Abstract
The lipid mediator sphingosine 1-phosphate (S1P) affects cellular functions in most systems. Interest in its therapeutic potential has increased following the discovery of its G protein-coupled receptors and the recent availability of agents that can be safely administered in humans. Although the role of S1P in bone biology has been the focus of much less research than its role in the nervous, cardiovascular and immune systems, it is becoming clear that this lipid influences many of the functions, pathways and cell types that play a key role in bone maintenance and repair. Indeed, S1P is implicated in many osteogenesis-related processes including stem cell recruitment and subsequent differentiation, differentiation and survival of osteoblasts, and coupling of the latter cell type with osteoclasts. In addition, S1P’s role in promoting angiogenesis is well-established. The pleiotropic effects of S1P on bone and blood vessels have significant potential therapeutic implications, as current therapeutic approaches for critical bone defects show significant limitations. Because of the complex effects of S1P on bone, the pharmacology of S1P–like agents and their physico-chemical properties, it is likely that therapeutic delivery of S1P agents will offer significant advantages compared to larger molecular weight factors. Hence, it is important to explore novel methods of utilizing S1P agents therapeutically, and improve our understanding of how S1P and its receptors modulate bone physiology and repair.
Keywords: Bone regeneration, bone defect, osteoblasts, osteoclasts, sphingosine 1-phosphate
Graphical abstract
1 Introduction
The incidence of non-union fractures is relatively low (20 per 100000 cases) (1). However, in severe fractures or in limb salvage following bone cancer, the incidence can be many fold higher (2). Current therapeutic options for non-union and other critical bone defects, mainly autologous grafts and allografts, suffer from drawbacks of both medical and logistical natures (3). There has been much hope that novel treatments based on the use of peptide or protein growth factors, mainly in combination with bone grafts or scaffolds, would show clinical benefit. Despite showing positive results, these strategies are limited by the need for high doses, as well as related ectopic growth (4–6). A potential promising alternative is the manipulation of lower molecular weight, non peptidic mediators, such as the bioactive lipid sphingosine 1-phosphate (S1P) (7).
S1P is the product of sphingosine kinase (SK)-mediated phosphorylation of sphingosine, itself derived from cell membrane sphingolipids (8, 9). S1P is an important player in cell death (10) and proliferation (11), with evidence that the balance between S1P and its pro-apoptotic precursors (sphingosine and ceramide) critically controls cell fate (12). Furthermore, S1P signalling is involved in cell adhesion and motility, smooth muscle contraction, and platelet aggregation (13).
S1P and its 5 known receptors (S1P1–5) are expressed in several systems, including the vascular, immune, nervous, and reproductive systems (14). S1P1 receptors have been detected in blood vessels and mesenchymal cells around day 12 of embryonic development (15). Their genetic deletion leads to defective limb chondrocyte development, and embryonic lethality from defective vasculature. Limb defects occur both following non-specific deletion and in mice specifically lacking endothelial S1P1 receptors, and there is evidence that S1P1 receptors may play a role in chondrocyte organization. Indeed, by day 16 of murine embryogenesis, S1P1 receptor mRNA expression is abundant in bones undergoing ossification (16). As will be seen throughout this review, S1P receptors have also been identified in the key cells involved in bone remodelling and repair, including S1P1–3 receptors expressed in osteoblasts, and S1P1 and S1P2 receptors in osteoclast precursor cells. Under basal conditions, the expression of S1P4 and S1P5 receptors seems to be limited to hematopoietic and lymphatic tissues (S1P4) and the central nervous system (S1P5) (17) and there is currently little evidence that either subtype plays a direct role in bone remodelling or repair. Semi-quantitative RT-PCR studies failed to detect mRNA for these two subtypes in primary rat osteoblasts (18), while they detected mRNA for all known S1P receptors except S1P5 in bone marrow-derived macrophages and differentiating osteoclasts (19). However, a more recent quantitative RT-PCR study found mainly S1P1, S1P2, and S1P3 receptor mRNA, with much lower levels of S1P4 receptor mRNA, and no detectable S1P5 receptor mRNA in primary osteoclasts or osteoblasts (20). Current pharmacological evidence for a lack of S1P4/5 receptor involvement should be interpreted with caution due to the poor characterization and/or selectivity of available drugs (see Table 1). Studies using novel agents specific for S1P4 and S1P5 receptors (21) are needed to rule out, or possibly uncover, a role of these subtype in bone (patho)physiology.
Table 1.
List of S1P associated agents mentioned in the review. Of note, many of these agents only show subtype selectivity with a narrow range of concentrations, and have known non S1P receptor targets (for review see ((29))).
| AGENT | SELECTIVITY/SPECIFICITY | NOTES |
|---|---|---|
| S1P | S1P1–5 Agonist | Endogenous agonist |
|
| ||
| FINGOLIMOD | Activates all S1P subtypes except S1P2, although recent evidence suggests S1P2 might also be a target (32). | Fingolimod is a prodrug (activated by sphingosine kinase 2). Phosphorylated fingolimod is likely to act as a functional antagonist of S1P1 in its approved therapeutic role, as it rapidly downregulates S1P1 receptors. The extent and the kinetics of fingolimod-induced receptor internalization and of their recycling to the cell membrane seem to differ between various S1P receptor subtypes. Furthermore, the extent of receptor downregulation may also depend on fingolimod concentration, the concentration of endogenous S1P and the level of S1P receptor expression, possibly explaining why the functional effects of fingolimod in various systems can either resemble the effects of agonists or of antagonists (30). |
| It is also a potent protein phosphatase 2A (PP2A)– activating drug. Effects of sphingosine kinases and S1P lyase have also been shown. | ||
|
| ||
| SEW2871 | S1P1 Agonist | First described S1P1-selective agonist. At variance with fingolimod, it demonstrates S1P1 agonist activity without long-term decrease in surface receptor expression (33). It is 10 to 50 times less potent than CYM5442 and poorly water-soluble (34). |
|
| ||
| JTE013 | S1P2 Antagonist | Most commonly used S1P2 receptor antagonist, but its selectivity is questionable (35). |
|
| ||
| VPC23019 | S1P1, S1P3 Antagonist | pKB values of 7.5 and 6.0 for S1P1 and S1P3 receptors, respectively (36). |
|
| ||
| VPC01091 | S1P1 partial agonist, S1P3 antagonist | The 1R,3S diastereomer is a conformationally constrained fingolimod analogue activated by sphingosine kinase 2 (37). |
|
| ||
| W146 | S1P1 Antagonist | W146 is an antagonist, but its in vivo effect often mimic those of S1P receptor agonists (38). |
|
| ||
| CAY10444 | S1P3 Antagonist | Also known as BML-241. Low potency and aqueous solubility agent. May also non-selectively inhibit increases in intracellular [Ca2+] (39). |
The therapeutic potential of interfering with S1P signalling has mostly been explored in the immune (22), nervous (23), and cardiovascular systems (24). The function of S1P receptors in the immune system especially is increasingly better understood, with apparent roles in cell trafficking (25), allergic responses (26), and coagulation secondary to inflammatory conditions (27). The role of S1P in maintaining vascular integrity is also linked to inflammatory cell trafficking (28), suggesting that the effect of S1P on the immune and vascularization responses could contribute to bone repair, and could be exploited for therapeutic purposes in this context.
This review will focus on the role of S1P in bone regeneration, teasing out its interaction with the various cellular components of bone repair. It will evaluate whether the manipulation of S1P signalling has been effective in cases of critical bone defects, bearing in mind the complexity of S1P signalling, and the uncertainty regarding the specificity of the pharmacological tools used in the studies in question (29). Table 1 lists the S1P receptor agonists and antagonists frequently mentioned in this review, with their presumed subtype selectivity/specificity.
Other agents activating or blocking S1P receptors, or interfering with S1P metabolism have been described (30, 31). To the best of our knowledge, they have not yet been used to characterize the role of S1P signalling in bone biology and are therefore not listed here.
2 Bone repair
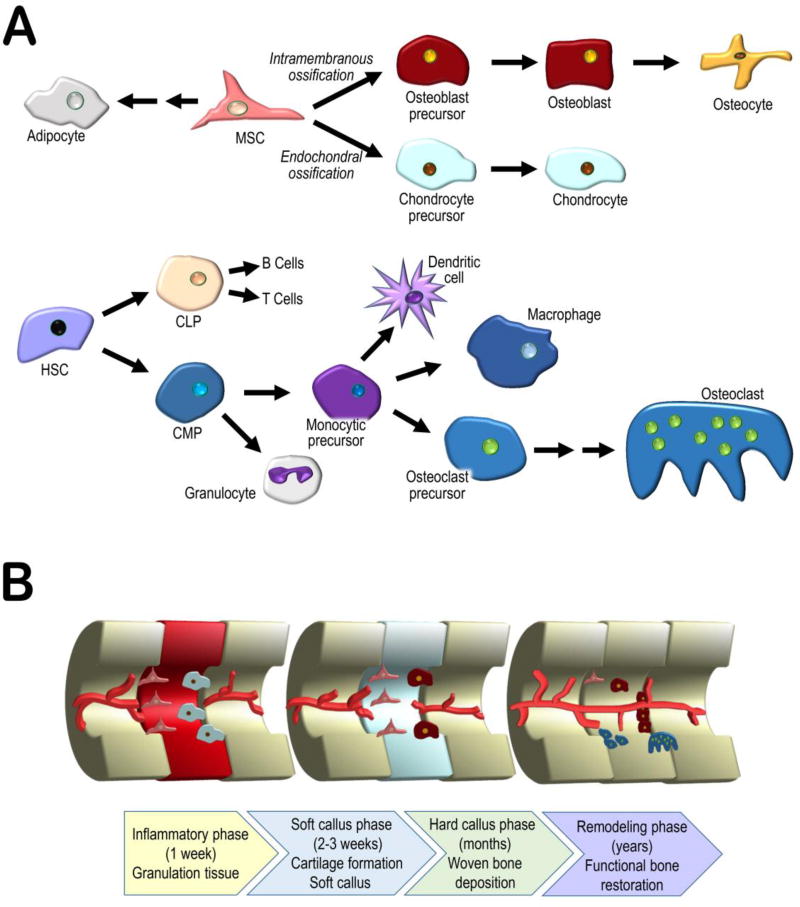
Bone is exceptionally proficient at self-repair, often able to avoid the formation of fibrous scar tissue in favour of complete regeneration (40). The cells responsible for bone development and repair are the same. Stem cells of mesenchymal origin are the source of bone forming osteoblasts and cartilage forming chondrocytes (41) whereas haematopoietic stem cells are the source of the monocytes and macrophages that differentiate into multinucleated osteoclasts, responsible for bone resorption (42). These cells collaborate in the formation of functional bone through intramembranous and endochondral ossification (43). Intramembranous ossification (IO) involves the direct differentiation of mesenchymal stem cells into osteoblasts and the deposition of bone, as occurs during the formation of bones of the skull. Endochondral ossification (EO), typical of long bone formation, involves an intermediary step, the formation of chondrocytes, and the deposition of cartilage, which acts as a template for osteoblasts as cartilage is systematically replaced by bone (44).
