Abstract
Introduction Olfactory groove meningiomas (OGMs) are often associated with loss of smell following resection. Loss of smell has a measurable impact on quality of life. Smell preservation has been previously described in open approaches for early stage or unilateral OGMs. Evidence of smell preservation in endoscopic approaches is lacking.
Design A multi-institutional retrospective review was performed on consecutive patients who underwent unilateral endoscopic endonasal resection of OGM. A gross total resection was achieved with preservation of the contralateral olfactory cleft and bulb. Olfactory function was assessed with a six-point olfactory symptom score and the Sniffin' Sticks 12-item smell identification test (SS-12). Contralateral olfactory bulb volume was measured on postoperative magnetic resonance imaging.
Results Four patients (age 42.0 ± 7.5, 75% female) were assessed. Olfactory function was assessed at 21.8 ± 5.6 months following surgery. All patients reported some degree of smell preservation (75% described a slight/mild impairment in smell or better). Olfactory identification was preserved with an SS-12 score of 9 ± 1.4 (anosmia defined as ≤6). The olfactory bulb volume was calculated to be 47.4 ± 15.9 mm 3 (normal >40 mm 3 ).
Conclusion Smell preservation is possible following unilateral endoscopic endonasal resection of carefully selected OGM.
Keywords: endoscopic, skull base, surgery, olfactory groove meningioma, smell, smell preservation
Introduction
Olfactory groove meningiomas (OGMs) have historically been excised via open craniotomy approaches and are typically associated with postoperative smell loss due to frontal lobe retraction and/or sacrifice of the olfactory bulbs. Anosmia has been shown to have a significant negative impact on quality of life. 1 2 3 Postoperative smell preservation has been demonstrated in open approaches via frontolateral (65% with preservation) or bifrontal (55% smell preservation) craniotomies. 4 Tumor size and preoperative olfactory function were found to be the primary predictors for smell preservation postoperatively.
Endoscopic endonasal techniques have been developed in recent years showing similar or improved tumor resection outcomes. 5 6 7 8 Endoscopic techniques are considered minimally invasive due to the fact that there is no external scar. Additionally, the endonasal approach results in less brain retraction to expose the tumor, and rather approaches the tumor from the ventral skull base where no elevation of brain from the associated bone is necessary. Cadaver studies and one surgical case has further demonstrated that a purely endonasal, unilateral approach is feasible; however, evidence of smell preservation in endoscopic approaches is lacking. 9
Methods
A multi-institutional retrospective review was performed on consecutive patients who underwent unilateral endoscopic endonasal resection of OGM. The primary outcomes were a six-point olfactory symptom score (“loss of sense of smell”) and Sniffin' Sticks 12-item smell identification test (SS-12). 10 11 12 13 Secondary outcomes were local recurrence and contralateral olfactory bulb volume on postoperative T2-weighted magnetic resonance imaging (MRI). This study received approval from the St Vincent's Hospital Human Research Ethics Committee (SVH HREC 09/083) and the Institutional Review Board of the University of Pennsylvania, and patients provided informed consent for research data collection.
Patient Population
Consecutive patients who underwent unilateral endoscopic endonasal resection of OGM within tertiary rhinology practices in Sydney, Australia and at the University of Pennsylvania were assessed at least 1-year postsurgery. Patients were excluded if the OGM extended past midline, which would require a more extensive surgery, exposing the contralateral olfactory bulb. In these cases, smell preservation would not be expected or attempted to not compromise gross total resection for tumor outcomes.
Patient Characteristics
Data retrieved included gender, age, and history of sinusitis or nasal allergies. Lund–Mackay score, a method of radiographic staging that ranges from 0 to 24, was calculated from the preoperative computed tomography scan. 14 Delineation of the patient's allergy status was determined via history in the case of two patients and immunoassay in the case of two patients. Perioperative blood samples underwent in vitro testing for allergen-specific immunoglobulin E, with four allergen mixes being employed (house dust mix, mold mix, and grass mix).
