Background:
Acellular dermal matrices (ADMs) were first incorporated into direct-to-implant (DTI) breast reconstruction by the senior author in 2001 and have since become foundational to implant-based reconstruction. ADM composition has evolved recently and now includes perforated types, which some speculate decrease the likelihood of seroma. The authors performed a retrospective review of perforated (P-ADM) and nonperforated (NP-ADM) ADM-assisted direct-to-implant breast reconstruction patients to evaluate differences in complication rates.
Methods:
Retrospective review of direct-to-implant breast reconstruction patients operated on by a single surgeon (CAS) from 2011 to 2018 was conducted. Patient and operative characteristics, including ADM type, were recorded. A propensity score matching algorithm accounting for potentially confounding variables was developed, followed by univariate analysis to evaluate the association between ADM perforation and postoperative complications.
Results:
The review began with 409 patients (761 breasts). Following exclusion of patients with missing demographic information, lack of ADM in their reconstruction, and follow-up times of less than 4 weeks, 364 patients (680 breasts) were included for analysis. A total of 530 (77.94%) and 150 (22.06%) breasts received NP-ADM and P-ADM, respectively. After propensity score matching, there were 294 breasts, composed of equal numbers of P-ADM and NP-ADM recipients. Univariate analysis showed no association between ADM type and any postoperative complication.
Conclusions:
The complication profile of direct-to-implant breast reconstruction appears to be unaffected by the use of P-ADM or NP-ADM. Current understanding of the association between ADM type and clinical outcomes would benefit from multi-institution, prospective, randomized trials.
INTRODUCTION
The rate of breast reconstruction has grown by almost 30% in the last two decades with more than 101,000 women undergoing breast reconstruction in 2018.1 Implant-based reconstruction is the most common type of reconstructive operation performed, and immediate reconstruction is preferred to delayed reconstruction for appropriate candidates due to economic and psychosocial benefits, coupled with the improved cosmetic outcomes facilitated by skin-sparing mastectomies.2–8 Direct-to-implant (DTI) breast reconstruction has been furthered by the use of acellular dermal matrices (ADMs), which are human-, bovine-, or porcine-derived biotechnologically engineered tissues that were first used in the management of full-thickness burns in 1995,9 and subsequently were found to have a variety of clinical applications in plastic surgery including abdominal hernia repair10 and facial soft tissue augmentation.11,12 ADMs were first used by the senior author in breast reconstruction in 2001,13 and have since become foundational to implant-based breast reconstruction with studies showing optimal cosmetic outcomes and low rates of complication using both subpectoral and prepectoral operative techniques.14,15
The composition of ADMs employed in breast reconstruction has evolved in recent years with ADMs currently used in a variety of forms including sterile prehydrated and aseptic freeze-dried compositions,16,17 derived from human and animal sources,18–20 and perforated versus nonperforated forms. While fenestrations or perforations in ADMs have been thought to decrease seroma formation and improve outcomes in breast reconstruction due to their improved recellularization and revascularization as shown in vivo,21 few clinical investigations have been performed, with prior studies limited to analysis of variable surgeon-created perforations18,22–25 as opposed to the standardized manufactured perforations that are currently widely used. Furthermore, outcomes have only been evaluated for two-stage reconstructive techniques as opposed to direct-to-implant prosthetic reconstructions.22–25 Thus, the authors performed a retrospective review of patients undergoing DTI breast reconstruction with either nonperforated (NP-ADM) or perforated (P-ADM) ADM and used propensity score analysis to evaluate differences in rates of postoperative complications. We hypothesized that DTI patients with P-ADM would have decreased risk of seroma formation.
