SYNOPSIS
Imaging in Radiation Oncology is essential for the evaluation of treatment response in tumors and organs at risk. This influences further treatment decisions and could possibly be used to adapt therapy. This review article focuses on the currently used imaging modalities for response assessment in Radiation Oncology and gives an overview of new and promising techniques within this field.
Keywords: CT, MRI, PET, functional imaging, response assessment, tumor, organs at risk, Radiation Oncology
1. Introduction
Computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) play a major role in the assessment of tumor response. Moreover, these imaging modalities are increasingly used to evaluate treatment-related changes in normal tissue and correlate these findings with treatment-related toxicity. Especially in Radiation Oncology, the prediction of tumor response and radiation-induced normal tissue damage arouses more and more interest. The aim is to adapt therapy in cases of more aggressive tumors or severe changes in organs at risk (OARs). Because CT is increasingly used to dictate patient positioning, many CT images are produced, which has prompted many investigators to search for predictors of tumor control and normal tissue toxicity in CT. In contrast, MRI has not been afforded the same attention as CT owing to the fact that MRI cannot be directly used for modeling dose deposition. However, with advantages of no radiation dose and superior soft tissue contrast compared to CT, and with the rising availability of combined MR linear accelerators (MR Linacs) that facilitate online imaging during irradiation, MRI is also a promising alternative for response assessment already during the course of radiotherapy.
In the next sections we will give an overview of the current and emerging imaging techniques using CT, MRI and PET for response assessment of tumor and OARs.
2. Imaging Applications for Tumor Response Assessment
2.1. CT Approaches
Tumor response in CT is principally assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) criteria1. Simplified, a complete response is therein defined as disappearance of all so-called target lesions. Stable disease is the proper term if the sum of the longest diameter of target lesions is less than 30% decreased (otherwise partial response) and less than 20% increased (otherwise progressive disease). It is an inherent and important limitation, however, that’s RECIST’s response evaluation is only two-dimensional.
Another use for CT imaging is quantitative characterization of tumor function using dynamic contrast enhanced CT (DCE-CT). DCE-CT scans are repeatedly acquired for approximately 40 seconds after injecting iodine-based contrast agents2. DCE-CT has gained popularity among radiation oncologists as a valuable tool to spatially map tumor vascular attributes like perfusion, permeability, and blood flow3. DCE-CT has shown promise in early and longitudinal assessment of tumor response to radiation therapy4, multi-parametric functional imaging characterization of radioresistant tumor subvolumes and adaptive radiotherapy2,5,6. Studies assessing chronological changes in quantitative DCE-CT metrics like transfer coefficient (K-trans) and relative tumor blood volume (rTBV) have reported a dose and treatment modality-dependent correlation in various cancer sites such as head and neck cancers (HNC) and non-small cell lung cancer (NSCLC)7,8. Nonetheless, as with all quantitative imaging biomarkers, the development of clinically applicable DCE-CT biomarker profiles in Radiation Oncology hinges on standardization and reproducibility assurance efforts that address technical validation and robustness issues9.
Dual energy CT (DE-CT) employs two x-ray sources of non-identical energies to acquire signal, rather than a single x-ray source used in traditional, single-energy CT (SE-CT)10. Because tissue x-ray attenuation varies with photon energy, dual-photon energies capture greater information about tissue density than does SE-CT11. The most common use for DE-CT is to extract iodine contrast maps and correlate them with a variable of interest12. For example, Bahig et al. described the first attempt to correlate DE-CT-derived iodine tissue concentration with oncologic outcomes in HNC13. Primary gross tumor volume (GTV) maximum voxel-wise iodine concentration, primary GTV kurtosis, nodal GTV volume, and nodal GTV iodine concentration standard deviation each predicted worse locoregional recurrence risk in a cohort of 25 laryngeal and hypopharyngeal cancer patients. DE-CT-derived iodine content also has the ability to differentiate metastatic, inflamed, and healthy lymph nodes14. Iodine maps derived by DE-CT may complement RECIST’s two-dimensional limitation by identifying cases where tumor viability has indeed decreased despite no change in tumor size12. Furthermore, they may also prove useful for risk-stratifying radiotherapy patients prior to radiation15.
An evolving approach is the use of radiomics features, which encompass a wide range of mineable multi-dimensional values that quantitatively describe tumor shape, intensity, and texture16. Predictive radiomics biomarkers have been demonstrably informative in HNC, gastric cancer and lung cancer, among others17. In a study by Ramella et al.18, a combined signature of semantic and radiomics features derived from baseline CT could be correlated to rate of volumetric changes at subsequent time points in NSCLC. A similar study found a grey level co-occurrence matrix (GLCM) texture feature improved the capacity of a clinicopathological model to predict tumor shrinkage at mid-treatment course19. Other studies tracked the kinetics of radiomics features from sequential in-treatment CT images and demonstrated an association with subsequent response to radiation in HNC and NSCLC20,21.
2.2. MRI Approaches
MRI is superior to CT for tumor response assessment in soft tissues when motion artifacts and tissue/air interfaces leading to susceptibility artifacts are negligible. As is the case for CT, the RECIST criteria1 are commonly used for response assessment of solid tumors on MRI.
