Supplemental Digital Content is available in the text.
Background:
During reconstructive breast surgery, intraoperative assessment of tissue perfusion has been solely based on subjective clinical judgment. However, in the last decade, intraoperative indocyanine green angiography (ICGA) has become an influential tool to visualize blood flow to the tissue of interest. This angiography technique produces real-time blood flow information to provide an objective assessment of tissue perfusion.
Methods:
A comprehensive literature search of articles pertaining to ICGA in breast reconstruction surgery was performed. The overall findings of the articles are outlined here by surgical procedure: skin-sparing and nipple-sparing mastectomy, implant-based reconstruction, and autologous reconstruction.
Results:
Overall, there were 133 articles reviewed, describing the use of ICGA in breast reconstruction surgery. We found that ICGA can provide valuable information that aids in flap design, anastomotic success, and perfusion assessment. We also included example photographs and videos of ICGA use at our institution.
Conclusions:
ICGA can reduce postoperative tissue loss and aid in intraoperative flap design and inset. Despite the benefits of ICGA, its technical use and interpretation have yet to be standardized, limiting its widespread acceptance.
INTRODUCTION
The success of a reconstructive flap is dependent on adequate perfusion and drainage of the tissue. Surgeons continuously analyze skin paddle color, capillary refill time, and bleeding from the flap edges to assess the blood flow intraoperatively. However, the rate of flap necrosis with clinical assessment alone is 4%–41%.%1 As a result, many modalities to assess flap perfusion perioperatively have been utilized, including indocyanine green angiography (ICGA).%2 Indocyanine green (ICG) is a fluorescent chemical that becomes tightly bound to plasma protein once injected intravenously. When ICG is activated by a laser or light-emitting diode, the compound has a maximum absorption of 805 nm and a minimum emission of 835 nm. Dye emission is captured by real-time camera through patients’ skin via a near-infrared detection device. Water molecules and hemoglobin do not absorb wavelengths of light above 800 nm and the wavelength is not scattered by surrounding tissue, making it ideal for visualizing blood vessels and their perfusion zones.%2 ICG is cleared by the bile system and has a half-life of 3–4 minutes in healthy adults. This rapid clearance allows for multiple uses of ICG in a short time.%2 The dye is contraindicated in patients with iodine hypersensitivity, despite only a small number of reactions reported.%2 Devices available for capturing and recording ICG emission are listed in supplemental materials (see table, Supplemental Digital Content 1, which displays a list of ICGA recording devices and programs, http://links.lww.com/PRSGO/B323).
Over the last decade, the use of ICGA to evaluate perfusion has become more common in the field of reconstructive surgery. ICGA has been reported to decrease rates of necrosis in mastectomy and reconstructive flaps compared with clinical judgment alone.3–13 In addition to qualitative assessment, the light detection device can quantitatively assess dye emission using computer algorithms to calculate different parameters representing perfusion.%14 Relative perfusion values are based on a reference region on the ICG image selected by the surgeon that represents a standard for comparison of other regions. The surgeon selects an area on the device display screen of good perfusion to represent 100%. Numbers that appear on the screen will represent a percentage of the selected region.%14 Analysis systems can also create a contour map over the area of interest. The contour map creates lines on the image, representing lines of isofluorescence.%14 Relative values and contour lines will change as the image sequence plays, representing changes in signal intensity over time. Absolute perfusion is derived directly from the intensity of fluorescence, or pixel values of the grayscale, without the use of a reference marker. The increasing number of value indicates higher absolute perfusion and vice versa.%15 Quantification systems are able to automatically calculate the rates of arterial inflow, ingress, and venous outflow, egress, during video surveillance. These values are presented graphically by the system for interpretation by the surgeon.%16 Lastly, intrinsic transit time (ITT) can be extrapolated by measuring the time it takes for the dye to flow from arterial anastomosis to venous anastomosis.%17
Despite the number of different qualitative and quantitative parameters available with ICGA, there is no gold standard method to measure flap perfusion in breast reconstruction. This article aims to evaluate the current uses of ICGA in the arena of breast reconstruction. We aim to determine whether there is a consensus on ICGA use and interpretation. Intraoperative images at our institution are presented to illustrate the utility of ICG and to clarify the techniques employing this technology.
METHODS
Systematic Review
We followed the Preferred Reporting Items for Systemic Reviews and Meta-analysis Statement guidelines in performing and reporting this review. We determined a search strategy in collaboration with a certified medical librarian. The search keywords were indocyanine green angiography, breast, reconstruction, implant, and mastectomy. The following databases were queried until August 2019: PubMed, Medline, Cochrane Library, Embase, and Web of Science. The inclusion criteria for this review were peer-reviewed published articles, articles written in English, and articles covering the use or comparison of ICGA in reconstructive breast surgery. Articles that did not focus on breast reconstruction were excluded. Preclinical and cadaver studies were also excluded. After completion of full-text screening, the database list was independently evaluated for potentially missed literature. The following study characteristics were extracted: sample size, type of reconstruction, imaging system, and any reported clinical outcome.
RESULTS
A total of 134 peer-reviewed articles were found using the search strategy. Figure 1 illustrates the methodology used for identification, screening, eligibility, and inclusion of articles. A total of 43 articles were included in the systematic review. All other sources cited were to provide background and discussion of this article. Tables 1, 2, 3, and 4 summarize all articles reviewed with following details: authors, number of subjects, imaging system used with assessment parameter studied, and a summary of findings.
Fig. 1.
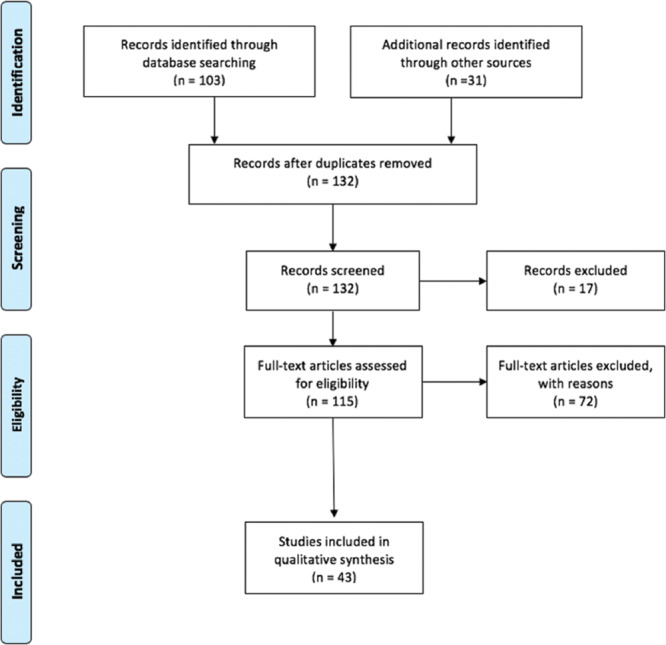
Preferred Reporting Items for Systemic Reviews and Meta-analysis Statement flow diagram depicting the flow of information through the different phases of this systematic review.
Table 1.
