Abstract
Background There is no consensus exists regarding which reconstructive approach, if any, should be used after performing transcranial lateral orbital wall resections. Rigid reconstruction is often done to prevent enophthalmos; however, it is not clear if this is a risk with extensive orbital wall resections for transcranial surgery.
Objective To assess globe position dynamics in patients that underwent transcranial lateral and superior orbital wall resections without rigid reconstruction to determine if enophthalmos is a significant risk.
Methods Preoperative (PO) and postoperative data were retrospectively collected from the electronic medical records of 55 adult patients undergoing lateral and superior orbital wall resections as part of a skull base approach. The globe positions were assessed radiologically at all available time points and used to track relative globe displacements over time.
Results An evaluation of PO variables identified a relationship between maximum lesion diameters and globe positions dynamics. The composition of globe position presentations in the population remained relatively stable over time, with only 1 out of 55 patients (1.81%) developing postoperative enophthalmos. An assessment of mean globe displacements revealed improvements in the patients presenting with PO exophthalmos, and stability in the patients presenting with normal PO globe positions.
Conclusions Excellent results in long-term postoperative globe position dynamics can be achieved without the use of rigid reconstruction after transcranial lateral and superior orbital wall resections, regardless of the PO globe positioning.
Keywords: enophthalmos, exophthalmos, lateral and superior orbital wall resection, meningioma, proptosis
Introduction
Orbital lesions, such as spheno-orbital meningiomas, present unique surgical challenges because of the relationships with an array of delicate structures housed within the orbit. As reviewed by Boulos et al, orbital involvement has been related to a clinical presentation that may include vision loss, optic disc changes, exophthalmos, diplopia, headaches, nausea, and vomiting. 1 Surgical interventions for orbital lesions stand to dramatically improve the quality of life by addressing related disruptions in the visual system. Accordingly, it is essential to optimize the surgical approaches that are implemented. These approaches may be influenced by several case-specific factors like lesion size, or region of origin. A transorbital approach is generally favored for orbital lesions that are restricted to the anterior orbit, while a transcranial approach is favored for orbital lesions with significant intracranial involvement. 1 There are several routes of access associated with each approach which require an orbitotomy to address the underlying pathology. Nonetheless, there is still no consensus regarding which form of orbital reconstruction, if any, should be used following resection of orbital walls. 2 In the present study, we aimed to assess long-term globe position dynamics in patients who underwent transcranial lateral and superior orbital wall resections without rigid reconstruction to determine if the development of enophthalmos was a significant risk.
Methods
Patient Population and Data Collection
The patient population consisted of adult patients undergoing transcranial lateral and superior orbital wall resections without rigid reconstruction between September 2013 and December 2017 at Mayo Clinic, Rochester, MN, United States. The electronic medical records of patients were retrospectively reviewed. Data were collected with regard to patient population factors including patient age at diagnosis, surgery, sex, lesion classification, and lesion size. Additionally, patient globe positions were calculated from preoperative (PO) and postoperative radiological images as described below in the Globe Position Assessment subsection. Patients who presented preoperatively with either normal globe positions or exophthalmos were included in the study. The postoperative calculations were based on radiological images obtained at the 3-month postoperative follow-up, 12-month postoperative follow-up, and/or last postoperative follow-up.
Globe Position Assessment
Globe positions were quantitatively assessed using magnetic resonance images (MRIs), or computed topography (CT) scans when MRI series were not available. The calculations were made on axial image series by first measuring the distance in mm from the most anterior aspect of each globe to an interzygomatic line that was drawn between the most anterior aspects of each zygomatic frontal process ( Fig. 1 ). Landmarks including the ethmoid sinuses, lateral rectus muscles, medial rectus muscles, and optic nerves were used to identify the ideal plane for analysis in each image series. The resulting values were then used to calculate the relative anterior globe displacement (A Δ ) by taking the distance measured on the transcranial lateral and superior orbital wall resection side and subtracting the distance measured on the contralateral side. A similar approach was utilized to calculate the relative posterior globe displacements (P Δ ) which were based on distances to the most posterior aspect of each globe. All calculations were performed in technical triplicates to identify the mean A Δ and P Δ for each individual case in the series, which could be used to classify the cases into three groups. Patients with either an A Δ ≤ −2 mm or a P Δ ≥ 2 mm were classified as having enophthalmos, patients with either an A Δ ≥ 2 mm or a P Δ ≤ −2 mm as having exophthalmos, and patients with an −2 mm < A Δ < 2 mm and a −2 mm < P Δ < 2 mm as having normal globe positioning. These criteria were used to partition patients into groups based on PO presentations of either normal globe displacement (normal PO) or exophthalmos (exophthalmos PO) and to evaluate the subsequent postoperative presentations.