Other MRI response criteria have been implemented, including criteria that are specific to certain anatomic subsites and/or tumors (such as glioma). Similar to the RECIST criteria, they are based on lesion sizes as well. For example, the Macdonald criteria22 assess the size of contrast-enhancing tumor in T1 sequences, while an updated version of the Response Assessment for Neuro-Oncology (RANO) criteria were introduced in 201023 to allow for incorporation of T2/Flair changes as well.
Newer imaging techniques, like MR perfusion and spectroscopy, are still not adopted by these criteria although they are more accurate at differentiating between true progression and treatment-induced changes24. This is especially a problem with the increasing use of immunotherapy25. High-grade glioma usually presents with increased vasculature, and the relative blood volume is greater in tumor progression or recurrence than in necrosis or pseudoprogression. This can be measured by dynamic susceptibility contrast (DSC) perfusion MRI26. Machine learning approaches have achieved some success at differentiating disease progression from pseudoprogression using diffusion-weighted imaging (DWI)27 and DSC perfusion MRI28. Arterial spin labeling measures perfusion using magnetically labeled blood as an endogenous tracer. Using MRS, active tumor tissue is defined as having an elevated choline level29; lipid-lactate peaks, a decrease in N-acetyl-aspartate and a lack of choline elevation, however, is suspect for radiation necrosis30,31.
Functional MRI has further potential to predict for tumor response. For example, high apparent diffusion coefficient (ADC) values pre-treatment derived from DWI associate with poor outcomes in HNC32,33. Treatment responders showed a higher increase in ADC during the first weeks of radiotherapy than non- or partial-responders32. Dynamic contrast enhanced (DCE) imaging is comparably more invasive because it uses an intravenous contrast agent and is inconsistent in its response prediction for most of the parameters. K-trans seems most reliable, with higher values correlating with better tumor response34–36. Other approaches include Blood Oxygen Level Dependent (BOLD) imaging which leverages the paramagnetic nature of deoxyhemoglobin to visualize a decrease in T2*37. As tumor hypoxia is known to adversely affect the outcome after radio- and/or chemotherapy, BOLD imaging may also be a predictive imaging biomarker.
MRI radiomics features may become a convenient tool for phenotyping tumors and normal tissues and integrating spatially-driven parameters to guide therapy and outcome38. In breast cancer, radiomics models have predicted tumor response to neoadjuvant and adjuvant therapy39,40. Similarly in HNC radiomics models have predicted local control and survival41,42. MRI has more acquisition parameters than CT (such as pulse sequence, echo time, relaxation time, etc.), which means that the task of standardizing reproducible features is even more challenging for MRI than CT43. Different vendor sequences or perhaps different Tesla magnets could complicate the reproducibility of extracted features44. Because MRI is more susceptible to motion artifact than CT, feature reproducibility may also depend on immobilization during acquisition. Effort is clearly needed to standardize different inconsistencies in MRI radiomics starting from imaging acquisition, imaging processing, model building, and model validation. This is a necessary step to integrate functional quantitative imaging data into clinical decision making tools toward personalized medicine.
2.3. PET Approaches
Utilization of PET for tumor response assessment has become a key component for monitoring disease progression and for restaging45,46. PET visualizes metabolic activity within the body in ways that CT and MRI may be limited47. The addition of this functional imaging modality presents a need for new standardized guidelines and criteria in tumor response assessment in order to provide patients with consistent quality care.
The first set of standardized tumor response guidelines for PET was defined by the European Organization for Research and Treatment of Cancer (EORTC) in 199948. These recommendations consider both clinical and subclinical measurements in treatment response interpretation. In 2009 the PET Response Criteria in Solid Tumors (PERCIST) criteria were introduced, which modifies the RECIST criteria to include metabolic activity with 18F-FDG-PET as an additional criterion to anatomical findings49. PET standardized uptake values (SUV) are a quantitative metric for categorizing tumor response, progression, or stabilization of disease.
A review of the EORTC and PERCIST response criteria showed strong agreement between the two despite their different approaches50. The review was limited by small patient cohorts, a heterogeneous patient populations, heterogenous equipment, and other factors, so it is still necessary to investigate differences between the two on a larger, homogeneous patient population in order to validate possible interchangeability and use for practice.
PET/CT has become standard for response assessment in most lymphomas51. According to the Lugano Classification, a complete response is defined as complete metabolic response of initially FDG-avid lymphoma independent of residual masses, whereas progressive disease is considered in case of increased FDG uptake compared to baseline values or the appearance of new FDG-avid lesions consistent with lymphoma51.
To limit the incidence of false positive PET readings due to normal-tissue damage and immune response caused by radiation therapy, response assessment must be delayed for some period after treatment52. Local inflammation and radiotherapy changes will be FDG-avid and limit the positive predictive value for local residual tumor. However, PET remains a strong diagnostic tool for identifying distant metastatic disease and progression. A 16-week FDG PET reassessment in locally advanced HNC showed good diagnostic accuracy and can be used to guide management with the high negative probability of complete response53. Although FDG is the most commonly used and studied radiotracer to monitor tumor response, others have shown some utility. 18F-fluorothymidine (18F-FLT), is promising as a more reliable tracer in measuring tumor proliferation than 18F-FDG, but is limited by its relatively low uptake in cells and is even more hindered by radiotherapy and cytotoxic chemotherapy54.