Publications on Use of ICGA in Mastectomy Flap Assessment
| Authors | Sample Size (N) | Imaging System and Measurement | Summary |
|---|---|---|---|
| Mastectomy Skin Flap Assessment | |||
| Newman et al.%15 | 20 patient images | SPY Elite Quantitative (Spy-Q) | The study compared 10 ICGA images of viable skin and 10 images of necrosed skin and the absolute and relative perfusion scores of each. Relative perfusion values were more objective and reproducible for augmenting surgical judgment. |
| Duggal et al.%5 | 368 patients | SPY Elite Qualitative |
After implementation of ICGA, a 10.4% reduction in mastectomy skin necrosis and an 8.2% reduction in reoperations due to perfusion-related complications were appreciated. The study reported 160$ in patient savings when using ICGA for intraoperative assessment. |
| Gorai et al.%23 | 181 patients | PDE Qualitative and Quantitative |
Rate of necrosis reaching the subcutaneous fat layer was significantly lower in the ICGA group than that in the no-ICGA group. The study found that maximum luminance cutoff value of 34 was 98.5% specific in predicting necrosis, and rate of luminance was more sensitive in predicting necrosis. |
| Rinker%22 | 60 patients | SPY Elite Qualitative |
Intraoperative ICGA reduced rates of postoperative mastectomy necrosis compared to clinical judgment alone. Also, the study compared fluorescein dye with ICGA and found that fluorescein dye had the lowest rates of complications. |
| Mattison et al.%14 | 31 patients | SPY Elite Quantitative (Spy-Q) |
Contour setting of 10 in SPY Elite system had 100% sensitivity and 68.1% specificity. The study found that ICGA is most useful when clinical examination produced marginal results, stated ICGA overly conservative in low-risk patients. |
| Sood et al.%19 | 91 patients | SPY Elite Qualitative |
The study retrospectively compared complications in patients pre-SPY and post-SPY implementation. Mean number of repeat visits to the operating room was significantly higher in pre-SPY group. No areas of poor perfusion were identified by clinical judgment alone. |
| Moyer et al.%64 | 14 patients | SPY Elite Quantitative (Spy-Q) |
Perfusion score of <25% is 90% positive predictive value of nonviable skin, <33% is 88% positive predictive value of nonviable skin, and a perfusion score of ≥45% survived 98% of the time. |
| Munabi et al.%63 | 42 patients | SPY Elite Quantitative (Spy-Q) |
Spy Elite absolute perfusion value of ≤7 predicted flap necrosis. If false positive cases (smoking history, epinephrine containing tumescent injection) were removed from the study, a value of ≤7 had even higher specificity. |
| Phillips et al.%21 | 51 breasts | SPY 2001 Qualitative and Quantitative (Spy-Q) |
Prospective comparison of clinical judgment, ICGA, and fluorescein angiography: Clinical judgment alone predicted 41% of necrosis. Both angiography methods predicted necrosis in 19 of 21 cases. ICGA had a higher specificity but predicted necrosis in 15 of 30 cases that had no resulting necrosis. Absolute perfusion score was more reliable because of its ability to be extrapolated across patients. |
| Newman et al.%20 | 12 patients | SPY Elite Qualitative |
The study found a 95% correlation between intraoperative imaging with ICGA and clinical course with 100% sensitivity and 91% specificity. False positive rate is 9%. |
| Diep et al.%6 | 135 patients | SPY Elite Quantitative (Spy-Q) |
When patients were grouped retrospectively into 3 chronological groups from 2012 to 2016, the study fowund that rates of ischemic complications decreased significantly as time went on, from 36% to 22% to 11%, and mean time to TE to implant exchange. Perfusion mapping was used to define and excise skin flap with <20% perfusion. |
PDE, photodynamic eye; TE, tissue expander.
ICGA in Mastectomy
Clinical assessment of mastectomy skin flap and nipple-areolar complex (NAC) perfusion can be augmented with ICGA to improve rates of postoperative ischemic complications%5,%6,14,15,18–23 (Table 1). Sood and Glat%19 reported that ICGA identified 5 cases of poor perfusion that were not recognized by clinical evaluation. Several studies have noted that the use of ICG imaging is more important when clinical examination is equivocal.%14 A clinical trial by Komorowska-Timek and Gurtner%24 reported a reduction in overall complication rates from 15.1% to 4% when they used ICG technology to guide excision of poorly perfused portions of mastectomy flaps. Newman et al.%20 also reported a 95% correlation between intraoperative ICGA imaging and postoperative outcomes of the mastectomy flap, with a sensitivity of 100% and specificity of 91%. A retrospective study by Diep et al.%11 assessed the use of quantitative ICGA analysis over 4 years and found that the mastectomy flap ischemic complication rates decreased consistently over time (Fig. 2). Lastly, Gorai et al. showed in their patient cohort that necrosis to deeper tissue was decreased when ICGA was used in implant based reconstruction.%23 These results show the utility of ICGA when assessing mastectomy flap perfusion to predict the survival of the skin; however, each study has a unique method of interpreting ICGA results.
Fig. 2.
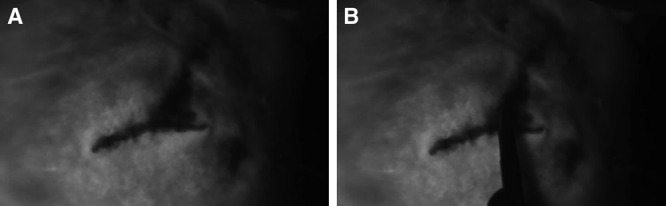
Skin-sparing mastectomy flap assessment intraoperatively. After placement of deflated tissue expander in the subpectoral pocket, the mastectomy skin flap was stapled, and ICGA was used to visualize perfusion. ICG images demonstrated hypoperfusion at the very superior portion of the lateral incision (A), which was marked (B) and excised. The patient had no postoperative complications.
Advances in cancer research have led to an increase in nipple-sparing mastectomies (NSM). However, NAC necrosis rates are reported up to 15%.25 ICGA can help visualize NAC perfusion to guide intraoperative decisions (Table 2) (Fig. 3). In preoperative planning of NSM, ICG imaging can determine the source of blood and perfusion patterns of the NAC and inform the surgeon of appropriate incision.26–28 A recent clinical trial used ICGA to show that an infraareolar incision had superior ingress and egress rates compared with supraareolar incision.16 Bertoni et al. used ICGA to plan devascularization and surgical delay mastectomies to prevent nipple necrosis in high-risk patients who otherwise would not be candidates for NSM. In doing so, they were able to convert perfusion patterns to a new pattern of diffuse capillary fill coursing toward NAC from peripheral skin. This 2-stage surgery resulted in no nipple loss in their cohort.29 Lastly, ICGA imaging is especially useful in NACs with very light or very dark complexions.30
Table 2.