The process of bone repair echoes osteogenesis and resembles either EO or IO, depending on the size and location of the defect encountered. When the defect is sufficiently small and rigid, and adjacent bone cortices are in contact, deposition of bone may take place directly via IO, without intermediate cartilage formation. This direct, or primary, repair process requires the recruitment of osteoprogenitor cells, osteoclasts and undifferentiated mesenchymal stem cells to the fracture site. In contrast, indirect repair is similar to EO and involves the formation of a cartilaginous template (soft callus) that undergoes calcification into a hard callus and is eventually replaced by new woven bone (44). This process typically involves an acute inflammatory phase, which includes haematoma formation at the defect site, an early response by platelets, and neutrophils, followed soon after by monocytes and macrophages, resulting in thrombus formation, debris removal and the eventual formation of granulation tissue. Inflammation is continuously supported by positive feedback from the release of interleukins (primarily IL-1, and −6, along with −11, and −18) and tumor necrosis factor α (TNF-α) mainly in the first 24 hours after injury (45). Other important factors include platelet derived growth factor (PDGF) and macrophage colony-stimulating factor (M-CSF), which, together with stromal cell-derived factor 1 (SDF1, CXCL12) contribute to the recruitment of stem cells from the immediate bone environment and from the circulation (44, 45). These stem cells are essential for the next stage of regeneration, the formation of the soft callus. Hypoxic conditions in the haematoma may contribute to the promotion of chondrocyte differentiation from progenitor stem cells, and subsequent cartilage deposition (46, 47). Angiogenesis and blood vessel infiltration controlled by angiopoetin-1 and −2 and by vascular endothelial growth factor (VEGF) increase until hypoxic conditions begin to resolve (45). Improved circulation, as well as the activation of M-CSF, receptor activator of nuclear factor kappa B ligand (RANKL) and TNF-α, stimulate chondroclastogenesis and cartilage mineralization (48). The resolution of hypoxic conditions is followed by osteoblast proliferation and differentiation, leading to the deposition of woven bone. Cytokines such as transforming growth factors β2 and 3 (TGF-β) and bone morphogenetic proteins (BMP) −2, −5, and −6 exert control over the healing process by supporting continued proliferation, differentiation, and activity of osteoblasts, as well as the long term remodelling and restoration of woven bone into lamellar, functional bone (45, 49). The cell types and processes involved in bone repair are shown in Figure 1.
Fig. 1.
(a) A simplified representation of the lineages of the cells involved in bone repair. Mesenchymal stem cells (MSC) differentiate into the major bone and cartilage forming cells, osteoblasts and chondrocytes (later replaced by osteoblasts), depending on whether ossification occurs through the intramembranous or endochondral pathways. Haematopoietic stem cells (HSC) differentiate into bone resorbing osteoclasts through the myeloid pathway. (b) Process of bone repair divided into 4 phases: inflammatory, soft callus, hard callus, and remodelling. Briefly, an early inflammatory response results in the removal of debris and the eventual recruitment of mesenchymal stem cells, initiating the soft callus phase and cartilage deposition. Improving vascularization leads to cartilage mineralization and deposition of bone, which is then slowly remodelled, restoring function.
The role of several mediators and signalling pathways in bone repair (e.g., BMPs, VEGF, Wnt and Notch pathways) and therapeutic attempts at harnessing them to improve bone repair have been the subject of various reviews (4, 41, 50–52). Less attention has been paid to the role of S1P signalling in bone disorders and repair (53). This review will therefore summarise the key findings in this field, with emphasis on the effects of S1P on the migration, differentiation and survival of the cellular components of bone repair and their respective precursors. In addition to the well-known role of S1P in vascularization and immune cell trafficking, these effects are likely to underlie any observed improvement in repair of bone defects following pharmacological intervention targeting S1P signalling.
3 S1P effect on progenitor stem cells
After injury, bone healing relies not only on differentiated bone cells but also on the recruitment of undifferentiated cells from bone and adjacent tissues. S1P regulates cell trafficking through surface receptors that respond to the S1P gradient between tissues (where S1P is found in nanomolar concentrations) and the blood (where it is found at micromolar concentrations), a gradient which may arise due to high levels of S1P degrading enzymes in the tissue compared to the blood (54). In general S1P functions as a chemoattractant for quiescent stem cell populations (55), and also participates in their differentiation into specialist bone forming and bone resorbing cells, as will be explored in more detail in the forthcoming sections.
3.1 S1P and stem cell migration
The balance between the major chemo-attractants CXCL12 (also known as SDF-1), predominantly found in bone marrow, and S1P, mainly found in the blood, dynamically regulates haematopoietic stem cell recruitment to the circulation versus their retention in the bone marrow. The principal chemoattractant retaining progenitor stem cells in a quiescent state in the bone marrow is CXCL12. Dissipating the S1P gradient between the blood and bone marrow by inhibiting S1P degradation in tissues or downregulating stem cell S1P1 receptors using fingolimod both reduce the number of circulating progenitor stem cells (56). The S1P3 receptor has been shown to have the reverse effect, whereby S1P3 agonism stimulates CXCL12-based retention of haematopoietic stem cells within the bone marrow, and S1P3 antagonism contributes to increased stem cell egress (57). Stress, such as that occurring in a fractured bone, induces the downregulation of CXCL12 in the bone marrow and an increase in circulating S1P levels, leading to stem cell mobilization and migration into the blood stream (58). These observations support a role for S1P in the exit of cells from the bone marrow, a finding reminiscent of S1P–mediated lymphocyte egress from lymph nodes (22). Therefore, by manipulating S1P levels in the local environment of a tissue injury site, it may be possible to draw more of the local progenitor resources into the repair process.
S1P–treated stromal cells show increased expression of extracellular matrix protease (e.g., MMP1) (59), which are important in bring down collagen during the cell migration process (60). S1P also induces stromal cell migration and formation of capillary-like structures (59) and Rho-dependent formation of stress fibres, followed by lamellipodia and filopodia, in bone marrow derived cells. MMP or MEK1-ERK1/2 inhibition reduces S1P–induced actin stress fibre formation, with no impact on lamellipodia or filopodia. MMP inhibition also interferes with S1P activation of RhoA and ERK, while Rho kinase blockage produces sustained S1P activation of ERK. This shows the intricate interplay downstream of S1P stimulation in the pathways involved in cell migration (61).
Medium conditioned by RANKL-differentiated bone marrow cells contains S1P that stimulates chemotaxis of mesenchymal stem cells (MSC) (62). Two parallel signalling pathways seem to be involved in this MSC migratory response: S1P1 receptors activating the JAK/STAT pathway and S1P2 receptors activating the FAK/PI3K/AKT pathway (62). Contrasting with these findings, a recent study showed that S1P2 receptors played a critical role in the inhibition of MSC migration through ERK phosphorylation (63), an effect more in line with the more commonly observed inhibition of migration by S1P2 receptors (64). Confirming the effects of S1P signalling on the recruitment of endogenous stem cells, exposure of bone marrow derived MSCs to the S1P agonist fingolimod released from biodegradable polymer scaffolds enhanced MSC migration toward CXCL12 (65), but the pharmacological profile of this response was not assessed. In these experiments fingolimod also led to cellular mineralization, an indicator of differentiation into the osteoblast lineage, and promoted vascularization (65).
3.2 S1P and stem cell differentiation
MSCs can differentiate into osteoblasts and adipocytes; commitment to one lineage inhibits commitment to the other due to the existence of negative feedback loops. S1P reduced adipogenic differentiation in MSCs (66) and increased their differentiation into osteoblasts as shown by increases in alkaline phosphatase and osteocalcin mRNAs, and the appearance of calcified deposits (66). While the MSC cell line expressed both S1P1 and S1P2 receptors, the inhibition of C/EBPβ expression by S1P was sensitive to pertussis toxin, suggesting that S1P1 receptors played a key role (66). A recent study further defined the nature of the Wnt pathway involved in S1P–induced osteogenic differentiation of MSCs, implicating the Wnt5a ligand and LRP5/6 receptor (67). In another study, S1P–functionalized titanium oxide coated stainless steel used as a growth substrate for human adipose derived stem cells also fostered their osteogenic differentiation (68). Both the S1P1/3 receptor antagonist VPC23019 and blocking of BMP6 with a neutralising antibody, polyclonal IgG reduced the mineralization response of human MSC to osteoclast-conditioned media, and similarly interferes with MSC migration. Indicating that osteoclasts and associated S1P release (among other osteoblast-osteoclast coupling factors) stimulate MSC differentiation and migration (69).
4 S1P and osteoblasts
4.1 Proliferative effect
Short (10–45 min) but not protracted (24 hr) treatment with S1P induces ERK-dependent proliferation of both rat and human osteoblasts (70, 71). This time dependence has been tentatively explained by the possibility that S1P might first induce an early phase of cell growth, but, upon longer stimulation, lead to a phase of differentiation in which proliferation stops. Alternatively, the differential increase in the PKCα isoform following short- vs. long-term exposure to S1P might also have played a role (71). This possibility is supported by the observation that, in response to a 10-minute S1P stimulation, PKCα immunoreactivity was redistributed from the cytosol to the nucleus (72). Osteoblasts are known to express S1P1, S1P2 and S1P3 receptors (18–20), but none of the studies mentioned above addressed the identity of the receptor involved in the proliferation response; while pertussis toxin sensitivity pointed to an S1P1-mediated effect (71), the S1P concentration used (10 µM) was higher than usually needed to activate S1P receptors. A more recent study reported increased DNA synthesis at S1P concentrations of 1 µM (18); S1P induced activation of p42/44 MAP kinases, in a Gi- and calcium-dependent manner, but independently of PKC, and proliferation was observed in response to 24-hour S1P treatment. When the effects of S1P were studied in human primary osteoblastic cells and the human osteosarcomal cell lines, G292 and MG-63, 10 minute incubations with 10 nM S1P increased proliferation in a pertussis toxin-sensitive manner, while the effect of 24-hr incubation were less consistent. In G292 cells, this longer exposure produced significant increases only with subnanomolar S1P, while higher doses had no effects; no proliferation was observed at any concentration in the other cell types (73). Both proliferation and apoptosis control the number of osteoblasts, and Gi proteins are not only involved in S1P–induced osteoblast proliferation but also in their survival. However, the role of PI3K appears to be restricted to the latter effect, since PI3K inhibition does not prevent the proliferative actions of S1P in osteoblastic cells (74).
4.2 Osteoblast differentiation
Differentiation of osteoblast precursors into mature osteoblasts is accompanied by an increase in SK 1 expression and enzyme activity, decreased levels of S1P1 and S1P2 receptor proteins, and increased levels of S1P3 receptor proteins (75). Sphingosine kinase inhibitor (SKI-II), an anti-S1P antibody and the S1P1/3 receptor antagonist VPC23019 all reduce alkaline phosphatase activity, while blocking S1P1 receptors with W146, or S1P2 receptors with JTE013, has no effect (75). A similar pharmacological profile was observed with RUNX2 expression (a key transcription factor associated with osteoblast differentiation), suggesting the existence of an autocrine SK1/S1P/S1P3 signalling pathway during osteoblastic differentiation (75).