METHODS
Data Collection
This retrospective, institutional review board-approved study included all patients who underwent DTI breast reconstruction by a single surgeon (CAS) from January 2011 to December 2018. During this timeframe, there was no change in operative procedure or postoperative protocol, including drain protocol: two 15 French Blake suction drains per breast inferiorly, both subcutaneously and subpectorally through the ADM, removed when output is less than 20–30 mL over 24 hours. Antibiotic protocol included administration of cephalosporin antibiotics intraoperatively and postoperatively until drain removal. Patients who had missing demographic/comorbid information, whose reconstructions were not ADM-assisted, or who failed to follow-up for more than 4 weeks were excluded. Retrospective chart review was conducted to collect the following information: patient age, body mass index, comorbidities, mastectomy type (prophylactic versus oncologic), breast radiation history (preoperative, intraoperative, postoperative, or none), mastectomy weight, implant size, ADM type (P-ADM versus NP-ADM), implant placement (subpectoral versus prepectoral), and postoperative complications (skin flap necrosis, infection, hematoma, seroma, implant loss). Skin flap necrosis was defined as necrosis requiring operative debridement. Infections were defined as those required intravenous antibiotics or operative debridement. Hematomas were defined as those requiring operative intervention, and seromas were defined as those requiring ultrasound-guided aspiration in the office. Implant loss was defined as implant removal secondary to postoperative complication. Statistical analyses were conducted using SAS 9.4 (Cary, N.C.), and significance was achieved with P < 0.05.
Univariate Analyses
The primary outcome in this study was the incidence of postoperative complication. First, descriptive statistics were performed to characterize the patient cohort (Table 1). Chi-square tests were used to determine the association between ADM type and patient characteristics (Table 1). Chi-square and Fisher’s exact tests were then conducted to elucidate the association between ADM type and incidence of postoperative complication (Table 2).
Table 1.
Associations between Clinical Covariates and ADM Type
| Characteristics | Entire DTI Cohort | Propensity Score-matched Groups | ||||
|---|---|---|---|---|---|---|
| P-ADM N = 150 n (%) |
NP-ADM N = 530 n (%) |
P | P-ADM N = 147 n (%) |
NP-ADM N = 147 n (%) |
P | |
| Age, y | 0.0620 | 0.8855 | ||||
| 20–29 | 14 (9.33) | 32 (6.04) | 14 (9.52) | 20 (13.61) | ||
| 30–39 | 40 (26.67) | 104 (19.62) | 37 (25.17) | 32 (21.77) | ||
| 40–49 | 57 (38.00) | 222 (41.89) | 57 (38.78) | 54 (36.73) | ||
| 50–59 | 27 (18.00) | 122 (23.02) | 27 (18.37) | 30 (20.41) | ||
| 60–69 | 8 (5.33) | 45 (8.49) | 8 (5.44) | 7 (4.76) | ||
| 70–79 | 4 (2.67) | 5 (0.94) | 4 (2.72) | 4 (2.72) | ||
| Diabetes | 0 (0) | 18 (3.40) | 0.0222 | 0 (0) | 0 (0) | 1.0000 |
| Current smoker | 0 (0) | 26 (4.91) | 0.0057 | 0 (0) | 0 (0) | 1.000 |
| Hypertension | 4 (2.67) | 43 (8.11) | 0.0202 | 4 (2.72) | 3 (2.04) | 0.7021 |
| Obesity | 11 (7.33) | 57 (10.75) | 0.2175 | 11 (7.48) | 7 (4.76) | 0.3305 |
| Chemo recipient | 10 (6.67) | 91 (17.17) | 0.0014 | 10 (6.80) | 8 (5.44) | 0.6266 |
| Radiation | 0.1488 | 0.7286 | ||||
| Preop | 9 (6.00) | 37 (6.98) | 9 (6.12) | 6 (4.08) | ||
| Postop | 4 (2.67) | 4 (0.75) | 4 (2.72) | 4 (2.72) | ||
| Prepectoral mastectomy | 10 (6.67) | 4 (0.75) | <0.0001 | 7 (4.76) | 4 (2.72) | 0.3566 |
| Oncologic mastectomy | 36 (24.00) | 144 (27.32) | 0.4161 | 36 (24.49) | 35 (23.81) | 0.8916 |
Univariate analysis (chi-square) of the association between clinical covariates by breast and ADM type among the entire patient cohort and among the propensity score-matched groups.