Cancer cells have been noted to adapt metabolically to hypoxic environments despite functioning mitochondria and the presence of oxygen. This phenomenon, known as the Warburg Effect55, contributes to therapy resistance and tumor progression56. Measurement of hypoxia has historically been performed by assessing the oxygen partial pressure (pO2) through a polarographic needle electrode which is invasive and limited to a small portion of the tumor57. However, cellular hypoxia measurements can now be performed with PET/CT using radiotracers like 18F-luoroazomycin arabinoside (FAZA) and 18F-fluoromisonidazole (FMISO). Both tracers fall under the nitroimidazole family, which work by the reduction of its NO2-group in the hypoxic environment, leading to covalent binding with macromolecules in the cell58. As a second-generation hypoxia PET radiotracer, FAZA has improved contrast when compared to the more widely used FMISO. The potential of both tracers to predict treatment outcome has been shown in several studies59, but must be further examined before implementing into clinical use.
3. Imaging Applications for Organ at risk Assessment
3.1. CT Approaches
Advanced treatment options such as proton therapy60,61 and MR-guided radiation62 create new opportunities to spare normal tissues. As life expectancy for cancer survivors increases, so does demand for predicting the development of treatment-induced side effects. OAR morphologic changes may be informative for this goal63,64, but so may other image parameters, such as intensity on CT65,66.
Just as radiomics has been investigated for monitoring tumor response, it may also be serviceable for monitoring OARs16,67. Aerts et al.68 was the first to perform a large extraction of radiomics features from tumors – including intensity, morphological and texture features – to predict survival. Since then, a wealth of radiomics studies have investigated tumor features, yet radiomics is comparatively less utilized to investigate normal tissue response or toxicity. Van Dijk et al. showed that radiomics texture features were associated with radiation-induced side effects in addition to dosimetric parameters and could improve current normal tissue complication probability (NTCP) models69. Radiomics features from images taken during treatment have been exploited to predict radiation-induced xerostomia63,65,70, and Cunliffe et al.66 showed that the change in lung texture during treatment correlated with radiation-induced pneumonitis. Colen et al. showed in pilot study that radiomics analysis may also predict immunotherapy-induced toxicities71. As previously mentioned in the above discussion of radiomics for tumor response assessment, the development of radiomics for predicting or normal tissue toxicity is still in its early stages. Larger, more robust image validation sets and feature parameter standardization are needed. Nevertheless, current and past studies have highlighted the potential of radiomics to improve conventional toxicity modeling.
3.2. MRI Approaches
Like CT, MRI can be used to identify radiation-induced changes in size of the structures. For example, several studies have reported on a volume loss of the salivary glands during radiotherapy72. Muscles have likewise been described to shrink in a dose-dependent manner73. Volumetric changes can be easily measured in standard anatomical T1 or T2 sequences. Functional MRI like DWI or intravoxel incoherent motion imaging (IVIM) can quantify free extracellular water as a biomarker for edema. DWI is one of the most reported functional sequences used for estimation of damage in OAR. Exemplarily, the ADC of the salivary glands increased during the course of radiotherapy and could be correlated with the degree of xerostomia74. Another functional MR sequence commonly used is DCE imaging, which gives details about vasculature that can be affected in the case of radiation-induced inflammation, but more research is necessary to consistently report on the changes and correlate them with outcomes. A dedicated sequence to describe the fatty degeneration of tissue is DIXON, but this sequence has only rarely been used for OAR assessment so far75. To better compare study results between different institutions, reporting percentage changes could be helpful. This could compensate for some inter-institutional variability caused by different MRI machines, radiofrequency coils and image acquisition parameters. Nevertheless, segmentation of OARs can also influence normal tissue complication probability outputs76, as can post-processing methods77.
MRI radiomics has been explored for predicting normal tissue toxicities as well. One study correlated fat content in parotid glands and xerostomia at 12 months post-therapy78. Another study investigated femoral bone damage post-radiotherapy by exploring radiomics features extracted from different MRI sequences79. As extensively discussed in the MRI for tumor response assessment section, standardization of radiomics acquisition and post-processing is crucial.
3.3. PET/SPECT Approaches
PET/CT and SPECT/CT are imaging modalities that are not currently used clinically for OAR toxicity assessment. However, there is potential for both modalities to quantify toxicity using the anatomical information from the CT and the functional information from the PET or SPECT to help clinicians ascertain the damage to normal tissue by providing metabolic80 or perfusion81 information.
For example, 18F-FDG-PET has been used to investigate radiation-induced normal tissue toxicity to the parotid gland; a decrease in 18F-FDG-uptake post-radiotherapy correlated with the development of late xerostomia82.
68Ga-PSMA is another radionuclide that can be used to quantify metabolic activity in prostate cancer and in salivary and lacrimal glands83. PSMA-PET/CT further allows for visualization of seromucous glands in the soft palate, pharyngeal wall, nasal mucosa, and suparaglottic larynx, which is not possible with other imaging modalities83. This is a significant step along the path to quantifying radiation-induced metabolic activity changes in these minor glands.
