Abstract
Tumor immune cell compositions play a major role in response to immunotherapy but the heterogeneity and dynamics of immune infiltrates in human cancer lesions remain poorly characterized. Here we identify conserved intratumoral CD4 and CD8 T cell behaviors in scRNA-seq data from 25 melanoma patients. We discover a large population of CD8 T cells showing continuous progression from an early effector “transitional” into a dysfunctional T cell state. CD8 T cells that express a complete cytotoxic gene set are rare, and TCR sharing data suggest their independence from the transitional and dysfunctional cell states. Notably, we demonstrate that dysfunctional T cells are the major intratumoral proliferating immune cell compartment and that the intensity of the dysfunctional signature is associated with tumor-reactivity. Our data demonstrate that CD8 T cells previously defined as exhausted, are in fact a highly proliferating, clonal and dynamically differentiating cell population within the human tumor microenvironment.
Introduction
T cell checkpoint blockade therapies that aim to reactivate tumor-specific T cell responses have revolutionized cancer treatment, resulting in durable responses in patients with advanced disease (Ribas and Wolchok, 2018; Sharma and Allison, 2015). Nevertheless, many patients do not achieve long-term clinical benefit, and our understanding of the mechanisms underlying response or resistance to these therapies are still incomplete (Reading et al., 2018; Sharma et al., 2017). Recent single cell RNA sequencing-based studies of tumor infiltrating immune cell populations in melanoma and other tumor types provide evidence for a highly heterogeneous make-up of immune cell infiltrates, and this heterogeneity is likely to form a determining factor in therapy outcome (Azizi et al., 2018; Guo et al., 2018; Lavin et al., 2017; Sade-Feldman et al., 2018; Savas et al., 2018; Tirosh et al., 2016; Zhang et al., 2018; Zheng et al., 2017). Continuous increases in sample size and quality, diversity of patients sampled, and analysis methodology are required to uncover the mechanisms that underlie successful immunotherapy response.
Within the heterogeneous tumor microenvironment, T cells make up a considerable part of the immune infiltrate. The intratumoral T cell compartment comprises effector, memory, and regulatory T cells. In addition, a subset of CD8 T cells that has acquired a state of ‘dysfunction’ or ‘exhaustion’ is frequently observed. Such dysfunctional T cells are characterized by a loss of classical CD8 T cell effector functions, such as cytotoxicity (Hashimoto et al., 2018; Pauken and Wherry, 2015; Wherry and Kurachi, 2015). In addition, the dysfunctional T cells in human tumors display a unique T cell cytokine secretion signature (Guo et al., 2018; Sade-Feldman et al., 2018; Savas et al., 2018; Thommen and Schumacher, 2018; Thommen et al., 2018). Whereas T cell exhaustion was previously associated with a loss of proliferative capacity, recent studies are providing evidence for a proliferative potential of T cells with high levels of PD-1 expression in human tumors (Guo et al., 2018; Savas et al., 2018; Thommen et al., 2018; Zhang et al., 2018). In addition to high levels of PD-1 expression, the dysfunctional T cell compartment is characterized by increased expression of inhibitory checkpoint molecules such as TIM-3 and LAG3 (Thommen and Schumacher, 2018; Wherry and Kurachi, 2015). Furthermore, characterization of dysfunctional T cell populations in murine tumor and chronic viral infection models has demonstrated that dysfunctionality of T cells in these models is associated with the expression of transcriptional regulators such as Prdm1, Maf1, and Eomes (Chihara et al., 2018; Paley et al., 2012; Shin et al., 2009). To what extent these and other factors drive T cell dysfunction in human melanoma, and how their expression is induced, remain open and important questions.
The role and predictive potential of T cells with different levels of expression of exhaustion markers is presently a matter of debate. In murine models, T cells with high expression of markers of T cell exhaustion appear refractory to reinvigoration by PD-1 blockade (Blackburn et al., 2008; Im et al., 2016; Pauken et al., 2016; Philip et al., 2017; Schietinger et al., 2016). Nevertheless, the frequency of dysfunctional T cells expressing high levels of PD-1 has been shown to correlate with clinical response to anti-PD-1 therapy in NSCLC patients (Thommen et al., 2018). As a second issue, a significant complication in the analysis of T cell states in human tumors is that T cell infiltrates at tumor sites express a variable degree of tumor-reactivity (Scheper et al., in press; Simoni et al., 2018). For this reason, detailed characterization of dysfunctional T cells in a setting in which TCR clonality and the level of tumor-reactivity is known would be of value.
Here we performed extensive transcriptional profiling of intratumoral immune infiltrates of 25 melanoma patients by massively parallel single cell RNA sequencing of 46,612 immune cells which passed quality control filtering, yielding profiles of 29,825 T and NK cells. While immune cell subtypes were largely shared across most patients, their relative abundance differed considerably between patients, even when disease stage and treatment background were matched. Notably, in spite of this variability in abundance, conserved trajectories of CD8 cells were observed, consistent with an ongoing differentiation process driven by interactions within the tumor microenvironment.
In particular, CD8 positive T cells were observed in two separate subsets, with only one of these transitioning into a dysfunctional T cell pool that is characterized by both known (PD-1, LAG3) and novel (e.g., ETV1, AKAP5) regulatory molecules, and that includes molecules shared with CD4 Treg (CSF-1, ZBED2). Coupled single cell T cell receptor (TCR) sequencing and transcriptional profiling revealed that dysfunctional T cells display the highest level of clonal expansion. Furthermore, analysis of a cell cycle transcriptional signature, as well as flow cytometric analysis of Ki-67 expression and cell cycle progression, provided evidence for ongoing proliferation within this dysfunctional T cell compartment. This proliferative capacity was mainly associated with initial buildup of the dysfunctional program, whereas more advanced dysfunctional cells lost this proliferative signature. In contrast, a discrete pool of cytotoxic CD8 cells showed little evidence of proliferation and was unlinked to the dysfunctional T cell pool, as based on both TCR sharing data and gene module analysis. Collectively, our data suggest that the dysfunctional CD8 T cell pool should be considered a dynamically differentiating and active cell compartment that is likely to drive tumor-reactivity across patients. In-depth models of regulation of this T cell compartment should lead to the identification of novel immune modulatory pathways and optimization of T cell-based cancer therapies.
Results
Transcriptional states of immune cells in human melanoma
In order to better understand the heterogeneity of immune cells within and across melanoma patients, we designed a protocol for single cell transcriptomic and protein index characterization of immune (CD45+) cells, and in particular T (CD3+) cells, in melanoma tumors (Figure 1A and S1A). Design of the study was focused on maintaining the in situ RNA composition of tumor infiltrating immune cells, by immediate dissociation of tumor material for MARS-seq analysis (Jaitin et al., 2014). We collected data on a total of 29,825 QC positive tumor infiltrating T and NK cells from 25 melanoma patients, including patients with stage 2, stage 3, and stage 4 melanoma with a diverse treatment history, and 9 treatment-naïve, stage 3, subcutaneous melanomas (Table S1). We developed a variant of MARS-seq that provides information on both the TCR and the transcriptome of individual T cells (Methods). We used the MetaCell algorithm (Baran et al., 2018) to identify homogeneous and robust groups of cells (“metacells”; Methods) from scRNA-seq data, resulting in a detailed map of 324 metacells organized into seven broad lineages, including T cells (characterized by expression of CD3), NK cells (KLRD1), dendritic cells (CD1C), macrophages (C1Q), monocytes (VCAN), B cells (CD19), and plasma cells (Ig) (Figure 1B and S1B-D; Tables S2 and S3).
Figure 1. Profiling immune infiltrates in human melanoma with scRNA-seq and scTCR-seq.
A. Graphical overview of the experimental setting. Single immune cells were collected from human melanoma, and processed by MARS-seq for transcriptional profiling, and scTCR-seq for clonotype analysis of T cells. B. Two-dimensional (2D) projection of expression profiles of 47,772 single cells (46,612 immune cells and 1,160 malignant cells) from tumor lesions partitioned into 324 metacells. Single cells are shown in dots. Metacells consisting of related cells are connected with edges and are positioned in proximity, broad immune cell subsets are annotated and marked by color code. C. 2D projection of sub-clustered T and NK cells. A total of 29,825 cells are represented in 218 metacells from the model shown in B, annotated in 9 groups and marked by color code. D. Expression (molecules/1,000 UMIs) of select genes across the T/NK metacell model. E. 2D projection of a selected set of marker genes over the metacell model. F. Distribution of the number of patients contributing to each metacell. Only patients with at least 2 cells in the metacell are considered as contributors.
T cells form a gradient of transcriptional states within tumors
Both T and NK cells were characterized by a diverse group of transcriptional states that we annotated broadly using analysis of the metacell similarity matrix (similarity between 218 metacells, Figure S1E) and its 2D projection (Figure 1C). This mapping revealed naïve-like T cells, but also CD4 and CD8 T cell pools with different degrees of differentiation. T cells in the naïve-like subset were metabolically inactive cells that showed weak transcriptional activity (Figure 1D-E). FACS-based index analysis allowed subdivision of some of the naïve-like subset into CD4+ and CD8+ (Figure S1F), but apart from high level expression of the IL7R, CCR7, and the transcription factor TCF7, limited subset specific transcriptional activity was observed relative to other T cell pools (Figure 1E and S1G-H). In contrast, the non-naïve-like T cell metacells showed remarkable transcriptional heterogeneity. One small group of cells that was made up of CD4 and CD8 T cells was putatively annotated as a memory T cell population (Figure S1I). The large number (n=145) of CD8 metacells were initially subdivided into a transitional CD8 effector T (GZMK+) pool, a cytotoxic T effector (GZMH+) pool and a large cluster of dysfunctional CD8 T cells, marked by high expression of immune checkpoint molecules such as PD-1 and LAG3. The border between these different CD8 classes was diffuse, even though metacell resampling analysis supported the robustness of the model. This observation suggested that transcriptional gradients contribute to T cell heterogeneity, as further discussed below. The CD4 T cell subset was dominated by FOXP3 expressing regulatory T cells (Treg) but also included a distinct subset with characteristics of follicular helper T cells (marked by CXCL13), and another smaller group of cells expressing various immune checkpoint molecules, similar to those observed in CD8 T cells (Figure S1J). Finally, although CD3 negative, NK cells expressed many gene modules that were also observed in cytotoxic T cells (Figure S1K).