Fig. 1.
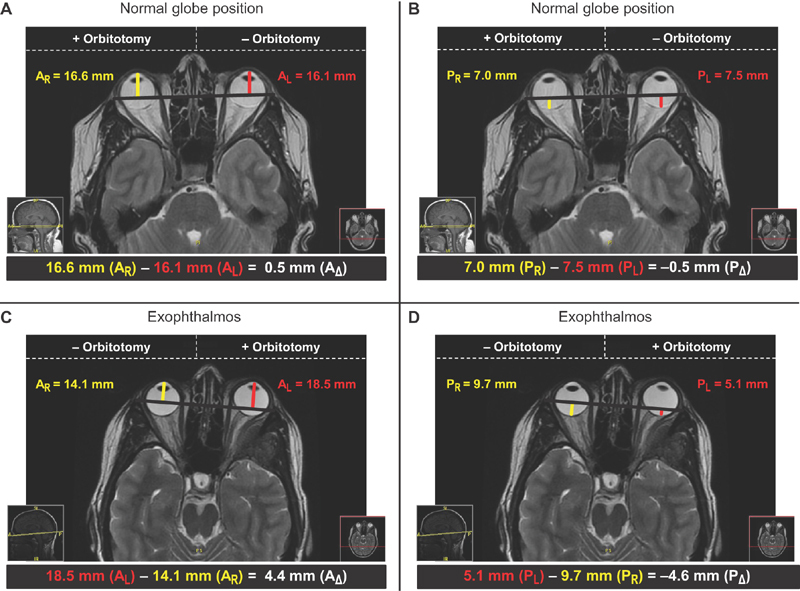
Representative radiological assessments of globe position. ( A ) The relative anterior globe displacement (A Δ ) was calculated on a preoperative T2-weighted MRI by taking the right anterior globe displacement (A R ) measured on the orbitotomy side (+orbitotomy) and subtracting the left anterior globe displacement (A L ) measured on the contralateral side (−orbitotomy). The calculated value for A Δ of 0.5 mm is representative of a normal globe position (−2 mm < A Δ < 2 mm). ( B ) The relative posterior globe displacement (P Δ ) was calculated on a preoperative T2-weighted MRI by taking the right globe displacement (P R ) measured on the orbitotomy side (+orbitotomy) and subtracting the left globe displacement (P L ) measured on the contralateral side (−orbitotomy). The calculated value for P Δ of −0.5 mm is representative of a normal globe position (−2 mm < P Δ < 2 mm). ( C ) The relative anterior globe displacement (A Δ ) was calculated on a preoperative T2-weighted MRI by taking the left anterior globe displacement (A L ) measured on the orbitotomy side (+orbitotomy) and subtracting the right anterior globe displacement (A R ) measured on the contralateral side (−orbitotomy). The calculated value for A Δ of 4.4 mm is representative of exophthalmos (A Δ ≥ 2 mm). ( D ) The relative posterior globe displacement (P Δ ) was calculated on a preoperative T2-weighted MRI by taking the left globe displacement (P L ) measured on the orbitotomy side (+orbitotomy) and subtracting the right globe displacement (P R ) measured on the contralateral side (−orbitotomy). The calculated value for P Δ of −4.6 mm is representative of exophthalmos (P Δ ≤ −2 mm). MRI, magnetic resonance imaging.
