnosis of CTS.23 Likewise, specific examination maneuvers have well-defined sensitivities—89% for Durkan’s test, and 83% for Semmes-Weinstein monofilament testing after Phalen’s maneuver.24 These diagnostic scoring systems have the advantage of minimal additional cost, when compared with EDX, MRI, or US.
In certain situations, one diagnostic technique may have clear advantages over the alternatives. In diabetic patients, or those with amyloid or postchemotherapy neuropathy, for example, polyneuropathy can mimic the symptoms of CTS. In other patients, cervical radiculopathy can mimic CTS. EDX can distinguish between mono- and poly-neuropathy25,26 and radiculopathy in these patients. In patients with amyloid deposition syndromes, like those on hemodialysis, imaging modalities like MRI and US can demonstrate increased CSA27 or altered mechanical properties28 of the median nerve.
Nonoperative Management of CTS
Nonoperative management remains the first-line treatment for CTS. The 2016 AAOS CPGs state that “strong evidence supports that the use of immobilization should improve patient-reported outcomes.”14 Likewise, corticosteroid injection is recommended as a nonoperative means of treating CTS. A 2019 study found that nerve conduction studies performed 3 months after corticosteroid injection show improvement in conduction velocity, motor latency, and sensory latency following injection with methylprednisolone.29 Direct head-to-head comparison of splinting versus steroid injection was performed in the INSTINCTS trial, an open-label trial of 234 patients. This trial demonstrated a statistically significant increased improvement in outcomes in the corticosteroid group, as measured by the Boston Carpal Tunnel Questionnaire (BCTQ) at 6 weeks in the injection group than the splinting group.30
OPEN CTR
Open CTR surgeries continue to be routinely performed and include extensile, standard, and mini-open techniques. A 2015 meta-analysis found a 1.1% reoperation rate and a 1.0% major complication rate for open CTR.31 Because >50% of patients presenting with CTS present with bilateral disease, there has been research into the risks and benefits of simultaneous bilateral open CTR. A 2014 prospective cohort study found no difference between bilateral and unilateral CTR groups in terms of ability to perform self-care postoperatively and only demonstrated increased difficulty opening jars, cooking, and conducting household chores in the bilateral group on postoperative days 1 and 2.32 Performing bilateral CTR has been demonstrated to have lower total costs than staged release.33
A study on the outcomes of CTR in end-stage median nerve dysfunction demonstrated that patients with unrecordable sensory and motor nerve potentials nonetheless demonstrated excellent outcomes following CTR, with average BCTQ symptom scores of 1.4 after 5–9 years.34 A study of patients with diabetes evaluated EDX studies in patients with and without diabetes at 1 and 5 years after CTR and found that nerve conduction velocity continues to improve from years 1–5 after release.35 This is consistent with prior research demonstrating complete resolution of numbness at 9 years after surgery in 94% of patients with severe CTS36 and indicates that CTR may result in nerve function improvements beyond the traditionally stated 1- to 2-year time frame after surgery.
Recent research has emphasized the effect on patient characteristics and experience on postoperative outcomes. A study of 1,607 patients demonstrated that a more positive patient experience (including communication, information provided, facilities, etc.) was correlated with improved BCTQ scores after surgery.37 Another study, of 809 patients, found a correlation between poor 12-item short form health survey mental health scores and worsened postoperative quick disabilities of the arm, shoulder, and hand (QuickDASH) scores and satisfaction (although total satisfaction remained high).38 A similarly focused study identified patients with depression before and after CTR and found higher BCTQ scores in depressed patients both before and after surgery, but that both depressive symptoms and carpal tunnel symptoms are improved after CTR in these patients.39 New research has also reinforced preexisting data that CTR significantly improves sleep quality, generally within 24 hours of surgery.40
In summary, despite copious evidence that CTS symptom severity correlates with depression, catastrophization, and other patient psychological factors, CTR has nonetheless been demonstrated to be effective in this group of patients.
