Abstract
Background
Fatigue ranks among the most common and disabling symptoms in MS. Recent theoretical works have surmised that this trait might be related to alterations across interoceptive mechanisms. However, this hypothesis has not been empirically evaluated.
Objectives
To determine whether fatigue in multiple sclerosis patients is associated with specific behavioral, structural and functional disruptions of the interoceptive domain.
Methods
Fatigue levels were established via the modified fatigue impact scale. Interoception was evaluated through a robust measure indexed by the heartbeat detection task. Structural and functional connectivity properties of key interoceptive hubs were tested by MRI and resting-state functional MRI. Machine learning analyses were employed to perform pairwise classifications.
Results
Only patients with fatigue presented with decreased interoceptive accuracy alongside decreased gray matter volume and increased functional connectivity in core interoceptive regions, the insula and the anterior cingulate cortex. Each of these alterations was positively associated with fatigue. Finally, machine-learning analysis with a combination of the above interoceptive indices (behavioral, structural, and functional), successfully discriminated (area under the curve > 90%) fatigued patients from both non-fatigued and healthy controls.
Conclusions
This study offers unprecedented evidence suggesting that disruptions of neurocognitive markers subserving interoception may constitute a signature of fatigue in MS.
Keywords: multiple sclerosis, fatigue, interoception, heartbeat detection task, voxel based morphometry, functional connectivity, machine learning
Introduction
Fatigue (i.e., extreme and persistent mental and/or physical exhaustion or lack of energy) is the most invalidating and frequent symptom of MS, affecting 50 to 90% of patients (1). Although the pathophysiology of this dysfunction is poorly understood, a recent hypothesis suggests that its varied manifestations may be related to core interoceptive deficits –i.e., disruptions in inner-body-signal processing (2, 3).
Interoception refers to the sensing and monitoring of inner bodily states, as mapped through hormonal, immunological, metabolic, thermal, nociceptive, and visceromotor signals (4). These inner cues are integrated into the brain stem and relayed to the insula and anterior cingulate cortex (ACC) to maintain homeostasis and shape cognition and behavior (4).
Abundant indirect evidence suggests that subjective fatigue in MS could reflect a disruption of interoceptive mechanisms (3, 5, 6). For instance, in healthy subjects, fatigue induced by inflammation correlates with hyperactivity in the insula and the ACC (7). Likewise, atrophy (8) and altered functional connectivity (9–12) of interoceptive regions such as the insula and the ACC has been repeatedly observed in multiple sclerosis patients as well as association between fatigue and atrophy (13) and fatigue and structural connectivity (14) of these areas. Compatibly, Salamone et al. have provided pioneering evidence of interoceptive alterations in MS at electrophysiological, neuroanatomical, and FC levels (12). These antecedents emphasize the potential link between body-sensing alterations (interoceptive domain) and disease-specific manifestations (fatigue).
In order to empirically test this premise, we performed a systematic and multidimensional evaluation of interoceptive processing in MS patients with and without fatigue (F-MS and nF-MS, respectively), integrating behavioral and neuroimaging evidence. To measure neurovisceral interactions, we profited from the heartbeat detection (HBD) task, a widely validated method related to well-defined anatomical and functional signatures (12, 15, 16), in which the subject must monitor their own heartbeats. Structural and functional connectivity properties of key interoceptive hubs were tested by MRI and resting-state functional MRI.
In MS, fatigue is a critical symptom cutting across vastly heterogeneous patterns of clinical and brain alterations (1, 17, 18). Accordingly, and since the only difference between our patient groups consisted in fatigue symptoms, we predicted that the greatest differences between them would emerge at the behavioral level (interoceptive accuracy, and association of fatigue with interoception), following the pattern controls = nF-MS > F-MS. On the other hand, we expected to find less robust differences in neural measures (atrophy and FC), given that interoceptive-sensitive brain regions are also implicated in several other functions (19–24). Accordingly, they afford less specific markers of interoceptive disturbances and, as such, they might discriminate each patient group from controls, but not necessarily from each other, following a graded pattern FMS < nF-MS < controls.
As predicted, we showed that, only F-MS patients, as opposed to nF-MS and controls, exhibit behavioral interoceptive deficits. Structural and functional disruptions of key interoceptive areas were observed in F-MS patients but only compared to controls and not to nF-MS. In addition, fatigue levels were associated with behavioral, structural, and FC disturbances. A supervised learning model using these multimodal metrics allowed classifying F-MS patients from nF-MS patients and healthy controls with high-performance (AUC = 0.92, area under de curve). Moreover, in line with our predictions, the most relevant feature for classification was afforded by behavioral performance, with atrophy and FC metrics of key interoceptive areas yielding less discriminatory contributions. Thus, our behavioral results suggest a link between fatigue and disrupted interceptive processing in MS.