Statistical Analyses
All statistical analyses were performed using JMP software (SAS Institute Inc., Cary, NC, United States). Descriptive statistics were used when appropriate to assess factors including measures of frequency (i.e., number of males and number of females), or measures of central tendency and variation (i.e., mean patient age at diagnosis ± standard error of the mean). Pearson's correlation coefficient was used to examine linear relationships of variables. Paired t -tests were used to examine relationships between the A Δ mean and the P Δ mean of all patients at each time point. Two-sample t -tests were used to examine relationships between the A Δ mean or the P Δ mean of the normal PO and exophthalmos PO groups at each time point, using two tails and assuming unequal variances. Multiple comparisons were accounted for by applying Bonferroni's corrections to an α level of 0.05.
Surgical Approach
All neurological surgeries were performed by the service of the senior author (J.J.V.G.). Zygomatic sparing tailored pterional craniotomies were performed with resection of the lateral and superior orbital walls as well as the anterior clinoid with decompression of the optic canal. In all cases, Dolenc's dural openings or dural resection was performed, reconstruction was done in the same way in all patients, an inlay of DuraGen (Integra LifeSciences, Plainsboro, NJ, United States) was done to cover the dural opening to several mm past the dural opening. The substitute inlay was placed over the optic nerve and care was taken not to pack the decompressed optic nerve. The dura was then approximated, however, due to the opening and dural resections, this was never water tight. Then a second piece of DuraGen was placed extradurally over the dural opening, this could all typically be accomplished with one 3 × 3 cm implant. The bone flap was replaced, and the wound was closed, however, interfacial sutures were placed between the galea and bone to prevent a painful pseudomeningocele. The head was always wrapped after the procedure and was kept wrapped for at least a day, and if the patient tolerated it out to 2 days.
Results
The patient population consisted of 55 cases including males ( n = 22) and females ( n = 33). In 42 of the cases, the orbital wall resections were meningioma-associated, while a variety of lesions were involved with the other 13 cases ( Table 1 ). The mean maximum lesion diameter in this patient series was 37.1 mm ± 2.3. There was a positive correlation between the maximum lesion diameter and the degree of PO relative anterior globe displacement (A Δ ; [ r (54): 0.4054; p = 0.0035; CI: 0.1432–0.6144]). Similar correlations involving the A Δ were observed across the 3-month follow-up (3M), 12-month follow-up (12M), and last follow-up (LFU) postoperative time points (3M: r [51]: 0.3486, p = 0.0131, CI: 0.0715–0.5750; 12M r [38]: 0.5585, p = 0.0002, CI: 0.2817–0.7495; LFU: r [24]: 0.5797, p = 0.0024, CI: 0.2093–0.8047). There was a negative correlation between the maximum lesion diameter and the degree of PO relative posterior globe displacement (P Δ ; [ r (54): −0.4266, p = 0.0020, CI: −0.6301 to −0.1682]). Similar correlations involving the P Δ were observed across the 3-month follow-up (3M), 12-month follow-up (12M), and last follow-up (LFU) postoperative time points (3M: r [51]: −0.4120, p = 0.0030, CI: −0.6232 to −0.1448; 12M r [38]: −0.5779, p = 0.0001, CI: −0.7618 to −0.3079; LFU: r [24]: −0.6438, p = 0.0005, CI: −0.8380 to −0.3050). Additionally, the maximum lesion diameters were depicted in the context of primary lesion locations and globe position statuses for each individual case ( Supplementary Tables S1 , S2 ; available online only).