Other S1P receptors and signalling pathways may also mediate osteoblastogenesis. Activation of S1P receptors in C2C12 myoblasts enhanced BMP-2-induced expression markers of osteoblast differentiation (76). The expression of RUNX2 was likewise increased in the presence of S1P or fingolimod, as were Smad transcription factors and ERK1/2 (76). S1P and fingolimod also enhanced BMP-2-stimulated Smad1/5/8 phosphorylation in C2C12 cells, and cell differentiation was sensitive to Pertussis toxin, to a MEK1/2 inhibitor, to the S1P1 receptor antagonist W146, and, to a smaller extent, to the S1P2 antagonist JTE013, whereas an S1P3 antagonist (CAY10444) had no effect. A similar pharmacological profile was observed for the effects of S1P on other osteoblast-like cell lines (human SaOS-2 and murine MC3T3-E1). In these cells, S1P activated PI3K/Akt signalling, inhibiting GSK-3β, promoting nuclear translocation of β-catenin and expression of osteoprotegerin (that inhibits osteoclastogenesis by acting as a soluble decoy receptor for RANKL), and enhancing ALP activity (77). In a more recent study by the same group, S1P stimulation of Smad1/5/8 phosphorylation was attributed to S1P2-G12/13-RhoA activity, leading to the nuclear translocation of the Smad complex, up-regulation of RUNX2 leading to increased ALP (78). Of note, this (77) and another study (19) found that S1P also increased RANKL mRNA in osteoblasts, but the OPG/RANKL ratio was higher after S1P treatment, which should lead to an overall inhibition of osteoclast maturation (77). Increased SK activity indeed reduces osteoclastogenesis in a monoculture of osteoclast precursors; however, in an osteoblast/osteoclast co-culture system, which better reflects the reality of a healing bone, S1P stimulated osteoclastogenesis (19).
As mentioned above, S1P seems to act as a coupling factor between osteoclasts and osteoblasts, and is referred to as a clastokine (79). Osteoclasts lacking the bone degrading enzyme cathepsin K show increased SK 1 expression and culture media conditioned by these cells were shown to induce a larger increase in ALP and mineralized nodules in osteoblast cultures, due to their higher S1P content. This response was blocked by the S1P1/3 antagonist VPC23019, in agreement with the studies described above (80).
4.3 Osteoblast precursor migration
Together with its activity on their proliferation and differentiation (18, 70–78, 80), S1P also affects the migration of osteoblast precursors (81). Treatment of mouse primary pre-osteoblasts with S1P drives cells toward the bone surface environment (81). However, when precursors differentiate into mature osteoblasts, they become insensitive to S1P, although they retain their chemotaxis to PDGF (81). The response to S1P is not sensitive to pertussis toxin, suggesting that a subtype other than S1P1 is involved in the chemorepellent response to S1P. Indeed, expression studies and experiments with JTE-013 or with anti S1P2 siRNA point to a developmental stage specific role of S1P2 receptors. The chemorepellent effect of S1P2 receptors is typical of this subtype in various cell types, whereas S1P1 receptors are associated with chemotaxis to S1P in other cells important for bone repair: MSCs that give rise to cells of the osteoblast lineage (see (62) above), endothelial cells (82) or osteoclasts (see below). The lack of S1P1-mediated positive chemotactic response in osteoblasts, despite high S1P1 expression levels in these cells, is therefore unusual.
4.4 Other effect of S1P signalling in osteoblasts
S1P has long been known to release calcium from intracellular stores in pre-osteoblasts (83, 84). Because of calcium’s central role in cell signalling, it is therefore not surprising that S1P is implicated in many osteoblast functions. Indeed, S1P stimulates IL-6 synthesis in these cells in a p42/p44 MAPK dependent manner (85), induces the synthesis of heat-shock protein 27 (HSP27) via p38 activation (86), and enhances PGF2α-induced phosphoinositide hydrolysis by phospholipase C through p38 MAPK (87, 88).
Administration of epidermal growth factor, a known mitogenic factor for osteoblasts, increased S1P levels which coincided with increased cell proliferation (89). There is also evidence for the involvement of S1P signalling in calcitonin activity (90). Calcitonin is an anti-resorptive hormone previously indicated in osteoporosis, however it may also influence bone formation through its interactions in S1P signalling. By decreasing the expression of the S1P transporter Spns2 in osteoclasts (20), limiting the cross-talk between osteoclasts and osteoblasts, and so also limiting S1P- or fingolimod-induced bone formation by osteoblasts which was found to be mediated by S1P3 receptors (20).
S1P may also influence mature osteoblasts following their entombing as osteocytes in the bone matrix, as S1P signalling via the S1P2 receptor has been shown to affect mechanotransduction in an osteocyte-like cell line (MLOY4) (91).
5 S1P and osteoclasts
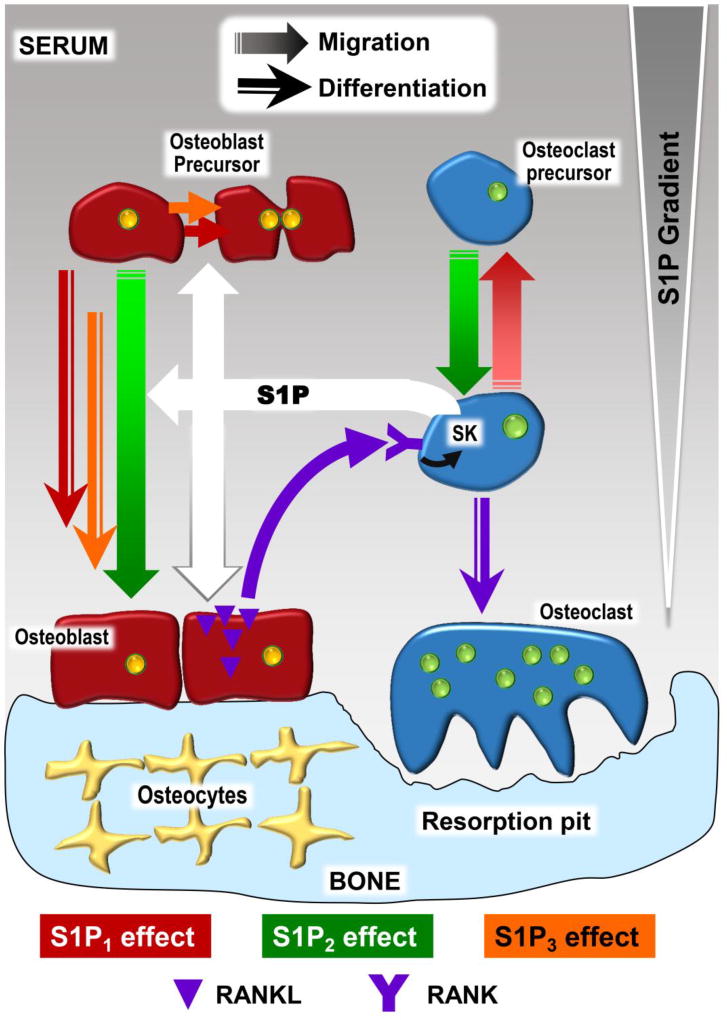
Osteoclasts are multinucleated, resorptive cells whose development is influenced by osteoblast lineage cells (92). Osteoclasts are responsible for the continuous remodelling of bone, working in tandem with bone forming osteoblasts (93). The coupling between osteoclasts and osteoblasts in osteoclastogenesis is a clear example of the functional relationship between the two cell populations, and S1P seems to play a role in the crosstalk between these two cell populations and their differentiation, as represented in Figure 2.
Fig. 2.
Simplified illustration of the effects of S1P and its receptors on osteoblasts, osteoclasts, their respective precursors, and the role of S1P in osteoblast-osteoclast coupling. The involvement of the 3 major S1P receptor subtypes (red: S1P1, green: S1P2, orange: S1P3) in particular responses is indicated by different arrow shapes. Briefly, osteoclast and osteoblast precursor migration is influenced by S1P1-mediated chemoattraction and S1P2-mediated chemorepulsion in response to the the S1P concentration gradient (larger quantities of S1P are generated in serum mainly by red blood cells and endothelial cells, while lower S1P concentrations predominate in tissue compartments, such as bone). S1P, produced locally by osteoclasts or osteoclast precursors (20, 69, 80), directly stimulates the proliferation of osteoblast precursors and their differentiation into mature osteoblasts, while increasing RANKL mRNA in osteoblasts, indirectly stimulating osteoclast precursor differentiation via RANK. The RANKL/RANK signalling pathway also upregulates SK in osteoclast precursors.
5.1 S1P and osteoclast recruitment
S1P can regulate the migration of osteoclast precursors both in vitro and in vivo. Bone marrow derived monocytes (an in vitro model of osteoclast precursors) express both S1P1 and S1P2 receptors. Upon exposure to RANKL, these cells differentiate into osteoclast-like cells and show decreased S1P1 expression, with concomitant loss of chemotactic response to S1P (94). Knockout mice with specific S1P1 deletion in the monocyte lineage are osteoporotic, a phenotype that has been attributed to the loss of S1P1 control of osteoclast precursor migration and increased residency time at the bone surface (94). The potential therapeutic significance of these findings was confirmed in an ovariectomy-induced osteoporosis model: fingolimod prevented bone loss in ovariectomized mice, but had no effects in sham-operated mice. This effect was due to a reduction of osteoclast deposition onto bone surfaces (94). In a rat model of periodontitis, fingolimod was found to reduce the number of osteoclast precursors and mature osteoclasts at the defect site, and increase the number of precursors in blood, an effect attributed to S1P1-induced positive chemotaxis (95).
S1P2 receptor deficient mice show higher bone density than control mice (96), and S1P2 receptors seem to antagonize the effect of S1P1 receptors on osteoclast precursor migration. Positive and negative chemotaxis are attributed to S1P1-mediated activation of Rac via Gi, and S1P2-mediated activation of Rho via G12/13, respectively (96). An in vitro migration assay of osteoclast precursors expressing both receptors subtypes showed that lower S1P concentrations stimulate positive chemotaxis, while higher concentrations stimulate negative chemotaxis, or chemorepulsion, suggesting that S1P2 receptors may only be active at high S1P concentrations. S1P1-deficient osteoclast precursor cells show very little motility, while S1P2-deficient cells showed positive chemotaxis, even at high S1P concentration (96). Intravital imaging confirmed the chemotactic effect of S1P2 by showing that the antagonist JTE013 mobilised a small subset of monocytic lineage cells from the calvarium and led them to enter the blood circulation (96).
5.2 Therapeutic manipulation of osteoclast trafficking
While approved or investigational anti-resorptive agents (e.g., bisphosphonate or cathepsin K inhibitors) target mature osteoclasts, manipulating osteoclast precursors would provide a novel therapeutic modality for bone loss. Indeed, the opposing roles of S1P1 and S1P2 receptors on precursor recruitment might underlie therapeutic interventions (i.e., activation of S1P1 or blockade of S1P2 receptors) that could prevent bone loss in conditions associated with inflammation and/or remodelling imbalance. This potential was ascertained using murine models of rheumatoid arthritis (in which fingolimod was as effective as prednisolone) and osteoporosis (fingolimod improved bone loss, but prednisolone had no effect) (97). In a model of periodontitis, a bacteria-driven inflammatory bone loss disease, fingolimod inhibited osteoclastogenesis and pro-inflammatory cytokines involved in osteoclast precursor recruitment (98).
Vitamin D analogues are used for the treatment of osteoporosis, but their mechanism of action is not completely clear. For instance, in vitro calcitriol increased RANKL expression in bone marrow stromal cells, thereby activating osteoclasts and bone resorption (99). A recent study showed that vitamin D’s effect on osteoclast precursor migration might underlie its anti-resorptive activity. Indeed, calcitriol and its analogue eldecalcitol were found to uniquely reduce S1P2 receptor expression in monocytic osteoclast precursors (99), while circulating monocytes expressed fewer S1P2 receptors in mice treated with calcitriol or eldecalcitol, and monocyte mobility was observed to increase in eldecalcitol-treated mice after treatment with JTE013 (99).