Importantly, despite the high diversity in T cell types and states, data from multiple patients contributed to the definition of all Metacells (Figure 1F). This observation demonstrates that a robust universal regulatory process controls the T cell states that occur within human tumors. As such, we can use the transcriptional T cell states that are observed pan-patient for in-depth analysis of the possible gene regulatory mechanisms that give rise to the diversity of T cell states in melanoma immune infiltrates.
A gradient of checkpoint expression intensities within the CD8 T cell populations
Analysis of CD8 metacells resulted in the de novo identification of a rich set of co-regulated gene modules (Figure 2A and S2A), including programs associated with basic cellular functions (ACTB and MHC-I genes), naïve T cell regulation (TCF7), and specific groups of genes linked with effector functions (GZMH, GNLY, FGFBP2, and CX3CR1), or with the dysfunctional state (TIGIT, PD-1, LAG3, and CXCL13). Based on these data, we computed two transcriptional scores, one quantifying the activity of a dysfunctional gene module (with the gene module being anchored to LAG3) and the other assessing overall intensity of the expression of a cytotoxic gene module (with the gene module being anchored to FGFBP2) (Figure 2B-C, validated in S2B-F). We then performed a quantitative comparison of the distribution of the two programs over all CD8 metacells and observed a spectrum of transcriptional intensities, demonstrating the presence of a transitional CD8 state that forms a continuum with the dysfunctional T cell state. In contrast, weaker support for a continuum between either the transitional and the cytotoxic state, or between the cytotoxic and the dysfunctional state, was observed (Figure 2D and S2G). Importantly, we found multiple transcription factors (TFs) that correlated with the dysfunctional program, including known regulators as well as several TFs that have not previously been associated with a dysfunctional phenotype in human T cells. These included the Notch signaling TF RBPJ (also observed in T cells in lung cancer (Guo et al., 2018)) as well as ZBED2, ETV1, ID3, MAF, PRDM1, and EOMES (Figure 2E). In addition, we observed distinct transcription factors that correlated specifically with the cytotoxic program (KLF2 and TBX21/T-bet) (Figure 2E). Using the expression of these TFs, we inferred a simple linear regulatory model, predicting the contrasting dysfunctional and cytotoxic programs with high accuracy (R2 = 0.93) using lasso-regularized cross validation on 145 metacells (Figure 2F).
Figure 2. Transcriptional gradients of tumor infiltrating T cells.
A. Gene-gene correlation heatmap of top variable genes within CD8 T metacells. B. Bar graph showing the top 30 genes that are most correlated with LAG3 across CD8 T metacells. This set of 30 “dysfunctional” genes is subsequently used to calculate dysfunctional scores. Scatter plots depict the dysfunctional score per metacell versus log enrichment of a selected set of dysfunctional genes. C. Similar to panel B, but showing the top 30 genes most correlated with FGFBP2, defining the cytotoxic score. The correlation of a selected set of cytotoxic genes with the cytotoxic score per metacell is shown. D. Cytotoxic score versus dysfunctional score on CD8 metacells. E. Top transcription factors correlated with the dysfunctional score (right) and cytotoxic score (left). F. Shown are differences of dysfunctional and cytotoxic scores per metacell (X axis) versus the prediction (Y axis) of a linear model using TF expression alone (10-fold cross validation, lasso regularized). Inferred non-zero coefficients for TF variables are shown to the right. G. Bar graph showing the top 30 genes with the highest correlation to IL2RA (Treg score). H. Transcription factors with the highest correlation to the Treg score. I. Linear model using transcription factors to predict the Treg score, similar to panel F. J. Gene enrichment in dysfunctional and Treg cells over naïve-like cells. A selected set of highly expressed genes (mean molecules per cell >= 0.05) is depicted, highlighting key genes either distinct or shared among the two groups. K. Treg score versus dysfunctional score on all metacells. Different groups of metacells are color-coded as in panel D.
See also Figure S2.
A similar analysis of CD4 Treg metacells identified a co-regulated gene module that includes IL2RA, ICOS and GITR (Figure 2G-H and S2H). Furthermore, analysis of this gene module and TFs that were correlated with it across Treg metacells (BATF, FOXP3, and IKZF2) suggested that Tregs within tumors can be organized along a gradient of intensifying expression of characteristic Treg genes and TFs (Figure 2I). Interestingly, some of the gene modules activated in Tregs overlapped with those characteristic of the dysfunctional program in CD8 cells. In addition, a number of TFs, including PRDM1, VDR, MAF and ZBED2, were correlated with both programs, indicating the presence of shared regulatory gene modules in CD4 Treg and dysfunctional CD8 T cells. To further examine transcriptional overlap between dysfunctional CD8 T cells and regulatory T cells, we compared gene expression enrichment of both T cell types as compared to the naïve-like cell population (Figure 2J). Genes enriched in both the dysfunctional and the Treg cells included regulatory molecules and many co-inhibitory and co-stimulatory receptors (e.g. TNFRSF9/CD137, CSF-1 and TIGIT). In contrast, FOXP3, IL2RA (CD25), and IKZF4 were only enriched in regulatory T cells, while PD-1, CXCL13, IFNG, and EOMES were preferentially enriched in the dysfunctional cells. Visualization of the dysfunctional and Treg scores for metacells confirmed the overlap between these two differentiation programs (Figure 2K). Interestingly, CD4 Tfh metacells shared genes with the dysfunctional CD8 program that were distinctively not expressed in Tregs - including CXCL13 (Figure S2I). In conclusion, we observed a dominant group of dysfunctional CD8 T cells that is characterized by gradual rather than discrete activation of immune checkpoint gene expression, and that also partially shares regulatory mechanisms with regulatory CD4 T and Tfh cell populations. In contrast, the observed CD8 cytotoxic cell pool stands out as a more distinct population.
Monocyte differentiation and bifurcation is observed within tumors
We performed in-depth analysis of the metacell map consisting of 16,412 QC positive CD45+ cells, across all patients, following in silico removal of T/NK cells (Figure S3A-B). Within this model we identified diverse myeloid cell types, including macrophages (C1Q), monocytes (VCAN), dendritic cells (DC; enriched for CLEC10A and CD1C), plasmacytoid dendritic cells (LILRA4), and also a small group of osteoclast-like (MMP9) cells (Figure S3C-D; Table S3). In addition, metacells defining B and plasma cells were observed. To characterize potential myeloid differentiation trajectories, we defined transcriptional signatures for monocyte, macrophage, and DC metacells (Figure S3E-F). We then used these signatures to compute scores for each metacell in the myeloid model, resulting in a bifurcation like structure that connects these three programs through metacells expressing these pathways with transitional intensity (Figure S3G-H). These data, and in particular the existence of monocytes in different stages of differentiation within tumors, suggest an ongoing development of monocytes within tumors, rather than the sole infiltration of mature myeloid types.
The presence of various myeloid cell populations has previously been suggested as a potential modifier of T cell activity in tumors (Binnewies et al., 2018; Lavin et al., 2017; Salmon et al., 2016). However, the relationship between these cell states in human tumor immune infiltrates is poorly understood, prompting us to subsequently characterize potential correlations between patient-specific transcriptional states not only within the T cell compartment, but also between this compartment and other infiltrating immune cells.
Inter-patient variation of dysfunctional CD8 T cells
To map conserved and patient-specific patterns in infiltrating immune cells, we systematically analyzed the immune composition of each patient (Figure 3A-B; Table S4). Globally, the majority of the observed T cell transcriptional states were shared across many patients, with relatively few cases of individual patients contributing more than half of the cells in a metacell. A more heterogeneous distribution was observed for myeloid cells, with many metacells composed by cells from a small number of patients. These patient-specific myeloid cell states included several macrophage and monocyte metacells highly enriched for type-I interferon signaling (Figure 3B and S4A). While the model showed that the transcriptional states of T cells were conserved between patients, the frequency of these states in different patients was remarkably diverse. In particular, we observed that the CD8 dysfunctional state constituted a highly variable fraction (ranging from 3.6% to 72.1%, median of 28.9%) of the tumor-infiltrating T cells (Figure 3C). Analysis of possible correlations between dysfunctional CD8 T cell load and gene expression signatures in monocytes or in B cells showed no significant results, indicating that such correlations, if existing, are insufficiently strong to allow detection in our patient cohort (Figure S4B). In contrast, we did observe the fraction of dysfunctional T cells to be negatively correlated to the fraction of naïve-like T cells (p<0.001; Figure 3D), and positively correlated to the fraction of Tfh cells (p<0.05; Figure 3D). To start examining the possible connections between this differential representation of T cell states and treatment history or site of metastasis, we studied heterogeneity in T cell states either within a set of stage 3 and 4 tumors with different treatment history, or within a set of 9 treatment-naïve, stage 3, subcutaneous melanomas. Notably, substantial heterogeneity was observed even when restricting analysis to treatment-naïve patients at the same anatomical site (Figure S4C). Furthermore, analysis of 2 independent lesions for 2 patients revealed a similar composition (Figure S4D), suggesting composition variability is not driven by technical biases. In summary, our data define a universal spectrum of dysfunctional T cell states within melanoma patients but show that the abundance of cells within this spectrum is remarkably different between patients. The current cohort does not support the existence of a strong link between myeloid cell compositions and dysfunctional or other T cell states, but does suggest that the load of CD8 dysfunctional T cells is an intrinsic, and possibly key feature, of melanoma tumors.
Figure 3. Inter-patient variation in the composition of dysfunctional CD8 T cell and other immune cell populations.
A. 2D projections of the composition of T and NK cell populations (top), and non-T/NK immune cell populations (bottom) in different patients. Six representative patients ordered by their fraction of dysfunctional CD8 T cells within all T cells are depicted. B. Metacells (columns) are ordered by groups and clustered within each group. Top panel shows the number of cells in the metacell. Second panel shows the top contributing patients to each metacell. Bar height is the fraction of cells, top patient in dark green, second patient in light green, remaining patients in white. Patients are ordered based on the frequency of dysfunctional CD8 T cells. Compositions of T cells and other immune cells are shown on the right (colors as in Figure 1 for T/NK cells and Figure S3 for non-T/NK cells). C. Patients are ordered by the fraction of dysfunctional CD8 T cells within all T cells and binned into 3 groups (low, intermediate, and high fraction of dysfunctional CD8 T cells). Disease stage, tumor location (LN: Lymph node; MSC: muscle; (S)C: (sub)cutaneous; p(S)C: primary (sub)cutaneous), treatment background (N: naïve; T: treated; IT: immunotherapy treated), percentage of CD3+ cells within total immune cells measured by flow cytometry, and percentage of tumor immune infiltrates measured by histology are depicted (Methods), grey bars represent missing data. D. Frequency of different immune populations within the three groups of patients defined in panel (C) with each circle representing a patient and horizontal line the group median. Spearman correlation between the fraction of the group and the fraction of the dysfunctional group are shown on top, with stars marking significant p-value of a Mann-Whitney test between patients in the low and high groups (*P < 0.05; **P < 0.01; ***P < 1 × 10−3).