Boldface indicates significance.
Table 2.
Associations between Postoperative Outcomes and ADM Type
| Postoperative Complication | Entire DTI Cohort | Propensity Score-matched Groups | ||||
|---|---|---|---|---|---|---|
| P-ADM N = 150 n (%) |
NP-ADM N = 530 n (%) |
P | P-ADM N = 147 n (%) |
NP-ADM N = 147 n (%) |
P | |
| Any complication | 7 (4.67) | 24 (4.53) | 0.9428* | 7 (4.76) | 4 (2.72) | 0.5410 |
| Necrosis | 3 (2.00) | 7 (1.32) | 0.4661 | 3 (2.04) | 1 (0.68) | 0.6224 |
| Infection | 2 (1.33) | 7 (1.32) | 1.0000 | 2 (1.36) | 1 (0.68) | 1.0000 |
| Hematoma | 0 (0) | 2 (0.38) | 1.0000 | 0 (0) | 1 (0.68) | 1.0000 |
| Seroma | 0 (0) | 8 (1.51) | 0.2105 | 0 (0) | 1 (0.68) | 1.0000 |
| Implant loss | 2 (1.33) | 7 (1.32) | 1.0000 | 2 (1.36) | 0 (0) | 0.4983 |
Univariate analyses of the association between postoperative complications by breast and ADM type among the entire patient cohort and among the propensity score-matched groups. All calculations done with Fisher’s exact test unless P-value is marked with a * to indicate use of chi-square test.
Propensity Score Matching
A propensity score matching algorithm was used to eliminate unwanted bias associated with clinical covariates in the assessment of the association between ADM type and the primary outcome. A 1:1 greedy-matching algorithm26 using the following clinically relevant variables was written using the PSMATCH procedure: age, diabetes, current tobacco use, hypertension, obesity, chemotherapy and radiation history, mastectomy type, and prepectoral implant placement. This algorithm generated pairs of matched breasts and scored breasts with P-ADM as the treated cohort. The pwr package27 in R was utilized to assess the ability of this statistical approach to detect effect sizes with power = 0.8. Chi-square tests were run to ensure that the P-ADM and NP-ADM groups were appropriately balanced (Table 1). Following confirmation of group balancing, Fisher’s exact tests were used to elucidate the association between ADM type and postoperative complications (Table 2).
RESULTS
Patient Characteristics
There were 409 DTI patients (761 breasts) included for review. Four patients (8 breasts) were excluded due to missing demographic or comorbid information, 8 patients (12 breasts) were excluded because their DTI reconstructions were not ADM-assisted, and 33 patients (61 breasts) were excluded because they did not follow up for at least 4 weeks. Thus, 364 patients (680 breasts) were included for analysis. Of these, 530 (77.94%) breasts were reconstructed using NP-ADM and 150 (22.06%) breasts were reconstructed using P-ADM (Table 1). The senior author’s preferred ADM thickness and size are 2.0–2.4 mm, 8 × 16 and contour medium size, respectively. All ADMs were human-derived: P-ADM patients received perforated Alloderm (Allergan, Dublin, Ireland), and NP-ADM patients received either Alloderm (474 breasts) or DermACELL (56 breasts; Stryker, Kalamazoo, MI). The mean follow-up time was 141.72 ± 42.35 weeks (range: 4.71–442.57) and 64.27 ± 42.35 weeks (range: 6.00–232.14) for the NP-ADM and P-ADM cohorts, respectively.
On univariate analysis, there was no association between ADM type and incidence of any postoperative complication (4.67% P-ADM versus 4.72% NP-ADM, P = 0.9795) or incidence of individual postoperative complications: necrosis (2.00% P-ADM versus 1.32% NP-ADM, P = 0.4661), infection (1.33% P-ADM versus 1.32% NP-ADM, P = 1.0000), hematoma (0% P-ADM versus 0.38% NP-ADM, P = 1.0000), seroma (0% P-ADM versus 1.51% NP-ADM, P = 0.2105), or implant loss (1.33% P-ADM versus 1.32% NP-ADM, P = 1.0000) (Table 2).