Whereas vitamin D analogues reduce S1P2 receptor expression, a recent study showed that the inflammatory cytokine IL-6 induced S1P2 mRNA, but not S1P1 mRNA expression in osteoclast precursor cells (100). This effect was associated with a decrease in S1P–induced chemotaxis and an increased number of precursors in tibial bone marrow. Systemic treatment with an anti-IL-6 receptor antibody prevented bone loss and decreased the number of precursors in tibial bone marrow via S1P2 receptor down-regulation (100), further validating the potential therapeutic value of S1P2 antagonists.
The following table summarises some of the effects of S1P receptors on the cellular components of bone repair.
6 S1P in the vasculature and the role of angiogenesis
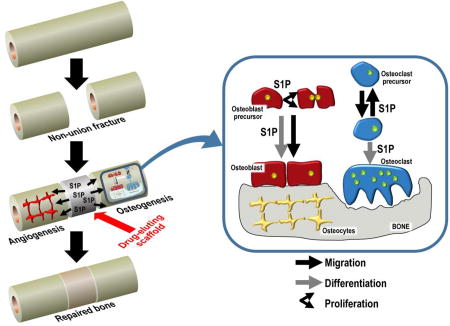
The repair of cranial bone defects by scaffold-mediated delivery of S1P agents involves not only the recruitment of bone cell progenitors, but also production of new vessels in the defect space (105, 106). Hence, while the previous sections focused on bone cells and their interactions, it is important to remember that bones are highly vascularized, perfused by up to 20ml of blood/100g of bone every minute (107). Blood vessels are not only an essential conduit for blood, providing minerals, nutrients, growth factors and osteoprogenitors, but the endothelium also acts as a paracrine and endocrine organ involved in growth factor production, coagulation, inflammation and the immune response (108). Fracture disrupts the bone’s vasculature, leading to hypoxia and necrosis of adjacent tissue. Reestablishment of the circulation and neovascularization in the tissue formed in response to injury are critical for successful fracture healing (109). Unfortunately, bone repair strategies based on bone grafts or scaffolds have so far shown limited success due in part to the lack sufficient blood vessel supply during the early stages of the repair process (20, 21).
There are three main mechanisms for producing new vessels (110). Vasculogenesis refers to the de novo generation of blood vessels that occurs for instance during embryogenesis. It differs from angiogenesis, which is the generation of new vessels from pre-existing ones. Angiogenesis occurs during physiological (e.g., wound healing or menstrual cycle) or pathological processes (e.g., neovascular disorders, rheumatoid arthritis and cancer). It can result from the formation of a new vessel branching off an existing vessel (sprouting angiogenesis) or from the splitting of a blood vessel into two or more vessels (intussusceptive angiogenesis). Finally, arteriogenesis is the remodelling of an existing artery to increase its luminal diameter. While arteriogenesis, and possibly angiogenesis (111–113), occurs in response to physical forces such as increased blood flow, angiogenesis is initiated in poorly perfused tissues when low oxygen levels lead to increased levels of the transcription factor Hypoxia-Inducible Factor (HIF)-1α in parenchymal cells.
VEGF is the main HIF-1α–dependent pro-angiogenic factor, and inhibiting VEGF signalling impairs healing of femoral fractures and cortical bone defects in mice (114). Although VEGF is the archetypical pro-angiogenic factor, it promotes by itself the formation of immature and leaky vessels (115). In contrast, angiopoetin-1 produces vessels that are resistant to leak (116), suggesting that different vascular growth factors play complementary and coordinated roles in new vessel formation, and that therapeutic strategies aimed at promoting angiogenesis should target more than one mediator. Indeed, when surgically implanted in the ear of mice, chemically modified hyaluronan hydrogels pre-loaded with both VEGF and angiopoetin-1 promote a larger angiogenic response than delivery of single growth factors (117). More recently, sequential delivery of VEGF and S1P using a porous hollow fibre in a skin Matrigel plug assay was shown to lead to more endothelial cell recruitment and a higher maturation index than single factor delivery, reverse sequential delivery or even co-delivery (118). The concept that temporal control of growth factor release produces more mature new vessels, able to integrate with the existing vasculature, was validated in similar experiments using Basic Fibroblast Growth Factor and Platelet-Derived Growth Factor (119).
These sequential release experiments were conducted over the course of a week, but the bone repair process takes months. Scaffold-mediated delivery of a low molecular weight, more lipophilic factor such as an S1P agent might be preferable to the delivery of recombinant proteins. The role of S1P in the vasculature and new vessel formation is well documented and has been the subject of numerous reviews (120–122). Endothelial cells express the same S1P receptor subtypes as intrinsic bone cells (S1P1>S1P2≈S1P3); these receptors mediate generally similar cellular responses (proliferation, differentiation and migration), in addition to effects more specific to endothelial cells (modulation of cell adhesion and of the inflammatory/immune response). S1P seems to play a key role in both vasculogenesis and angiogenesis. In a mouse hind limb ischemia model, S1P stimulates angiogenesis (123), while postischemic blood flow recovery and angiogenesis are accelerated in transgenic mice overexpressing SK1 (124). At variance with the effects of VEGF however, the angiogenic response to S1P is not associated with increased vascular permeability in the ischemic limb, and many studies have shown that S1P actually enhances endothelial barrier integrity (120). In fact, in this model, S1P–containing Poly(lactic-co-glycolic-acid) (PLGA) microparticles not only stimulated post-ischemic angiogenesis at 28 days but also blocked edema induced when VEGF was co-administered (125). The effects of S1P1 and S1P3 receptors on adherens junctions in endothelial cells were documented soon after the identification of these receptors (126). While S1P1 and S1P3 receptors strengthen the formation of endothelial cell junctions (28, 127–129), S1P2 receptors increase vascular permeability in vitro via disruption of adherens junctions (130, 131). In vivo, S1P1 receptor activation inhibit VEGF-induced vascular leakage in skin capillaries (132), whereas S1P1 receptor antagonists have shown that they induce capillary leakage in the lung, kidney, skin, and intestine (133–135). S1P1 receptors promote vascular stabilization by regulating the interactions between endothelial and mural cells during the maturation process (136, 137), and, in apparent contradiction with their pro-angiogenic effects mentioned above, S1P1 receptors were recently shown to inhibit sprouting angiogenesis during vascular development (138), by stabilizing VE-cadherin at endothelial junctions and inhibiting VEGFR2 (111, 112), suggesting the existence of an alternative mechanism that helps stabilize the newly formed vascular network and improves its barrier function.
These data showing that S1P plays a role both at the early stages of angiogenesis and at the stage of new vessel stabilization, taken together with the effects of this lipid on bone cells, suggest that scaffold-mediated delivery of S1P (most likely S1P1) agonists might promote bone repair via pleiotropic and possible synergistic mechanisms.
7 Current efforts in S1P delivery
The importance of S1P as a chemoattractant, and in coupling the activity of osteoblasts and osteoclasts suggests it could be utilized systemically in bone repair, and in disorders such as osteoporosis (53). However, a study of daily subcutaneous fingolimod (6mg/kg) did not lead to any improvement in fracture healing of a murine femoral defect (139), indicating that a more localised approach of delivering S1P and related analogue, may lead to more promising results.
Local administration of S1P has typically involved the use of scaffolds, which often have the dual role of acting as drug delivery device, and mimicking native tissue to elicit functional tissue development. Hence a range of biocompatible materials, including natural polymers (collagen, chitosan, silk), synthetic organic polymers PLGA and poly-ε-caprolactone [PCL]) and inorganic materials (ceramics and glasses) have been investigated to fabricate scaffolds that are conducive to tissue regeneration, and allow temporal control over the release of therapeutic cargoes (140). Biodegradable PLGA is among the commonest copolymers investigated (141) and has been used to control the release of S1P (105) and fingolimod (142), resulting in increased new bone formation post-implantation in a rat cranial defect model, an effect that was attributed to increased development of vasculature and the possible dose-dependent initiation of bone progenitor cell migration towards the defect site (142). The underlying mechanism was probed in a similar study investigating the delivery of S1P agonists and antagonists (S1P, fingolimod or VPC01091) from PLGA scaffold implants in a rat cranial defect model (106). Although S1P is subject to much more rapid in vivo degradation than fingolimod, scaffolds loaded with either agonist were equally effective in generating new bone over 6 weeks, while VPC01091-loaded scaffolds did not differ from unloaded controls (106). This study suggests that sustained release from scaffolds may offset the challenges of employing therapeutic cargoes (e.g. S1P) with short half-lives, and that S1P3 receptors synergize with S1P1 receptors to influence the various processes underlying repair (i.e., vascular remodelling, cell proliferation and migration, inflammation), albeit to differing extents. fingolimod has been incorporated into electrospun nanofibers composed of PLGA and biodegradable PCL and showed significant improvement in defect healing and vascularization in a rat critical mandibular defect (143). These fingolimod-loaded nanofibers increased neovascularization and enhanced the proportion of macrophages with an anti-inflammatory phenotype (M2) (143), a cell population that is also known to play an important role in tissue repair (144), and had been previously shown to be selectively attracted by fingolimod (145). A similar result of anti-inflammatory macrophage stimulation was found in another study using a PLGA coated allograft (146), and whilst SEW2871 was also observed to stimulate macrophage recruitment, details regarding phenotype were not reported (147). An electrospun amphiphilic copolymer was developed to act as a carrier for S1P to promote vascularization in tissue repair applications, the amphiphilic nature of the copolymer was anticipated to mimic the binding of S1P to apolipoprotein M. S1P was first applied directly to endothelial cells (HUVEC), and showed pro-angiogenic effects in a tube formation assay. Tube length and uniformity were then improved when S1P was administered as part of the amphiphilic scaffold, additional evidence of new vessel formation was shown in a 3-day chorioallantoic membrane assay (148).
Whether small molecule delivery alone will achieve sufficient and effective bone repair remains to be established, but it is worth noting that fingolimod PLGA microspheres in a chitosan gel improved bone regeneration in a rat cranial defect study, with no substantial improvement upon addition of BMP-2 to fingolimod-loaded microspheres (65), despite fingolimod being known to enhance BMP-2 mediated osteoblast differentiation in vitro (76). Conversely, SEW2871 alone failed to improve bone regeneration, but co-administration with platelet rich plasma improved the latter’s performance, by enhancing macrophage recruitment and cell debris clearance (149). Combining S1P with low-cost, biocompatible, biodegradable polymers represents an enticing alternative prospect for current bone graft treatments. Unfortunately, results to date still show most polymeric biomaterials cannot match the efficacy of bone grafts, because they lack both the osteogenic and osteoinductive properties that make grafts so successful. Consequently, bioactive polymer-graft composites are a potential solution to recapitulate mechanical and biological properties of host tissue in an effort to repair critical-sized defects. In one case, fingolimod elution from a PLGA-coated devitalized-bone allograft in a critical rat tibial defect improved elastic modulus and ultimate compressive strength of the bone, outcomes attributed to evidence of enhanced active remodelling at the defect site (150). The same procedure was investigated further, and similarly attributed tissue regeneration to improved vascularization, while also presenting a more detailed discussion of the role of bone marrow derived cells in immune modulation (146). Another PLGA coated allograft delivery system for fingolimod showed a dose-dependent increase in bone volume in a cranial defect model at 2 and 4 weeks. Although differences in bone volumes were no longer significant at 8 weeks, fingolimod still enhanced host-graft integration at this time point (151). Notably, direct adsorption of fingolimod onto implanted allograft improved bone deposition and vascularisation (152). Predictably, this method produced higher local concentrations of fingolimod, but lower increases in bone density compared to polymer based delivery discussed above (151, 152).