WIDE-AWAKE, WITH LOCAL ANESTHESIA ONLY, AND NO TOURNIQUET ECONOMICS AND SAFETY WITH CTR SURGERY
More recently, significant research is being put forward examining hand surgeries performed Wide-Awake, with Local Anesthesia only, and No Tourniquet (WALANT), including for CTR surgeries. When compared with sedation, the WALANT technique has been demonstrated to be significantly less expensive saving $1,320 to $1,613 per CTR case when performed in an operating room,41 while also being equally effective.44 Moreover, a 2019 study found improved pain control for 24 hours postoperatively with WALANT versus standard anesthesia.43 Performing open CTR surgery using the WALANT method in the office setting provides even further savings, with a 2017 study reporting 85% cost savings in a military population.44 Moreover, performing CTR in the office under WALANT anesthesia and field sterility has also been demonstrated to be safe. A multicenter study of 1,504 consecutive CTRs performed in the office with WALANT from 2008 to 2010 found a superficial infection rate of only 0.4% and a deep infection rate of 0%, which was comparable to the CTR being performed in an operating room.45 The cost savings, convenience to the patient, and convenience for the surgeon have led to wide-awake surgery becoming the method of choice for the majority of CTRs performed in Canada.46
The traditional argument against WALANT—that the use of epinephrine in the hand and fingers risks ischemic necrosis—has essentially been refuted through a number of recent studies.47 A 2005 study of 3,110 consecutive cases using local anesthesia alone with epinephrine found no incidents of tissue ischemia.48 A similar 2018 study of 488 cases using local anesthesia alone with epinephrine similarly found no incidents of tissue ischemia.49 A 2007 literature review of digits injected with high-dose (1:1,000) epinephrine also found no incidents of tissue necrosis.50 Yet, recent case reports have reported on cases of digital necrosis after epinephrine injection.51–53 These case reports have emphasized the importance of obtaining an adequate preprocedure history to evaluate for the presence of vascular disorders including Raynaud’s syndrome, and the importance of having phentolamine available as a reversal agent.
ENDOSCOPIC CTR
Endoscopic CTR continues to evolve as a surgical technique and continues to grow in popularity. A 2017 study of the Medicare database found an annual growth rate of 5% in endoscopic CTR from 2005 to 2012, versus 0.9% for open CTR.54 Prior studies noted increased rates of nerve injury in endoscopic CTR,55 and more recent population-level data have supported a 125% increase in risk of nerve injury requiring repair following endoscopic CTR versus open CTR.56 However, data are conflicted regarding the safety of endoscopic CTR versus open CTR. A 2014 Cochrane systematic review demonstrated a 45% lower rate of complications with endoscopic CTR versus open CTR.57 A recent database study of over 571,000 American patients between 2000 and 2014 found no statistically significant increase in reported complications with endoscopic CTR.58 Morphometric studies have also failed to demonstrate differences in postoperative carpal tunnel volume following open versus endoscopic CTR, reducing concerns that the endoscopic technique does not allow for full release of nerve constriction.59 Despite this evidence of equivalence, some experienced caution against endoscopic CTR in situations of aberrant anatomy, including nerve variants, cysts, amyloidosis, and rheumatoid tenosynovitis.60
Endoscopic CTR continues to demonstrate advantages in terms of immediate postoperative pain. A prospective study of patients undergoing bilateral CTR, with open and endoscopic techniques compared in each patient as an internal control, found that 80% of patients preferred the endoscopic surgery, citing postoperative pain as the reason.61 The subjectively improved patient experience following endoscopic CTR is of particular interest, as it contradicts a prior meta-analysis of intraindividual endoscopic CTR and open CTR comparisons that found similar pain scores between the two groups,62 instead being in keeping with prior research demonstrating improved pain and decreased analgesia requirements following endoscopic CTR versus open CTR.63 Ultimately, as surgeons have become more familiar with endoscopic technique for CTR, the weight of the evidence demonstrates that endoscopic techniques now rival open techniques for safety and likely provide an easier early postoperative recovery. However, studies examining differences in pain experience are challenged by the generally low pain in either endoscopic or open techniques and variations in the surgical technique employed for the endoscopic and open arms of the various studies.