Published Literature on the Use of ICGA in NAC Perfusion Assessment
| Authors | Sample Size (N) | Imaging System and Measurement | Summary |
|---|---|---|---|
| NAC Assessment | |||
| Wapnir et al.32 | 39 NACs | SPY Elite Qualitative |
The study used relative perfusion scores to assess perfusion patterns of NAC: 18% of breasts demonstrated perfusion from underlying breast tissue pattern, 46% demonstrated perfusion from surrounding skin, and 36% had a combination of perfusion from underlying breast tissue and surrounding skin. Nipples perfused by surrounding breast skin had the highest rates of necrosis postmastectomy. |
| Dua et al.28 | 93 NACs | SPY Elite Qualitative |
Larger cohort of previous study—the study found that 19% of breasts demonstrated perfusion from underlying breast tissue pattern, 45% demonstrated perfusion from surrounding skin, and 29% had a combination of perfusion from underlying breast tissue and surrounding skin. |
| de Vita et al.26 | 38 patients | Quest Spectrum Qualitative |
The study used ICGA to prove safety of NAC perfusion over implant after NSM. ICGA was used in all patients undergoing reconstruction and adequate perfusion was shown in all patients. |
| Bertoni et al.29 | 28 patients | SPY Elite Qualitative |
The study assessed using a 2-stage approach to NSM in high-risk patients who would not otherwise be good candidates for NSM. The first operation entailed separating the NAC and surrounding skin from the underlying breast tissue, definitive surgery occurred 3–6 wk later. After devascularization, 35.8% nipples converted to a new perfusion pattern and they did not observe nipple loss. |
| Wang et al.16 | 17 breasts | SPY Elite Quantitative (Spy-Q) |
Using ICG-calculated ingress and egress rates, it was determined that the infraareolar incision provides superior blood flow than supraareolar incisions. The study opted for smaller implants if the NAC egress rate was ≤2. |
| Brunworth et al.42 | 1 patient | SPY Elite Qualitative |
ICGA is useful for patients with dark skin tone and recent smoking history, and women with long dermal pedicels. |
| Murray et al.30 | 12 patients | SPY 2001 Qualitative |
ICGA is most useful for very light or dark skin toned NAC. ICGA is useful to visualize venous outflow 10 mins after injection of the dye; if outflow was compromised, surgeons would revise the pedicle or graft the NAC. |
Fig. 3.
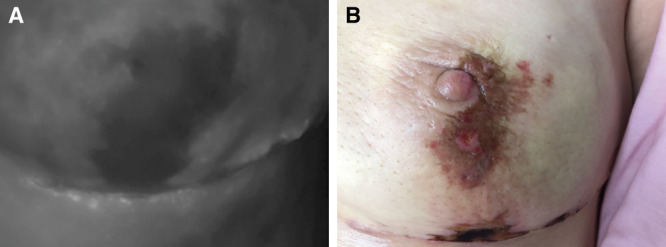
A, Assessment of nipple perfusion after NSM. A prepectoral tissue expander was placed with acellular dermal matrix sling. B, Clinical pictures correlating to intraoperative ICG images. Area of concern was treated conservatively with no need for intervention.
ICGA in Breast Reconstruction
Implant-based Reconstruction
ICGA has applications for use during the placement of a breast prosthetics (tissue expander or permanent implant) (Table 3). Its use can determine skin flap and NAC perfusion after the implant has been placed.12,%%16,%%24,%%26,%%31,%%32 Komorowska-Timek et al. completed a clinical trial of 19 implant-based breast reconstructions using ICGA intraoperatively to assess mastectomy flap perfusion after implant placement. When larger implants were associated with decreased perfusion of the flap, they decreased the implant volume to restore adequate skin flap perfusion24 (Fig. 4). The tension–perfusion relationship has been quantified, using ingress and egress rates with increasing implant volume.16,%%31 Yang et al.31 stated that the ingress rate was more strongly affected by increased skin tension from larger implant fills. However, Wang et al.16 focused on egress rates and stated that an egress rate of ≤2 would signify that a smaller implant was needed to avoid decreased perfusion to the NAC. Lastly, Jones et al. described an ICG imaging protocol using qualitative ICGA results to determine whether direct-to-implant prepectoral reconstruction was feasible, regardless of other patient factors. Using this protocol, they had no ischemic complications.33 Similarly, Harless and Jacobson7 reported mastectomy flap necrosis rates decreased by 84% and an overall reduction in complication rate once ICGA protocol was implemented in implant-based reconstruction (Figs. 3 and 4). These studies illustrate the number of different ways ICGA can improve implant-based reconstruction outcomes; however, each study has published unique ICGA interpretation methods (see appendix, Supplemental Digital Content 2, which displays ICGA Protocol, http://links.lww.com/PRSGO/B324).
Table 3.
Publications on the Use of ICGA in Implant-based Breast Reconstruction
| Clinical Studies | Sample Size (N) | Imaging System and Measurement | Summary |
|---|---|---|---|
| Implant-based Reconstruction | |||
| Mirhaidari et al.%%12 | 126 patients | SPY Elite Quantitative (Spy-Q) |
This prospective study used absolute perfusion values after placement of TE to determine whether expander size, fill, and placement allowed adequate perfusion. Absolute perfusion values were interpreted as >30 (well perfused), 16–30 (adequately perfused), 10–15 (marginal), and <10 (poorly perfused). The study found that ICGA decreased the rate of mastectomy flap necrosis and infection, implant loss, and overall reoperation rate. |
| Venturi et al.32 | 20 patients | SPY Elite Quantitative (Spy-Q) |
Intraoperative ICGA was used to assess nipple perfusion before and after TE or implant placement. Relative perfusion scores were used to determine poor perfusion; a perfusion score <5% was defined as ischemic. No intraoperative adjustments were made. ICGA correctly predicted postoperative nipple necrosis. |
| Komorowska-Timek et al.24 | 20 patients | SPY Elite Qualitative |
The study used ICGA during implant-based reconstruction to tailor initial size and fill of implant. Intraoperative ICGA to assess mastectomy skin flap and autologous flap perfusion produced a 4% complication rate, compared with the 15.1% complication rate of previous breast reconstructions without ICG imaging. |
| Yang et al.31 | 10 patients | SPY Elite Quantitative (Spy-Q) |
Tension of skin flap in implant-based reconstruction effects the egress rate as measured by SPY Elite system. This can be used to determine the correct fill volume to avoid low perfusion of the covering skin. |
| Harless et al.7 | 296 patients | SPY Elite Qualitative |
This is a retrospective review of breast reconstruction before and after implementation of ICGA with a single surgeon. Rate of skin flap necrosis decreased by 86%, overall complication rate decreased from 13.8% to 6.6%, and single-stage reconstruction increased from 12% to 32% after introduction of ICGA to their surgical practice. |
| Jones et al.33 | 50 patients | SPY Elite Quantitative (Spy-Q) |
Authors employed ICGA to assess skin flap perfusion in implant-based reconstruction. If adequate perfusion was determined by ICGA, they proceeded with single-stage breast reconstruction. This technique resulted in no full-thickness skin necrosis. |
| Hammer-Hansen et al.60 | 92 patients | SPY Elite Quantitative (Spy-Q) |
Areas with perfusion <33% were excised or marked intraoperatively. They found no significant decrease in rates of necrosis requiring conservative treatment or reoperation. |
TE, tissue expander.
Fig. 4.