8 Conclusion
Although the role of S1P in bone biology has been the focus of much less research than its role in the cardiovascular and immune systems, it is becoming clear that this lipid influences many of the functions, pathways and cell types that play a key role in bone repair. Indeed, S1P has a well-established role in promoting angiogenesis (14, 105, 148, 153, 154), but is also implicated in many other bone related processes including stem cell recruitment (59, 62, 155) and subsequent differentiation (66). S1P stimulates the differentiation and survival of osteoblasts (76, 77), and contributes to their intricate coupling with osteoclasts (19). S1P is not only a key factor in its own right, it also seems to mediate the functions of critical bone growth factors, such as BMPs (69, 76). Although the use of growth factors for bone repair has been widely explored, some issues remain, such as those related to supra-physiologic doses (156), short half-lives (157), an inability to maintain osteogenicity due to slow vascular integration of grafts (2), not to mention high costs (158). As summarized in earlier sections, various groups have therefore begun to explore the use of non peptidic agents, such as S1P and analogues, to promote bone repair in vivo, with generally promising results. Remaining issues regarding pleiotropic activity (159), solubility (147) and the need to maintain local concentrations over a number of weeks (159) may be addressed by using more specific agents and/or novel delivery options. A number of such delivery methods have been studied in the field of bone repair to enhance delivery of growth factors (158, 160, 161), small molecule drugs, and stem cell therapies (48, 162, 163). They have generally involved biomaterials for controlled release of drugs including biocompatible, biodegradable polymers, and bio-ceramics (4, 163) and the use of high affinity delivery systems, which have led to reductions in required doses (5).
The use of S1P agents for bone repair is likely to be greatly accelerated by the much more active translational and clinical research of the role of S1P signalling in other fields, such as inflammation or cancer. The number of active clinical trials involving S1P receptor ligands in inflammatory conditions ranges from 2 and 3% of trials for inflammatory bowel disease and psoriasis, up to 32% of all trials for new multiple sclerosis therapies (164). S1P1 receptors have been the focus of most research in this field, as evidenced by the great emphasis placed on the development of agents such as ponesimod, siponimod, and ozanimod, with improved specificity compared to fingolimod. Whilst other possible targets, such as S1P lyase inhibition have been less well investigated (165). In the field of bone repair, further basic and translational research will be needed to better define which S1P metabolic enzymes or receptors should be targeted, when and for what duration, and whether an agonist or an antagonist would be preferable. The latter issue is particularly critical considering that S1P1 receptor agonists seem to exert their action as functional antagonists, with S1P1 agonists and antagonists showing similar therapeutic effects (166). Furthermore, some of the work quoted in this review has been based on qualitative or semi-quantitative data, and the pharmacological profile of the response was sometimes unclear, either due to incomplete dose response studies, or the use of agents with questionable specificity (29, 35) .
To conclude, the manipulation of S1P signalling using systemic administration of therapeutic agents seems promising for the management of inflammatory or hormonally-related bone loss, as S1P agents can be used to affect osteoblast/osteoclast coupling, the unbalancing of which manifests as conditions such as osteoporosis. In contrast, local administration of S1P agents has shown more compelling results in bone defect studies, and so improving local delivery of these agents will be key to optimising their regenerative potential. Critically, this may be achieved by not only increasing the recruitment of osteogenic cell precursors but also by inducing and supporting vascularization and modulating the immune response; S1P agents may be unique in that they are known to possess all three activities (106, 142, 143, 146, 149–152).
Table 2.
Cell types involved in bone regeneration and some S1P receptor related effects.
| CELL TYPE | AGENT | RECEPTOR | STUDY | EFFECT | REF. |
|---|---|---|---|---|---|
| OSTEOBLAST CELL MODELS | S1P as part of osteoclast conditioned medium | S1P1/3 involvement determined using VPC23019 (2µM and 10µM) | Murine long bone osteoblasts cultured in osteoclast conditioned medium | ↑ALP | (80) |
| ↑Mineralization | |||||
|
| |||||
| S1P (1–30µM) | No S1P receptors were investigated | MC3T3-E1 cell line, treated with 1–30µM S1P, media contained 0.01% bovine serum albumin | ↑IL-6 | (85) | |
|
| |||||
| S1P, various doses ranging from 1nM to 10µM | S1P1 as determined by pertussis toxin (73). The remaining articles do not identify individual receptors. | Human osteoblast explant (71–73), Foetal rat osteoblasts (18, 70, 74), SaOS2 cell line (18), thymidine incorporation proliferation assays | ↑Proliferation | (18, 70–74) | |
|
| |||||
| S1P (100nM) added to top and/or bottom compartments of migration chamber | S1P2 as determined using pertussis toxin (200ng/mL), JTE013 (10−8–10−5M), and RNA interference | MC3T3-E1 cell line, migration assay for PDGF and S1P pre-and post-differentiation | Negative chemotaxis | (81) | |
|
| |||||
| Endogenous S1P | S1P3 as determined using W146, JTE013, and VPC23019 (All 2µM) | MC3T3-E1 cell line cultured in osteoblast differentiation media, contained 10% serum | ↑Maturation | (75) | |
|
| |||||
| S1P (0.01–0.1µM) or fingolimod* (0.01–0.1µM) | S1P1 as determined by the pertussis toxin (100ng/mL), W146, JTE013, and CAY10444 (All 10µM) | C2C12 murine osteoblast precursor cultured in media containing 10% serum. S1P and fingolimod used supplementary to BMP-2 | ↑ALP (↑↑*) | (76) | |
| ↑Osteocalcin (↑↑*) | |||||
| ↑RUNX2(↑*) | |||||
|
| |||||
| S1P (0.1–2µM) | S1P1 as determined using W146, JTE013, and CAY10444 (BML-241) | Human SaOS2 and murine MC3T3-E1 cell lines, cultured in media containing 10% serum | ↑ALP | (77) | |
| ↑Mineralization | |||||
| ↑Osteoprotegerin | |||||
| ↑RANKL mRNA | |||||
| Nuclear localization of β-catenin | |||||
|
|
|||||
| OSTEOBLAST CELL MODELS | S1P (0.1µM and 1µM) | No receptors were investigated | Osteoclast from minced rabbit bones incubated on dentine slices. Treated for 16 hours with S1P in media containing 10% serum | ↓ Resorption | (101) |
|
| |||||
| S1P (10−10–10−7) | S1P1 as determined by osteoclast lineage specific conditional S1P1 knockout | Murine monocyte cell line migration assay Cells cultured in media containing 10% serum | Positive chemotaxis | (94, 96) | |
|
| |||||
| fingolimod (3mg/Kg) intraperitoneal injection | S1P1 determined from S1P1 knockout osteoclasts collected from transgenic mice | Murine model of osteoporosis | ↓Bone density loss | (94, 97) | |
| Positive chemotaxis | |||||
|
| |||||
| S1P2 receptor deficiency or blockade | S1P2 as determined in vitro by targeting with RNA interference. And in vivo by use of JTE013 3mg/Kg | In vitro and in vivo investigation of the role of S1P2 in the migration of osteoclast precursors | Osteopetrosis | (96) | |
| ↑Bone density | |||||
| ↓Negative chemotaxis (osteoclast precursors remain in circulation) | |||||
|
| |||||
| fingolimod (3mg/Kg/Day) intraperitoneal injections | S1P1 as determined using immunohistochemistry and an anti-S1P1 receptor antibody | Rat model of periodontitis | Positive chemotaxis | (95) | |
|
| |||||
| Calcitriol and eldecalcitol (In vitro: 10−9–10−8M | S1P2 receptor expression as determined by PCR | Monocytoid cell line migration assay | ↓S1P2 receptor expression | (99) | |
| In vivo osteoporosis model | Positive chemotaxis | ||||
| ↑Bone mineral density | |||||
| In vivo: 50ng/Kg) effect on S1P (10−6M) chemotaxis | |||||
|
| |||||
| IL-6 (1–10ng/mL) effect on S1P (10−7M) chemotaxis | S1P2 receptor expression as determined by PCR | Murine osteoclast precursors cultured in media containing fatty-acid free bovine serum albumin, migration assay. In vivo arthritis model | ↑S1P2 Receptor expression | (100) | |
| Negative chemotaxis | |||||
| ↓Bone volume | |||||
|
| |||||
| MESENCHYMAL STEM CELLS | S1P (1µM) | No receptors were investigated | Murine bone marrow stromal cells cultured in 10% inactivated serum | ↑Stress fibre formation | (61) |
| ↑Migration | |||||
|
| |||||
| S1P as part of murine osteoclast conditioned medium | S1P1 as determined using VPC23019 (1µM), without any discussion of S1P3 antagonism | Human mesenchymal stem cells cultured in media containing 10% serum and 10-fold concentrated conditioned media | ↑Mineralization | (69) | |
| ↑Migration | |||||
|
| |||||
| S1P as part of osteoclast conditioned medium, and S1P1 agonist VPC24191 (5µM) | S1P1/2 as determined using VPC23019 (100nM), and JTE013 (20nM), and S1P1 antagonist W143 (1µM) | Human bone marrow derived MSCs, cultured in media containing 10% serum | ↑Migration | (62) | |
| (Both S1P1/2 led to increased migration although through different pathways) | |||||
|
| |||||
| S1P (1µM) | S1P1 as determined by pertussis toxin (100ng/mL), and W146 (10µM) receptor blockade | C3H10T1/2 murine MSCs incubated with S1P for 15 minutes to 24 hours. Media contained 10% serum | ↑ALP | (102) | |
| ↑Osteocalcin | |||||
| ↑Mineralization | |||||
| ↓Adipogenic differentiation | |||||
| No effect on proliferation | |||||
|
| |||||
| S1P (40mg/mL and 80mg/mL) | S1P1/2 as determined by changes in gene expression | Human adipose derived stem cells cultured on titanium oxide coated stainless steel doped in S1P, cells were exposed to S1P for 120 hours | ↑Proliferation | (68) | |
| ↑Mineralization | |||||
| ↑Expression of S1P1 and S1P2 at 80mg/mL | |||||
| ↑Expression of S1P2 only at 40mg/mL | |||||
|
| |||||
| CHONDROCYTES | S1P (0.1–3µM) | S1P1–3 receptors exhibit increased expression as determined by PCR | Bovine and human cartilage explants (monolayer culture), proliferation assay | ↑Proliferation | (103) |
|
| |||||
| S1P (0.1–10µM) | Broad S1P receptor expression, though no specific receptor roles were identified, although Gi protein blockade with pertussis toxin reduced PGE2 induction by S1P | Human articular chondrocytes from osteoarthritis patients. Treated following serum starving (0.5% serum) | ↑PGE2 release | (104) | |
| ↑Cartilage degradation | |||||
| No effect on proliferation and viability | |||||
|
|
|||||
| OSTEOCYTES | Mechanical stimulation-S1P (100nM) | S1P2 as determined by pre-treatment with JTE013 (10µM) | MLO-Y4 cell line, oscillatory fluid flow, JTE013 | ↑PGE2 release | (91) |
| ↓RANKL/OPG | |||||