Another possible application for PET/CT in OAR assessment was described for the prediction of radiation induced lung toxicity (RILT)84. Petit et al. found that patients were more likely to develop RILT if the lung voxels with the highest 18F-FDG uptake in the pre-treatment scan received 2 Gy or more84. In another study, SPECT/CT was used to evaluate the association between perfusion and radiation dose in patients who developed radiation pneumonitis post-treatment81.
Furthermore, PET/CT, using 15O-H2O as tracer, can be utilized to detect perfusion changes in the heart after breast radiotherapy and thus also help to identify radiation-induced heart damage85. Similarly, patients showed a significantly higher 18F-FDG uptake in myocardial regions within the radiation field as opposed to non-irradiated regions86.
4. Conclusion
CT, MRI, PET or a combination of these modalities, is commonly used for response assessment after radiotherapy, depending on treatment site. With the recent technical advances, newer imaging methods and quantitative approaches have become increasingly available such as DE-CT, functional MRI and radiomics. Despite promising results regarding the prediction of tumor response and toxicity with newer techniques, larger studies are yet lacking to clinically validate them and thereafter scale them to the standard of care.
Figure 1:

Example of a patient with a lung adenocarcinoma metastasis at the left skull base (red arrow). Left: 140 keV DE-CT (window level: −100 – 250 HU), middle: 60 keV DE-CT (window level: −100 – 250 HU), right: Iodine map of DE-CT (window level: −10 – 100 HU).
Figure 2:
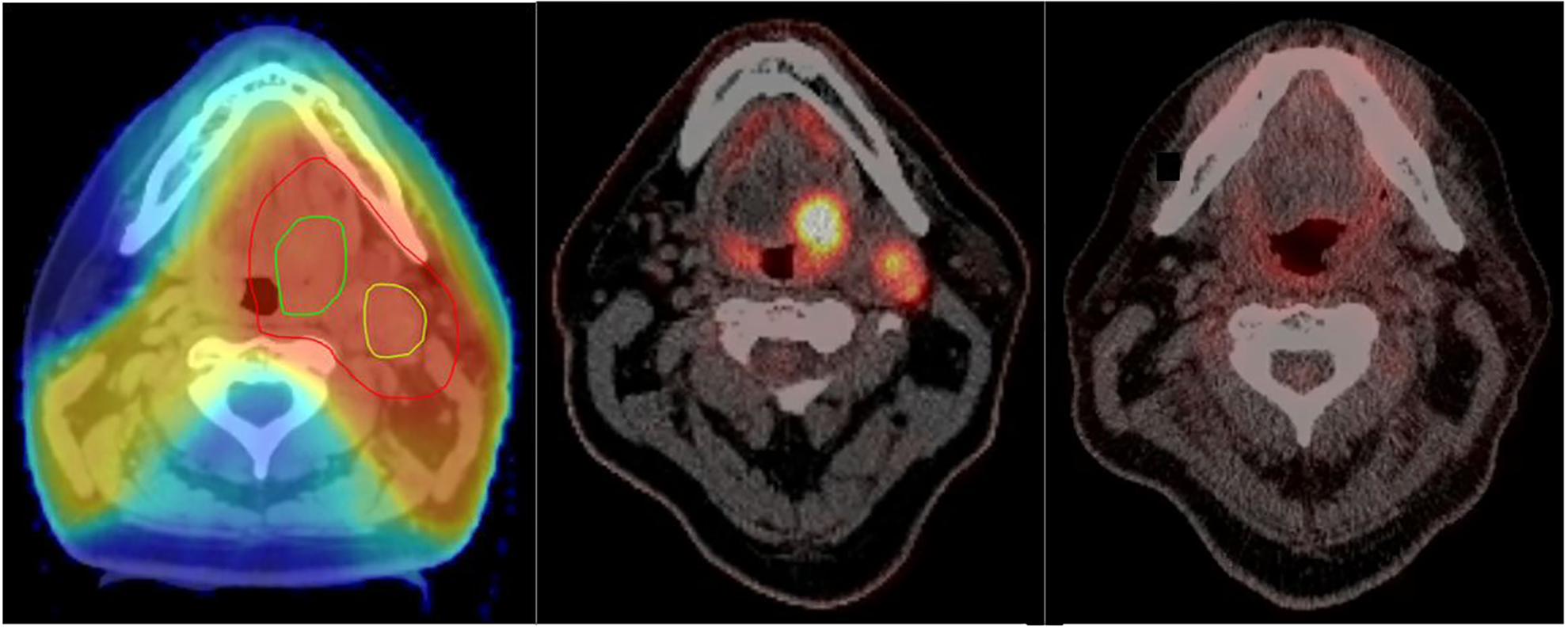
Example of a patient with T2 base of tongue (BOT) tumor and ipsilateral neck lymph node. Left: Treatment plan with BOT tumor delineated in dark green, lymph node in light green and clinical target volume (CTV) in red; middle: PET/CT before treatment (window level for PET: 0.5 – 22 SUV); right: PET/CT 3 months after radiochemotherapy (window level for PET: 0.5 – 22 SUV).