PERCUTANEOUS CTR
In the effort to further reduce the invasiveness and cost of CTR surgery, recent research has examined percutaneous or US-guided techniques. An open-label study of 129 patients examined the safety and efficacy of US-guided percutaneous CTR performed by an interventional radiologist. At 1 month, the average BCTQ symptom severity score had decreased from 3.3 to 1.7 in these patients. No complications were reported.64 A separate study of 20 patients using an alternative percutaneous release technique also demonstrated improved BCTQ and electrophysiologic measurements without complications up to 6 months postoperatively.65 A similar technique was used in a 2017 industry-funded study on 159 hands and demonstrated rapid improvement of BCTQ scores, with a 5% rate of pillar pain-type symptoms and a zero reported rate of neurovascular injuries.66 Proponents of this technique have emphasized a short learning curve, with junior radiologists able to perform the technique adequately on cadavers after training on just 4 specimens.67 Given the push toward removing CTR from the operating room and for nonsurgical physicians to perform CTR procedures, it is likely that more research will continue to accumulate regarding the safety and efficacy of these percutaneous techniques, including much-needed head-to-head comparisons with established techniques as well as larger series powered to evaluate the risk of low-incidence, high-severity complications like nerve laceration or incomplete release.
CTS AND THE OPIOID EPIDEMIC
Given the severity of the opioid epidemic in the United States, a great deal of recent research has focused on pain control and minimizing opioid need after CTR surgery. A recent double-blinded, prospective, randomized trial examining postoperative pain experience and medication use demonstrated a trend toward patients consuming less pain medications after endoscopic compared with open CTR, although this was not statistically significant. The same study randomized patients to either receive an opioid (5 mg oxycodone), an NSAID (600 mg ibuprofen), or a nonopioid analgesic (500 mg acetaminophen) and found that patients used similar numbers of pills (less than 5 pills on average) regardless of the type with no difference in total consumption, pain experience, or need for refills whether they contained a non-steroidal antiinflammatory drug (NSAID), acetaminophen, or an opioid.68 A separate trial randomizing patients to opioid versus nonopioid postoperative regimens demonstrated that no patients in the nonopioid group required supplementation with opioids and that the nonopioid group had lower early postoperative pain scores and improved quickDASH scores.69 A third recent study compared acetaminophen/hydrocodone with acetaminophen/ibuprofen in soft tissue hand procedures and found no statistical difference in pain between groups, although medication side effects were more common in the acetaminophen/hydrocodone group.70 Although treatment must be tailored to the individual patient, these studies serve as strong evidence that opioids are not required following CTR surgeries and may in fact be less effective than an NSAID or acetaminophen regimen.
Revision CTR
Revision CTR may be required for persistent, recurrent, or new symptoms. A study of 97 revision surgeries found that symptoms were persistent in 43%, recurrent in 19%, and new in 37%. In those with persistent or recurrent symptoms, scarring of the median nerve to the flexor retinaculum and incomplete release were the most common findings. In those with new symptoms, nerve injuries were a common finding.71 These nerve injuries, while potentially severe, occur in only 0.2%–0.3% of cases.72
Although likely underreported, estimated recurrence rate after CTR ranges from 3% to 25%.73 A prospective single-center study of 14 patients found a median time to revision of 13.3 years (range: 3.9–35.4 years).74
Surgical procedure in revision CTR varies greatly with the findings in each individual case. Providing vascularized coverage for the median nerve is often recommended. A prospective study of the hypothenar fat flap in 34 patients found high rates of paresthesia resolution and pain improvement and persistent effects at 5-year follow-up.75 Alternative coverage methods include an abductor digiti minimi flap76 or a radial artery perforator fascial flap.77 Due to the etiological heterogeneity—and relative rarity—of failed CTR, large-scale studies are currently lacking in this field.