Dysfunctional CD8 cells have proliferative capacity and form large clones
To understand the clonal structure of different T cell states, we generated a modified version of the MARS-seq protocol that allows coupled analysis of single cell transcriptomes and TCR sequences (Figure 1A; Methods). With this strategy, we were able to recover TCRβ sequence information from 6,306 T cells, consisting of 3,492 unique TCRβ sequences. As expected, TCR clonotype composition was highly variable across patients, and the only case where we identified the same TCR clones in different biopsies was for the two independent metastases from patient p12, revealing a remarkably similar clonal composition in the two lesions (Figure 4A-B; Table S5). Whereas some T cell infiltrates showed a diverse TCR repertoire with minimal clonal expansion, others were strongly dominated by a small number of T cell clones (Figure 4B-C). Interestingly, larger clones showed a non-uniform distribution of functional states (Figure 4C), with an enrichment for dysfunctional states and depletion of naïve-like states. Conversely, stratification of patients by their inferred dysfunctional T cell score showed clonality to be positively correlated with the fraction of dysfunctional cells (Figure 4D). Projection of clonal composition data on the T cell metacells (Figure 4E), and quantification of the fraction of cells linked with a singleton or with a clone for which more than a single cell was sampled, highlighted dysfunctional metacells as being strongly enriched for larger clones (additional controls for TCR expression intensity are shown in Figure S5A-B).
Figure 4. Clonal expansion within the dysfunctional CD8 T cell compartment.
A. Graphical overview of the use of scTCR-seq to identify shared clones between two independent lesions from the same patient. B. Overview of TCR clonality for all patients, showing the size of each T cell clone (largest at the bottom, smallest at the top) in each tumor. Corresponding TCRs from two lesions of the same patient are marked. C. Clonal composition of T cells in patients, showing from top to bottom the number of distinct clones per patient, the number of T cells of which the TCR was retrieved, the distribution of clones by size (size =1, size =2, and size >2 cells), and pie charts showing the cell type composition of each patient clones stratified by clone size (shown are data representing at least 10 cells). Patients are ordered by the fraction of their size-one clones. D. The fraction of clones with >2 cells (left) and patient clonality score (right; defined by Pielou’s evenness) are shown for patients grouped by their fraction of dysfunctional CD8 cells, as in Figure 3D. E. Metacells composition. Showing per metacell the fraction of cells with a unique TCR (clone size=1, x-axis) versus fraction of cells with a recurring TCR (clone size >1, y-axis).
See also Figure S5.
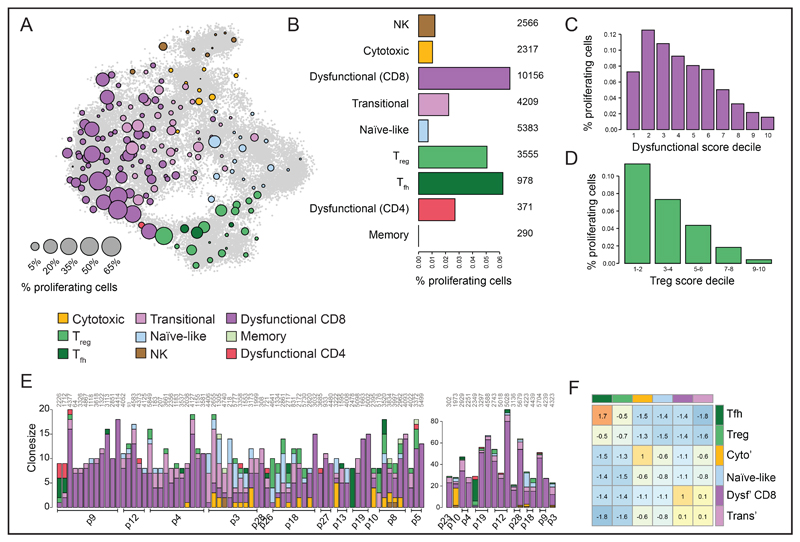
To understand whether the observed clonal expansion could be due to T cell proliferation at the tumor site, we computed a proliferation score for each cell, by pooling the expression of cell cycle genes (Figure S5C; Table S6), and then used the resulting bimodal distribution to classify the proliferative state of individual cells (Figure S5D; Methods). Notably, to avoid interference of parallel gene modules with analysis of cell state, proliferation genes were excluded during metacell derivation in the model described in Figure 1. A significant fraction of T cells was observed to be cycling (in total 3.7%), and the fraction of proliferating cells was strongly correlated with specific T cell states (Figure 5A-B and S5E). Specifically, the highest fraction of proliferative cells was observed in dysfunctional T cells, which were nearly 10 fold more likely to proliferate than naïve-like T cells (Figure 5B and S5E). Flow cytometry analysis of Ki-67, a nuclear protein marking cellular proliferation, validated the proliferative capacity of dysfunctional T cells (Figure S5F). Notably, dysfunctional T cells were observed to occupy all stages of the cell cycle (G0/G1, S, and G2/M), arguing against the possibility of cell cycle arrest (Figure S5G). Within the dysfunctional CD8 T cell pool, proliferation was most profound in the earlier stages of acquisition of the dysfunctional program (Figure 5C and S5H). This indicates that progression of CD8 T cells from the transitional state is accompanied – and perhaps driven by – their proliferation. The reduction in proliferative capacity of highly dysfunctional cells may either be interpreted as an intrinsic impairment in proliferative capacity, or by the quenching of proliferation-promoting signals by the now highly expressed inhibitory receptors. Treg and Tfh CD4 T cell populations also showed higher fractions of cells expressing proliferation-associated genes as compared to naïve-like or cytotoxic T cells, or as compared to NK cells. Analysis of the fraction of cells that show a proliferation signature along the Treg transcriptional gradient demonstrated that, similarly to dysfunctional CD8 T cells, most profound proliferation occured in the early stages of their differentiation trajectory (Figure 5D and S5I). In summary, both the proliferation dynamics and distribution of clone sizes support the model that dysfunctional T cells in human melanoma form a highly proliferative and dynamic cell compartment.
Figure 5. CD8 T cells in early dysfunctional state are highly proliferative.
A. Fraction of proliferating cells per metacell, calculated by defining a cell as proliferative by its fraction of cell cycle genes expression out of total expression. Circle size reflects the percentages of proliferating cells in the metacell, using the 2D projection as in Figure 1C. B. Percentage of proliferating cells in the different immune cell types and subtypes, number of cells per metacell group are shown on the right. C-D. Fraction of proliferating cells in dysfunctional (C) and Treg (D) groups of cells, stratifying cells by their dysfunctional score (C) and Treg score (D). Scores defined as in Figure 2. E. Cell subtype composition of T cell clones of intermediate size (shared by 8-20 cells, left) or large size (shared by more than 20 cells, right). Clones are hierarchically clustered, patient ID are shown on the bottom and clone ID on top. F. Pairwise clone sharing propensity by different T cell groups. Data depict the enrichment of the observed number of cell pairs sharing TCRs by their associated group over a control generated by multiple repetitions of random sampling of cells from individual patients, taking into account the TCR detection probability (shown in Figure S5A), while preserving the number of clones and clone sizes per patient.
Clonal linkage of transitional and dysfunctional T cells
The clonal identifiers obtained by TCR analysis provide a unique data set to infer the lineage structure of T cells in tumors. As shown in Figure 5E-F, the composition of clones shows high functional coherence, with the individual cells within a clone being allocated to the same functional class or to related classes. In general, cells within large clones (8 cells or more) were linked with CD8 or CD4 metacells, but not both. Only 5.7% of the cells in these clones were inconsistent with the majority of cells in their clone (e.g. attributed to TCR or metacell misclassification). To systematically analyze all available information on intra-clonal structure, including data from small clones, we identified all pairs of cells sampled from the same TCR clone and then estimated lineage relationship between cell types using pooled statistics of paired types, as compared to shuffled controls (Methods). As expected, that data show a very clear separation between CD4 cells and CD8 cells. More interestingly, a strong clonal sharing of transitional CD8 and dysfunctional cells was observed, whereas cytotoxic cells formed a distinct group (Figure 5F). While this approximated lineage structure cannot support a deterministic clonal structure in the data, it is consistent with the existence of a differentiation gradient that links transitional cells to the dysfunctional spectrum. In contrast, cytotoxic CD8 T cells are likely to originate from external inputs. To further examine this, we profiled an additional 7,680 (7,410 QC positive) PBMCs from 3 melanoma patients (Figure S6A-D). This analysis demonstrated a complete lack of dysfunctional cells within these samples but ample evidence for naïve-like, transitional and cytotoxic CD8 T cells (Figure S6E-F). This is consistent with a model of ongoing differentiation and proliferation of dysfunctional T cells at the tumor site, and suggests that their development may be distinct (in time and space) from the dynamics of cytotoxic T cell differentiation.
Dysfunctional T cell programs and their variance in lung cancer and melanoma
To address to what extent these characteristics of T cell infiltrates would be shared across human cancer types, we performed a comparative analysis with the T cell infiltrates observed in the recently published lung adenocarcinoma dataset of Guo et al., profiling 11,769 QC positive T cells in 14 samples (Guo et al., 2018). Application of the metacell algorithm uncovered a map of lung transcriptional states (Figure S7A-B) analogous to the melanoma map, with a distribution of cell states between tumor, normal and blood that is consistent with the analysis presented by Guo et al. (Figure S7C). Notably, a number of aspects of T cell infiltrates in melanoma and lung cancer showed qualitative and quantitative differences (Table S7). Several ligands such as CSF1, OX40 ligand/TNFSF4 and 4-1BB ligand/TNFSF9 were enriched more strongly in melanoma than in lung cancer (Figure S7D). Furthermore, a subset of putative dysfunctional regulatory molecules, including the transcription factors ZBED2 and ID3, were more enriched in melanoma, together suggesting a differential mode of regulation in melanoma tumors. In line with this, the fraction of dysfunctional T cells in lung tumors was small compared to melanoma; 1.1% - 25.2%, median 11.6% of captured T cells, as compared to 3.6% - 72.1%, median 28.9% in melanoma. We confirmed that similarly to melanoma, dysfunctional T cells in lung tumors were characterized by multiple checkpoint molecules expression and specific enrichment of proliferation signatures amongst the dysfunctional T cells (Figure S7E-F). In conclusion, meta-analysis of human lung cancer and melanoma lesions suggests that the regulation of the dysfunctional gene program can vary with tumor type. Nevertheless, most of the dysfunctional program, its dynamics and association with intratumoral T cell proliferation are to a large extent universal between tumors.