Table 1. Indications for lateral and superior orbital wall resections.
| Lesion type | Number of cases |
|---|---|
| Adenoid cystic carcinoma (grade II) | 1 |
| Aneursym; basilar caput | 1 |
| Eperimoid cyst | 1 |
| Fibrous dysplasia; McCune–Albright syndrome | 1 |
| IgG4-related disease | 1 |
| Meningioma | 42 |
| Meningothelial (grade I) | 19 |
| Secretory (grade I) | 2 |
| Transitional (grade I) | 6 |
| Unspecified (grade I) | 7 |
| Atypical (grade II) | 7 |
| Unspecified (grade II) | 1 |
| Neurosarcoidosis | 1 |
| Pituitary adenoma | 4 |
| Corticotroph | 2 |
| Null cell | 1 |
| Pleurihormonal | 1 |
| Sarcoma | 3 |
| Chondrosarcoma (grade I) | 1 |
| Chondrosarcoma (grade II) | 1 |
| Ewing's sarcoma (metastatic) | 1 |
Abbreviation: IG, immunoglobulin.
Note : A complete list of lesions in the patient population that were associated with lateral and superior orbital wall resections. When available, the World Health Organization (WHO) classification of lesion grade was included.
Subsequently, the compositions of globe position presentations in the patient population were quantitatively evaluated over time ( Figs. 1 and 2 ). Among the 42 patients presenting with a meningioma, normal PO globe displacement was observed in 31 of the cases and exophthalmos PO globe displacement was observed in 11 of the cases. Among the 13 patients presenting with other lesions, normal PO globe displacement was observed in 11 of the cases and exophthalmos PO globe displacement was observed in two of the cases. The patient presenting with a basilar caput aneurysm accounted for the single instance of postoperative enophthalmos that developed. The globe position dynamics were then further assessed by evaluating the A Δ means and the P Δ means of all patients over time ( Fig. 3A ). Significant differences were initially observed between the A Δ and P Δ at the PO ( t [54]: −2.800, p = 0.0071), 3M ( t [51]: −4.770, p < 0.0001), and 12M ( t [38]: −3.426, p = 0.0015) time points; however, no significant differences were observed at the LFU ( t [24]: −1.307, p = 0.2036) time point.
Fig. 2.
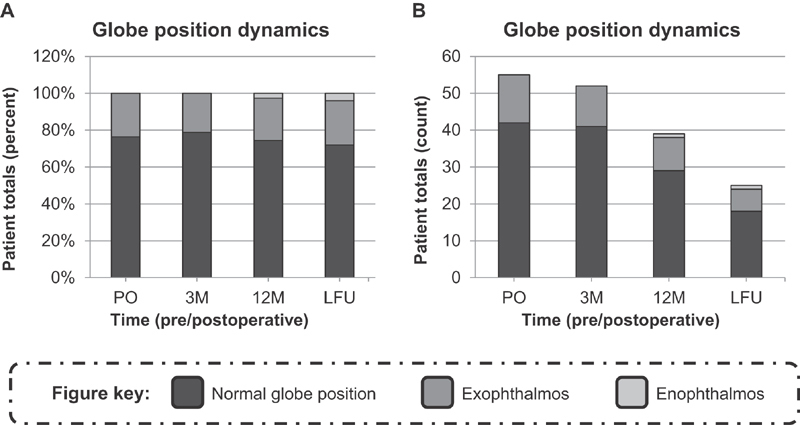
Composition of globe position presentations in the patient population over time. ( A ) The percent of patients in the population presenting with either normal global positions, exophthalmos, or enophthalmos. The compositions were assessed by analyzing preoperative (PO), 3-month postoperative follow-up (3M), 12-month postoperative follow-up (12M), and last postoperative follow-up (LFU) radiological images. ( B ) The number of patients in the population presenting with either normal global positions, exophthalmos, or enophthalmos. The compositions were assessed by analyzing preoperative (PO), 3-month postoperative follow-up (3M), 12-month postoperative follow-up (12M), and last postoperative follow-up (LFU) radiological images.
Fig. 3.