Footnotes
Published online 19 March 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Luckhaupt SE, Dahlhamer JM, Ward BW, et al. Prevalence and work-relatedness of carpal tunnel syndrome in the working population, United States, 2010 national health interview survey. Am J Ind Med. 2013;56:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fnais N, Gomes T, Mahoney J, et al. Temporal trend of carpal tunnel release surgery: a population-based time series analysis. PLoS One. 2014;9:e97499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer DH, Hanrahan LP. Social and economic costs of carpal tunnel surgery. Instr Course Lect. 1995;44:167–172. [PubMed] [Google Scholar]
- 4.Zhang D, Collins JE, Earp BE, et al. Surgical demographics of carpal tunnel syndrome and cubital tunnel syndrome over 5 years at a single institution. J Hand Surg Am. 2017;42:929.e1–929.e8. [DOI] [PubMed] [Google Scholar]
- 5.Amadio PC. The first carpal tunnel release? J Hand Surg Br. 1995;20:40–41. [DOI] [PubMed] [Google Scholar]
- 6.Phalen GS. The carpal-tunnel syndrome: seventeen years’ experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Jt Surg Am. 1966;48:211–228. [PubMed] [Google Scholar]
- 7.Zuo D, Zhou Z, Wang H, et al. Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2008;90:2587–2593. [DOI] [PubMed] [Google Scholar]
- 9.Fowler JR. Nerve conduction studies for carpal tunnel syndrome: gold standard or unnecessary evil? Orthopedics. 2017;40:141–142. [DOI] [PubMed] [Google Scholar]
- 10.Zyluk A, Szlosser Z. The results of carpal tunnel release for carpal tunnel syndrome diagnosed on clinical grounds, with or without electrophysiological investigations: a randomized study. J Hand Surg Eur Vol. 2013;38:44–49. [DOI] [PubMed] [Google Scholar]
- 11.Glowacki KA, Breen CJ, Sachar K, et al. Electrodiagnostic testing and carpal tunnel release outcome. J Hand Surg Am. 1996;21:117–121. [DOI] [PubMed] [Google Scholar]
- 12.Nathan PA, Takigawa K, Keniston RC, et al. Slowing of sensory conduction of the median nerve and carpal tunnel syndrome in japanese and american industrial workers. J Hand Surg Br. 1994;19:30–34. [DOI] [PubMed] [Google Scholar]
- 13.Sears ED, Swiatek PR, Hou H, et al. Utilization of preoperative electrodiagnostic studies for carpal tunnel syndrome: an analysis of national practice patterns. J Hand Surg Am. 2016;41:665, 672–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham B, Peljovich A, Afra R, et al. The American Academy of Orthopaedic Surgeons evidence-based clinical practice guideline on: management of carpal tunnel syndrome. J Bone Joint Surg Am. 2016;98:1750, 1754, 10.2106/JBJS.16.00719 [DOI] [PubMed] [Google Scholar]
- 15.Fowler JR, Gaughan JP, Ilyas AM. The sensitivity and specificity of ultrasound for the diagnosis of carpal tunnel syndrome: A meta-analysis. Clin Orthop Relat Res. 2011;469:1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler JR, Hirsch D, Kruse K. The reliability of ultrasound measurements of the median nerve at the carpal tunnel inlet. J Hand Surg Am. 2015;40:1992–1995. [DOI] [PubMed] [Google Scholar]
- 17.Ng AWH, Griffith JF, Lee RKL, et al. Ultrasound carpal tunnel syndrome: additional criteria for diagnosis. Clin Radiol. 2018;73:214.e11–214.e18. [DOI] [PubMed] [Google Scholar]
- 18.Pulikkottil BJ, Schub M, Kadow TR, et al. Correlating median nerve cross-sectional area with nerve conduction studies. J Hand Surg Am. 2016;41:958–962. [DOI] [PubMed] [Google Scholar]
- 19.Tulipan JE, Kachooei AR, Shearin J, et al. Ultrasound evaluation for incomplete carpal tunnel release. Hand (N Y). 20191558944719832040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crasto JA, Scott ME, Fowler JR. Ultrasound measurement of the cross-sectional area of the median nerve: the effect of teaching on measurement accuracy. Hand (N Y). 2019;14:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarvik JG, Yuen E, Haynor DR, et al. MR nerve imaging in a prospective cohort of patients with suspected carpal tunnel syndrome. Neurology. 2002;58:1597–1602. [DOI] [PubMed] [Google Scholar]
- 22.Bagga B, Sinha A, Khandelwal N, et al. Comparison of magnetic resonance imaging and ultrasonography in diagnosing and grading carpal tunnel syndrome: a prospective study. Curr Probl Diagn Radiol. 2019;pii:S0363-0188(18)30319-0 [DOI] [PubMed] [Google Scholar]