High levels of dysfunctionality in CD8 T cells is associated with tumor-reactivity
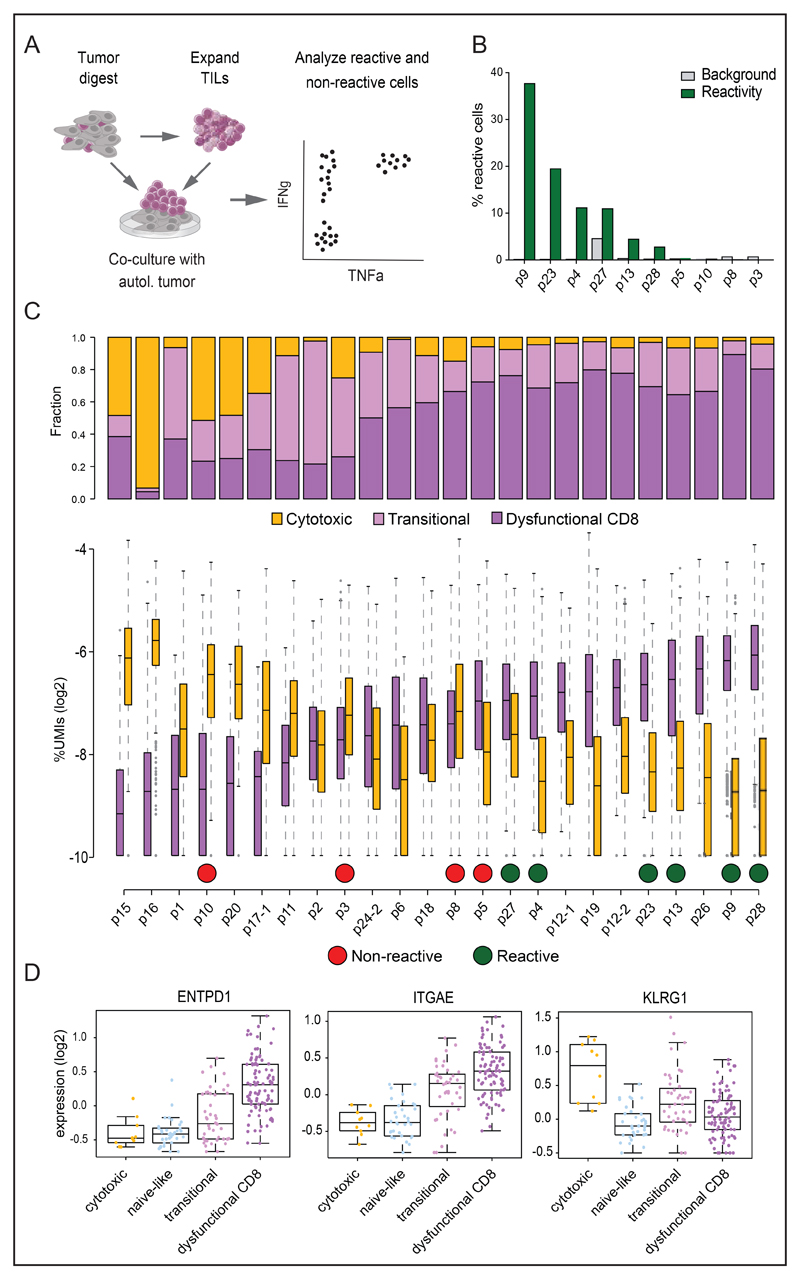
Finally, to understand the links between T cell states as characterized above and tumor-reactivity of the intratumoral T cell pool, T cells from 10 patients were expanded ex vivo, and reactivity of expanded T cells towards autologous single cell tumor digest was tested (Figure 6A). Tumor-reactivity, as measured by IFNg and TNFa production, was highly variable between tumor infiltrating lymphocytes (TILs) derived from different patients. In six out of ten patients, 2%-38% of the T cells showed tumor-reactivity, and for the remaining patients no reactivity above background could be detected (Figure 6B). As expected, T cell reactivity was dependent on interaction with MHC class I molecules (n=2, Figure S7G). Of the 4 samples that did not show reactivity, tumor cells from one patient (p8) were HLA class I negative, thereby preventing proper analysis of CD8 T cell reactivity. Interestingly, when ordering patients by the distribution of dysfunctional scores within CD8 T cells, we observed that T cell pools with detectable reactivity against autologous tumor cells generally displayed a more prominent CD8 dysfunctional state (Figure 6C). In line with recent data that demonstrate increased tumor-reactivity of T cell populations marked by expression of CD39 (ENTPD1) and CD103 (ITGAE) and by the absence of KLRG1 (Duhen et al., 2018; Simoni et al., 2018), the dysfunctional T cell compartment in our data set showed increased expression of both CD39 and CD103 as compared to the cytotoxic T cell compartment, which showed increased expression of KLRG1 (Figure 6D). Due to limited sample size, independent statistical support for the linkage between tumor-reactivity and dysfunctional T cell load could not be derived. However, when combined with the data on transcriptional gradients that characterize dysfunctional states, the clonal composition of the dysfunctional T cell pool, and the evidence for ongoing proliferation of this subset, these data support a model in which an ongoing intratumoral T cell differentiation towards dysfunctionality is induced by antigen-driven interactions with surrounding tumor cells.
Figure 6. Relationship between cell states and tumor-reactivity of T cells.
A. Graphical overview of the method to assess T cell reactivity. Ex vivo expanded TILs were co-cultured with autologous tumor cells to examine the presence of a tumor-specific TCR repertoire by analysis of IFNg and TNFa secretion. B. tumor-reactivity is depicted as the percentage of T cells that secrete IFNg and/or TNFa after co-culture with autologous tumor material. Tumor-reactivity of expanded TIL was assayed for 10 patients. C. The fraction of UMIs from genes of the dysfunctional program and cytotoxic program were calculated for each CD8 T cell, and the distribution of these fractions across all CD8 T cells are shown per patient. Patients are ordered by the median of the UMI percentage of the dysfunctional gene program. T cell subtype composition within CD8 T cells is shown on top for each patient. Green and red circles mark patients with reactive and non-reactive TIL, respectively. D. Expression enrichment per metacell of tumor-specific and bystander T cell marker genes, including ENTPD1, ITGAE, and KLRG1, in different T cell subtypes.
See also Figure S7.
Discussion
We combined single cell RNA-seq and TCR-seq to infer models for the transcriptional states of tumor-infiltrating immune cells in 25 melanoma patients across a spectrum of disease stages and treatment strategies. Several recent studies have utilized scRNA-seq to describe tumor infiltrates in melanoma, non-small cell lung cancer, breast cancer, and colorectal cancer (Azizi et al., 2018; Guo et al., 2018; Sade-Feldman et al., 2018; Tirosh et al., 2016; Zhang et al., 2018). These studies have led to a better understanding of some of the intrinsic transcriptional programs that regulate T cell infiltrates. Although scRNA-seq alone cannot inform us on the longitudinal dynamics and complex cellular interactions between different T cells and surrounding immune and stromal cells in the tumor microenvironment, the generation of large scRNA-seq data sets containing coupled TCR information facilitates more in depth T cell analyses making it feasible to test which transcriptional states are linked. Consistent with expectations, T cells that express genes associated with T cell dysfunction were observed in many patients, and sometimes in large numbers. In addition to the expression of checkpoint molecules such as PD-1 and LAG3, these dysfunctional CD8 T cells produced CSF-1 and CXCL13 transcripts, suggesting the possibility of interaction with other intratumoral cell populations, and consistent with data in NSCLC (Guo et al., 2018; Thommen et al., 2018).
In line with previous observations (Azizi et al., 2018; Guo et al., 2018), our data show that the dysfunctional T cell pool does not form a discrete cell population but is part of a wide differentiation spectrum, spanning from transitional, through early dysfunctional, towards highly dysfunctional T cells. This proposed differentiation trajectory was not a patient-specific phenomenon but rather a universal feature of CD8 T cells within melanoma. Notably, while this trajectory was also observed in NSCLC, it appears more abundant in melanoma patients. In addition, a subset of putative dysfunctional regulatory molecules, including ZBED2, EOMES and ID3, were more enriched in melanoma. This suggests a differential impact of the tumor microenvironment on the development of T cell dysfunction across tumor types, conceivably reflecting differential availability of antigen or differential exposure to inhibitory factors.
Importantly, the observed sharing of TCR sequences between dysfunctional T cells and transitional T cells provided independent evidence for the existence of this differentiation trajectory. The dysfunctional signature that was dominant at tumor sites was not observed in peripheral blood of patients, suggesting a locally induced differentiation process. Indirect support for this model was provided by the observation that clone size, as based on TCR-seq data, was increased in dysfunctional metacells. More directly, we found dysfunctional CD8 T cells, in particularly the early-dysfunctional population, to form the most actively proliferating cell state as based on scRNA-seq analysis. This property appears shared between tumor types, as proliferation signatures have been shown for T cells in various tumor types, in part using different experimental approaches (Guo et al., 2018; Savas et al., 2018; Thommen et al., 2018; Zhang et al., 2018). Of note, the clear proliferative signal in dysfunctional T cells in many patients is suggestive of a doubling time of a few days in the dysfunctional niche. It is noted that this extensive proliferation in the niche must be counterbalanced by rapid turnover of dysfunctional T cells (and also Treg cells), when assuming an overall infiltrate burden that is stable through time. Collectively these data characterize the dysfunctional CD8 T cell state, previously associated with an “exhausted” phenotype, as a de facto extremely dynamic and active T cell state. Interestingly, CD4 T cells, including Treg and Tfh populations, expressed specific combinations of checkpoint genes that partially overlapped with those observed in the dysfunctional CD8 pool. In future work, the complex interactions between these different cell pools will have to be interpreted within a broader context of tumor and stromal cell populations and their potential immunomodulatory functions. In addition to determining the interactions between different intratumoral cell subsets, the linking of transcriptional profiles and functional characteristics of T cells in the tumor will become increasingly important. Our data show that tumor-reactivity of T cells expanded from tumor lesions is associated with a higher level of dysfunctionality in the tumor. Emerging techniques should make it feasible to directly profile the reactivity of TCRs in a setting that is independent of prior cell state and tumor microenvironment (Scheper et al., in press), thereby allowing one to couple cell state to tumor-reactivity of the associated TCR.