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Mills LA, Simpson AH. The relative incidence of fracture non-union in the Scottish population (5.17 million): a 5-year epidemiological study. BMJ Open. 2013;3(2) doi: 10.1136/bmjopen-2012-002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, Gerbhard F. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101. doi: 10.1016/j.bone.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3(1):49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, Tabata Y. Dual-controlled release system of drugs for bone regeneration. Adv Drug Deliv Rev. 2015;94:28–40. doi: 10.1016/j.addr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Martino MM, Briquez PS, Maruyama K, Hubbell JA. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv Drug Deliv Rev. 2015;94:41–52. doi: 10.1016/j.addr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Curry AS, Pensa NW, Barlow AM, Bellis SL. Taking cues from the extracellular matrix to design bone-mimetic regenerative scaffolds. Matrix Biol. 2016;52–54:397–412. doi: 10.1016/j.matbio.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder BY, Williams PA, Silva EA, Leach JK. Lysophosphatidic Acid and Sphingosine-1-Phosphate: A Concise Review of Biological Function and Applications for Tissue Engineering. Tissue Eng Part B Rev. 2015;21(6):531–42. doi: 10.1089/ten.teb.2015.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277(29):25851–4. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4(5):397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 10.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365(6446):557–60. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114(1):155–67. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–3. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 13.Takuwa Y. Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2002;1582(1–3):112–20. doi: 10.1016/s1388-1981(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 14.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15(5):513–20. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Chae SS, Paik JH, Allende ML, Proia RL, Hla T. Regulation of limb development by the sphingosine 1-phosphate receptor S1p1/EDG-1 occurs via the hypoxia/VEGF axis. Dev Biol. 2004;268(2):441–7. doi: 10.1016/j.ydbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Liu CH, Hla T. The mouse gene for the inducible G-protein-coupled receptor edg-1. Genomics. 1997;43(1):15–24. doi: 10.1006/geno.1997.4759. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Mao J, Redfield S, Mo Y, Lage JM, Zhou X. Systemic distribution, subcellular localization and differential expression of sphingosine-1-phosphate receptors in benign and malignant human tissues. Experimental and molecular pathology. 2014;97(2):259–65. doi: 10.1016/j.yexmp.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grey A, Xu X, Hill B, Watson M, Callon K, Reid IR, et al. Osteoblastic cells express phospholipid receptors and phosphatases and proliferate in response to sphingosine-1-phosphate. Calcified Tissue International. 2004;74(6):542–50. doi: 10.1007/s00223-003-0155-9. [DOI] [PubMed] [Google Scholar]
- 19.Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO Journal. 2006;25(24):5840–51. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller J, Catala-Lehnen P, Huebner AK, Jeschke A, Heckt T, Lueth A, et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nature Communications. 2014;5 doi: 10.1038/ncomms6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chew WS, Wang W, Herr DR. To fingolimod and beyond: The rich pipeline of drug candidates that target S1P signaling. Pharmacological research. 2016;113(Pt A):521–32. doi: 10.1016/j.phrs.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Hisano Y, Nishi T, Kawahara A. The functional roles of S1P in immunity. J Biochem. 2012;152(4):305–11. doi: 10.1093/jb/mvs090. [DOI] [PubMed] [Google Scholar]
- 23.Choi JW, Chun J. Lysophospholipids and their receptors in the central nervous system. Biochim Biophys Acta. 2013;1831(1):20–32. doi: 10.1016/j.bbalip.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waeber C, Walther T. Sphingosine-1-phosphate as a potential target for the treatment of myocardial infarction. Circ J. 2014;78(4):795–802. doi: 10.1253/circj.cj-14-0178. [DOI] [PubMed] [Google Scholar]
- 25.Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci. 2011;32(1):16–24. doi: 10.1016/j.tips.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207(3):465–74. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452(7187):654–8. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 28.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. The Journal of clinical investigation. 2009;119(7):1871–9. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salomone S, Waeber C. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front Pharmacol. 2011;2:9. doi: 10.3389/fphar.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigaud M, Guerini D, Billich A, Bassilana F, Brinkmann V. Second generation S1P pathway modulators: research strategies and clinical developments. Biochimica et biophysica acta. 2014;1841(5):745–58. doi: 10.1016/j.bbalip.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Sanllehi P, Abad JL, Casas J, Delgado A. Inhibitors of sphingosine-1-phosphate metabolism (sphingosine kinases and sphingosine-1-phosphate lyase) Chem Phys Lipids. 2016;197:69–81. doi: 10.1016/j.chemphyslip.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Sobel K, Monnier L, Menyhart K, Bolinger M, Studer R, Nayler O, et al. FTY720 Phosphate Activates Sphingosine-1-Phosphate Receptor 2 and Selectively Couples to Galpha12/13/Rho/ROCK to Induce Myofibroblast Contraction. Mol Pharmacol. 2015;87(6):916–27. doi: 10.1124/mol.114.097261. [DOI] [PubMed] [Google Scholar]
- 33.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12(6):703–15. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P–like headgroup interactions. Mol Pharmacol. 2008;74(5):1308–18. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adada M, Canals D, Hannun YA, Obeid LM. Sphingosine-1-phosphate receptor 2. FEBS J. 2013;280(24):6354–66. doi: 10.1111/febs.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280(11):9833–41. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 37.Zhu R, Snyder AH, Kharel Y, Schaffter L, Sun Q, Kennedy PC, et al. Asymmetric synthesis of conformationally constrained fingolimod analogues--discovery of an orally active sphingosine 1-phosphate receptor type-1 agonist and receptor type-3 antagonist. J Med Chem. 2007;50(25):6428–35. doi: 10.1021/jm7010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarrason G, Auli M, Mustafa S, Dolgachev V, Domenech MT, Prats N, et al. The sphingosine-1-phosphate receptor-1 antagonist, W146, causes early and short-lasting peripheral blood lymphopenia in mice. Int Immunopharmacol. 2011;11(11):1773–9. doi: 10.1016/j.intimp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Jongsma M, Hendriks-Balk MC, Michel MC, Peters SL, Alewijnse AE. BML-241 fails to display selective antagonism at the sphingosine-1-phosphate receptor, S1P(3) Br J Pharmacol. 2006;149(3):277–82. doi: 10.1038/sj.bjp.0706872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petite H, Viateau V, Bensaid W, Meunier A, de Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18(9):959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 41.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 42.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed Res Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karaplis AC. Embryonic Development of Bone and the Molecular Regulation of Intramembranous and Endochondral Bone Formation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 1. San Diego, California 92101–4495, USA: ACADEMIC PRESS; 2002. pp. 33–58. [Google Scholar]
- 44.Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–30. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–5. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134(21):3917–28. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 47.Mangiavini L, Merceron C, Araldi E, Khatri R, Gerard-O’Riley R, Wilson TL, et al. Fibrosis and hypoxia-inducible factor-1alpha-dependent tumors of the soft tissue on loss of von Hippel-Lindau in mesenchymal progenitors. Am J Pathol. 2015;185(11):3090–101. doi: 10.1016/j.ajpath.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leijten J, Chai YC, Papantoniou I, Geris L, Schrooten J, Luyten FP. Cell based advanced therapeutic medicinal products for bone repair: Keep it simple? Adv Drug Deliv Rev. 2015;84:30–44. doi: 10.1016/j.addr.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 49.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19(5):459–66. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Alman BA. Wnt pathway, an essential role in bone regeneration. J Cell Biochem. 2009;106(3):353–62. doi: 10.1002/jcb.22020. [DOI] [PubMed] [Google Scholar]
- 52.Secreto FJ, Hoeppner LH, Westendorf JJ. Wnt signaling during fracture repair. Curr Osteoporos Rep. 2009;7(2):64–9. doi: 10.1007/s11914-009-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meshcheryakova A, Mechtcheriakova D, Pietschmann P. Sphingosine 1-phosphate signaling in bone remodeling: multifaceted roles and therapeutic potential. Expert Opin Ther Targets. 2017;21(7):725–37. doi: 10.1080/14728222.2017.1332180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510(7503):58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Hsu A, Lee JF, Cramer DE, Lee MJ. To stay or to leave: Stem cells and progenitor cells navigating the S1P gradient. World J Biol Chem. 2011;2(1):1–13. doi: 10.4331/wjbc.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bendall LJ, Basnett J. Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr Opin Hematol. 2013;20(4):281–8. doi: 10.1097/MOH.0b013e3283606090. [DOI] [PubMed] [Google Scholar]
- 57.Ogle ME, Olingy CE, Awojoodu AO, Das A, Ortiz RA, Cheung HY, et al. Sphingosine-1-Phosphate Receptor-3 Supports Hematopoietic Stem and Progenitor Cell Residence Within the Bone Marrow Niche. Stem Cells. 2017;35(4):1040–52. doi: 10.1002/stem.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golan K, Kollet O, Lapidot T. Dynamic Cross Talk between S1P and CXCL12 Regulates Hematopoietic Stem Cells Migration, Development and Bone Remodeling. Pharmaceuticals (Basel) 2013;6(9):1145–69. doi: 10.3390/ph6091145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Annabi B, Thibeault S, Lee YT, Bousquet-Gagnon N, Eliopoulos N, Barrette S, et al. Matrix metalloproteinase regulation of sphingosine-1-phosphate-induced angiogenic properties of bone marrow stromal cells. Exp Hematol. 2003;31(7):640–9. doi: 10.1016/s0301-472x(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 60.Ho IA, Chan KY, Ng WH, Guo CM, Hui KM, Cheang P, et al. Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells. 2009;27(6):1366–75. doi: 10.1002/stem.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meriane M, Duhamel S, Lejeune L, Galipeau J, Annabi B. Cooperation of matrix metalloproteinases with the RhoA/Rho kinase and mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase signaling pathways is required for the sphingosine-1-phosphate-induced mobilization of marrow-derived stromal cells. Stem Cells. 2006;24(11):2557–65. doi: 10.1634/stemcells.2006-0209. [DOI] [PubMed] [Google Scholar]
- 62.Quint P, Ruan M, Pederson L, Kassem M, Westendorf JJ, Khosla S, et al. Sphingosine 1-phosphate (S1P) receptors 1 and 2 coordinately induce mesenchymal cell migration through S1P activation of complementary kinase pathways. J Biol Chem. 2013;288(8):5398–406. doi: 10.1074/jbc.M112.413583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Price ST, Beckham TH, Cheng JC, Lu P, Liu X, Norris JS. Sphingosine 1-Phosphate Receptor 2 Regulates the Migration, Proliferation, and Differentiation of Mesenchymal Stem Cells. International journal of stem cell research and therapy. 2015;2(2) doi: 10.23937/2469-570x/1410014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong Y, Wang H, Lin T, Wang S. Sphingosine-1-phosphate/S1P receptors signaling modulates cell migration in human bone marrow-derived mesenchymal stem cells. Mediators of inflammation. 2014;2014:565369. doi: 10.1155/2014/565369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das A, Barker DA, Wang T, Lau CM, Lin Y, Botchwey EA. Delivery of bioactive lipids from composite microgel-microsphere injectable scaffolds enhances stem cell recruitment and skeletal repair. PLoS One. 2014;9(7):e101276. doi: 10.1371/journal.pone.0101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hashimoto Y, Matsuzaki E, Higashi K, Takahashi-Yanaga F, Takano A, Hirata M, et al. Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells into adipocyte. Molecular and Cellular Biochemistry. 2015;401(1–2):39–47. doi: 10.1007/s11010-014-2290-1. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto Y, Kobayashi M, Matsuzaki E, Higashi K, Takahashi-Yanaga F, Takano A, et al. Sphingosine-1-phosphate-enhanced Wnt5a promotes osteogenic differentiation in C3H10T1/2 cells. Cell Biology International. 2016;40(10):1129–36. doi: 10.1002/cbin.10652. [DOI] [PubMed] [Google Scholar]