Figure 3:
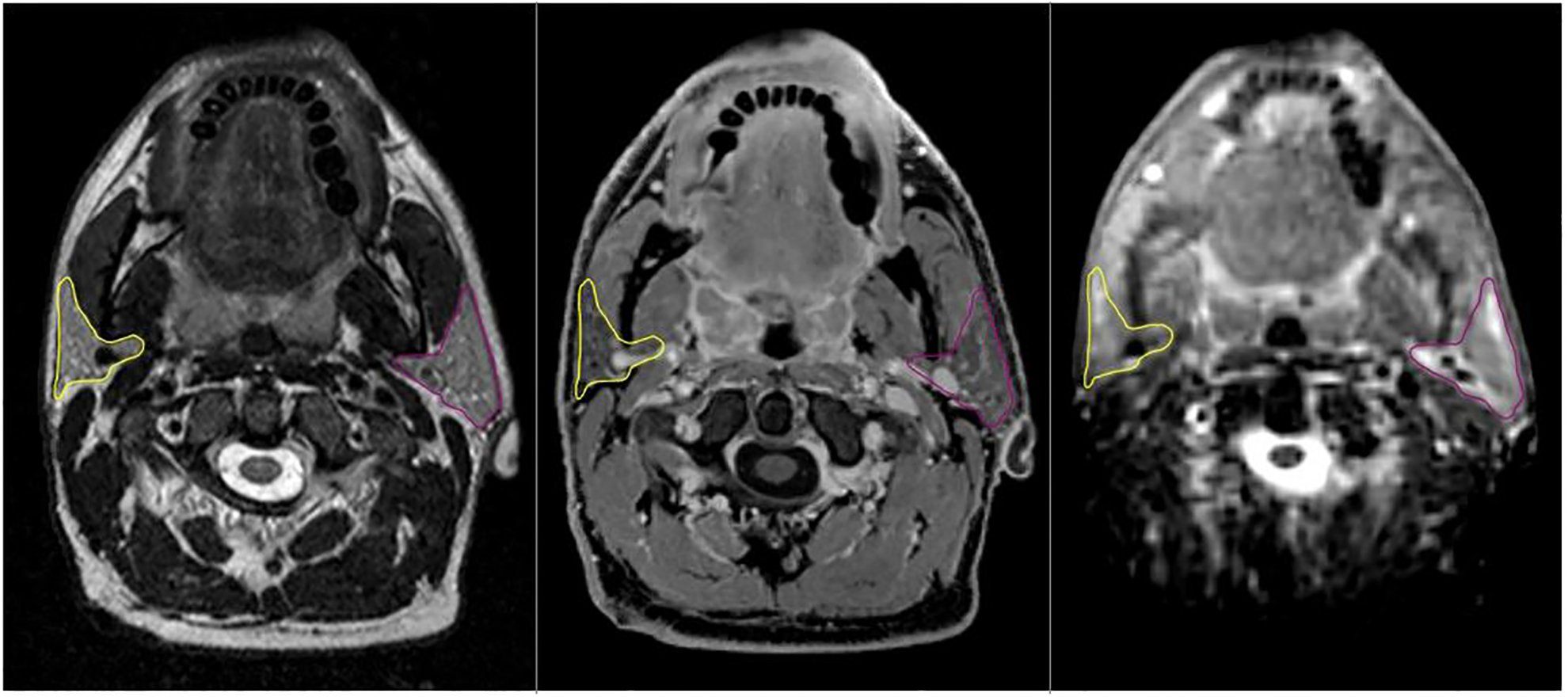
54-year old patient with bilateral tonsil cancer stage IVA. Parotid gland structures are highlighted in yellow and pink and were rigidly propagated from T2 image (left) to DIXON (middle) and ADC map (right side).
Key Points.
CT, MRI and PET are the current state-of-the-art imaging methods in Radiation Oncology for treatment response assessment, depending on cancer site.
New and promising imaging techniques like functional MRI and radiomics are currently investigated for prediction of treatment response in tumors and organs at risk.
Still, more research needs to be conducted to implement these new imaging techniques into clinical routine.
DISCLOSURE STATEMENT
Sonja Stieb is funded by the Swiss Cancer League (BIL KLS-4300-08-2017). Lisanne van Dijk received funding from the NOW Rubicon Award and Nadia Roxanne Livingstone a NIH/NIDCR diversity supplement (3R01DE025248-03). Clifton David Fuller received funding from the National Institute for Dental and Craniofacial Research Award (1R01DE025248-01/R56DE025248) and Academic-Industrial Partnership Award (R01 DE028290), the National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679), the NIH Big Data to Knowledge (BD2K) Program of the National Cancer Institute (NCI) Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825), the NCI Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (1R01CA218148), the NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672), the NIH/NCI Head and Neck Specialized Programs of Research Excellence (SPORE) Developmental Research Program Award (P50 CA097007) and the National Institute of Biomedical Imaging and Bioengineering (NIBIB) Research Education Program (R25EB025787). Dr. Fuller has received direct industry grant support, speaking honoraria and travel funding from Elekta AB.