Preoperative smell evaluation was performed using smell symptom scores on a six-point Likert scale (from “no problem” to “problem as bad as it can be”) and SS-12 testing. Tumor volume was calculated from the most recent preoperative MRI. Contralateral olfactory bulb volume was measured from the T2 MRI sequence with 3 mm cuts using standard methods. 15 World Health Organization (WHO) tumor grade was determined from histopathologic analysis. The incidence of postoperative radiation treatment was recorded.
Surgical Approach
The endoscopic endonasal tumor resection was performed entirely through one nasal cavity, leaving the contralateral nasal cavity undisturbed. The tumor was resected without visualizing the contralateral olfactory bulb. The septal bone and cartilage were removed if necessary, but the contralateral septal mucosa was preserved. In all cases, a gross total resection was achieved with preservation of the contralateral olfactory cleft and bulb, and septal mucosa (including the olfactory strip). Reconstruction was performed on an individual basis with a dural substitute with or without fat as an underlay layer. A nasoseptal flap was used in all cases to resurface the operative site and provide additional coverage of the skull base defect as an overlay.
Outcome Measures
At the patients' most recent follow-up appointment, at least 1 year following surgery, olfactory function was assessed. A six-point olfactory symptom score was obtained rating the patient's loss of smell, ranging from 0 (“no problem”) to 5 (“problem as bad as it can be”). Scores were assessed as rating over the past 2-week period. The SS-12 smell identification test was performed.
The most recent postoperative MRI was obtained as dictated by hospital protocol to evaluate for residual or recurrent tumor ( Fig. 1 ). Contralateral olfactory bulb volume was measured from the T2 MRI sequence with 3 mm cuts using standard methods.
Fig. 1.
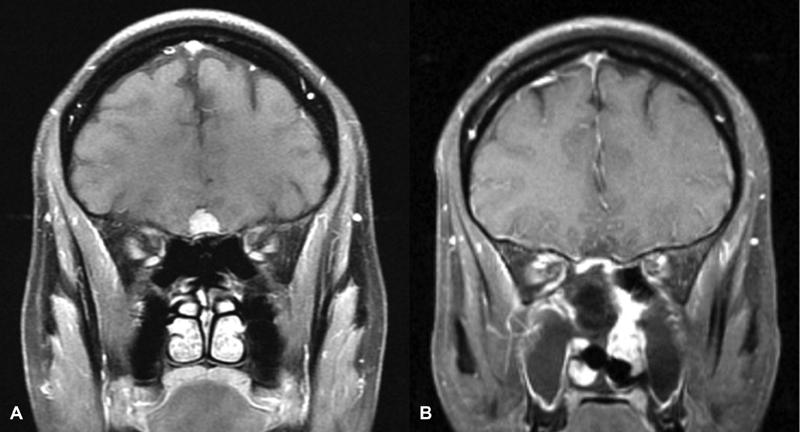
Patient 3 (A) preoperative T1 postcontrast MRI demonstrating a small unilateral OGM. (B) Postoperative T1 postcontrast MRI demonstrating interval resection of OGM and enhancing nasoseptal flap reconstruction. MRI, magnetic resonance imaging; OGM, olfactory groove meningioma.
Data Analysis
Statistical analysis was performed using SPSS version 25 (SPSS, Inc., Chicago, Illinois, United States). Descriptive data were displayed as percentages for categorical variables and means with standard deviations for parametric continuous variables. Statistical significance was determined at the p < 0.05 level.
Results
Four patients were identified for recruitment (age 43.39 ± 7.54; 75% female). No patients were found to have a history of sinusitis or nasal allergies. Patient demographics, tumor characteristics, and smell testing are detailed in Table 1 .