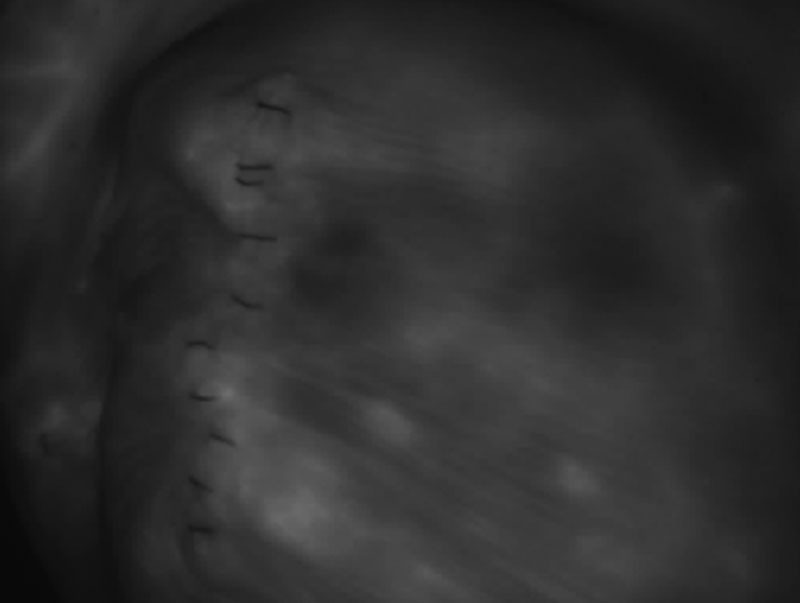
Prepectoral implant breast reconstruction after skin-sparing mastectomy. Assessment of perfusion of the mastectomy skin after tissue expander placement under acellular dermal matrix pocket. Image shows good perfusion of the mastectomy skin.
Autologous Reconstruction
Autologous breast reconstruction encompasses complex procedures that require assessment of multiple blood flow variables for optimal outcomes. These variables include perforator selection, flap perfusion, anastomotic patency, and venous outflow assessment. The use of ICGA has been successfully applied at each step of microsurgical breast reconstruction (Table 4).
Table 4.
Publications of the Use of ICGA in Autologous Breast Reconstruction
| Clinical Studies | Sample Size (N) | Type of Reconstruction | Imaging System and Measurement | Summary |
|---|---|---|---|---|
| Autologous Reconstruction | ||||
| Onoda et al.36 | 50 patients | Free flaps | PDE Qualitative |
Flaps were assessed with MDCT first, then Doppler flowmetry, then ICGA to confirm intensity and position of perforators. Free flaps survived without complications. |
| Pestana et al.8 | 18 patients | DIEP | SPY Elite Quantitative (Spy-Q) |
Preoperative ICGA skin blush location, size, and intensity did not correlate to preoperative CTA imaging of perforating vessels. BMI was not associated with increased skin blush when using ICGA. CTA and SPY informed surgical decisions in 74% of patients. Decreased perfusion shown in ICGA images informed flap resection in 46% of patients. |
| Wu et al.37 | 16 patients | DIEP | SPY Elite Quantitative (Spy-Q) |
ICGA images correlated with CTA image in 85% of cases of perforator flap breast reconstruction. However, ICG underestimated and overestimated perfusion in 7% and 7%, respectively, of those cases. |
| Azuma et al.38 | 14 patients | DIEP | PDE Qualitative |
Qualitative assessment of ICGA was able to identify 2–6 perforators preoperatively. The perforators were confirmed during surgical dissection at the deep fascial level in all flaps. |
| Douglas et al.40 | 13 patients | DIEP | SPY Elite Qualitative |
Based on ICGA images, the study stated that perfusion of zone IV in DIEP flap was significantly higher when 1 perforator was used instead of 2. |
| Holm et al.41 | 25 patients | SIEA | Laser light source, digital video camera Qualitative |
In this study, SIEA flap was originally intended for all patients; however, intraoperative perfusion measurements with ICGA changed the surgical plan in 44% of patients. Partial flap necrosis was seen in 1 case (4%). |
| Yamaguchi et al.44 | 10 patients | TRAM | IC-View Qualitative |
The study found individual pattern “perfusion map” using ICGA and noted that Hartrampf zone II is sometimes less perfused than zone III. |
| Holm et al.45 | 15 patients | DIEP | IC-View Qualitative |
Hartrampf zone IV was not perfused in 5 patients, describe the abdomen perfusion pattern as 2 halves, separated by midline, with variable blood supply. |
| Girard et al.46 | 40 patients | DIEP | SPY Elite Qualitative and Quantitative (Spy-Q) |
Authors defined 3 perfusion zones of the unilateral DIEP flap based on qualitative color analysis. Then the study used ingress and ingress rate to confirm adequate perfusion of each region of the flap. Also, it found that young patients with diabetes mellitus and tamoxifen hormone therapy effected results of the SPY imaging. |
| Hembd et al.52 | 409 patients | DIEP | Not stated Qualitative |
Authors conducted a retrospective review of DIEP cases and compared odds ratios of different surgical variables. They found that a decrease in the odds of fat necrosis was seen if ICGA was used to assess flap perfusion (OR, 0.46; P = 0.04). |
| Malagon-Lopez et al.53 | 61 patients | DIEP | PDE Qualitative |
Incidence of fat necrosis was reduced to 29% from 59.5% when ICGA was used in this prospective study. Number of secondary surgeries due to fat necrosis also reduced from 45.9% to 20.8%. |
| Ludolph et al.43 | 32 patients | DIEP and ms-TRAM | SPY Elite Qualitative |
The study compared ICGA and combined laser Doppler spectrophotometry in assessment of flap perfusion. ICGA was found to be useful intraoperatively when clinical signs of perfusion were unclear because of its high sensitivity of poorly perfused flap areas. Combined laser Doppler spectrophotometry, however, was superior to ICGA in topographical mapping of well-perfused areas. |
| Schrogendorfer et al.55 | 41 patients | DIEP, TRAM, TMG | IC-View IC-Clac |
Microscopic ICG was able to detect vessel occlusion after anastomosis and led to immediate revision. No flap loss occurred. Authors note that this relationship is not necessarily a causation relationship. |
| Holm et al.56 | 50 patients | Random, pedicle, and free flaps | Modified microscope Qualitative |
The study used ITT to assess blood flow through the flap. A transit time greater than 90 s signified venous delay, and surgeons would then revise the anastomosis. Angiographic results were confirmed 100% during revisions and all flaps that were revised survived. Flaps that were not revised despite increased blood transit time resulted in necrosis. |
| Koonce et al.51 | 53 patients | ms-LDF | Not stated Qualitative |
The study used a laterally extended skin paddle on ms-LDF and defined 3 perfusions zones. All zones were adequately perfused. |
| Khansa et al.57 | 442 patients | Pedicle and free flaps | Not stated Qualitative |
ICGA was used intraoperatively to assess arterial insufficiency and venous congestion based on “turbulence” of flow through the anastomosis. This method of ICGA interpretation was 37.5% sensitive of predicting vascular compromise and 100% specific. They then propose a new systematic algorithm for assessing flaps when complications occur postoperatively. |
| Holm et al.17 | 86 patients | Random, pedicle, and free flaps | Modified microscope Quantitative (Spy-Q) |
Using ITT as previously described, a blood transit time of greater than 50 s had the strongest association with flap complications. |
| Hitier et al.58 | 20 patients | Pedicle and free flaps | Floubeam Quantitative (Fluobeam v1.47 Software) |
ITT and fluorescence intensity were used intraoperatively to assess blood flow of the flap. Slope of fluorescence intensity rise and amplitude of fluorescence were used postoperatively. The mean fluorescence intensity, slope of fluorescence rise, and amplitude of fluorescence all produced a significantly lower signal in flaps that resulted in vascular complications. Authors state that ICGA detected complications sooner than physical examination. |
| Alstrup et al.%%61 | 191 patients | LDF, ms-LDF, TRAM | SPY Elite Quantitative (Spy-Q) |
Authors found no significant difference in complication rates, minor or major, between patient groups that received intraoperative ICGA and those who did not. Within the ICGA group, immediately reconstructed breasts had significantly less complications. |
| Krishnan et al.59 | 9 patients | Random, pedicle, and free flaps | IC-View Quantitative (IC-Clac) |
The authors used relative perfusion, time to maximum fluorescence, and time of fluorescence fall to determine venous inflow and venous congestion, respectively, postoperatively. They found a delay in ICG uptake and clearance in 6 flaps (1 random pattern, 2 axial pattern, and 3 free flaps). However, clinical signs of congestion were only seen in 2 of the 6 flaps. |
BMI, body mass index; LDF, latissimus dorsi flap; MDCT, multiple-detector computed tomography; ms, muscle sparing, OR, odds ratio; PDE, photodynamic eye; TMG, transverse myocutaneous gracilis flap; TRAM, transverse rectus abdominis myocutaneous; SIEA, superficial inferior epigastric artery-based flap.