Comparison of patient cohorts that either included treatment-naïve patients with the same disease stage and site of metastasis, or patients who varied in disease stage, prior treatment and disease location was used to determine to what extent the relative abundance of different immune cell states is a tumor intrinsic property. For the cohort analyzed here, we observed large variability in the abundance of dysfunctional T cells even between treatment-naïve tumors at the same organ site. This indicates that immune infiltrates of human melanomas show a heterogeneity that is largely intrinsic, independent of prior treatment or metastatic site. Parameters that may determine this setpoint of the intratumoral immune pool may potentially include driver oncogenes but also tumor mutational burden.
In contrast to the pool of dysfunctional T cells that appeared linked to the transitional cell state, a discrete pool of cytotoxic T cells was observed that shows no evidence of a link towards either the transitional or the dysfunctional pool, as both determined by transcriptional gradient analysis and by TCR sharing. It will be interesting to determine which factors in human tumors drive the separate formation of the transitional – dysfunctional axis on the one hand, and the cytotoxic T cell pool on the other hand. As a first possibility, the cell populations that together form the observed differentiation trajectory may be undergoing antigen recognition at the tumor site, thereby potentially driving – together with intratumoral factors – both the proliferation and the differentiation process that is observed. In this model, the cytotoxic pool should be considered as bystanders that are irrelevant to the tumor site at which they reside, and recent work has provided evidence for the frequent occurrence of such bystanders in human tumors (Scheper et al., in press; Simoni et al., 2018). As a second possibility, the transitional - dysfunctional and the cytotoxic T cell pool may both be equally tumor-reactive, but with the progressive dysfunction seen in the former reflecting the genetic imprint of suboptimal T cell priming (Ahrends et al., 2017; Borst et al., 2018). While more data are required to settle this issue, the observation of an association between the strength of the dysfunctional signature and the detection of tumor-reactivity amongst TIL provides evidence in favor of the former model as a driver of T cell heterogeneity in human cancers.
Star Methods
Subject details
Human tumor specimens
Human tumor tissue and blood material, as summarized in Table S1, were obtained in accordance with national guidelines, where applicable following opt-out procedure or signed informed consent and after approval by the local medical ethical committee (institutional review board, IRB) of The Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital (NKI-AVL). Tumor tissue was collected from surgical specimens after macroscopical examination of the tissue by a pathologist. For each specimen, a fragment was fixed in Formalin-Fixed Paraffin-Embedded (FFPE) for histology. The remainder of the tissue was directly processed for single cell RNA-seq. Alternatively, the tissue was dissected into 1x1x1mm fragments and frozen in 90% Fetal Bovine Serum (FBS) and 10% Dimethyl Sulfoxide (DMSO).
Method Details
Tumor dissociation
Fresh tumor tissue was dissociated by manual mincing followed by an incubation of 20 minutes at 37C in RPMI with collagenase IV and pulmozyme, alternated with 3 rounds of dissociation with a gentlemacs dissociator. After dissociation, cell suspensions were filtered with a 100μm filter, washed in RPMI 1640 medium with penicillin, streptomycin and human serum. Cell suspensions were frozen down in 90% FBS and 10% DMSO.
PBMC isolation
PBMCs were isolated from blood using a Ficoll gradient. PBMCs were stored in liquid N2 in 90% FBS and 10% DMSO directly after collection.
Single cell sorting of tumor and blood materials
Tumor cell suspensions and PBMCs were thawed in RPMI with 10% human serum, penicillin and streptomycin, and 250U/ml benzonase. Cells were stained in PBS with 0.5% BSA and 2mM EDTA containing fluorochrome-conjugated antibodies. Cells were stained with propidium iodide (PI) immediately prior to sorting with a FACSaria Fusion. Forward and side scatter settings were used to select for immune cells and to exclude doublets. Viable cells were identified based on low PI staining, and immune cells were sorted based on CD45 expression. For each tumor, multiple plates were sorted with CD3+ and CD3- immune cells. Immune cells were single cell sorted using index sorting into 384 wells plates containing 2μL of lysis solution with barcoded poly(T) reverse-transcription (RT) primers. Four empty wells were kept in each 384-well plate as ‘no-cell controls’. Plates were briefly centrifuged, snap frozen on dry ice, and stored at -80 degrees. Antibodies used for sorting were anti-CD3-FITC and anti-CD45-APC or anti-CD45-BV510, and for index sorting combinations of anti-CD4-BV421, anti-CD4-BV510, anti-CD8-AF700, anti-CD8-Pacific Orange, anti-PD-1-PE, anti-CD103-BV711, anti-PD-L1-APC, anti-CD11b-BV650, anti-CD56-PE, anti-TIM-3-BV421, anti-LAG3-AF700, anti-OX40-BV711, anti-CD137-APC. For PBMC sorting, anti-CD45-FITC, anti-CD3-FITC, anti-CD8-AF700, anti-PD-1-PE, anti-CD103-BV711, anti-CCR7-PECF594, and anti-CD45RA-PECY5.5 were used.
Single cell library preparation
Single cell libraries were prepared with Massively Parallel Single-Cell RNA-seq method(MARS-seq) (Jaitin et al., 2014). In brief, mRNA from single cells sorted into cell capture plates was barcoded and converted into cDNA and pooled using an automated pipeline. Subsequently, the pooled sample was linearly amplified by T7 in vitro transcription, and resulting RNA was fragmented and converted into a sequencing-ready library by tagging the samples with pool barcodes and illumina sequences during ligation, reverse transcription, and PCR. Each pool of cells was tested for library quality and library concentration was assessed.
Single cell TCR-seq (scTCR-seq) library preparation
After linear amplification by T7 RNA polymerase-mediated in vitro transcription in the MARS-seq library process, half of the resulting RNA material was reverse transcribed with a primer set that is specific to the different Vβ segments of the human T cell receptor β chain (Han et al., 2014). The resulting complementary DNA was then amplified with a set of nested primers (Han et al., 2014) and partial rd-2 primer. The PCR product was then cleaned up and used for a final amplification step with P7-rd1 and P5-rd2 primers. resulting TCR libraries were sequenced on an Illumina Miseq, with 165bp of read1 sequence and 15bp of read2 sequence. Primer sequences are listed in Table S8.
scTCR-seq protocol validation
Five different human T cell clones with known TCR sequences were stained with either anti-CD3-APC, anti-CD3-FITC, anti-CD3-PE, anti-CD3-PerCP, and anti-CD3-AF700 and subsequently mixed in a 1:1 ratio, and four single cell 384 well plates were sorted while recording index values. Resulting plates were then processed using the scTCR-seq method. Obtained TCRβ sequences were then compared with the known reference sequence per cell to determine sensitivity and specificity. This analysis revealed that the method was able to identify a TCRβ sequence in 32% (490/1520) of sorted cells, with the correct TCRβ sequence being assigned in 97% (442/455) of cells with clear flow cytometry index data to serve as a reference.
Ex vivo T cell expansion
T cells were expanded by culturing tumor fragments or single cell tumor suspensions in RPMI with 10% human serum, penicillin, streptomycin and 6000 IU/ml IL-2 for 14 days. Every 3 days, fresh medium with IL-2 was added to the culture. Subsequently, T cells were further expanded in RPMI with human serum, penicillin, streptomycin, 3000 IU/ml IL-2, 30ng/ml anti-CD3 antibody, and 1:200 irradiated PBMCs (40Gy) for an additional 14 days. Fresh medium with IL-2 was added every three days.
Cell cycle staining
Tumor single cell suspensions were stained with Live/dead fixable near-IR dead cell stain kit, anti-CD45-APC, anti-CX3CR1-PE, anti-PD-1-PeCy7, anti-CD8-AF700 and anti-CD3-FITC. After fixation with the FOXP3/transcription factor staining buffer set and additional fixation with 70% ethanol, cells were permeabilized and stained with anti-KI67-PerCPCy5.5. Prior to read-out, 1ug/ml DAPI was added.
Tumor-reactivity assays
Ex vivo expanded T cells were thawed and rested in RPMI with 10% human serum, penicillin, streptomycin, and 60 IU/ml IL-2. Tumor suspensions were thawed and incubated in RPMI with human serum, penicillin, streptomycin, and 250U/ml benzonase for 45-60min. To assess HLA class I dependency of observed T cell reactivity, tumor digests were incubated with anti-HLA class I antibody (W2/36) for 60min. T cells were labeled with cell trace violet according to the manufacturer’s protocol. T cells and tumor cells were co-cultured in a 1:1 ratio in RPMI with human serum, penicillin and streptomycin. After 60 minutes, golgiplug (BD Biosciences) was added, followed by a 12-15 hour incubation. Cell suspensions were then stained with anti-CD3-APC, anti-CD8-AF700, and IRDye in PBS with 0.5% BSA and 2mM EDTA. Subsequently, cells were fixed and permeabilized in 1% PFA and 0.1% Triton-X100. Staining with anti-IFNg-PE, anti-TNFa-AF499, and anti-CD137-BV650 was subsequently performed in 1% PFA, 0.1% Triton X-100, and 1% BSA.
Histological analysis
3μm paraffin sections were cut from FFPE tumor material. Slides were stained with Hematoxylin and Bluing Reagent, scanned on the Aperio Scanscope, uploaded on Slide Score (www.slidescore.com) and manually scored for the percentage of immune infiltrate.