Table 1. Patient demographics, tumor characteristics, and smell testing.
| Patient ID | Age | Gender | WHO grade | Tumor volume (cm 3 ) | Preoperative smell score | Preoperative smell testing (SS-12) | Preoperative olfactory bulb volume (mm 3 ) | Time to follow-up (mo) | Postoperative smell score | Postoperative smell testing (SS-12) | Residual olfactory bulb volume (mm 3 ) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | Male | 1 | 19.4 | 0 | N/A | 37.4 | 28.1 | 2 | 8 | 48.1 |
| 2 | 50 | Female | 2 | 1.4 | 0 | N/A | 89.1 | 14.5 | 0 | 8 | 61.8 |
| 3 | 32 | Male | 1 | 1.4 | N/A | 12 | 72.3 | 22.9 | 2 | 11 | 54.5 |
| 4 | 41 | Male | 1 | 3.8 | N/A | 12 | 26.6 | 21.8 | 4 | 9 | 25.1 |
Abbreviations: N/A, not available; SS-12, Sniffin' Sticks 12-item smell identification test; WHO, World Health Organization.
Preoperative Characteristics
Two patients underwent preoperative SS-12 smell identification testing (both scored 12 out of 12, anosmia defined as ≤6). The other two patients had a preoperative smell symptom score documented as 0, demonstrating “no problem” with loss of smell. Tumor volume was found to be 6.5 ± 8.6 cm 3 . The preoperative contralateral olfactory bulb volume was calculated to be 56.3 ± 29.3 mm 3 (normal >40 mm 3 ). 16 17 Three patients were found to have a WHO grade of 1 (benign). One patient had a WHO grade of 2 (atypical). No patients underwent postoperative radiation treatment.
Postoperative Outcome Measures
Olfactory function was assessed at a time point of 21.8 ± 5.6 months following surgery. All patients reported some degree of smell preservation on smell symptom score testing (75% described a slight/mild impairment in smell or better). Olfactory identification was preserved with a SS-12 score of 9 ± 1.4.
All patients were found be free of tumor on postoperative imaging at 16.3 ± 3.9 months following surgery. The contralateral olfactory bulb volume was calculated to be 47.4 ± 15.9 mm 3 .
Discussion
Though anosmia has long been an anticipated and accepted outcome of OGM resection, it has a significant impact on quality of life that should not be overlooked. 1 2 18 19 20 21 When 1,407 patients with abnormal smell test results were surveyed by Miwa et al, patients with persistent smell dysfunction had significantly reduced quality-of-life scores when compared with patients who reported improvement in olfactory impairment. 18 These quality-of-life differences were especially pronounced in the areas of safety and eating. Patients with persistent dysfunction reported significant disabilities in activities of daily living compared with patients with improvement in olfaction. Additionally, anosmia has a major impact on taste and the enjoyment of food. 3
Anosmia leads to significant anxiety associated with the inability to smell dangerous gas or fire, as well as regarding personal hygiene in social interactions. 3 Santos et al investigated the risk of cooking accidents, undetected gas leaks, undetected fires, and ingestion of harmful substances in a retrospective review of 445 patients who had undergone olfactory testing. 19 It was found that cooking impairments were most common among patients with olfactory impairment (45%), followed by ingestion of spoiled food (25%), inability to detect a gas leak (23%), and inability to smell a fire (7%). At least one hazardous event was reported by 45.2% of patients with anosmia. Pence et al conducted a follow-up study in 1,047 patients in which a significant positive correlation was found between the frequency of hazardous events and degree of olfactory impairment. 20 These findings together highlight the importance of even a minor degree of residual smell in both quality of life and patient safety.