Propensity Score Matching
After propensity score matching, there were 294 breasts, half with P-ADM-assisted DTI reconstruction and half with NP-ADM-assisted DTI reconstruction, which enabled detection with power = 0.8 of difference in any complication with medium and large effect sizes as defined by Cohen’s convention.28 On univariate analysis, ADM type was not significantly associated with incidence of any postoperative complication (4.76% P-ADM versus 2.72% NP-ADM, P = 0.5410): necrosis (2.04 % P-ADM versus 0.68 % NP-ADM, P = 0.6224), infection (1.36% P-ADM versus 0.68% NP-ADM, P = 1.0000), hematoma (0% P-ADM versus 0.68% NP-ADM, P = 1.0000), seroma (0% P-ADM versus 0.68% NP-ADM, P = 1.0000), or implant loss (1.36% P-ADM versus 0% NP-ADM, P = 0.4983) (Table 2).
DISCUSSION
In 2001, the senior author pioneered the use of ADMs to assist DTI breast reconstruction.13 ADMs have since become foundational to implant-based breast reconstruction using both subpectoral and prepectoral techniques,14,15 with estimates showing the majority of prosthetic breast reconstructions are carried out with ADM-assistance.29 The rising popularity of ADM use has been met with an increase in the amount of products available. Presently, the plastic surgeon can choose from a myriad of ADM products with diverse shapes, sizes, sourcing, and processing characteristics.30
The present study analyzes the association between ADM perforation and development of complications in a large cohort of patients with DTI reconstruction operated on by a single surgeon. It has been found that compared to two-stage expander reconstruction, DTI breast reconstruction is associated with improved psychosocial morbidity, optimized cosmetic outcomes, and decreased medical system costs.2–8 ADM assistance in DTI reconstruction has proven to be helpful, as it enables the full utilization of mastectomy skin flaps while simultaneously providing coverage of the inferolateral pole, which allows for greater control of the inframammary fold and improved lower pole projection.13,31–35 Therefore, understanding the effect of ADM characteristics on postoperative complications in DTI breast reconstruction is crucial.
While the benefits of ADM-assisted breast reconstruction are well established, there have been concerns over possible increase in seroma rates and time for drain removal associated with ADM use.36–38 As a result, surgeons began manually creating perforations in NP-ADM in an effort to decrease seroma formation and improve biointegration,22–24 and manufacturers eventually followed suit and began producing P-ADMs. This rationale has been validated by Cottler et al.21 in their demonstration of improved host cell integration and angiogenesis in P-ADMs in vivo. Cottler et al. suggest that the increased border zone surface area allotted by perforations eases the inflammatory process that precedes angiogenesis and collagen matrix remodeling, and that the perforations themselves facilitate the free movement of fluid. By utilizing photoacoustic microscopy to monitor changes in NP-ADM and P-ADM over time, Cottler et al. demonstrated that by day 10, the P-ADM had developed visible vascular ingrowth at both the periphery and the perforations’ border zones, while vasculature was only visible at the periphery of the NP-ADM. By day 21, the P-ADM showed evidence of more advanced integration into the skin flap (such as pinkish hue and wound contracture) as compared to the NP-ADM and perforations were no longer visible. Figure 1 depicts successful biointegration of a sheet of P-ADM in a breast reconstruction patient of the senior author.
Fig. 1.