References
- 1.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 2.Thorwarth D Functional imaging for radiotherapy treatment planning: current status and future directions-a review. Br J Radiol. 2015;88(1051):20150056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connor JP, Tofts PS, Miles KA, Parkes LM, Thompson G, Jackson A. Dynamic contrast-enhanced imaging techniques: CT and MRI. Br J Radiol. 2011;84 Spec No 2:S112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coolens C, Driscoll B, Chung C, et al. Automated voxel-based analysis of volumetric dynamic contrast-enhanced CT data improves measurement of serial changes in tumor vascular biomarkers. International journal of radiation oncology, biology, physics. 2015;91(1):48–57. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Pan C, Balter JM, et al. Liver function after irradiation based on computed tomographic portal vein perfusion imaging. International journal of radiation oncology, biology, physics. 2008;70(1):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Even AJG, Reymen B, La Fontaine MD, et al. Clustering of multi-parametric functional imaging to identify high-risk subvolumes in non-small cell lung cancer. Radiother Oncol. 2017;125(3):379–384. [DOI] [PubMed] [Google Scholar]
- 7.Abramyuk A, Hietschold V, Appold S, von Kummer R, Abolmaali N. Radiochemotherapy-induced changes of tumour vascularity and blood supply estimated by dynamic contrast-enhanced CT and fractal analysis in malignant head and neck tumours. Br J Radiol. 2015;88(1045):20140412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang SH, Yoo MR, Park CH, Jeon TJ, Kim SJ, Kim TH. Dynamic contrast-enhanced CT to assess metabolic response in patients with advanced non-small cell lung cancer and stable disease after chemotherapy or chemoradiotherapy. Eur Radiol. 2013;23(6):1573–1581. [DOI] [PubMed] [Google Scholar]
- 9.Shukla-Dave A, Obuchowski NA, Chenevert TL, et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Elmpt W, Landry G, Das M, Verhaegen F. Dual energy CT in radiotherapy: Current applications and future outlook. Radiother Oncol. 2016;119(1):137–144. [DOI] [PubMed] [Google Scholar]
- 11.Grajo JR, Patino M, Prochowski A, Sahani DV. Dual energy CT in practice: basic principles and applications. Appl Radiol. 2016;45(7):6–12. [Google Scholar]
- 12.Agrawal MD, Pinho DF, Kulkarni NM, Hahn PF, Guimaraes AR, Sahani DV. Oncologic applications of dual-energy CT in the abdomen. Radiographics. 2014;34(3):589–612. [DOI] [PubMed] [Google Scholar]
- 13.Bahig H, Lapointe A, Bedwani S, et al. Dual-energy computed tomography for prediction of loco-regional recurrence after radiotherapy in larynx and hypopharynx squamous cell carcinoma. Eur J Radiol. 2019;110:1–6. [DOI] [PubMed] [Google Scholar]
- 14.Tawfik AM, Razek AA, Kerl JM, Nour-Eldin NE, Bauer R, Vogl TJ. Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol. 2014;24(3):574–580. [DOI] [PubMed] [Google Scholar]
- 15.Lapointe A, Bahig H, Blais D, et al. Assessing lung function using contrast-enhanced dual-energy computed tomography for potential applications in radiation therapy. Med Phys. 2017;44(10):5260–5269. [DOI] [PubMed] [Google Scholar]
- 16.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Wang S, Dong D, et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics. 2019;9(5):1303–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramella S, Fiore M, Greco C, et al. A radiomic approach for adaptive radiotherapy in non-small cell lung cancer patients. PLoS One. 2018;13(11):e0207455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sicilia R, Cordelli E, Ramella S, et al. Exploratory Radiomics for Predicting Adaptive Radiotherapy in Non-Small Cell Lung Cancer. Paper presented at: 2018 IEEE 31st International Symposium on Computer-Based Medical Systems (CBMS); 18–21 June 2018, 2018. [Google Scholar]
- 20.Paul J, Yang C, Wu H, et al. Early Assessment of Treatment Responses During Radiation Therapy for Lung Cancer Using Quantitative Analysis of Daily Computed Tomography. International journal of radiation oncology, biology, physics. 2017;98(2):463–472. [DOI] [PubMed] [Google Scholar]
- 21.Elhalawani HE, Mohamed ASR, Volpe S, et al. PO-0991: Serial tumor radiomic features predict response of head and neck cancer treated with Radiotherapy. Radiother Oncol. 2018;127:S551. [Google Scholar]
- 22.Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 23.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 24.van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017;27(10):4129–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30(3):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu X, Wong KK, Young GS, Guo L, Wong ST. Support vector machine multiparametric MRI identification of pseudoprogression from tumor recurrence in patients with resected glioblastoma. J Magn Reson Imaging. 2011;33(2):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cha J, Kim ST, Kim HJ, et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol. 2014;35(7):1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamada K, Houkin K, Abe H, Sawamura Y, Kashiwaba T. Differentiation of cerebral radiation necrosis from tumor recurrence by proton magnetic resonance spectroscopy. Neurol Med Chir (Tokyo). 1997;37(3):250–256. [DOI] [PubMed] [Google Scholar]