Perforator Selection
Recently, surgeons have used imaging modalities such as computed tomography angiography (CTA) or magnetic resonance angiography to identify the quality and location of the perforator preoperatively.34 ICGA can provide additional information about the flow and perfusion area but cannot demonstrate the course of the vessel as the technology is limited to 2-dimensional images and 2-cm depth of visualization.2,%%35,%%36 As such, there are reports of ICGA correctly identifying perforators seen on CTA in thin fasciocutaneous flaps preoperatively.37,%%38 However, in deep inferior epigastric perforator (DIEP) flaps, the study of Pestana et al.39 was not able to demonstrate a direct correlation between perforator size as identified by CTA preoperatively and the ICGA skin blush intensity before dissection of the flap tissue. Authors of these articles acknowledge that ICGA utility lies within perfusion assessment intraoperatively, not perforator selection preoperatively.37–39
Intraoperatively, ICG images can identify the perforators with the most robust perfusion during flap dissection.40,%%41 Holm et al. assessed the perfusion zones of the superficial inferior epigastric artery with qualitative ICGA. Once dominant perforators of the flap were dissected, the surgeons clamped the perforators and assessed the vascular territory of the superficial inferior epigastric arteries and determined the ultimate source vessel of the flap based on ICG imaging results41 (Fig. 5). (See Video 1 [online], which displays an assessment of right DIEP flap perfusion in situ. Two perforators were originally harvested during dissection. The bilateral flaps were imaged with the SPY with 1 perforator open and 1 perforator clamped. In the video, the right flap has decreased perfusion of the medial flap. After unclamping the second perforator and reimaging, the flap was fully perfused by SPY images. The surgeons proceeded with a 2-perforator flap based on these images.)
Fig. 5.
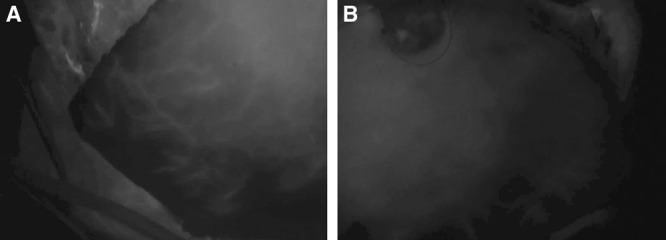
Assessment of unilateral DIEP flap perfusion in situ. During dissection of flap, a large perforator in the midflap was noted, and a superior perforator that appeared in-line with the larger one noted on preoperative CTA. The remaining perforators were clamped with atraumatic clamps. The SPY imaging device was brought into the field and the patient received ICG as per manufacturers’ guidelines: (A) right side of flap, (B) left side of flap. Zone 4 did not perfuse as well, and it was marked for removal as was the small tip of the right flap, as it perfused more slowly than the rest of the flap.
Video 1. Video 1 from “Indocyanine Green Angiography in Breast Reconstruction”.
Among these studies, each used different ICGA parameters, qualitative and quantitative, for assessing perforators within a flap.
Flap Perfusion
ICG is highly useful in visualizing perfusion of an area of interest of flaps especially when clinical signs of perfusion are ambiguous, because of ICGA’s high sensitivity.43 ICGA can reveal each patient’s unique anatomy and lead to a more individualized approach to reconstruction.21,%%41 Several articles state that the Hartrampf perfusion zones vary from patient to patient and may need to be redefined.39–41 ICGA has also been shown to be reliable in the visualization of flap perfusion despite previous liposuction, recontouring, and other abdominal scars.47–49 Furthermore, ICGA can aid in flap design by providing real-time information on the perfusion of the tissue of interest. This is also helpful in the validation of novel procedures where perfusion is not well characterized.50,%%51
ICGA has also been attributed to decreasing fat necrosis of the autologous flap postoperatively. Hembd et al.52 retrospectively reviewed 409 DIEP flaps for breast reconstruction and found that qualitative ICGA assessment of flap perfusion decreased the odds ratio of fat necrosis compared with cases where ICGA was not used. In another study, Malagón-López et al.53 showed that rates of fat necrosis decreased from 59.5% to 29% when qualitative ICG was used in their prospective study. Lastly, Varela et al.54 report a fat necrosis incidence of 8.3% in the ICGA group compared with 59.8% in the clinical assessment group. Patients in the ICGA group had no incidence of reoperation due to fat necrosis and scored higher in all categories of the BREAST-Q. The qualitative assessment of ICGA was not standardized between flap perfusion studies using ICGA.
Anastomotic Patency
Anastomosis of donor to recipient vessel can also be assessed with ICGA.55–57 Using ICGA qualitatively, it has been reported up to 100% sensitivity and 100% specificity at detecting vascular problems after anastomosis in free flaps.56,%%57 Qualitative assessment of the flap can also be performed after flap inset to ensure anastomotic patency and good perfusion of the superficial tissue (Fig. 6). Many parameters are described to assess inflow and outflow via ICGA SPY technology. Holm et al. used the ITT from arterial anastomosis to venous anastomosis to predict the success of free flaps. They reported that an ITT of 50 seconds or greater was suggestive of flap necrosis and reexploration with 92% and 98% sensitivity and specificity, respectively.17 Hitier et al. also used ITT, slope of the fluorescence intensity rise, and amplitude of the maximum fluorescence intensity and compared these with flap outcomes. They found that uncomplicated flaps had a shorter blood transit time, steeper slope of fluorescence intensity rise, and higher fluorescent amplitude than flaps that resulted in complications. This study used a handheld ICGA imaging system that could be used at bedside postoperatively.58
Fig. 6.