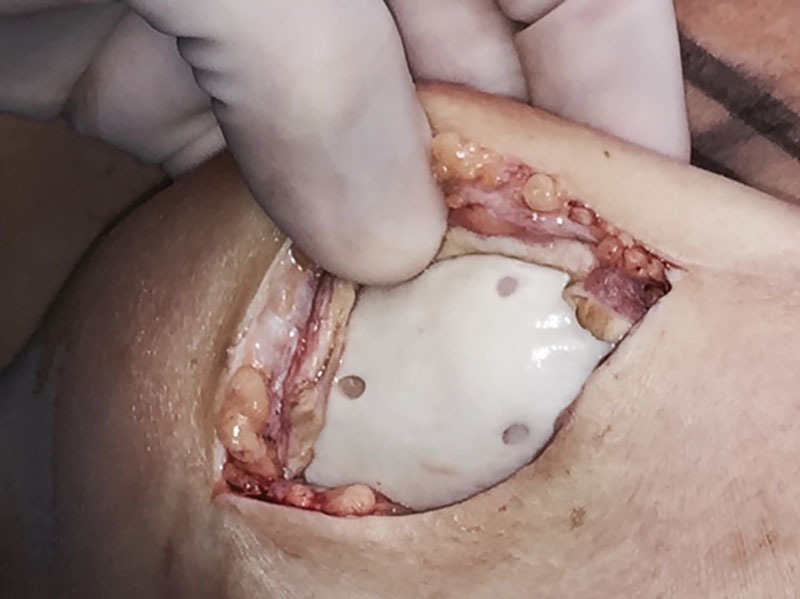
Photograph of a healed sheet of P-ADM 5 years after its placement.
There exists a limited body of literature describing the association between P-ADM and postoperative complications in breast reconstruction. ADM perforation has been shown to be associated with decreased pain, length of stay, incidence of seroma, and length of time needed for drain removal in two-stage breast reconstruction.18,21–25 Many of these researchers have echoed the suggestion put forward by Cottler et al.21 and speculate that their observations are the result of the free movement of fluid allotted by perforations, which facilitate lymphatic uptake.18,22–25 These clinical studies, however, are limited by factors associated with multiple surgeons22–25 and by the variability of surgeon-created perforations as opposed to the available standardized manufactured perforations.18,22–25 Adding further complexity to the issue, Wilson and Varnadore39 retrospectively compared 29 patients with NP-ADM-assisted two-stage breast reconstruction to 24 patients with P-ADM-assisted two-stage breast reconstruction and found no significant association between ADM perforation and seroma development. Instead, they determined that P-ADMs were associated with reduced likelihood of capsular thickening requiring capsulectomy. However, Mowlds et al.40 found that the ability of P-ADMs to suppress rates of capsular contraction is similar to what is observed among NP-ADMs.
In contrast to prior studies, the present study found no significant difference in the use of P-ADM versus NP-ADMs on postoperative rates of necrosis, infection, hematoma, seroma, or implant loss. Capsular contracture was not included in the present analysis due to the mean follow-up time of under 3 years and under 2 years for the NP-ADM and P-ADM cohorts, respectively. Departing from traditional statistical methodologies, the present study employed propensity score matching, a method of statistical analysis designed to decrease the bias conferred by uncontrolled patient characteristics that often limit retrospective studies. Indeed, prior studies analyzed patient cohorts that included discordant numbers of patients receiving P-ADM versus NP-ADMs.22–25 Additionally, others have compared fetal bovine and human cadaveric ADMs with inconsistent use of P-ADM and NP-ADM types in addition to changes in drain protocol, thus restricting the ability of the results to inform understanding on the effect of ADM characteristics on patient outcomes.18
Furthermore, in contrast to prior studies evaluating P-ADMs in prosthetic breast reconstructions, the present study is the first analysis of standardized manufactured ADM perforations on postoperative complications in DTI breast reconstruction. ADM-assisted DTI breast reconstruction in properly selected patients has been shown to have a similar complication profile to non-ADM-assisted two-stage expander-based breast reconstruction.41,42 Although revisional surgeries are sometimes needed following DTI reconstruction, it has been suggested that the one-stage nature of DTI reconstruction may benefit appropriate patients by eliminating the morbidities associated with undergoing multiple operations.41
The majority of prior studies failed to report what percent of their cohort underwent prophylactic or oncologic mastectomy.22–25 Butterfield18 reported a cohort of 89 breasts reconstructed with ADM, 38 (43%) of which were reconstructed prophylactically, as compared to the cohort of 680 breasts in the present study, 500 (73.53%) of which were reconstructed prophylactically. It follows that the present study further distinguishes itself from the existing literature in its relatively high number of prophylactic prosthetic reconstructions, which have been shown to have improved outcomes as compared to oncologic prosthetic reconstructions with or without radiation and/or chemotherapy.43 Additionally, the authors underscore the importance of the placement of a second subpectoral drain on postoperative outcomes. While the placement of the second drain in the subpectoral plane did not influence the comparison of P-ADM and NP-ADM outlined in the present study due to the fact that drain protocol remained consistent throughout the study period, the presence of drains both superficial and deep to the ADM plane may have influenced the overall number of seromas. Therefore, although the placement of only a suprapectoral drain is commonplace in many surgical practices, the authors suggest that the placement of a second subpectoral drain is a crucial step in the reduction of seroma rate among breast reconstruction patients.