- 30.Kazda T, Bulik M, Pospisil P, et al. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: Single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. Neuroimage Clin. 2016;11:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura T, Sako K, Tohyama Y, et al. Diagnosis and treatment of progressive space-occupying radiation necrosis following stereotactic radiosurgery for brain metastasis: value of proton magnetic resonance spectroscopy. Acta Neurochir (Wien). 2003;145(7):557–564; discussion 564. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Loevner L, Quon H, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15(3):986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombardi M, Cascone T, Guenzi E, et al. Predictive value of pre-treatment apparent diffusion coefficient (ADC) in radio-chemiotherapy treated head and neck squamous cell carcinoma. Radiol Med. 2017;122(5):345–352. [DOI] [PubMed] [Google Scholar]
- 34.Ng SH, Lin CY, Chan SC, et al. Clinical utility of multimodality imaging with dynamic contrast-enhanced MRI, diffusion-weighted MRI, and 18F-FDG PET/CT for the prediction of neck control in oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiation. PLoS One. 2014;9(12):e115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng SH, Lin CY, Chan SC, et al. Dynamic contrast-enhanced MR imaging predicts local control in oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiotherapy. PLoS One. 2013;8(8):e72230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S, Loevner LA, Quon H, et al. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2010;31(2):262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buxton RB. The physics of functional magnetic resonance imaging (fMRI). Reports on progress in physics Physical Society (Great Britain). 2013;76(9):096601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avanzo M, Stancanello J, El Naqa I. Beyond imaging: The promise of radiomics. Phys Med. 2017;38:122–139. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Li Z, Qu J, et al. Radiomics of multi-parametric MRI for pretreatment prediction of pathological complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res. 2019. [DOI] [PubMed] [Google Scholar]
- 40.Xiong Q, Zhou X, Liu Z, et al. Multiparametric MRI-based radiomics analysis for prediction of breast cancers insensitive to neoadjuvant chemotherapy. Clin Transl Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 41.Zhang LL, Huang MY, Li Y, et al. Pretreatment MRI radiomics analysis allows for reliable prediction of local recurrence in non-metastatic T4 nasopharyngeal carcinoma. EBioMedicine. 2019;42:270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuo EH, Zhang WJ, Li HJ, et al. Radiomics on multi-modalities MR sequences can subtype patients with non-metastatic nasopharyngeal carcinoma (NPC) into distinct survival subgroups. Eur Radiol. 2019. [DOI] [PubMed] [Google Scholar]
- 43.Duron L, Balvay D, Vande Perre S, et al. Gray-level discretization impacts reproducible MRI radiomics texture features. PLoS One. 2019;14(3):e0213459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B, Tan Y, Tsai WY, et al. Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep. 2016;6:23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med. 2009;50(1):88–99. [DOI] [PubMed] [Google Scholar]
- 46.Yao M, Graham MM, Smith RB, et al. Value of FDG PET in assessment of treatment response and surveillance in head-and-neck cancer patients after intensity modulated radiation treatment: a preliminary report. International journal of radiation oncology, biology, physics. 2004;60(5):1410–1418. [DOI] [PubMed] [Google Scholar]
- 47.Bussink J vHC, Kaanders JH, Oyen WJ. PET-CT for response assessment and treatment adaptation in head and neck cancer. Lancet Oncol. 2010;11(7):661–669. [DOI] [PubMed] [Google Scholar]
- 48.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–1782. [DOI] [PubMed] [Google Scholar]
- 49.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JH. Comparison of the EORTC criteria and PERCIST in solid tumors: a pooled analysis and review. Oncotarget. 2016;7(36):58105–58110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laing RE, Nair-Gill E, Witte ON, Radu CG. Visualizing cancer and immune cell function with metabolic positron emission tomography. Curr Opin Genet Dev. 2010;20(1):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prestwich RJ, Subesinghe M, Gilbert A, Chowdhury FU, Sen M, Scarsbrook AF. Delayed response assessment with FDG-PET-CT following (chemo) radiotherapy for locally advanced head and neck squamous cell carcinoma. Clin Radiol. 2012;67(10):966–975. [DOI] [PubMed] [Google Scholar]
- 54.Weber WA. Monitoring tumor response to therapy with 18F-FLT PET. J Nucl Med. 2010;51(6):841–844. [DOI] [PubMed] [Google Scholar]
- 55.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41(3):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. [DOI] [PubMed] [Google Scholar]
- 57.Sun X, Niu G, Chan N, Shen B, Chen X. Tumor hypoxia imaging. Mol Imaging Biol. 2011;13(3):399–410. [DOI] [PubMed] [Google Scholar]
- 58.Lopci E, Grassi I, Chiti A, et al. PET radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence. Am J Nucl Med Mol Imaging. 2014;4(4):365–384. [PMC free article] [PubMed] [Google Scholar]
- 59.Stieb S, Eleftheriou A, Warnock G, Guckenberger M, Riesterer O. Longitudinal PET imaging of tumor hypoxia during the course of radiotherapy. Eur J Nucl Med Mol Imaging. 2018;45(12):2201–2217. [DOI] [PubMed] [Google Scholar]
- 60.Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol. 2013;107(3):267–273. [DOI] [PubMed] [Google Scholar]
- 61.Lomax A Intensity modulation methods for proton radiotherapy. Phys Med Biol. 1999;44(1):185–205. [DOI] [PubMed] [Google Scholar]