- 68.Marycz K, Krzak J, Maredziak M, Tomaszewski KA, Szczurek A, Moszak K. The influence of metal-based biomaterials functionalized with sphingosine-1-phosphate on the cellular response and osteogenic differentaion potenial of human adipose derived mesenchymal stem cells invitro. Journal of Biomaterials Applications. 2016;30(10):1517–33. doi: 10.1177/0885328216628711. [DOI] [PubMed] [Google Scholar]
- 69.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105(52):20764–9. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carpio LC, Stephan E, Kamer A, Dziak R. Sphingolipids stimulate cell growth via MAP kinase activation in osteoblastic cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 1999;61(5):267–73. doi: 10.1054/plef.1999.0100. [DOI] [PubMed] [Google Scholar]
- 71.Lampasso JD, Kamer A, Margarone J, Dziak R. Sphingosine-1-phosphate effects on PKC isoform expression in human osteoblastic cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2001;65(3):139–46. doi: 10.1054/plef.2001.0302. [DOI] [PubMed] [Google Scholar]
- 72.Lampasso JD, Marzec N, Margarone J, Dziak R. Role of protein kinase C alpha in primary human osteoblast proliferation. Journal of Bone and Mineral Research. 2002;17(11):1968–76. doi: 10.1359/jbmr.2002.17.11.1968. [DOI] [PubMed] [Google Scholar]
- 73.Dziak R, Yang BM, Leung BW, Li S, Marzec N, Margarone J, et al. Effects of sphingosine-1-phosphate and lysophosphatidic acid on human osteoblastic cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2003;68(3):239–49. doi: 10.1016/s0952-3278(02)00277-6. [DOI] [PubMed] [Google Scholar]
- 74.Grey A, Chen Q, Callon K, Xu X, Reid IR, Cornish J. The phospholipids sphingosine-1-phosphate and lysophosphatidic acid prevent apoptosis in osteoblastic cells via a signaling pathway involving G(i) proteins and phosphatidylinositol-3 kinase. Endocrinology. 2002;143(12):4755–63. doi: 10.1210/en.2002-220347. [DOI] [PubMed] [Google Scholar]
- 75.Brizuela L, Martin C, Jeannot P, Ader I, Gstalder C, Andrieu G, et al. Osteoblast-derived sphingosine 1-phosphate to induce proliferation and confer resistance to therapeutics to bone metastasis-derived prostate cancer cells. Molecular Oncology. 2014;8(7):1181–95. doi: 10.1016/j.molonc.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato C, Iwasaki T, Kitano S, Tsunemi S, Sano H. Sphingosine 1-phosphate receptor activation enhances BMP-2-induced osteoblast differentiation. Biochemical and Biophysical Research Communications. 2012;423(1):200–5. doi: 10.1016/j.bbrc.2012.05.130. [DOI] [PubMed] [Google Scholar]
- 77.Matsuzaki E, Hiratsuka S, Hamachi T, Takahashi-Yanaga F, Hashimoto Y, Higashi K, et al. Sphingosine-1-phosphate promotes the nuclear translocation of beta-catenin and thereby induces osteoprotegerin gene expression in osteoblast-like cell lines. Bone. 2013;55(2):315–24. doi: 10.1016/j.bone.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Higashi K, Matsuzaki E, Hashimoto Y, Takahashi-Yanaga F, Takano A, Anan H, et al. Sphingosine-1-phosphate/S1PR2-mediated signaling triggers Smad1/5/8 phosphorylation and thereby induces Runx2 expression in osteoblasts. Bone. 2016;93:1–11. doi: 10.1016/j.bone.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 79.Teti A. Mechanisms of osteoclast-dependent bone formation. BoneKEy reports. 2013;2:449. doi: 10.1038/bonekey.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lotinun S, Kiviranta R, Matsubara T, Alzate JA, Neff L, Luth A, et al. Osteoclast-specific cathepsin K deletion stimulates S1P–dependent bone formation. Journal of Clinical Investigation. 2013;123(2):666–81. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roelofsen T, Akkers R, Beumer W, Apotheker M, Steeghs I, van de Ven J, et al. Sphingosine-1-Phosphate Acts as a Developmental Stage Specific Inhibitor of Platelet-Derived Growth Factor-induced Chemotaxis of Osteoblasts. Journal of Cellular Biochemistry. 2008;105(4):1128–38. doi: 10.1002/jcb.21915. [DOI] [PubMed] [Google Scholar]
- 82.Waeber C, Blondeau N, Salomone S. Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors. Drug news & perspectives. 2004;17(6):365–82. doi: 10.1358/dnp.2004.17.6.829028. [DOI] [PubMed] [Google Scholar]
- 83.Lyons JM, Karin NJ. A role for G protein-coupled lysophospholipid receptors in sphingolipid-induced Ca2+ signaling in MC3T3-E1 osteoblastic cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(11):2035–42. doi: 10.1359/jbmr.2001.16.11.2035. [DOI] [PubMed] [Google Scholar]
- 84.Liu R, Farach-Carson MC, Karin NJ. Effects of sphingosine derivatives on MC3T3-E1 pre-osteoblasts: psychosine elicits release of calcium from intracellualr stores. Biochemical and biophysical research communications. 1995;214(2):676–84. doi: 10.1006/bbrc.1995.2339. [DOI] [PubMed] [Google Scholar]
- 85.Kozawa O, Tokuda H, Matsuno H, Uematsu T. Activation of mitogen-activated protein kinase is involved in sphingosine 1-phosphate-stimulated interleukin-6 synthesis in osteoblasts. Febs Letters. 1997;418(1–2):149–51. doi: 10.1016/s0014-5793(97)01366-5. [DOI] [PubMed] [Google Scholar]
- 86.Kozawa O, Niwa M, Matsuno H, Tokuda H, Miwa M, Ito H, et al. Sphingosine 1-phosphate induces heat shock protein 27 via p38 mitogen-activated protein kinase activation in osteoblasts. Journal of Bone and Mineral Research. 1999;14(10):1761–7. doi: 10.1359/jbmr.1999.14.10.1761. [DOI] [PubMed] [Google Scholar]
- 87.Kozawa O, Kawamura H, Uematsu T. Sphingosine 1-phosphate amplifies phosphoinositide hydrolysis stimulated by prostaglandin f2 alpha in osteoblasts: involvement of p38MAP kinase. Prostaglandins Leukot Essent Fatty Acids. 2000;62(6):355–9. doi: 10.1054/plef.2000.0166. [DOI] [PubMed] [Google Scholar]
- 88.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 89.Carpio LC, Shiau H, Dziak R. Changes in sphingolipid levels induced by epidermal growth factor in osteoblastic cells. Effects of these metabolites on cytosolic calcium levels. Prostaglandins Leukot Essent Fatty Acids. 2000;62(4):225–32. doi: 10.1054/plef.2000.0147. [DOI] [PubMed] [Google Scholar]
- 90.Martin TJ, Sims NA. Calcitonin physiology, saved by a lysophospholipid. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30(2):212–5. doi: 10.1002/jbmr.2449. [DOI] [PubMed] [Google Scholar]
- 91.Zhang JN, Zhao Y, Liu C, Han ES, Yu X, Lidington D, et al. The role of the sphingosine-1-phosphate signaling pathway in osteocyte mechanotransduction. Bone. 2015;79:71–8. doi: 10.1016/j.bone.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 92.Alford AI, Kozloff KM, Hankenson KD. Extracellular matrix networks in bone remodeling. Int J Biochem Cell Biol. 2015;65:20–31. doi: 10.1016/j.biocel.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Walsh JS. Normal bone physiology, remodelling and its hormonal regulation. Surgery -Oxford International Edition. 33(1):1–6. [Google Scholar]
- 94.Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458(7237):524–8. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee DE, Kim JH, Choi SH, Cha JH, Bak EJ, Yoo YJ. The sphingosine-1-phosphate receptor 1 binding molecule FTY720 inhibits osteoclast formation in rats with ligature-induced periodontitis. J Periodontal Res. 2017;52(1):33–41. doi: 10.1111/jre.12366. [DOI] [PubMed] [Google Scholar]
- 96.Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207(13):2793–8. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kikuta J, Iwai K, Saeki Y, Ishii M. S1P–targeted therapy for elderly rheumatoid arthritis patients with osteoporosis. Rheumatol Int. 2011;31(7):967–9. doi: 10.1007/s00296-010-1634-8. [DOI] [PubMed] [Google Scholar]
- 98.Yu H, Herbert BA, Valerio M, Yarborough L, Hsu LC, Argraves KM. FTY720 inhibited proinflammatory cytokine release and osteoclastogenesis induced by Aggregatibacter actinomycetemcomitans. Lipids Health Dis. 2015;14:66. doi: 10.1186/s12944-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kikuta J, Kawamura S, Okiji F, Shirazaki M, Sakai S, Saito H, et al. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc Natl Acad Sci U S A. 2013;110(17):7009–13. doi: 10.1073/pnas.1218799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tanaka K, Hashizume M, Mihara M, Yoshida H, Suzuki M, Matsumoto Y. Anti-interleukin-6 receptor antibody prevents systemic bone mass loss via reducing the number of osteoclast precursors in bone marrow in a collagen-induced arthritis model. Clinical and Experimental Immunology. 2014;175(2):172–80. doi: 10.1111/cei.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takeda H, Ozaki K, Yasuda H, Ishida M, Kitano S, Hanazawa S. Sphingomyelinase and ceramide inhibit formation of F-actin ring in and bone resorption by rabbit mature osteoclasts. FEBS Lett. 1998;422(2):255–8. doi: 10.1016/s0014-5793(98)00005-2. [DOI] [PubMed] [Google Scholar]
- 102.Hashimoto Y, Matsuzaki E, Higashi K, Takahashi-Yanaga F, Takano A, Hirata M, et al. Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells into adipocyte. Mol Cell Biochem. 2015;401(1–2):39–47. doi: 10.1007/s11010-014-2290-1. [DOI] [PubMed] [Google Scholar]
- 103.Stradner MH, Hermann J, Angerer H, Setznagl D, Sunk I, Windhager R, et al. Spingosine-1-phosphate stimulates proliferation and counteracts interleukin-1 induced nitric oxide formation in articular chondrocytes. Osteoarthritis Cartilage. 2008;16(3):305–11. doi: 10.1016/j.joca.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Masuko K, Murata M, Nakamura H, Yudoh K, Nishioka K, Kato T. Sphingosine-1-phosphate attenuates proteoglycan aggrecan expression via production of prostaglandin E2 from human articular chondrocytes. BMC Musculoskelet Disord. 2007;8:29. doi: 10.1186/1471-2474-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials. 2008;29(19):2869–77. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petrie Aronin CE, Sefcik LS, Tholpady SS, Tholpady A, Sadik KW, Macdonald TL, et al. FTY720 promotes local microvascular network formation and regeneration of cranial bone defects. Tissue Eng Part A. 2010;16(6):1801–9. doi: 10.1089/ten.tea.2009.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tondevold E, Eliasen P. Blood flow rates in canine cortical and cancellous bone measured with 99Tcm-labelled human albumin microspheres. Acta Orthop Scand. 1982;53(1):7–11. doi: 10.3109/17453678208992171. [DOI] [PubMed] [Google Scholar]
- 108.Inagami T, Naruse M, Hoover R. Endothelium as an endocrine organ. Annu Rev Physiol. 1995;57:171–89. doi: 10.1146/annurev.ph.57.030195.001131. [DOI] [PubMed] [Google Scholar]