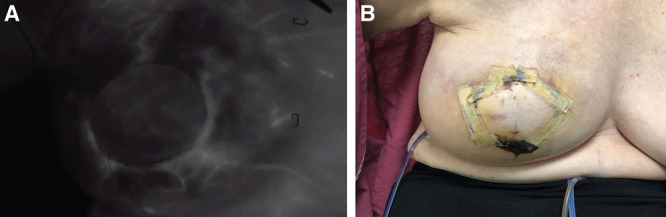
Assessment of perfusion after flap inset in DIEP procedure. A, Intraoperative ICGA imaging of DIEP flap at time of inset. B, Postoperative day 8 clinical image of reconstructed breast. The patient suffered no clinical complications.
Venous Outflow
Venous outflow is a strong contributor to flap failure, and an increased duration of venous stasis can increase the incidence of fat necrosis.56 Venous stasis can be assessed clinically by cyanotic color. It can also be qualitatively and quantitatively assessed by ICG.57 (See Video 2 [online], which displays venous congestion of a flap after venous anastomosis. ICG-carrying blood was not draining from flap after sufficient time had passed. Retrograde venous flow was then attempted without improvement of venous drainage. Ultimately, a brachiocephalic turndown was used to establish venous outflow in the flap. The intraoperative findings with SPY imaging led to necessary additional procedure to ensure survival of the flap.)
Video 2. Video 2 from “Indocyanine Green Angiography in Breast Reconstruction”.
Hitier et al. monitored free flaps intraoperatively and during the postoperative period with the handheld device. They found that the quantitative mean fluorescence intensity, slope of fluorescence rise, and amplitude of fluorescence all produced a significantly lower signal in flaps that resulted in vascular complications. Additionally, the authors state that ICGA detected complications sooner than the physical examination.58
DISCUSSION
ICGA has a wide variety of applications in the assessment of perfusion in reconstructive breast surgery. It has been shown to be superior to clinical judgment alone and has many reports of positive outcomes in reconstructive surgery.3–13 A recent meta-analysis of ICGA use in mastectomy skin flap assessment by Liu et al.18 reported that the incidence of necrosis and unexpected reoperations was significantly lower when ICGA was used. In implant-based reconstruction, the perfusion of the mastectomy flap can be assessed qualitatively with ICGA, and there has been a reported 84% decrease in skin flap necrosis rates.7 ICGA is instrumental in the intraoperative setting to determine individual perforator flow and zone of perfusion.40,%%41 Autologous flap perfusion can also be assessed with ICGA. Specifically, reports in the literature state that ICGA is useful with physical exam assessment of perfusion, is equivocal and reliable when scarring from previous surgical procedures is present, and is instrumental in reducing the rates of fat necrosis postoperatively.43,47–49 Lastly, anastomotic patency has been assessed with ICGA intraoperatively and postoperatively, leading to increased survival of flaps.56,%%58
All technologies have limitations. Articles reporting on the limitations of ICGA focus on concerning perfusion results that do not correlate with clinical findings.10,14,20,%%21,%%41,%%59, Krishnan et al. reported that 4 of 6 autologous flaps showing venous congestion by ICGA did not have clinical signs of venous congestion. These 4 flaps did not receive any intervention and had no postoperative complications.59 Other reports on ICGA assessment of mastectomy skin and autologous flaps show that there was no significant difference in complication rates when using ICGA compared with clinical examination.37,%%60,%%61 Despite these studies, authors have reported that the concerning ICGA findings do not impact their ability to complete surgical procedures.10,%%43 Phillips et al.21 reported that ICGA overpredicted poor perfusion areas in the mastectomy skin flap but noted that quantitative analysis with SPY-Q software improved ischemic prediction rates. When compared with ICGA predecessor, fluorescence dye, ICGA has a lower rate of overpredicting necrosis, but only modestly.21 There is a general paucity of prospective blinded studies to show the positive predictive value of ICGA. In addition, smoking, epinephrine containing tumescence, and certain patient comorbidities were reported to produce low perfusion scores by ICGA without resulting in tissue necrosis.46,%%62–64
A contributing factor to mixed reviews of ICGA is its lack of standardization. The protocol of ICG use and recording itself is not standardized, nor is the interpretation of the resulting images. Qualitative assessment of the ICG images is subjective, and different users can draw different conclusions. Analysis tool software is now available and can produce objective, quantitative values such as relative and absolute perfusion, contour maps, ingress, egress, and ITT. Nonetheless, the number of different quantitative measurements provided by analysis programs has created a controversy over which parameter is accurate and reliable. One example is the comparison of relative and absolute perfusion. Newman et al.15 reported that the difference in the relative perfusion values between necrosed and viable tissue was statistically significant, whereas the difference in absolute perfusion values was not statistically significant. Similarly, Munabi et al.63 have published their findings using absolute perfusion values. Phillips et al. preferred the use of absolute perfusion, as relative perfusion is relative solely to the individual patient.21,%%65 Moyer and Losken64 completed a retrospective study of SPY data of skin-sparing mastectomy flaps at their institution using SPY-Q software’s average perfusion. Moreover, Wang et al.16 used egress rates to determine the size of implants that were appropriate for patients, and Yang et al.31 published that ingress rates were more significantly affected by implant size and should be the primary parameter used. Though an institution can implement a specific parameter to use in their practice, results may not extrapolate across multiple institutions, and the debate on the most reliable parameter continues. Future directions for ICGA is moving toward handheld devices (see Supplemental Digital Content 1, http://links.lww.com/PRSGO/B323).10,%%38,%%59 Though a handheld device may be advantageous for monitoring outside of the operating room, it would introduce more variability when assessing flow and perfusion, such as the distance and angle to skin at which the probe is held.58
CONCLUSIONS
ICGA can aid in the assessment of perfusion for both implant- and autologous-based breast reconstructions. It is advantageous over clinical assessment alone, leading to decreased complication rates. However, the use of this technology cannot replace clinical judgment but instead serves to augment it. The future of this technology would benefit from improved standardization and continued refinement of the software to provide accurate values to the perfusion seen on the screen.