- 23.Fowler JR, Cipolli W, Hanson T. A comparison of three diagnostic tests for carpal tunnel syndrome using latent class analysis. J Bone Joint Surg Am. 2015;97:1958–1961. [DOI] [PubMed] [Google Scholar]
- 24.Szabo RM, Slater RR, Jr, Farver TB, et al. The value of diagnostic testing in carpal tunnel syndrome. J Hand Surg Am. 1999;24:704–714. [DOI] [PubMed] [Google Scholar]
- 25.Comi G, Lozza L, Galardi G, et al. Presence of carpal tunnel syndrome in diabetics: effect of age, sex, diabetes duration and polyneuropathy. Acta Diabetol Lat. 1985;22:259–262. [DOI] [PubMed] [Google Scholar]
- 26.Ito E, Sonoo M, Iwanami T, et al. Can palm stimulation differentiate diabetic polyneuropathy from carpal tunnel syndrome? J Clin Neurophysiol. 2014;31:169–174. [DOI] [PubMed] [Google Scholar]
- 27.Fujita K, Kimori K, Nimura A, et al. MRI analysis of carpal tunnel syndrome in hemodialysis patients versus non-hemodialysis patients: a multicenter case-control study. J Orthop Surg Res. 2019;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xin H, Hu HY, Liu B, et al. Ultrasound elastographic evaluation of the median nerve in hemodialysis with carpal tunnel syndrome. J Med Ultrason (2001). 2017;44:123–131. [DOI] [PubMed] [Google Scholar]
- 29.Ertem DH, Sirin TC, Yilmaz I. Electrophysiological responsiveness and clinical outcomes of local corticosteroid injection in the treatment of carpal tunnel syndrome. Arq Neuropsiquiatr. 2019;77:638–645. [DOI] [PubMed] [Google Scholar]
- 30.Chesterton LS, Blagojevic-Bucknall M, Burton C, et al. The clinical and cost-effectiveness of corticosteroid injection versus night splints for carpal tunnel syndrome (INSTINCTS trial): an open-label, parallel group, randomised controlled trial. Lancet. 2018;392:1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasiliadis HS, Nikolakopoulou A, Shrier I, et al. Endoscopic and open release similarly safe for the treatment of carpal tunnel syndrome. A systematic review and meta-analysis. PLoS One. 2015;10:e0143683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osei DA, Calfee RP, Stepan JG, et al. Simultaneous bilateral or unilateral carpal tunnel release? A prospective cohort study of early outcomes and limitations. J Bone Joint Surg Am. 2014;96:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park KW, Boyer MI, Gelberman RH, et al. Simultaneous bilateral versus staged bilateral carpal tunnel release: a cost-effectiveness analysis. J Am Acad Orthop Surg. 2016;24:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D, Ostergaard P, Cefalu C, et al. Outcomes of mini-open carpal tunnel release in patients with unrecordable preoperative nerve conduction potentials at a minimum of 5 years. Hand (N Y). 2019:1558944719857815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomsen NOB, Andersson GS, Björk J, et al. Neurophysiological recovery 5 years after carpal tunnel release in patients with diabetes. Muscle Nerve. 2017;56:E59–E64. [DOI] [PubMed] [Google Scholar]
- 36.Tang CQY, Lai SWH, Tay SC. Long-term outcome of carpal tunnel release surgery in patients with severe carpal tunnel syndrome. Bone Joint J. 2017;99-B:1348–1353. [DOI] [PubMed] [Google Scholar]
- 37.Schrier VJMM, Poelstra R, Selles RW, et al. Better patient-reported experiences with health care are associated with improved clinical outcome after carpal tunnel release surgery. Plast Reconstr Surg. 2019;143:1677–1684. [DOI] [PubMed] [Google Scholar]
- 38.Maempel JF, Jenkins PJ, McEachan JE. The relationship of mental health status to functional outcome and satisfaction after carpal tunnel release. J Hand Surg Eur Vol. 20191753193419866400. [DOI] [PubMed] [Google Scholar]
- 39.Datema M, Tannemaat MR, Hoitsma E, et al. Outcome of carpal tunnel release and the relation with depression. J Hand Surg Am. 2018;43:16–23. [DOI] [PubMed] [Google Scholar]
- 40.Erickson J, Polatsch D, Beldner S, et al. An assessment of sleep disturbance in patients before and after carpal tunnel release. J hand Surg Asian-Pacific Vol. 2019;24:144–146. [DOI] [PubMed] [Google Scholar]
- 41.Alter TH, Warrender WJ, Liss FE, et al. A cost analysis of carpal tunnel release surgery performed wide awake versus under sedation. Plast Reconstr Surg 2018;142:1532–1538. [DOI] [PubMed] [Google Scholar]