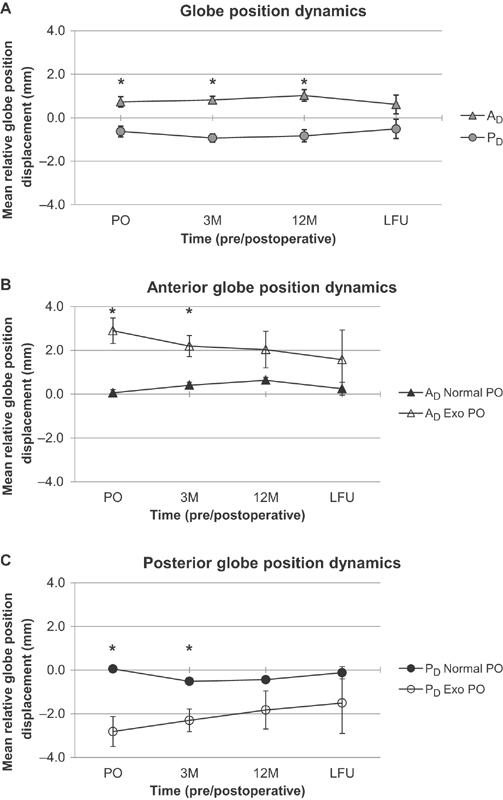
Globe position dynamics in the patient population over time. ( A ) The mean relative anterior (A Δ ) and posterior (P Δ ) globe displacements of all patients are represented over time. The means were calculated by analyzing preoperative (PO), 3-month postoperative follow-up (3M), 12-month postoperative follow-up (12M), and last postoperative follow-up (LFU) radiological images. All means are depicted with the associated standard error of the mean. Statistically significant differences between the A Δ and P Δ means at any given pre-/postoperative time are indicated with an asterisk ( * ; paired t -test, Bonferroni's corrected p ≤ 0.05). ( B ) The mean relative anterior globe displacements (A Δ ) of patients that preoperatively presented with either normal globe positions (Normal PO) or exophthalmos (Exo PO) are represented over time. The means were calculated by analyzing preoperative (PO), 3-month postoperative follow-up (3M), 12-month postoperative follow-up (12M), and last postoperative follow-up (LFU) radiological images. All means are depicted with the associated standard error of the mean. Statistically significant differences between the A Δ PO Normal and A Δ PO Exo means at any given pre-/postoperative time are indicated with an asterisk ( * ; two-sample t -test, Bonferroni's corrected p ≤ 0.05). ( C ) The mean relative posterior globe displacements (P Δ ) of patients that preoperatively presented with either normal globe positions (Normal PO) or exophthalmos (Exo PO) are represented over time. The means were calculated by analyzing preoperative (PO), 3-month postoperative follow-up (3M), 12-month postoperative follow-up (12M), and last postoperative follow-up (LFU) radiological images. All means are depicted with the associated standard error of the mean. Statistically significant differences between the P Δ PO Normal and P Δ PO Exo means at any given pre-/postoperative time are indicated with an asterisk ( * ; two-sample t -test, Bonferroni's corrected p ≤ 0.05).
The PO radiological measurements of globe positions were then used to partition the patient series into either the “Normal PO” or the “Exophthalmos PO” groups, as defined in the Methods Globe Position Assessment subsection. The A Δ means of the normal PO and exophthalmos PO groups were then assessed over time ( Fig. 3B ). Significant differences were initially observed between the normal PO and exophthalmos PO groups at the PO ( t [13.51]: 4.710, p = 0.0004) and 3M ( t [12.82]: 3.583, p = 0.0034) time points; however, no significant differences were observed by the 12M ( t [10.53]: 1.664, p = 0.1256) or LFU ( t [6.574]: 0.9649, p = 0.3687) time points. Additionally, the P Δ means of the normal PO and exophthalmos PO groups were assessed over time ( Fig. 3C ). Significant differences were initially observed between the P Δ of the normal PO and exophthalmos PO groups at the PO ( t [12.96]: −4.085, p = 0.0013) and 3M ( t [12.65]: −3.327, p = 0.0056) time points; however, no significant differences were observed by the 12M ( t [10.61]: −1.563, p = 0.1475) or LFU ( t [6.505]: −0.9763, p = 0.3638) time points.