Quantification and statistical analysis
Low-level MARS-seq processing
scRNA-seq libraries (pooled at equimolar concentration) were sequenced on an Illumina NextSeq 500 at a median sequencing depth of ~40,000 reads per cell. Sequences were mapped to the human genome (hg19), demultiplexed, and filtered as previously described (Jaitin et al., 2014) with the following adaptations. Mapping of reads was done using HISAT (version 0.1.6); reads with multiple mapping positions were excluded. Reads were associated with genes if they were mapped to an exon, using the UCSC genome browser for reference. Exons of different genes that shared a genomic position on the same strand were considered a single gene with a concatenated gene symbol. We estimated the level of spurious UMIs in the data using statistics on empty MARS-seq wells, and excluded rare cases with estimated noise >5% (median estimated noise over all experiment was 2%).
scTCR-seq raw data processing and analysis
TCR sequences were generated from cells sorted in 384 well plates, by inclusion of a barcode and UMI during subsequent processing, similar to MARS-seq. Initial filtering was performed on each plate independently. We corrected barcode sequencing errors by grouping reads with similar barcodes (hamming distance <=2) and filtering reads with similar UMIs but different barcodes. After extracting positions 80-130 base pairs in the sequence of the hypervariable region, typical sequencing coverage for TCR molecules was high (median of 350 reads), and we used filtering of low coverage UMIs since these were observed to be strongly enriched for errors and contaminations (i.e. as demonstrated previously in MARS-seq). These filtering stages provided us with a filtered table of candidate TCR sequences per barcode.
Remaining pre-processing was similar to Tracer (Stubbington et al., 2016). Fasta files from the first step were used as the input of IgBlast (ftp://ftp.ncbi.nlm.nih.gov/blast/executables/igblast/release/1.7.0/). Reads were then grouped according to the TCR sequence that represented them best, provided mapping was to the correct gene segments and the E-values for the reported V and J alignments were below 5e-3. A csv file with statistics from IgBlast was generated for all the productive reads (Table S5). Validation analysis showed that TCRs extracted from cells of different patients were never shared, while informative sharing was observed within patients.
Metacell modelling
We used the MetaCell package (Baran et al., 2018) with the following specific parameters (complete script reproducing all analyses from raw data will be available). We removed specific mitochondrial genes, immunoglobulin genes, high abundance lincRNA (Table S6), and genes linked with poorly supported transcriptional models (annotated with the prefix “RP-“). We then filtered cells with less than 500 UMIs or total fraction of mitochondrial gene expression exceeding 60%. Gene features were selected using the parameter Tvm=0.2 and minimal total umi > 200. We excluded gene features associated with the cell cycle, type I Interferon (IFN) response, and general cell stress (Table S6) using a clustering approach. To this end we identified first all genes with a correlation coefficient of at least 0.1 for one of the anchor genes MKI67, HIST1H1D, PCNA, SMC4, MCM3 (cell cycle), ISG15, OAS1, WARS, IFIT1 (type I IFN) and TXN, HSP90AB1, HSPA1A, FOS, HIF1A (stress). We then hierarchically clustered the correlation matrix between these genes (filtering genes with low coverage and computing correlation using a down-sampled UMI matrix) and selected the gene clusters that contained the above anchor genes.
For the metacell model in Figures 1-5, we used cells from pathologically confirmed tumor tissue from 25 patients (with cells from 2 metastases for two patients). We note that no patients were filtered before the metacell creation, but in some panels, only samples with a minimum of 100 T/NK cells per patient were used (Figure 3B-D and 6C). The gene selection strategy discussed above retained a total of 1,675 gene features for the computation of the Metacell balanced similarity graph. We used K=200, 500 bootstrap iterations and otherwise standard parameters. We did not apply outlier filtering, but performed the metacell splitting phase that clusters the cells within each metacell and splits it if distinct clusters are detected. This step marked a single metacells which contained 14 erythrocytes that were then discarded.
Annotation of the metacell model was done using the metacell confusion matrix and analysis of marker genes. We classified the metacells as T/NK or other, using straightforward analysis of known cell type markers (e.g. CD3D, CD3G, CD4, CD8A, CD8B, and more). Detailed annotation within the T/NK metacell model was performed using hierarchical clustering of the T/NK confusion matrix (Figure S1E) and supervised analysis of enriched genes, as described in the main text.
Analysis of PBMC data (Figure S6) was performed using similar parameters, with K=100, and analysis of marker genes shown to be enriched in the T/NK tumor infiltrates model.
Defining differentiation gene modules and gradients
To account for the complex gene expression patterns in dysfunctional CD8 T cells, cytotoxic CD8 T cells, regulatory T cells, macrophages, and monocytes, we combined metacell analysis with an approach aiming at identifying quantitative gene signatures. Given any list of signature genes for a certain differentiation state, we defined the signature’s scores for each metacell by averaging the metacell log enrichment scores (lfp values) of the genes in the set. Note that using this approach we limited the contribution of highly expressed genes to the score, and that we relied on the regularization of the metacell computation of gene enrichment scores to restrict the noise levels inflicted over the differentiation scores.
To define signatures gene sets for dysfunctional CD8 T cells, cytotoxic CD8 T cells, and regulatory T cells , we identified groups of 30 non-TF genes that were maximally correlated to selected anchor genes (LAG3, FGFBP2 and IL2RA), using linear correlation over metacells’ log enrichment scores. Genes associated with cell cycle, type I IFN and stress were filtered from these lists. Anchors were validated by using alternative anchor genes that yielded similar gene signatures (Figure S2E-F) and more systematically by testing correlation of the signature scores computed from their derived gene sets to all genes while excluding the gene itself from the score (Figure S2C-D), establishing consistency and robustness (genes remained top ranking even when omitting them from the score) to anchor selection. We note that we preferred the anchor approach for T-cells over the alternative approach of finding genes with maximal enrichment for a selected metacell, given the high complexity and multiple regulatory processes affecting the transcriptional space of T cells. Selecting and validating a consistent gene set starting from a well-defined anchor ensured that our score is based on the pathway of interest, and imitates some classical concepts from biclustering analysis (Bergmann et al., 2003).
To define myeloid signature gene sets we used the more simple approach of selecting the genes with the highest enrichment in metacells annotated as monocytes, DC, or macrophages (excluding one metacell annotated as non-classical monocytes, Table S3). To this end, we calculated per gene the log-ratio of the total UMI count in each group after scaling the total UMI count on all genes to be the same. We excluded genes for which only one metacell was enriched over the other metacells in the group by 8-fold or more.
To validate the significance of the transcriptional gradients that we observed when computing the various signature scores, we relied on the fact that metacells group cells into disjoint sets, and tested differential expression of genes that were not part of the signature gene set between bins of cells that were grouped into metacells with increasing ranges of signature scores (e.g. Figure S5H). To study potential transcriptional regulatory networks in T cells and myeloid cells, we used a list of annotated transcription factors genes (Lambert et al., 2018) with some specific additions (ID2, ID3 and TOX). As mentioned above, TFs were not considered when defining the signature scores, so modelling was not over-fitted a priori. For modelling the difference between the dysfunctional and cytotoxic signature scores in CD8 metacells, we identified candidate regulatory TFs as those enriched at least two fold over the background in at least one CD8 metacell and inferred a simple linear model aiming to predict the difference in signature scores using the enrichment scores of a subset of the TF candidates . This was done in the framework of a lasso-regularized cross validation scheme with the R package glmnet. A similar approach was applied to predict the Treg score in Treg metacells and the difference between monocyte and macrophage scores in myeloid metacells.
Analysis of TCR sharing
scTCR-seq provides TCR sequence information that may be used as clonal identifiers for T cells and, based on the absence of overlap in TCRs between patients, cells sharing a TCR sequence were considered to be derived from the same cell clone. Cells missing an identifier were considered as not observed (rather than not clonal). We observed a correlation between the intensity of TCR expression (as estimated by MARS-seq) and the success rate of scTCR-seq detection, which may suggest systematic bias in the analysis of clonality rates, enriching for signals in T-cell populations that show high levels of TCR expression (e.g. dysfunctional, Tfh, and Treg cells, Figure S5A-B). To control for these effects, as well as for the variable efficiency of TCR detection in different patients, we used a resampling strategy to generate randomized TCR-seq information and control for clonality statistics. Resampling was performed by randomly selecting a new cell for each observed TCR sequence, but forcing the cell to maintain the original patient source, and the original bin (one of 9 bins) of overall TCR-seq expression. This was repeated for 200 iterations and gave rise to a robust control dataset that preserves patient/clone size compositions and the overall relationship between TCR intensity and probability of TCR detection. Statistics on the metacell types of pairs of cells belonging to the same clone was thereby controlled for, using a comparison to the randomized data (Figure 5F). NK cells (51 cells) with retrieved TCR sequences were removed from this analysis as they represent misclassified T cells. Cells within the memory and dysfunctional CD4 metacells were also removed because their low frequency prevented robust statistical analysis of clonality signatures.
Supplementary Material
A. 2D projection of 11,769 cells partitioned into 68 metacells from human NSCLC (Guo et al, 2018). Single cells are shown in dots. Cells from tumor, normal tissue, and blood are projected separately below. B. Human NSCLC T cell subtype compositions from tumor, normal tissue, and blood are shown for different patients. Patients are ordered by their fraction of CD8-dysf cells in the tumor. C. Comparison of assignments of human NSCLC T cells to metacells (columns) and to Guo et al’s original clusters (rows). Fraction of cells per metacell is color coded. D. Scatter plot comparing enriched genes in dysfunctional T cells and Tregs from human melanoma (x-axes) with their matching subtypes in human NSCLC (y-axes); barplots showing the most enriched genes in melanoma as compared to NSCLC for dysfunctional T cells and Tregs. Each panel shows the enrichment of cells in a certain group over a reference group. Naïve-like population was used as a reference group for dysfunctional T cells from melanoma, and naive-CXCR6 cells (which is the most similar population to naïve-like population in melanoma) was used as a reference for dysfunctional T cells from NSCLC. E. Percentage of proliferating cells in different T cell subtypes of human NSCLC, numbers of proliferating cells per subtype are shown on the top. F. Fraction of proliferating cells in the human NSCLC dysfunctional group classified into bins according to their dysfunctional score. G. Expanded tumor infiltrating T cells (TILs) were either untreated, treated with PMA and ionomycin, or co-cultured with autologous single cell tumor suspensions in the absence or presence of HLA-I blocking antibodies. The percentage of cells secreting TNFa or IFNg after treatment are shown.