- 62.Pollard JM, Wen Z, Sadagopan R, Wang J, Ibbott GS. The future of image-guided radiotherapy will be MR guided. Br J Radiol 2017;90(1073):20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dijk LV, Brouwer CL, van der Laan HP, et al. Geometric Image Biomarker Changes of the Parotid Gland Are Associated With Late Xerostomia. International journal of radiation oncology, biology, physics. 2017;99(5):1101–1110. [DOI] [PubMed] [Google Scholar]
- 64.Broggi S, Fiorino C, Dell’Oca I, et al. A two-variable linear model of parotid shrinkage during IMRT for head and neck cancer. Radiother Oncol. 2010;94(2):206–212. [DOI] [PubMed] [Google Scholar]
- 65.Wu H, Chen X, Yang X, et al. Early Prediction of Acute Xerostomia During Radiation Therapy for Head and Neck Cancer Based on Texture Analysis of Daily CT. International journal of radiation oncology, biology, physics. 2018;102(4):1308–1318. [DOI] [PubMed] [Google Scholar]
- 66.Cunliffe A, Armato SG 3rd, Castillo R, Pham N, Guerrero T, Al-Hallaq HA. Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. International journal of radiation oncology, biology, physics. 2015;91(5):1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278(2):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Dijk LV, Brouwer CL, van der Schaaf A, et al. CT image biomarkers to improve patient-specific prediction of radiation-induced xerostomia and sticky saliva. Radiother Oncol. 2017;122(2):185–191. [DOI] [PubMed] [Google Scholar]
- 70.Rosen BS, Hawkins PG, Polan DF, et al. Early Changes in Serial CBCT-Measured Parotid Gland Biomarkers Predict Chronic Xerostomia After Head and Neck Radiation Therapy. International journal of radiation oncology, biology, physics. 2018;102(4):1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colen RR, Fujii T, Bilen MA, et al. Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Invest New Drugs. 2018;36(4):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stieb S, Elgohari B, Fuller CD. Repetitive MRI of organs at risk in head and neck cancer patients undergoing radiotherapy. Clin Transl Radiat Oncol. 2019;18:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Popovtzer A, Cao Y, Feng FY, Eisbruch A. Anatomical changes in the pharyngeal constrictors after chemo-irradiation of head and neck cancer and their dose-effect relationships: MRI-based study. Radiother Oncol. 2009;93(3):510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Ou D, Gu Y, He X, Peng W. Evaluation of Salivary Gland Function Using Diffusion-Weighted Magnetic Resonance Imaging for Follow-Up of Radiation-Induced Xerostomia. Korean J Radiol. 2018;19(4):758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou N, Chu C, Dou X, et al. Early evaluation of radiation-induced parotid damage in patients with nasopharyngeal carcinoma by T2 mapping and mDIXON Quant imaging: initial findings. Radiat Oncol. 2018;13(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brouwer CL, Steenbakkers RJ, Gort E, et al. Differences in delineation guidelines for head and neck cancer result in inconsistent reported dose and corresponding NTCP. Radiother Oncol. 2014;111(1):148–152. [DOI] [PubMed] [Google Scholar]
- 77.Zeilinger MG, Lell M, Baltzer PA, Dorfler A, Uder M, Dietzel M. Impact of post-processing methods on apparent diffusion coefficient values. Eur Radiol. 2017;27(3):946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Dijk LV, Thor M, Steenbakkers R, et al. Parotid gland fat related Magnetic Resonance image biomarkers improve prediction of late radiation-induced xerostomia. Radiother Oncol. 2018;128(3):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdollahi H, Mahdavi SR, Shiri I, Mofid B, Bakhshandeh M, Rahmani K. Magnetic resonance imaging radiomic feature analysis of radiation-induced femoral head changes in prostate cancer radiotherapy. J Cancer Res Ther. 2019;15(Supplement):S11–S19. [DOI] [PubMed] [Google Scholar]
- 80.Mawlawi OWR, Wong WH. Principles of PET/CT In: Kim EE, Lee MC, Inoue R, Wong WH (Eds.) Clinical PET. Springer; 2004; ISBN 9781441923554. [Google Scholar]
- 81.Farr KP, Khalil AA, Moller DS, et al. Time and dose-related changes in lung perfusion after definitive radiotherapy for NSCLC. Radiother Oncol. 2018;126(2):307–311. [DOI] [PubMed] [Google Scholar]
- 82.Cannon B, Schwartz DL, Dong L. Metabolic imaging biomarkers of postradiotherapy xerostomia. International journal of radiation oncology, biology, physics. 2012;83(5):1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klein Nulent TJW, Valstar MH, de Keizer B, et al. Physiologic distribution of PSMA-ligand in salivary glands and seromucous glands of the head and neck on PET/CT. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(5):478–486. [DOI] [PubMed] [Google Scholar]
- 84.Petit SF, van Elmpt WJ, Oberije CJ, et al. [(1)(8)F]fluorodeoxyglucose uptake patterns in lung before radiotherapy identify areas more susceptible to radiation-induced lung toxicity in non-small-cell lung cancer patients. International journal of radiation oncology, biology, physics. 2011;81(3):698–705. [DOI] [PubMed] [Google Scholar]
- 85.Zyromska A, Malkowski B, Wisniewski T, Majewska K, Reszke J, Makarewicz R. (15)O-H2O PET/CT as a tool for the quantitative assessment of early post-radiotherapy changes of heart perfusion in breast carcinoma patients. Br J Radiol. 2018;91(1088):20170653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Unal K, Unlu M, Akdemir O, Akmansu M. 18F-FDG PET/CT findings of radiotherapy-related myocardial changes in patients with thoracic malignancies. Nucl Med Commun. 2013;34(9):855–859. [DOI] [PubMed] [Google Scholar]