The expense of ADMs is often cited as a concern regarding its utility in breast reconstruction. Prior cost analyses have shown that ADM-assisted DTI breast reconstruction is the least expensive procedure when compared to ADM-assisted and non-ADM-assisted two-stage breast reconstruction.8 However, the additional manufacturing step required to create P-ADMs results in additional cost; for example, our practice is charged approximately 3% more per unit of P-ADM as compared to NP-ADM. At the same time, it may be that the expansion of a single ADM sheet allotted by perforations may translate into a potential reduction in the amount of material needed per procedure, thereby reducing the overall cost associated with ADM assistance in implant-based breast reconstruction.44 As the present study did not find a difference between the complication profiles of P-ADM and NP-ADM, additional investigation is required to weigh increased cost and potential clinical benefits.
A limitation of the present study is the difference in follow-up time among the P-ADM and NP-ADM groups, which is the result of the retrospective nature of the study alongside the recent advent of P-ADM. The authors also acknowledge that the present patient cohort is inclined toward uncomplicated postoperative healing, given the relatively high number of prophylactic prosthetic reconstructions (and therefore low rates of chemotherapy and radiation recipients) and low number of smokers. Additionally, our statistical methodology could detect medium and large effect sizes as defined by Cohen’s convention with a power = 0.8.28 Analysis via the pwr package27 in R revealed that the detection of a small effect size would require 785 breasts in each propensity score-matched group. Thus, further high-quality research is needed, and this area of research would benefit from prospective randomized control studies.
Among the strengths of this study is the large patient database. Additionally, our use of propensity score matching enables the generation of a kind of pseudorandomized controlled trial in a situation in which true randomization is not possible. Finally, as the entire patient cohort was operated on by a single surgeon, it follows that the results herein are not limited by variables associated with different surgeons.
CONCLUSIONS
Through the use of propensity score matching analysis, the present study found that DTI breast reconstruction with P-ADM has a complication profile comparable to that of DTI breast reconstruction with NP-ADM, including no change in the rate of seroma development. Further investigation is needed to evaluate complications after using commercially manufactured P-ADM in tissue expander-based reconstruction, as well as potential differences in cosmetic outcomes.
Footnotes
Published online 18 March 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.American Society of Plastic Surgeons. 2018 National Plastic Surgery Statistics. 2019:2018–2020. https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-report-2018.pdf. Accessed July 10, 2019.