Discussion
Developing standardized surgical approaches to orbital wall resections is imperative to ensure optimal postoperative outcomes for patients, including those outcomes related to visual system function and cosmetic satisfaction. An array of materials have been utilized for structural reconstruction of orbital defects; however, there is still controversy regarding which yield ideal long-term results, whether there is an increased risk of complications, such as infection or postoperative exophthalmos with rigid reconstruction, and if rigid reconstruction is a necessity. 3 4 In recent years, several studies have specifically focused on assessing postoperative outcomes of meningioma-associated transcranial lateral orbital wall resections that were completed with or without rigid reconstruction. 2 5 6 7 8 9 10 11 Both surgical approaches have been associated with a host of postoperative neurological and ophthalmological improvements. One improvement that has been consistently observed in the majority of patients is postoperative improvements in exophthalmos, otherwise known as proptosis. Notably, these improvements were documented in patient populations where significant exophthalmos presented preoperatively in most, if not all, of the cases. To our knowledge, no studies have focused on long-term postoperative outcomes in a patient population that predominately presented with normal PO globe positions, or assessed how the outcomes may differ from a population subset with PO exophthalmos.
In the present study, none of the patients that presented with a meningioma developed enophthalmos following the surgical intervention. Similar outcomes have been previously documented in studies where patients presented with PO exophthalmos; however, little is known about the expected outcomes in patients preoperatively presenting with normal globe positions. 2 5 6 8 9 10 The results of this study suggested that there is not a significant risk for the development of postoperative enophthalmos in patients undergoing meningioma-associated transcranial lateral and superior orbital wall resections without rigid reconstruction, regardless of PO globe position status. Similarly, enophthalmos did not develop postoperatively in most patients that presented with nonmeningioma lesions. However, in one case that involved a basilar caput aneurysm, the development of postoperative enophthalmos was observed. There was no direct involvement of the orbit by this lesion; the orbital wall resections were indicated to access and clip the aneurysm. The pathology of this lesion is markedly different from that of the other lesions in this case series, and thus it is quite possible that unique reconstructive approaches to the orbital wall may need to be utilized in such instances. Nonetheless, we are reticent to draw conclusions based on this isolated case given that favorable postoperative globe position outcomes were achieved in most patients with nonmeningioma lesions. Future studies are undoubtedly warranted to clarify the optimal reconstructive approaches that should be employed when orbital wall resections are indicated for access to aneurysms, and to elucidate the potential roles that lesion locations may have influencing surgical outcomes.
In addition to considering the qualitative composition of globe position presentations in this patient population, we also quantified the dynamics of these presentations. When the population was considered as a whole, the mean postoperative relative anterior globe displacement (A Δ ) and mean postoperative relative posterior globe displacement (P Δ ) remained relatively stable over the entire postoperative time course. However, disparate trends were noted when these dynamics were considered in the context of PO globe position presentations. Within the group that presented with PO exophthalmos, the mean relative globe displacements exhibited a postoperative shift toward normal positioning. To that end, by the last follow-up, the mean globe positions in this group no longer met criteria for exophthalmos and were statistically indistinct from the group that presented with normal PO globe positions. Similar shifts toward normal globe positions have been observed in previous studies, and are typically deemed favorable for both visual system function and cosmetic satisfaction. Correlations of maximum lesion diameter with either A Δ or P Δ , however, implied that increased lesion size may portend a long-term risk for residual postoperative exophthalmos. In contrast, no such posterior shifts were observed in the mean A Δ and P Δ of the group with normal PO globe positions; the mean postoperative values of this group remained normal throughout the entire time course.
Taken together, these data revealed that posterior postoperative shifts in the relative globe displacements after transcranial lateral and superior orbital wall resections without rigid reconstruction may be limited to patients presenting with PO exophthalmos. This finding has important implications for the safety of utilizing this surgical approach in patients that present with normal PO globe positions. If a similar shift had been observed in this group, it would have resulted in the unfavorable postoperative presentation of enophthalmos, and thus warranted an investigation into alternative approaches to orbital reconstruction. Although additional research is needed to establish a link between our findings related to globe positions dynamics and satisfactory postoperative improvements in visual function, we believe that nonrigid reconstruction can be safely utilized in a variety of scenarios. Indeed, the results of this study ultimately indicated that excellent results in long-term postoperative globe position dynamics can be achieved without the use of rigid reconstruction during transcranial lateral and superior orbital wall resections, regardless of the PO globe positioning.