- 42.Tulipan J, Kim N, Abboudi J, et al. Open carpal tunnel release outcomes: performed wide awake versus with aedation. J Hand Microsurg. 2017974–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang SW, Park HM, Park JK, et al. Open cubital and carpal tunnel release using wide-awake technique: reduction of postoperative pain. J Pain Res. 201912:2725–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhee PC, Fischer MM, Rhee LS, et al. Cost savings and patient experiences of a clinic-based, wide-awake hand surgery program at a military medical center: a critical analysis of the first 100 procedures. J Hand Surg Am. 2017;42:e139–e147. [DOI] [PubMed] [Google Scholar]
- 45.Leblanc MR, Lalonde DH, Thoma A, et al. Is main operating room sterility really necessary in carpal tunnel surgery? A multicenter prospective study of minor procedure room field sterility surgery. Hand (N Y). 2011;6:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leblanc MR, Lalonde J, Lalonde DH. A detailed cost and efficiency analysis of performing carpal tunnel surgery in the main operating room versus the ambulatory setting in canada. Hand (N Y). 2007;2:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalonde D, Martin A. Epinephrine in local anesthesia in finger and hand surgery: the case for wide-awake anesthesia. J Am Acad Orthop Surg. 2013;21:443–447. [DOI] [PubMed] [Google Scholar]
- 48.Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the dalhousie project clinical phase. J Hand Surg Am. 2005;30:1061–1067. [DOI] [PubMed] [Google Scholar]
- 49.Sardenberg T, Ribak S, Colenci R, et al. 488 hand surgeries with local anesthesia with epinephrine, without a tourniquet, without sedation, and without an anesthesiologist. Rev Bras Ortop. 2018;53:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzcharles-Bowe C, Denkler K, Lalonde D. Finger injection with high-dose (1:1,000) epinephrine: does it cause finger necrosis and should it be treated? Hand. 2007;2:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruiter T, Harter T, Miladore N, et al. Finger amputation after injection with lidocaine and epinephrine. Eplasty. 2014;14:ic43. [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang JX, Gray J, Lalonde DH, et al. Digital necrosis after lidocaine and epinephrine injection in the flexor tendon sheath without phentolamine rescue. J Hand Surg Am. 2017;42:e119–e123. [DOI] [PubMed] [Google Scholar]
- 53.Sama CB. Post-traumatic digital gangrene associated with epinephrine use in primary raynaud’s phenomenon: lesson for the future. Ethiop J Health Sci. 2016;26:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Law TY, Rosas S, Hubbard ZS, et al. Trends in open and endoscopic carpal tunnel release utilization in the medicare patient population. J Surg Res. 2017;214:9–13. [DOI] [PubMed] [Google Scholar]
- 55.Vasiliadis HS, Georgoulas P, Shrier I, et al. Endoscopic release for carpal tunnel syndrome. Cochrane database Syst Rev. 2014CD008265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trehan SK, Lyman S, Ge Y, et al. Incidence of nerve repair following endoscopic carpal tunnel release is higher compared to open release in new york state. HSS J. 2019;15:143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williamson ERC, Vasquez Montes D, Melamed E. Multistate comparison of cost, trends, and complications in open versus endoscopic carpal tunnel release. Hand (N Y). 20191558944719837020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters BR, Martin AM, Memauri BF, et al. Morphologic analysis of the carpal tunnel and median nerve following open and endoscopic carpal tunnel release. Hand (N Y). 20191558944719861711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michelotti BM, Vakharia KT, Romanowsky D, et al. A prospective, Randomized Trial comparing open and endoscopic carpal tunnel release within the same patient. Hand (N Y). 20181558944718812129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gould D, Kulber D, Kuschner S, et al. Our surgical experience: open versus endoscopic carpal tunnel surgery. J Hand Surg Am. 2018;43:853–861. [DOI] [PubMed] [Google Scholar]