- 2.Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet. 1983;1:459–462. [DOI] [PubMed] [Google Scholar]
- 3.Khoo A, Kroll SS, Reece GP, et al. A comparison of resource costs of immediate and delayed breast reconstruction. Plast Reconstr Surg. 1998;101:964–968; discussion 969. [DOI] [PubMed] [Google Scholar]
- 4.Losken A, Carlson GW, Bostwick J, 3rd, et al. Trends in unilateral breast reconstruction and management of the contralateral breast: the emory experience. Plast Reconstr Surg. 2002;110:89–97. [DOI] [PubMed] [Google Scholar]
- 5.Singletary SE. Skin-sparing mastectomy with immediate breast reconstruction: the M. D. Anderson Cancer Center experience. Ann Surg Oncol. 1996;3:411–416. [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, McGrath MH, Druss RG, et al. The psychological impact of immediate breast reconstruction for women with early breast cancer. Plast Reconstr Surg. 1984;73:619–628. [DOI] [PubMed] [Google Scholar]
- 7.Spencer KW. Significance of the breast to the individual and society. Plast Surg Nurs. 1996;16:131–132. [PubMed] [Google Scholar]
- 8.de Blacam C, Momoh AO, Colakoglu S, et al. Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg. 2012;69:516–520. [DOI] [PubMed] [Google Scholar]
- 9.Wainwright DJ. Use of an acellular allograft dermal matrix (Alloderm) in the management of full-thickness burns. Burns. 1995;21:243–248. [DOI] [PubMed] [Google Scholar]
- 10.Buinewicz B, Rosen B. Acellular cadaveric dermis (Alloderm): a new alternative for abdominal hernia repair. Ann Plast Surg. 2004;52:188–194. [DOI] [PubMed] [Google Scholar]
- 11.Achauer BM, VanderKam VM, Celikoz B, et al. Augmentation of facial soft-tissue defects with Alloderm dermal graft. Ann Plast Surg. 1998;41:503–507. [DOI] [PubMed] [Google Scholar]
- 12.Terino EO. Alloderm acellular dermal graft: applications in aesthetic soft-tissue augmentation. Clin Plast Surg. 2001;28:83–99. [PubMed] [Google Scholar]
- 13.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (Alloderm). Ann Plast Surg. 2006;57:1–5. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim AM, Koolen PG, Ganor O, et al. Does acellular dermal matrix really improve aesthetic outcome in tissue expander/implant-based breast reconstruction? Aesthetic Plast Surg. 2015;39:359–368. [DOI] [PubMed] [Google Scholar]
- 15.Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;140:432–443. [DOI] [PubMed] [Google Scholar]
- 16.Parikh R, Brown G, Sharma K, et al. Immediate implant-based breast reconstruction with acellular dermal matrix: a comparison of sterile and aseptic Alloderm in 2039 consecutive cases. Plast Reconstr Surg. 2018;142:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” Alloderm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. [DOI] [PubMed] [Google Scholar]
- 18.Butterfield JL. 440 consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between surgimend fetal bovine and Alloderm human cadaveric acellular dermal matrices. Plast Reconstr Surg. 2013;131:940–951. [DOI] [PubMed] [Google Scholar]
- 19.Scheflan M, Grinberg-Rashi H, Hod K. Bovine acellular dermal matrix in immediate breast reconstruction: a retrospective, observational study with surgimend. Plast Reconstr Surg. 2018;141:1e–10e. [DOI] [PubMed] [Google Scholar]
- 20.Glasberg SB, Light D. Alloderm and strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg. 2012;129:1223–1233. [DOI] [PubMed] [Google Scholar]
- 21.Cottler PS, Olenczak JB, Ning B, et al. Fenestration improves acellular dermal matrix biointegration: an investigation of revascularization with photoacoustic microscopy. Plast Reconstr Surg. 2019;143:971–981. [DOI] [PubMed] [Google Scholar]
- 22.Martin JB, Moore R, Paydar KZ, et al. Use of fenestrations in acellular dermal allograft in two-stage tissue expander/implant breast reconstruction. Plast Reconstr Surg. 2014;134:901–904. [DOI] [PubMed] [Google Scholar]