Recently, smell preservation has been described in open approaches 4 and phantosmia has been reported in endoscopic resection of OGM. 22 Theoretically, smell preservation has been described via endoscopic approaches in cadaver dissections and one case report; however, clinical evidence is lacking. 9 Olfactory strip preservation is well described; therefore, protecting contralateral septal mucosa is expected to maintain end organ function. 23 24
Tajudeen et al have previously demonstrated the ability to preserve olfaction following unilateral endoscopic approach to olfactory neuroblastoma. Kadish staging of these tumors included stages B through D; however, these tumors were all determined to be unilateral preoperatively and effort was made to preserve the entire contralateral olfactory apparatus. However, given this is a sinonasal malignancy, gross total resection was the primary goal of surgery. Additionally, all patients additionally underwent postoperative radiation treatment and four completed chemotherapy, which also could have impacted the patients' posttreatment smell function. In the population of 14 patients, 6 patients were found to have residual smell function (43%).
The reported data in this series demonstrate that smell preservation is possible with gross total resection of properly selected, unilateral OGMs. Of key importance is a meticulous approach, minimizing disturbance to the contralateral nasal cavity and olfactory cleft. All studied patients had residual smell on identification testing, although one patient had the subjective sense of severe loss of smell. It is important to mention that in any approach to OGM, insuring gross total resection is crucial, even if this means smell must be sacrificed.
Though these patients chose to undergo surgical resection, further investigation comparing outcomes of unilateral resection to stereotactic radiotherapy will be of future interest. Additionally, with the accrual of more patients amenable to this approach, further analysis could determine postoperative olfactory prognosis. In this study, all patients reported or tested to have normal preoperative olfactory function. Future study is necessary to determine the possibility of smell preservation in patients with preoperative hyposmia.
Conclusion
Smell preservation is possible following unilateral endoscopic endonasal resection of carefully selected OGMs.
Footnotes
Conflict of Interest Richard J. Harvey is consultant with Medtronic, Olympus and NeilMed pharmaceuticals. He has received research grant funding from Meda Pharmaceuticals and Stallergenes and has been on the speakers' bureau for BHR, Seqirus, AstraZeneca, GlaxoSmithKline, and ArthroCare. James N. Palmer is a consultant and stock holder in Optinose and a consultant for Acclarent and Medtronic. Mark Winder is a consultant for Nuvasive Pty Ltd. and Spinal Innovations Pty Ltd. Benjamin P. Jonker has received speaker fees from Integra Life Sciences Corporation. The remaining authors have no disclosure.
References
- 1.Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life--an updated review. Chem Senses. 2014;39(03):185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- 2.Neuland C, Bitter T, Marschner H, Gudziol H, Guntinas-Lichius O. Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope. 2011;121(04):867–872. doi: 10.1002/lary.21387. [DOI] [PubMed] [Google Scholar]
- 3.Patel Z M, DelGaudio J M. Olfaction following endoscopic skull base surgery. Curr Opin Otolaryngol Head Neck Surg. 2016;24(01):70–74. doi: 10.1097/MOO.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 4.Jang W Y, Jung S, Jung T Y, Moon K S, Kim I Y. Preservation of olfaction in surgery of olfactory groove meningiomas. Clin Neurol Neurosurg. 2013;115(08):1288–1292. doi: 10.1016/j.clineuro.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.de Almeida J R, Carvalho F, Vaz Guimaraes Filho F et al. Comparison of endoscopic endonasal and bifrontal craniotomy approaches for olfactory groove meningiomas: a matched pair analysis of outcomes and frontal lobe changes on MRI. J Clin Neurosci. 2015;22(11):1733–1741. doi: 10.1016/j.jocn.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 6.Liu J K, Hattar E, Eloy J A. Endoscopic endonasal approach for olfactory groove meningiomas: operative technique and nuances. Neurosurg Clin N Am. 2015;26(03):377–388. doi: 10.1016/j.nec.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Komotar R J, Starke R M, Raper D M, Anand V K, Schwartz T H.Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas World Neurosurg 201277(5-6):713–724. [DOI] [PubMed] [Google Scholar]