- 109.Tomlinson RE, Silva MJ. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013;1(4):311–22. doi: 10.4248/BR201304002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102(4):840–7. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 111.Gaengel K, Niaudet C, Hagikura K, Lavina B, Muhl L, Hofmann JJ, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Developmental cell. 2012;23(3):587–99. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 112.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Developmental cell. 2012;23(3):600–10. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duran CL, Kaunas R, Bayless KJ. S1P Synergizes with Wall Shear Stress and Other Angiogenic Factors to Induce Endothelial Cell Sprouting Responses. Methods in molecular biology. 2017 doi: 10.1007/7651_2017_26. [DOI] [PubMed] [Google Scholar]
- 114.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 116.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 117.Riley CM, Fuegy PW, Firpo MA, Shu XZ, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials. 2006;27(35):5935–43. doi: 10.1016/j.biomaterials.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tengood JE, Kovach KM, Vescovi PE, Russell AJ, Little SR. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials. 2010;31(30):7805–12. doi: 10.1016/j.biomaterials.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tengood JE, Ridenour R, Brodsky R, Russell AJ, Little SR. Sequential delivery of basic fibroblast growth factor and platelet-derived growth factor for angiogenesis. Tissue engineering Part A. 2011;17(9–10):1181–9. doi: 10.1089/ten.tea.2010.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waeber C. Sphingosine 1-Phosphate (S1P) Signaling and the Vasculature. In: Chun J, Hla T, Spiegel S, Moolenaar W, editors. Lysophospholipid Receptors: Signaling and Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc; 2013. pp. 313–47. [Google Scholar]
- 121.Takuwa Y, Du W, Qi X, Okamoto Y, Takuwa N, Yoshioka K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World journal of biological chemistry. 2010;1(10):298–306. doi: 10.4331/wjbc.v1.i10.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lucke S, Levkau B. Endothelial functions of sphingosine-1-phosphate. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2010;26(1):87–96. doi: 10.1159/000315109. [DOI] [PubMed] [Google Scholar]
- 123.Oyama O, Sugimoto N, Qi X, Takuwa N, Mizugishi K, Koizumi J, et al. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovascular research. 2008;78(2):301–7. doi: 10.1093/cvr/cvn002. [DOI] [PubMed] [Google Scholar]
- 124.Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochimica et biophysica acta. 2008;1781(9):483–8. doi: 10.1016/j.bbalip.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Qi X, Okamoto Y, Murakawa T, Wang F, Oyama O, Ohkawa R, et al. Sustained delivery of sphingosine-1-phosphate using poly(lactic-co-glycolic acid)-based microparticles stimulates Akt/ERK-eNOS mediated angiogenesis and vascular maturation restoring blood flow in ischemic limbs of mice. European journal of pharmacology. 2010;634(1–3):121–31. doi: 10.1016/j.ejphar.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 126.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99(3):301–12. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 127.Singleton PA, Moreno-Vinasco L, Sammani S, Wanderling SL, Moss J, Garcia JG. Attenuation of vascular permeability by methylnaltrexone: role of mOP-R and S1P3 transactivation. American journal of respiratory cell and molecular biology. 2007;37(2):222–31. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- 128.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(12):1646–56. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 129.Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, et al. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circulation research. 2009;104(8):978–86. doi: 10.1161/CIRCRESAHA.108.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(6):1312–8. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 131.Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, et al. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009;296(1):33–42. doi: 10.1152/ajpheart.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. The Journal of biological chemistry. 2003;278(47):47281–90. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 133.Rosen H, Gonzalez-Cabrera P, Marsolais D, Cahalan S, Don AS, Sanna MG. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunological reviews. 2008;223:221–35. doi: 10.1111/j.1600-065X.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 134.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nature chemical biology. 2006;2(8):434–41. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 135.Foss FW, Jr, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, et al. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorganic & medicinal chemistry. 2007;15(2):663–77. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochimica et biophysica acta. 2002;1582(1–3):222–7. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 137.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. The Journal of clinical investigation. 2000;106(8):951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ben Shoham A, Malkinson G, Krief S, Shwartz Y, Ely Y, Ferrara N, et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development. 2012;139(20):3859–69. doi: 10.1242/dev.078550. [DOI] [PubMed] [Google Scholar]
- 139.Heilmann A, Schinke T, Bindl R, Wehner T, Rapp A, Haffner-Luntzer M, et al. Systemic treatment with the sphingosine-1-phosphate analog FTY720 does not improve fracture healing in mice. J Orthop Res. 2013;31(11):1845–50. doi: 10.1002/jor.22426. [DOI] [PubMed] [Google Scholar]
- 140.Ahern E, Doody T, Ryan K. Bioengineered Nanomaterials. CRC Press; 2013. Bioinspired Nanomaterials for Bone Tissue Engineering; pp. 369–412. [Google Scholar]
- 141.Galvin P, Thompson D, Ryan KB, McCarthy A, Moore AC, Burke CS, et al. Nanoparticle-based drug delivery: case studies for cancer and cardiovascular applications. Cell Mol Life Sci. 2012;69(3):389–404. doi: 10.1007/s00018-011-0856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Das A, Tanner S, Barker DA, Green D, Botchwey EA. Delivery of S1P receptor-targeted drugs via biodegradable polymer scaffolds enhances bone regeneration in a critical size cranial defect. J Biomed Mater Res A. 2014;102(4):1210–8. doi: 10.1002/jbm.a.34779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Das A, Segar CE, Hughley BB, Bowers DT, Botchwey EA. The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages. Biomaterials. 2013;34(38):9853–62. doi: 10.1016/j.biomaterials.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ogle ME, Segar CE, Sridhar S, Botchwey EA. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp Biol Med (Maywood) 2016;241(10):1084–97. doi: 10.1177/1535370216650293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ogle ME, Sefcik LS, Awojoodu AO, Chiappa NF, Lynch K, Peirce-Cottler S, et al. Engineering in vivo gradients of sphingosine-1-phosphate receptor ligands for localized microvascular remodeling and inflammatory cell positioning. Acta Biomater. 2014;10(11):4704–14. doi: 10.1016/j.actbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Das A, Segar CE, Chu Y, Wang TW, Lin Y, Yang C, et al. Bioactive lipid coating of bone allografts directs engraftment and fate determination of bone marrow-derived cells in rat GFP chimeras. Biomaterials. 2015;64:98–107. doi: 10.1016/j.biomaterials.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murakami M, Saito T, Tabata Y. Controlled release of sphingosine-1-phosphate agonist with gelatin hydrogels for macrophage recruitment. Acta Biomater. 2014;10(11):4723–9. doi: 10.1016/j.actbio.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 148.Zhang J, Song J. Amphiphilic degradable polymers for immobilization and sustained delivery of sphingosine 1-phosphate. Acta Biomater. 2014;10(7):3079–90. doi: 10.1016/j.actbio.2014.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim YH, Furuya H, Tabata Y. Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels. Biomaterials. 2014;35(1):214–24. doi: 10.1016/j.biomaterials.2013.09.103. [DOI] [PubMed] [Google Scholar]
- 150.Petrie Aronin CE, Shin SJ, Naden KB, Rios PD, Jr, Sefcik LS, Zawodny SR, et al. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials. 2010;31(25):6417–24. doi: 10.1016/j.biomaterials.2010.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang C, Das A, Barker D, Tholpady S, Wang T, Cui QJ, et al. Local delivery of FTY720 accelerates cranial allograft incorporation and bone formation. Cell and Tissue Research. 2012;347(3):553–66. doi: 10.1007/s00441-011-1217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang T, Krieger J, Huang C, Das A, Francis MP, Ogle R, et al. Enhanced osseous integration of human trabecular allografts following surface modification with bioactive lipids. Drug Deliv Transl Res. 2016;6(2):96–104. doi: 10.1007/s13346-015-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sefcik LS, Aronin CEP, Awojoodu AO, Shin SJ, Mac Gabhann F, MacDonald TL, et al. Selective Activation of Sphingosine 1-Phosphate Receptors 1 and 3 Promotes Local Microvascular Network Growth. Tissue Engineering Part A. 2011;17(5–6):617–29. doi: 10.1089/ten.tea.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kono M, Mi YD, Liu YJ, Sasaki T, Allende ML, Wu YP, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. Journal of Biological Chemistry. 2004;279(28):29367–73. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 155.Ratajczak MZ, Suszynska M, Borkowska S, Ratajczak J, Schneider G. The role of sphingosine-1 phosphate and ceramide-1 phosphate in trafficking of normal stem cells and cancer cells. Expert Opinion on Therapeutic Targets. 2014;18(1):95–107. doi: 10.1517/14728222.2014.851671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine Journal. 2014;14(3):552–9. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 157.Yamamoto M, Takahashi Y, Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24(24):4375–83. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 158.Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess. 2007;11(30):1–150. doi: 10.3310/hta11300. iii-iv. [DOI] [PubMed] [Google Scholar]
- 159.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36(12):1392–404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 161.Hankenson KD, Gagne K, Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev. 2015;94:3–12. doi: 10.1016/j.addr.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 162.Klontzas ME, Kenanidis EI, MacFarlane RJ, Michail T, Potoupnis ME, Heliotis M, et al. Investigational drugs for fracture healing: preclinical & clinical data. Expert Opin Investig Drugs. 2016;25(5):585–96. doi: 10.1517/13543784.2016.1161757. [DOI] [PubMed] [Google Scholar]
- 163.Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, Dickinson IC, et al. Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective. Bone Research. 2013;1:216–48. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hanke T, Merk D, Steinhilber D, Geisslinger G, Schubert-Zsilavecz M. Small molecules with anti-inflammatory properties in clinical development. Pharmacology & Therapeutics. 2016;157:163–87. doi: 10.1016/j.pharmthera.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 165.Chew WS, Wang W, Herr DR. To fingolimod and beyond: The rich pipeline of drug candidates that target S1P signaling. Pharmacological Research. 2016;113:521–32. doi: 10.1016/j.phrs.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 166.Quancard J, Bollbuck B, Janser P, Angst D, Berst F, Buehlmayer P, et al. A Potent and Selective S1P(1) Antagonist with Efficacy in Experimental Autoimmune Encephalomyelitis. Chemistry & Biology. 2012;19(9):1142–51. doi: 10.1016/j.chembiol.2012.07.016. [DOI] [PubMed] [Google Scholar]