A. T cell subtype composition for T cells with different levels of TCR mRNA transcript (percentage of TRAC and TRBC2 genes UMIs out of total UMIs); black dot marks the probability of detecting the TCR within each group of cells. B. Fraction of cells with a detected TCR per metacell versus the gene enrichment difference of CD8A plus CD8B minus CD4. C. Correlation of genes from the cell cycle gene module on T and NK metacells. D. Frequency distribution of cell cycle scores on all T cells. Cell cycle scores were calculated as the percentage of cell cycle gene UMIs out of total UMIs. Dashed line indicates the threshold for marking a cell as proliferative. E. Empiric cumulative distribution plots of cell cycle scores per group. Note that the score is negated, hence cells below the dashed line are the proliferative ones. Fraction of proliferating cells per cell group is shown in the legend. F. FACS analysis of PD-1 and Ki-67 expression in tumor infiltrating CD8 T cells from three patients (p8, p28, p10). G. Cell cycle profile for PD-1 positive CD8 T cells from one representative tumor (p28), as measured by DNA staining with DAPI and distinguishing G0/G1 phase, S phase, and G2/M phase. H. Gene enrichment across dysfunctional CD8 T cells that are stratified by their dysfunctional score load. Data depict highly variable genes, sorted by the decile they peak in and then by their enrichment in that decile. I. Gene enrichments as in panel H, now for Tregs stratified by Treg score.
A. Sorting strategy of immune cells from tumor suspensions. Top panels show the gating on immune, single, and live cells respectively, followed by gating for T cells (CD45+/CD3+) and non-T cells (CD45+/CD3-) from tumor single cell suspensions, as shown for three patients (bottom). B. Confusion matrix of all metacells as shown in Figure 1B. C-D. Metacell size distribution (C) and metacell ribosomal load compared to mean total UMI (D). Metacells are colored as in panel B. E. Confusion matrix of T and NK metacells. F. Distribution of FACS indices (measured by index-sorting) for CD4 and CD8 across different T cell types and NK cells for a representative patient and staining panel. Values are logicle transformed (using the flowCore R package from Bioconductor). Colors represent cell types as in panel E. G. Scatter plot comparing mean absolute gene UMIs (log2) of naïve-like CD4 and naïve-like CD8 T cells, based on FACS indices for CD4 and CD8. H. Naïve gene enrichment as compared to non-naïve T cells versus naïve mean gene expression. I. Genes characterizing memory T cells, showing the top 70 enriched genes, averaged across the 3 memory T metacells. J. Genes characterizing dysfunctional CD4 T cells, showing the top 70 enriched genes of the dysfunctional CD4 metacell. K. Comparison of cytotoxic T cell and NK cell gene enrichment, showing the top 20 enriched genes within each group.
A. Type I IFN-induced gene expression intensity per patient. Showing the fraction of type I IFN-induced genes UMIs out of total UMIs across all patient cells, ordering patients by their median percentage. B. Genes in monocytes and B cells that are most highly correlated with dysfunctional load across patients. Using patients with more than 50 cells in each group, ending up with 14 patients for monocytes and 12 for B cells, showing total UMI count of genes in parentheses. C. T cell subtype composition in treatment-naïve patients. Percentage of CD3+ cells in total CD45+ cells (analyzed by FACS) and percentage of immune infiltrates (analyzed by histology, missing values shown in grey) are depicted D. Similar to panel C, comparison of T cell subtype composition between 2 metastases from the same patient. Two patients, p12 and p17, are shown.
A. 2D projection of 16,142 non-T/NK cells partitioned into 100 metacells and 7 metacell groups annotated and marked by color code. B. Confusion matrix for non-T/NK metacells. C. Marker gene expression enrichment heat map for non-T/NK cells. Each column is a cell and each row is a gene. Cells are separated into different groups, colors as in panel A. D. Expression (molecules/1,000 UMIs) of marker genes in B , plasma , and different myeloid metacells. E. Top differentially expressed genes in macrophages, monocytes, and dendritic cells, as compared to the other two groups. These genes comprise the macrophage, monocyte and DC scores used in panel G. F. Gene-gene correlation on gene enrichments in metacells over monocyte, macrophage, and dendritic cell metacells. G. Scatter plot comparing the monocyte, macrophage and DC scores per metacell as shown for myeloid metacells. H. Stratification of classical monocyte cells by the percentage of UMIs from the monocyte program, showing fraction of UMIs of the monocyte program genes, LYZ, and CEBPB in each quantile, difference between adjacent quantiles is tested for significance by Chisq-test (*P < 0.05; **P < 0.01; ***P < 1 × 10−3).
A. Gene-gene correlation matrix for the top variable genes in CD8 T cells, identical to Figure 2A. Shown here with all gene names. B. Gene correlation to LAG3 on single cells (using Spearman correlation on UMI counts, y-axis) and on metacells (using Pearson correlation on gene enrichment, x-axis). Genes comprising the dysfunctional score are marked in red. C-D. Sixty genes that correlated most strongly with the dysfunctional score (C) and cytotoxic score (D) over CD8 cells. Genes comprising these scores are colored, and were removed from the score when calculating their own correlation to it. Number in parenthesis is the rank of the gene in the metacell correlation to the anchor gene defining the dysfunctional and cytotoxic scores (LAG3 or FGFBP2). E. Similar to Figure 2, showing the top 30 genes that are most correlated with an alternative anchor gene from dysfunctional genes, HAVCR2, across CD8 T metacells. F. Similar to panel E, but showing the top 30 genes most correlated with an alternative anchor gene from cytotoxic genes, CX3CR1. G. Comparison of cytotoxic and dysfunctional loads in single cells. The fraction of cytotoxic UMIs and dysfunctional UMIs is depicted, separating sub-panel per group of metacells (transitional, dysfunctional, cytotoxic). In each panel all CD8 cells are in grey and the group cells overlay them in color. H. Gene enrichment in Treg metacells of several representative genes comprising the Treg score versus Treg score. I. Gene enrichment of Tfh and Treg cells over naïve-like cells. Showing data for strongly expressed genes (mean molecules per cell >= 0.05) and labelling genes of interest and strongly enriched genes.
A. 2D projection of 17,989 cells partitioned into 175 metacells. Single cells are shown in dots. Data depict cells from: PBMCs and tumor samples of 3 melanoma patients (p13, p17, p27). B. Confusion matrix for all metacells as shown in panel A. C. Expression (molecules/1,000 transcripts) of marker genes over metacells, same genes as in Figure 1D. D. Marker gene expression heat map for cells shown in panel A. Each column is a cell and each row is a gene. E. 2D projections of the composition of immune cells for tumor and PBMCs from p13, p17, and p27 F. Cell type compositions of CD3+; CD45+ and CD3-; CD45+ cells from PBMCs and tumor of p13 and p27.
Acknowledgements
This study is funded by Merck KGaA, Darmstadt, Germany. We thank Stephanie Blankenstein for help with patient inclusion and acquisition of clinical data, Raquel Gomez and Jan Hudecek for assistance with the scanning of tissue slides and data analysis, Noa Godin for artwork, the NKI- AVL flow cytometry facility for assistance with sorting and flow cytometric analyses, the NKI-AVL Core Facility Molecular Pathology & Biobanking for supplying and processing of NKI-AVL Biobank material, and members of the Schumacher, Tanay and Amit labs for input on experiment design and analyses I.A is supported by the Chan Zuckerberg Initiative (CZI), the HHMI International Scholar award, the European Research Council Consolidator Grant (ERC-COG) 724471-HemTree2.0, an MRA Established Investigator Award (509044), the Ernest and Bonnie Beutler Research Program for Excellence in Genomic Medicine,. T.N.S is supported by the KWF Queen Wilhelmina Award (NKI 2013-6122) and ERC AdG SENSIT. A.T is supported by the ERC (scAssembly), the CZI and the flight attendant medical research institute (FAMRI). I.A and A.T are supported by Helen and Martin Kimmel awards for innovative investigation and by the SCA award of the Wolfson Foundation and Family Charitable Trust. H.L is funded by the Marie Curie Individual Fellowship (EU project 746382 - SCALTIE). I.Y is funded by the Rising Tide Foundation. Raw and processed single cell RNA-sequencing data can be downloaded from EGA: EGAS00001003363 and NCBI GEO: GSE123139.
Footnotes
Data and software availability
The MetaCell R package and its open-source code are available from https://bitbucket.org/tanaylab/metacell/src/default/. The accession number for the raw sequence data reported in this paper is EGA: EGAS00001003363. The accession number for the processed data reported in this paper is NCBI GEO: GSE123139. Scripts reproducing the analysis will be available at http://compgenomics.weizmann.ac.il/tanay/?page_id=804
Author Contributions
H.L, A.v.d.L, I.Y, Y.L, T.N.S, A.T, I.A, conceived the project and designed the experiments. H.L, A.v.d.L, I.Y, performed the experiments. H.L, A.v.d.L, I.Y, Y.L, D.G.S, A.T, T.N.S, I.A, analyzed the data. Y.L, D.G.S, Y.B, A.B, A.L, developed computational algorithms. A.v.A, M.v.d.B, L.R, J.H, and C.B were involved in patient inclusion and sample acquisition. H.M.H performed histology scoring. H.L, A.v.d.L, I.Y, Y.L, T.N.S, A.T, I.A, wrote the manuscript. T.N.S, A.T, I.A, supervised the project.
Declaration of Interests
A patent application has been filed related to this work.