- 61.Hu K, Zhang T, Xu W. Intraindividual comparison between open and endoscopic release in bilateral carpal tunnel syndrome: a meta-analysis of randomized controlled trials. Brain Behav. 2016;6:e00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orak MM, Gümüştaş SA, Onay T, et al. Comparison of postoperative pain after open and endoscopic carpal tunnel release: a randomized controlled study. Indian J Orthop. 2016;50:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrover D, Silvera J, De Baere T, et al. Percutaneous ultrasound-guided carpal tunnel release: study upon clinical efficacy and safety. Cardiovasc Intervent Radiol. 2017;40:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burnham RS, Loh EY, Rambaransingh B, et al. A controlled trial evaluating the safety and effectiveness of ultrasound-guided looped thread carpal tunnel release. Hand (N Y). 20191558944719842199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo D, Guo D, Guo J, et al. A clinical study of the modified thread carpal tunnel release. Hand (N Y). 2017;12:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dekimpe C, Andreani O, Camuzard O, et al. Ultrasound-guided percutaneous release of the carpal tunnel: comparison of the learning curves of a senior versus a junior operator. A cadaveric study. Skeletal Radiol. 2019;48:1803–1809. [DOI] [PubMed] [Google Scholar]
- 67.Ilyas AM, Miller AJ, Graham JG, et al. Pain management after carpal tunnel release surgery: a prospective randomized double-blinded trial comparing acetaminophen, ibuprofen, and oxycodone. J Hand Surg Am. 2018;43:913–919. [DOI] [PubMed] [Google Scholar]
- 68.Grandizio LC, Zhang H, Dwyer CL, et al. Opioid versus nonopioid analgesia after carpal tunnel release: a Randomized, Prospective Study. Hand (N Y). 20191558944719836211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinheimer K, Michelotti B, Silver J, et al. A prospective, randomized, double-blinded controlled trial comparing ibuprofen and acetaminophen versus hydrocodone and acetaminophen for soft tissue hand procedures. J Hand Surg Am. 2019;44:387–393. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, Chen L, Wu L, et al. Value of superb microvascular imaging ultrasonography in the diagnosis of carpal tunnel syndrome. Med (United States). 2017;96e6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zieske L, Ebersole GC, Davidge K, et al. Revision carpal tunnel surgery: a 10-year review of intraoperative findings and outcomes. J Hand Surg Am. 2013;38:1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boeckstyns ME, Sørensen AI. Does endoscopic carpal tunnel release have a higher rate of complications than open carpal tunnel release? An analysis of published series. J Hand Surg Br. 1999;24:9–15. [DOI] [PubMed] [Google Scholar]
- 73.Mosier BA, Hughes TB. Recurrent carpal tunnel syndrome. Hand Clin. 2013;29:427–434. [DOI] [PubMed] [Google Scholar]
- 74.Stirling PHC, Yeoman TFM, Duckworth AD, et al. Decompression for recurrent carpal tunnel syndrome provides significant functional improvement and patient satisfaction. J Hand Surg Eur Vol. 2019175319341987594. [DOI] [PubMed] [Google Scholar]
- 75.Athlani L, Haloua JP. Strickland’s hypothenar fat pad flap for revision surgery in carpal tunnel syndrome: prospective study of 34 cases. Hand Surg Rehabil. 2017;36:202–207. [DOI] [PubMed] [Google Scholar]
- 76.Cheung K, Klausmeyer MA, Jupiter JB. Abductor digiti minimi flap for vascularized coverage in the surgical management of complex regional pain syndrome following carpal tunnel release. Hand (N Y). 2017;12:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahmoud M, El Shafie S, Coppola EE, et al. Perforator-based radial forearm fascial flap for management of recurrent carpal tunnel syndrome. J Hand Surg Am. 2013;38:2151–2158. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Suarez CB, Fidel BC, Cabrera JTC, et al. Diagnostic accuracy of ultrasound parameters in carpal tunnel syndrome: additional criteria for diagnosis. J Ultrasound Med. 2019;38:3043–3052. [DOI] [PubMed] [Google Scholar]
- 79.Oh WT, Kang HJ, Koh IH, et al. Morphologic change of nerve and symptom relief are similar after mini-incision and endoscopic carpal tunnel release: a randomized trial. BMC Musculoskelet Disord. 2017;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]