Supplementary Material
Footnotes
Published online 26 March 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
References
- 1.Khavanin N, Qiu C, Darrach H, et al. Intraoperative perfusion assessment in mastectomy skin flaps: how close are we to preventing complications? J Reconstr Microsurg. 2019;35:471–478. [DOI] [PubMed] [Google Scholar]
- 2.Liu DZ, Mathes DW, Zenn MR, et al. The application of indocyanine green fluorescence angiography in plastic surgery. J Reconstr Microsurg. 2011;27:355–364. [DOI] [PubMed] [Google Scholar]
- 3.Zenn MR. Evaluation of skin viability in nipple sparing mastectomy (NSM). Gland Surg. 2018;7:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhart MB, Huntington CR, Blair LJ, et al. Indocyanine green: historical context, current applications, and future considerations. Surg Innov. 2016;23:166–175. [DOI] [PubMed] [Google Scholar]
- 5.Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aestet Surg J. 2014;34:61–65. [DOI] [PubMed] [Google Scholar]
- 6.Diep GK, Hui JY, Marmor S, et al. Postmastectomy reconstruction outcomes after intraoperative evaluation with indocyanine green angiography versus clinical assessment. Ann Surg Oncol. 2016;23:4080–4085. [DOI] [PubMed] [Google Scholar]
- 7.Harless CA, Jacobson SR. Tailoring through technology: a retrospective review of a single surgeon’s experience with implant-based breast reconstruction before and after implementation of laser-assisted indocyanine green angiography. Breast J. 2016;22:274–281. [DOI] [PubMed] [Google Scholar]
- 8.Pestana IA, Coan B, Erdmann D, et al. Early experience with fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg. 2009;123:1239–1244. [DOI] [PubMed] [Google Scholar]
- 9.Bigdeli AK, Gazyakan E, Schmidt VJ, et al. Indocyanine green fluorescence for free-flap perfusion imaging revisited: advanced decision making by virtual perfusion reality in visionsense fusion imaging angiography. Surg Innov. 2016;23:249–260. [DOI] [PubMed] [Google Scholar]
- 10.Holm C, Tegeler J, Mayr M, et al. Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery. 2002;22:278–287. [DOI] [PubMed] [Google Scholar]
- 11.Diep GK, Marmor S, Kizy S, et al. The use of indocyanine green angiography in postmastectomy reconstruction: do outcomes improve over time? J Plast Reconstr Aesthet Surg. 2019;72:548–554. [DOI] [PubMed] [Google Scholar]
- 12.Mirhaidari SJ, Beddell GM, Orlando MV, et al. A prospective study of immediate breast reconstruction with laser-assisted indocyanine green angiography. Plast Reconstr Surg Glob Open. 2018;6:e1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips BT, Munabi NC, Roeder RA, et al. The role of intraoperative perfusion assessment: what is the current state and how can I use it in my practice? Plast Reconstr Surg. 2016;137:731–741. [DOI] [PubMed] [Google Scholar]
- 14.Mattison GL, Lewis PG, Gupta SC, et al. SPY imaging use in postmastectomy breast reconstruction patients: preventative or overly conservative? Plast Reconstr Surg. 2016;138:15e–21e. [DOI] [PubMed] [Google Scholar]
- 15.Newman MI, Jack MC, Samson MC. SPY-Q analysis toolkit values potentially predict mastectomy flap necrosis. Ann Plast Surg. 2013;70:595–598. [DOI] [PubMed] [Google Scholar]
- 16.Wang CY, Wang CH, Tzeng YS, et al. Intraoperative assessment of the relationship between nipple circulation and incision site in nipple-sparing mastectomy with implant breast reconstruction using the SPY imaging system. Ann Plast Surg. 2018;802S Suppl 1S59–S65. [DOI] [PubMed] [Google Scholar]
- 17.Holm C, Dornseifer U, Sturtz G, et al. The intrinsic transit time of free microvascular flaps: clinical and prognostic implications. Microsurgery. 2010;30:91–96. [DOI] [PubMed] [Google Scholar]
- 18.Liu EH, Zhu SL, Hu J, et al. Intraoperative SPY reduces post-mastectomy skin flap complications: a systematic review and meta-analysis. Plast Reconstr Surg Glob Open. 2019;7:e2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sood M, Glat P. Potential of the SPY intraoperative perfusion assessment system to reduce ischemic complications in immediate postmastectomy breast reconstruction. Ann Surg Innov Res. 2013;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman MI, Samson MC, Tamburrino JF, et al. Intraoperative laser-assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. J Reconstr Microsurg. 2010;26:487–492. [DOI] [PubMed] [Google Scholar]
- 21.Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg. 2012;129:778e–788e [DOI] [PubMed] [Google Scholar]
- 22.Rinker B. A comparison of methods to assess mastectomy flap viability in skin-sparing mastectomy and immediate reconstruction: a prospective cohort study. Plast Reconstr Surg. 2016;137(2)395–401. [DOI] [PubMed] [Google Scholar]
- 23.Gorai K, Inoue K, Saegusa N, et al. Prediction of skin necrosis after mastectomy for breast cancer using indocyanine green angiography imaging. Plast Reconstr Surg Glob Open. 2017;5(4)e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg. 2010;125:1065–1073 [DOI] [PubMed] [Google Scholar]
- 25.Agha RA, Al Omran Y, Wellstead G, et al. Systematic review of therapeutic nipple-sparing versus skin-sparing mastectomy. BJS Open. 2019;3:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vita R, Buccheri EM. Nipple sparing mastectomy and direct to implant breast reconstruction, validation of the safe procedure through the use of laser assisted indocyanine green fluorescent angiography. Gland Surg. 2018;7:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol. 2014;21(1)100–106. [DOI] [PubMed] [Google Scholar]
- 28.Dua M, Bertoni DM, Nguyen D, et al. Using intraoperative laser angiography to safeguard nipple perfusion in nipple-sparing mastectomy. Gland Surg. 2015;4(6)497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertoni DM, Nguyen D, Rochlin D, et al. Protecting nipple perfusion by devascularization and surgical delay in patients at risk for ischemic complications during nipple-sparing mastectomies. Ann Surg Oncol. 2016;23:2665–2672. [DOI] [PubMed] [Google Scholar]
- 30.Murray JD, Jones GE, Elwood ET, et al. Fluorescent intraoperative tissue angiography with indocyanine green: evaluation of nipple-areola vascularity during breast reduction surgery. Plast Reconstr Surg. 2010;126:33e–34e. [DOI] [PubMed] [Google Scholar]
- 31.Yang CE, Chung SW, Lee DW, et al. Evaluation of the relationship between flap tension and tissue perfusion in implant-based breast reconstruction using laser-assisted indocyanine green angiography. Ann Surg Oncol. 2018;25:2235–2240. [DOI] [PubMed] [Google Scholar]
- 32.Venturi ML, Mesbahi AN, Copeland-Halperin LR, et al. SPY elite’s ability to predict nipple necrosis in nipple-sparing mastectomy and immediate tissue expander reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones G, Yoo A, King V, et al. Prepectoral immediate direct-to-implant breast reconstruction with anterior AlloDerm coverage. Plast Reconstr Surg. 2017;1406S Prepectoral Breast Reconstruction31S–38S. [DOI] [PubMed] [Google Scholar]