Conclusion
There is still no consensus about what approaches to reconstruction should be utilized after accessing lesions that have involved the orbit. We described a case series of 55 patients that underwent transcranial lateral and superior orbital wall resections without rigid reconstruction. We concluded that excellent results in long-term postoperative globe position dynamics can be achieved without the use of rigid reconstruction during transcranial lateral and superior orbital wall resections, regardless of the PO globe positioning.
Footnotes
Conflict of Interest The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Supplementary Material
References
- 1.Boulos P T, Dumont A S, Mandell J W, Jane J A., Sr Meningiomas of the orbit: contemporary considerations. Neurosurg Focus. 2001;10(05):E5. doi: 10.3171/foc.2001.10.5.6. [DOI] [PubMed] [Google Scholar]
- 2.Bowers C A, Sorour M, Patel B C, Couldwell W T. Outcomes after surgical treatment of meningioma-associated proptosis. J Neurosurg. 2016;125(03):544–550. doi: 10.3171/2015.9.JNS15761. [DOI] [PubMed] [Google Scholar]
- 3.Dubois L, Steenen S A, Gooris P J, Bos R R, Becking A G. Controversies in orbital reconstruction-III. Biomaterials for orbital reconstruction: a review with clinical recommendations. Int J Oral Maxillofac Surg. 2016;45(01):41–50. doi: 10.1016/j.ijom.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Talacchi A, De Carlo A, D'Agostino A, Nocini P.Surgical management of ocular symptoms in spheno-orbital meningiomas. Is orbital reconstruction really necessary? Neurosurg Rev 20143702301–309., discussion 309–310 [DOI] [PubMed] [Google Scholar]
- 5.Scarone P, Leclerq D, Héran F, Robert G. Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. J Neurosurg. 2009;111(05):1069–1077. doi: 10.3171/2009.1.JNS081263. [DOI] [PubMed] [Google Scholar]
- 6.Chambless L B, Mawn L A, Forbes J A, Thompson R C. Porous polyethylene implant reconstruction of the orbit after resection of spheno-orbital meningiomas: a novel technique. J Craniomaxillofac Surg. 2012;40(01):e28–e32. doi: 10.1016/j.jcms.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Margalit N, Ezer H, Fliss D M, Naftaliev E, Nossek E, Kesler A. Orbital tumors treated using transcranial approaches: surgical technique and neuroophthalmogical results in 41 patients. Neurosurg Focus. 2007;23(05):E11. doi: 10.3171/FOC-07/11/E11. [DOI] [PubMed] [Google Scholar]
- 8.Heufelder M J, Sterker I, Trantakis C et al. Reconstructive and ophthalmologic outcomes following resection of spheno-orbital meningiomas. Ophthal Plast Reconstr Surg. 2009;25(03):223–226. doi: 10.1097/IOP.0b013e3181a1f345. [DOI] [PubMed] [Google Scholar]
- 9.Saeed P, van Furth W R, Tanck M et al. Surgical treatment of sphenoorbital meningiomas. Br J Ophthalmol. 2011;95(07):996–1000. doi: 10.1136/bjo.2010.189050. [DOI] [PubMed] [Google Scholar]
- 10.Essa A A, Hamdan A R. Sphenoid meningioma enplaque with proptosis: surgical excision, reconstruction and outcome. Clin Neurol Neurosurg. 2018;167:147–156. doi: 10.1016/j.clineuro.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Seiichiro M, Yoshinori H, Kentaro H, Naokatu S. Superolateral orbitotomy for intraorbital tumors: comparison with the conventional approach. J Neurol Surg B Skull Base. 2016;77(06):473–478. doi: 10.1055/s-0036-1583947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