References
- Ahrends T, Spanjaard A, Pilzecker B, Babala N, Bovens A, Xiao Y, Jacobs H, Borst J. CD4+T Cell Help Confers a Cytotoxic T Cell Effector Program Including Coinhibitory Receptor Downregulation and Increased Tissue Invasiveness. Immunity. 2017;47:848–861.e5. doi: 10.1016/j.immuni.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell. 2018;174:1293–1308.e36. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran Y, Sebe-Pedros A, Lubling Y, Giladi A, Chomsky E, Meir Z, Hoichman M, Lifshitz A, Tanay A. MetaCell: analysis of single cell RNA-seq data using k-NN graph partitions. BioRxiv. 2018 doi: 10.1186/s13059-019-1812-2. 437665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S, Ihmels J, Barkai N. Iterative signature algorithm for the analysis of large-scale gene expression data. Phys Rev E. 2003;67 doi: 10.1103/PhysRevE.67.031902. 031902. [DOI] [PubMed] [Google Scholar]
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J, Ahrends T, Babala N, Melief CJM, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, Nyman J, Marjanovic ND, Kowalczyk MS, Wang C, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558:454–459. doi: 10.1038/s41586-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05072-0. 2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang Y, Zheng L, Zheng C, Song J. Global characterization of T cells in non-small cell lung cancer by single-cell sequencing. Nat Med. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- Han A, Glanville J, Hansmann L, Davis MM. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol. 2014;32:684–692. doi: 10.1038/nbt.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, Araki K, Ahmed R. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu Rev Med. 2018;69:301–318. doi: 10.1146/annurev-med-012017-043208. [DOI] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitin DA, Kenigsberg E, Keren-Shaul H, Elefant N, Paul F, Zaretsky I, Mildner A, Cohen N, Jung S, Tanay A, et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343:776–779. doi: 10.1126/science.1247651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR, Weirauch MT. The Human Transcription Factors. Cell. 2018;172:650–665. doi: 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell. 2017;169:750–765.e17. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science (80-. ) 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading JL, Gálvez-Cancino F, Swanton C, Lladser A, Peggs KS, Quezada SA. The function and dysfunction of memory CD8 + T cells in tumor immunity. Immunol. Rev. 2018;283:194–212. doi: 10.1111/imr.12657. [DOI] [PubMed] [Google Scholar]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade-Feldman M, Yizhak K, Bjorgaard SL, Sullivan RJ, Getz G, Hacohen N. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell. 2018;175:998–1013. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, Salgado R, Byrne DJ, Teo ZL, Dushyanthen S, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. 2018;24:986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- Scheper W, Kelderman S, Fanchi LF, Linnemann C, Bendle G, Rooij MAJ, de Hirt C, Mezzadra R, Slagter M, Dijkstra K, et al. Low and variable tumor-reactivity of the intratumoral TCR repertoire in human cancers. Nat Med. doi: 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]
- Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, Lauer P, Brockstedt DG, Knoblaugh SE, Hämmerling GJ, et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity. 2016;45:389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al. Bystander CD8+T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- Stubbington MJT, Lönnberg T, Proserpio V, Clare S, Speak AO, Dougan G, Teichmann SA. T cell fate and clonality inference from single-cell transcriptomes. Nat Methods. 2016;13:329–332. doi: 10.1038/nmeth.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommen DS, Schumacher TN. T Cell Dysfunction in Cancer. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24:994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, Gao R, Kang B, Zhang Q, Huang JY, et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature. 2018 doi: 10.1038/s41586-018-0694-x. [DOI] [PubMed] [Google Scholar]
- Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342–1356.e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. 2D projection of 11,769 cells partitioned into 68 metacells from human NSCLC (Guo et al, 2018). Single cells are shown in dots. Cells from tumor, normal tissue, and blood are projected separately below. B. Human NSCLC T cell subtype compositions from tumor, normal tissue, and blood are shown for different patients. Patients are ordered by their fraction of CD8-dysf cells in the tumor. C. Comparison of assignments of human NSCLC T cells to metacells (columns) and to Guo et al’s original clusters (rows). Fraction of cells per metacell is color coded. D. Scatter plot comparing enriched genes in dysfunctional T cells and Tregs from human melanoma (x-axes) with their matching subtypes in human NSCLC (y-axes); barplots showing the most enriched genes in melanoma as compared to NSCLC for dysfunctional T cells and Tregs. Each panel shows the enrichment of cells in a certain group over a reference group. Naïve-like population was used as a reference group for dysfunctional T cells from melanoma, and naive-CXCR6 cells (which is the most similar population to naïve-like population in melanoma) was used as a reference for dysfunctional T cells from NSCLC. E. Percentage of proliferating cells in different T cell subtypes of human NSCLC, numbers of proliferating cells per subtype are shown on the top. F. Fraction of proliferating cells in the human NSCLC dysfunctional group classified into bins according to their dysfunctional score. G. Expanded tumor infiltrating T cells (TILs) were either untreated, treated with PMA and ionomycin, or co-cultured with autologous single cell tumor suspensions in the absence or presence of HLA-I blocking antibodies. The percentage of cells secreting TNFa or IFNg after treatment are shown.
A. T cell subtype composition for T cells with different levels of TCR mRNA transcript (percentage of TRAC and TRBC2 genes UMIs out of total UMIs); black dot marks the probability of detecting the TCR within each group of cells. B. Fraction of cells with a detected TCR per metacell versus the gene enrichment difference of CD8A plus CD8B minus CD4. C. Correlation of genes from the cell cycle gene module on T and NK metacells. D. Frequency distribution of cell cycle scores on all T cells. Cell cycle scores were calculated as the percentage of cell cycle gene UMIs out of total UMIs. Dashed line indicates the threshold for marking a cell as proliferative. E. Empiric cumulative distribution plots of cell cycle scores per group. Note that the score is negated, hence cells below the dashed line are the proliferative ones. Fraction of proliferating cells per cell group is shown in the legend. F. FACS analysis of PD-1 and Ki-67 expression in tumor infiltrating CD8 T cells from three patients (p8, p28, p10). G. Cell cycle profile for PD-1 positive CD8 T cells from one representative tumor (p28), as measured by DNA staining with DAPI and distinguishing G0/G1 phase, S phase, and G2/M phase. H. Gene enrichment across dysfunctional CD8 T cells that are stratified by their dysfunctional score load. Data depict highly variable genes, sorted by the decile they peak in and then by their enrichment in that decile. I. Gene enrichments as in panel H, now for Tregs stratified by Treg score.
A. Sorting strategy of immune cells from tumor suspensions. Top panels show the gating on immune, single, and live cells respectively, followed by gating for T cells (CD45+/CD3+) and non-T cells (CD45+/CD3-) from tumor single cell suspensions, as shown for three patients (bottom). B. Confusion matrix of all metacells as shown in Figure 1B. C-D. Metacell size distribution (C) and metacell ribosomal load compared to mean total UMI (D). Metacells are colored as in panel B. E. Confusion matrix of T and NK metacells. F. Distribution of FACS indices (measured by index-sorting) for CD4 and CD8 across different T cell types and NK cells for a representative patient and staining panel. Values are logicle transformed (using the flowCore R package from Bioconductor). Colors represent cell types as in panel E. G. Scatter plot comparing mean absolute gene UMIs (log2) of naïve-like CD4 and naïve-like CD8 T cells, based on FACS indices for CD4 and CD8. H. Naïve gene enrichment as compared to non-naïve T cells versus naïve mean gene expression. I. Genes characterizing memory T cells, showing the top 70 enriched genes, averaged across the 3 memory T metacells. J. Genes characterizing dysfunctional CD4 T cells, showing the top 70 enriched genes of the dysfunctional CD4 metacell. K. Comparison of cytotoxic T cell and NK cell gene enrichment, showing the top 20 enriched genes within each group.
A. Type I IFN-induced gene expression intensity per patient. Showing the fraction of type I IFN-induced genes UMIs out of total UMIs across all patient cells, ordering patients by their median percentage. B. Genes in monocytes and B cells that are most highly correlated with dysfunctional load across patients. Using patients with more than 50 cells in each group, ending up with 14 patients for monocytes and 12 for B cells, showing total UMI count of genes in parentheses. C. T cell subtype composition in treatment-naïve patients. Percentage of CD3+ cells in total CD45+ cells (analyzed by FACS) and percentage of immune infiltrates (analyzed by histology, missing values shown in grey) are depicted D. Similar to panel C, comparison of T cell subtype composition between 2 metastases from the same patient. Two patients, p12 and p17, are shown.
A. 2D projection of 16,142 non-T/NK cells partitioned into 100 metacells and 7 metacell groups annotated and marked by color code. B. Confusion matrix for non-T/NK metacells. C. Marker gene expression enrichment heat map for non-T/NK cells. Each column is a cell and each row is a gene. Cells are separated into different groups, colors as in panel A. D. Expression (molecules/1,000 UMIs) of marker genes in B , plasma , and different myeloid metacells. E. Top differentially expressed genes in macrophages, monocytes, and dendritic cells, as compared to the other two groups. These genes comprise the macrophage, monocyte and DC scores used in panel G. F. Gene-gene correlation on gene enrichments in metacells over monocyte, macrophage, and dendritic cell metacells. G. Scatter plot comparing the monocyte, macrophage and DC scores per metacell as shown for myeloid metacells. H. Stratification of classical monocyte cells by the percentage of UMIs from the monocyte program, showing fraction of UMIs of the monocyte program genes, LYZ, and CEBPB in each quantile, difference between adjacent quantiles is tested for significance by Chisq-test (*P < 0.05; **P < 0.01; ***P < 1 × 10−3).
A. Gene-gene correlation matrix for the top variable genes in CD8 T cells, identical to Figure 2A. Shown here with all gene names. B. Gene correlation to LAG3 on single cells (using Spearman correlation on UMI counts, y-axis) and on metacells (using Pearson correlation on gene enrichment, x-axis). Genes comprising the dysfunctional score are marked in red. C-D. Sixty genes that correlated most strongly with the dysfunctional score (C) and cytotoxic score (D) over CD8 cells. Genes comprising these scores are colored, and were removed from the score when calculating their own correlation to it. Number in parenthesis is the rank of the gene in the metacell correlation to the anchor gene defining the dysfunctional and cytotoxic scores (LAG3 or FGFBP2). E. Similar to Figure 2, showing the top 30 genes that are most correlated with an alternative anchor gene from dysfunctional genes, HAVCR2, across CD8 T metacells. F. Similar to panel E, but showing the top 30 genes most correlated with an alternative anchor gene from cytotoxic genes, CX3CR1. G. Comparison of cytotoxic and dysfunctional loads in single cells. The fraction of cytotoxic UMIs and dysfunctional UMIs is depicted, separating sub-panel per group of metacells (transitional, dysfunctional, cytotoxic). In each panel all CD8 cells are in grey and the group cells overlay them in color. H. Gene enrichment in Treg metacells of several representative genes comprising the Treg score versus Treg score. I. Gene enrichment of Tfh and Treg cells over naïve-like cells. Showing data for strongly expressed genes (mean molecules per cell >= 0.05) and labelling genes of interest and strongly enriched genes.
A. 2D projection of 17,989 cells partitioned into 175 metacells. Single cells are shown in dots. Data depict cells from: PBMCs and tumor samples of 3 melanoma patients (p13, p17, p27). B. Confusion matrix for all metacells as shown in panel A. C. Expression (molecules/1,000 transcripts) of marker genes over metacells, same genes as in Figure 1D. D. Marker gene expression heat map for cells shown in panel A. Each column is a cell and each row is a gene. E. 2D projections of the composition of immune cells for tumor and PBMCs from p13, p17, and p27 F. Cell type compositions of CD3+; CD45+ and CD3-; CD45+ cells from PBMCs and tumor of p13 and p27.