- 23.Palaia DA, Arthur KS, Cahan AC, et al. Incidence of seromas and infections using P versus nonP acellular dermal matrix in breast reconstructions. Plast Reconstr Surg - Glob Open. 2015;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan BKH. Pie crusting of acellular dermal matrix may help decrease incidence of seromas in breast reconstruction. J Plast Reconstr Aesthetic Surg. 2013;66:1629–1630. [DOI] [PubMed] [Google Scholar]
- 25.Hagarty SE, Yen L, Fosco C, et al. Positive impact of meshing Autogenous Dermal Matrix (ADM) on pain, length of stay and length of time required for post-operative drains in tissue expander based breast reconstruction. Plast Reconstr Surg. 2015;136:113–114. [Google Scholar]
- 26.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Champley S. pwr: Basic Functions for Power Analysis. 2018. R package version 1.2-2; https://cran.r-project.org/package=pwr. [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 1988New York: Routledge. [Google Scholar]
- 29.Ibrahim AMS, Koolen PGL, Ashraf AA, et al. Acellular dermal matrix in reconstructive breast surgery: survey of current practice among plastic surgeons. Plast Reconstr Surg - Glob Open. 2015;3:e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JYS, Mlodinow AS. What’s new in acellular dermal matrix and soft-tissue support for prosthetic breast reconstruction. Plast Reconstr Surg. 2017;140:30S–43S. [DOI] [PubMed] [Google Scholar]
- 31.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120:373–381. [DOI] [PubMed] [Google Scholar]
- 32.Breuing KH, Colwell AS. Inferolateral Alloderm hammock for implant coverage in breast reconstruction. Ann Plast Surg. 2007;59:250–255. [DOI] [PubMed] [Google Scholar]
- 33.Breuing KH, Colwell AS. Immediate breast tissue expander-implant reconstruction with inferolateral Alloderm hammock and postoperative radiation: a preliminary report. Eplasty. 2009;15:e.16. [PMC free article] [PubMed] [Google Scholar]
- 34.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. [DOI] [PubMed] [Google Scholar]
- 35.Salzberg CA. Focus on technique: one-stage implant-based breast reconstruction. Plast Reconstr Surg. 2013;130:95S–103S. [DOI] [PubMed] [Google Scholar]
- 36.Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Ann Surg Oncol. 2016;23:600–610. [DOI] [PubMed] [Google Scholar]
- 37.Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg. 2011;128:1162–1169. [DOI] [PubMed] [Google Scholar]
- 38.Jordan SW, Khavanin N, Kim JY. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg. 2016;137:1104–1116. [DOI] [PubMed] [Google Scholar]
- 39.Wilson H, Varnadore A. Evaluation of modifications to tissue-expander breast reconstruction, a quality improvement assessment within a private practice. Ann Plast Surg. 2018;806S Suppl 6S381–S387. [DOI] [PubMed] [Google Scholar]
- 40.Mowlds DS, Salibian AA, Scholz T, et al. Capsular contracture in implant-based breast reconstruction: examining the role of acellular dermal matrix fenestrations. Plast Reconstr Surg. 2015;136:629–635. [DOI] [PubMed] [Google Scholar]
- 41.Hunsicker LM, Ashikari AY, Berry C, et al. Short-term complications associated with acellular dermal matrix-assisted direct-to-implant breast reconstruction. Ann Plast Surg. 2017;78:35–40. [DOI] [PubMed] [Google Scholar]
- 42.Clarke-Pearson EM, Lin AM, Hertl C, et al. Revisions in implant-based breast reconstruction: how does direct-to-implant measure up? Plast Reconstr Surg. 2016;137:1690–1699. [DOI] [PubMed] [Google Scholar]
- 43.Spear SL, Schwarz KA, Venturi ML, et al. Prophylactic mastectomy and reconstruction: clinical outcomes and patient satisfaction. Plast Reconstr Surg. 2008;122:1–9. [DOI] [PubMed] [Google Scholar]
- 44.Paydar KZ, Wirth GA, Mowlds DS. Prepectoral breast reconstruction with P acellular dermal matrix: a novel design. Plast Reconstr Surg - Glob Open. 2018;6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