- 34.Keys KA, Louie O, Said HK, et al. Clinical utility of CT angiography in DIEP breast reconstruction. J Plast Reconstr Aesthet Surg. 2013;66:e61–e65. [DOI] [PubMed] [Google Scholar]
- 35.Mohan AT, Saint-Cyr M. Advances in imaging technologies for planning breast reconstruction. Gland Surg. 2016;5:242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onoda S, Azumi S, Hasegawa K, et al. Preoperative identification of perforator vessels by combining MDCT, doppler flowmetry, and ICG fluorescent angiography. Microsurgery. 2013;33:265–269. [DOI] [PubMed] [Google Scholar]
- 37.Wu C, Kim S, Halvorson EG. Laser-assisted indocyanine green angiography: a critical appraisal. Ann Plast Surg. 2013;70:613–619. [DOI] [PubMed] [Google Scholar]
- 38.Azuma R, Morimoto Y, Masumoto K, et al. Detection of skin perforators by indocyanine green fluorescence nearly infrared angiography. Plast Reconstr Surg. 2008;122:1062–1067. [DOI] [PubMed] [Google Scholar]
- 39.Pestana IA, Zenn MR. Correlation between abdominal perforator vessels identified with preoperative CT angiography and intraoperative fluorescent angiography in the microsurgical breast reconstruction patient. Ann Plast Surg. 2014;72:S144–S149. [DOI] [PubMed] [Google Scholar]
- 40.Douglas HE, Wilkinson MJ, Mackay IR. Effects of perforator number and location on the total pedicle flow and perfusion of zone IV skin and fat of DIEP flaps. J Plast Reconstr Aesthet Surg. 2014;67:212–218. [DOI] [PubMed] [Google Scholar]
- 41.Holm C, Mayr M, Höfter E, et al. Interindividual variability of the SIEA angiosome: effects on operative strategies in breast reconstruction. Plast Reconstr Surg. 2008;122:1612–1620. [DOI] [PubMed] [Google Scholar]
- 42.Brunworth LS, Samson MC, Newman MI, et al. Nipple-areola complex evaluation in long pedicled breast reductions with real-time fluorescent videoangiography. Plastic Reconstr Surg. 2011;128(2)585–586. [DOI] [PubMed] [Google Scholar]
- 43.Ludolph I, Arkudas A, Schmitz M, et al. Cracking the perfusion code?: laser-assisted indocyanine green angiography and combined laser doppler spectrophotometry for intraoperative evaluation of tissue perfusion in autologous breast reconstruction with DIEP or ms-TRAM flaps. J Plast Reconstr Aesthet Surg. 2016;69:1382–1388. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi S, De Lorenzi F, Petit JY, et al. The “perfusion map” of the unipedicled TRAM flap to reduce postoperative partial necrosis. Ann Plast Surg. 2004;53:205–209. [DOI] [PubMed] [Google Scholar]
- 45.Holm C, Mayr M, Höfter E, et al. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg. 2006;117:37–43. [DOI] [PubMed] [Google Scholar]
- 46.Girard N, Delomenie M, Malhaire C, et al. Innovative DIEP flap perfusion evaluation tool: qualitative and quantitative analysis of indocyanine green-based fluorescence angiography with the SPY-Q proprietary software. PLoS One. 2019;14:e0217698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Louges MA, Bellaiche J, Correia N, et al. Relevance of intraoperative indocyanine green injection in breast reconstruction using DIEP procedure for abdominal scars. Ann Chir Plast Esthet. 2016;61:231–234. [DOI] [PubMed] [Google Scholar]
- 48.Casey WJ, III, Connolly KA, Nanda A, et al. Indocyanine green laser angiography improves deep inferior epigastric perforator flap outcomes following abdominal suction lipectomy. Plast Reconstr Surg. 2015;135:491e–497e. [DOI] [PubMed] [Google Scholar]
- 49.Bank J, Pavone LA, Seitz IA, et al. Case report and review of the literature: deep inferior epigastric perforator flap for breast reconstruction after abdominal recontouring. Eplasty. 2012;12:e52. [PMC free article] [PubMed] [Google Scholar]
- 50.Satake T, Muto M, Kou S, et al. Contralateral unaffected breast augmentation using zone IV as a SIEA flap during unilateral DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg. 2019;72:1537–1547. [DOI] [PubMed] [Google Scholar]
- 51.Koonce SL, Barnavon Y, Newman MI, et al. Perfusion zones of extended transverse skin paddles in muscle-sparing latissimus dorsi myocutaneous flaps for breast reconstruction. Plast Reconstr Surg. 2019;143:920e–926e. [DOI] [PubMed] [Google Scholar]
- 52.Hembd A, Teotia SS, Zhu H, et al. Optimizing perforator selection: a multivariable analysis of predictors for fat necrosis and abdominal morbidity in DIEP flap breast reconstruction. Plast Reconstr Surg. 2018;142:583–592. [DOI] [PubMed] [Google Scholar]
- 53.Malagón-López P, Vilà J, Carrasco-López C, et al. Intraoperative indocyanine green angiography for fat necrosis reduction in the deep inferior epigastric perforator (DIEP) flap. Aesthet Surg J. 2019;39:NP45–NP54. [DOI] [PubMed] [Google Scholar]
- 54.Varela R, Casado-Sanchez C, Zarbakhsh S, et al. Outcomes of DIEP flap and fluorescent angiography: a randomized controlled clinical trial. Plast Reconstr Surg. 2020;145:1–10. [DOI] [PubMed] [Google Scholar]
- 55.Schrögendorfer KF, Nickl S, Keck M, et al. Viability of five different pre- and intraoperative imaging methods for autologous breast reconstruction. Eur Surg. 2016;48:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holm C, Mayr M, Höfter E, et al. Assessment of the patency of microvascular anastomoses using microscope-integrated near-infrared angiography: a preliminary study. Microsurgery. 2009;29:509–514. [DOI] [PubMed] [Google Scholar]
- 57.Khansa I, Chao AH, Taghizadeh M, et al. A systematic approach to emergent breast free flap takeback: clinical outcomes, algorithm, and review of the literature. Microsurgery. 2013;33:505–513. [DOI] [PubMed] [Google Scholar]
- 58.Hitier M, Cracowski JL, Hamou C, et al. Indocyanine green fluorescence angiography for free flap monitoring: a pilot study. J Craniomaxillofac Surg. 2016;44:1833–1841. [DOI] [PubMed] [Google Scholar]
- 59.Krishnan KG, Schackert G, Steinmeier R. The role of near-infrared angiography in the assessment of post-operative venous congestion in random pattern, pedicled island and free flaps. Br J Plast Surg. 2005;58:330–338. [DOI] [PubMed] [Google Scholar]
- 60.Hammer-Hansen N, Juhl AA, Damsgaard TE. Laser-assisted indocyanine green angiography in implant-based immediate breast reconstruction: a retrospective study. J Plast Surg Hand Surg. 2018;52:158–162. [DOI] [PubMed] [Google Scholar]
- 61.Alstrup T, Christensen BO, Damsgaard TE. ICG angiography in immediate and delayed autologous breast reconstructions: preoperative evaluation and postoperative outcomes. J Plast Surg Hand Surg. 2018;24:1–5. [DOI] [PubMed] [Google Scholar]
- 62.Xue EY, Schultz JJ, Therattil PJ, et al. Indocyanine green laser angiography in the setting of tumescence. Eplasty. 2019;19:e1. [PMC free article] [PubMed] [Google Scholar]
- 63.Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg. 2014;67:449–455. [DOI] [PubMed] [Google Scholar]
- 64.Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg. 2012;129:1043–1048. [DOI] [PubMed] [Google Scholar]
- 65.Phillips BT, Fourman MS, Rivara A, et al. Comparing quantitative values of two generations of laser-assisted indocyanine green dye angiography systems: can we predict necrosis? Eplasty. 2014;14:e44. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