- 8.Khan O H, Krischek B, Holliman D et al. Pure endoscopic expanded endonasal approach for olfactory groove and tuberculum sellae meningiomas. J Clin Neurosci. 2014;21(06):927–933. doi: 10.1016/j.jocn.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Youssef A S, Sampath R, Freeman J L, Mattingly J K, Ramakrishnan V R. Unilateral endonasal transcribriform approach with septal transposition for olfactory groove meningioma: can olfaction be preserved? Acta Neurochir (Wien) 2016;158(10):1965–1972. doi: 10.1007/s00701-016-2922-1. [DOI] [PubMed] [Google Scholar]
- 10.Albrecht J, Anzinger A, Kopietz R et al. Test-retest reliability of the olfactory detection threshold test of the Sniffin' Sticks. Chem Senses. 2008;33(05):461–467. doi: 10.1093/chemse/bjn013. [DOI] [PubMed] [Google Scholar]
- 11.Gudziol V, Lötsch J, Hähner A, Zahnert T, Hummel T. Clinical significance of results from olfactory testing. Laryngoscope. 2006;116(10):1858–1863. doi: 10.1097/01.mlg.0000234915.51189.cb. [DOI] [PubMed] [Google Scholar]
- 12.Hummel T, Sekinger B, Wolf S R, Pauli E, Kobal G. ‘Sniffin’ Sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(01):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 13.Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin' Sticks”: screening of olfactory performance. Rhinology. 1996;34(04):222–226. [PubMed] [Google Scholar]
- 14.Hopkins C, Browne J P, Slack R, Lund V, Brown P. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg. 2007;137(04):555–561. doi: 10.1016/j.otohns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Rombaux P, Duprez T, Hummel T. Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology. 2009;47(01):3–9. [PubMed] [Google Scholar]
- 16.Wang J, You H, Liu J F, Ni D F, Zhang Z X, Guan J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am J Neuroradiol. 2011;32(04):677–681. doi: 10.3174/ajnr.A2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rombaux P, Huart C, Deggouj N, Duprez T, Hummel T. Prognostic value of olfactory bulb volume measurement for recovery in postinfectious and posttraumatic olfactory loss. Otolaryngol Head Neck Surg. 2012;147(06):1136–1141. doi: 10.1177/0194599812459704. [DOI] [PubMed] [Google Scholar]
- 18.Miwa T, Furukawa M, Tsukatani T, Costanzo R M, DiNardo L J, Reiter E R. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg. 2001;127(05):497–503. doi: 10.1001/archotol.127.5.497. [DOI] [PubMed] [Google Scholar]
- 19.Santos D V, Reiter E R, DiNardo L J, Costanzo R M. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004;130(03):317–319. doi: 10.1001/archotol.130.3.317. [DOI] [PubMed] [Google Scholar]
- 20.Pence T S, Reiter E R, DiNardo L J, Costanzo R M. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg. 2014;140(10):951–955. doi: 10.1001/jamaoto.2014.1675. [DOI] [PubMed] [Google Scholar]
- 21.Hummel T, Whitcroft K L, Andrews P et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54(26):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 22.Venteicher A S, Kumar J I, Murphy E A, Gray S T, Holbrook E H, Curry W T. Phantosmia and dysgeusia following endoscopic transcribriform approaches to olfactory groove meningiomas. J Neurol Surg B Skull Base. 2017;78(03):245–250. doi: 10.1055/s-0036-1597925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey R J, Winder M, Davidson A et al. The olfactory strip and its preservation in endoscopic pituitary surgery maintains smell and sinonasal function. J Neurol Surg B Skull Base. 2015;76(06):464–470. doi: 10.1055/s-0035-1554905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajudeen B A, Adappa N D, Kuan E C et al. Smell preservation following endoscopic unilateral resection of esthesioneuroblastoma: a multi-institutional experience. Int Forum Allergy Rhinol. 2016;6(10):1047–1050. doi: 10.1002/alr.21794. [DOI] [PubMed] [Google Scholar]