Materials and methods
Participants
The study comprised 29 relapsing-remitting MS patients and 28 healthy controls. Patients were diagnosed by two experts (VS, FPC) based on McDonald’s criteria (25) and further assessed with the Expanded Disability Status Scale (26) and the Multiple Sclerosis Severity Score (27) (Table 1). The Ineco Frontal Screening (IFC) (28) test was employed to specifically assess executive functions among all groups (Table 1). Also, all participants completed the Modified Fatigue Impact Scale (MFIS) (29), a 21-item quantitative self-report questionnaire on physical, cognitive, and social aspects of fatigue. MFIS scores range from 0 to 84, with values above 37 signaling disabling levels of fatigue (14, 29). Accordingly, patients were divided into F-MS (n=16, mean=53.19, minimum=37, maximum=76) and nF-MS (n=13, mean=19.85, minimum=4, maximum=32) subjects. All controls presented low levels of fatigue (mean=9, minimum=0, maximum=35). The three groups were matched for age, gender, and education –and the patient subsamples were also matched for years since diagnosis (Table 1). The study was approved by the institutional ethics committee. All participants signed an informed consent in accordance with the Declaration of Helsinki.
Table 1:
Demographic, clinical information and HBD task results
| Post-hoc analysis (Tukey) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Controls vs F-MS | Controls vs nF-MS | NF-MS vs F-MS | ||||||
| F-MS | nF-MS | Controls | χ2 | p-value | ||||
| Sex (n) | F=14 / M=2 | F=9 / M=4 | F=23 / M=5 | 1.6 | .44 | - | - | - |
| F | ||||||||
| Age (years) | 42.19 (11) | 36.31 (9.42) | 35.82 (11.03) | 0,15 | .15 | - | - | - |
| Education (years) | 17.25 (3.64) | 16 (3.24) | 16.89 (2.55) | 0,63 | .53 | - | - | - |
| EDSS | 1.37 (1.91) | 1.04 (1.35) | - | - | .61 | - | - | - |
| MSSS | 1.84 (2.64) | 1.31 (1.96) | - | - | .56 | - | - | - |
| IFS | 23.38 (0.53) | 23.88 (1.06) | 27.23 (1.91) | 14.89 | p<.0001 | p<.0001*** | p<.0001*** | p=.65 |
| Years since diagnosis | 11.31 (9.3) | 7.92 (4.89) | - | - | .24 | - | - | - |
| IC-IA | −0.49 (0.83) | 0.64 (0.99) | 0.55 (0.87) | 7.69 | .001 | p<.001** | p=.77 | p<.001** |
| EC-IA | 1.09 (1.56) | 1.27 (1.63) | 2 (1.79) | 1.51 | .23 | - | - | - |
Pearson’s chi-square (χ2) test was used to assess sex statistics. Age, education, interoceptive accuracy and IFS (Ineco Frontal Screening) were assessed using one-way ANOVAs and post-hoc Tukey’s HSD tests when necessary. Additional between-group comparisons were performed via unpaired t-tests –for outcomes of the EDSS (16), MSSS (severity assessed by the relationship between EDSS and disease duration (17)), and years of disease evolution between MS patients. Values are expressed as mean SD (standard deviation). F: female; M: male; EDSS: Expanded Disability Status Scale; MSSS: Multiple Sclerosis Severity Score; IC-AS: Interoceptive Condition-Accuracy Score; EC-AS: Exteroceptive Condition-Accuracy Score. The asterisk (*) denotes a significant difference.
Interoceptive performance: Heartbeat detection task
Given that some participants underwent the MRI session but missed the behavioral evaluation, the HBD task included 15 F-MS patients, 12 nF-MS patients, and 22 controls–these groups were also matched for the abovementioned variables (Supplementary Table 1). Cardiac interoception was measured with a modified version of a validated HBD task (12, 15, 16), encompassing two conditions. In the interoceptive condition (IC), aimed to assess inner signal monitoring, participants tapped a key to follow their own heartbeats without any external cues. Each participant completed two 2.5-min blocks. The IC provides a measure of interoceptive accuracy –the subjects’ objective performance in following their heartbeats (12, 15, 16). Participants were requested to respond with their dominant hand, keep their eyes on a fixation cross, and avoid excessive blinking and moving. An accuracy score was estimated for each subject based on HBD outcomes via signal detection (30) –Supplementary material 1.1. In the exteroceptive condition (EC), a control measure of external monitoring skills, participants tapped a keyboard to follow binaurally presented heartbeats. This condition included two blocks of 2.5 min, featuring regularly timed and irregularly timed heartbeats, respectively.
Accuracy scores were compared among groups using one-way ANOVA and Tukey’s HSD test. The association between interoceptive accuracy and fatigue was examined through linear regression analyses. To increase behavioral variance and statistical power for the latter, each patient group was analyzed against the control sample, as in previous reports (15, 31).
Image acquisition, preprocessing, and analysis
MRI acquisition and preprocessing steps are reported following the practical guide from the Organization for Human Brain Mapping (for specific details see Supplementary material 1.2.1 and 1.2.2). We obtained 3D volumetric and ten-minute-long resting-state MRI sequences from all participants, following standard protocols (15). For FC analysis, one subject was excluded per group due technical problems during the acquisition.
SPM (Statistical Parametric Mapping) 12 was used to estimate whole brain atrophy pattern and the association between fatigue and brain volume, including -total intracranial volume (TIV) as a covariate, (in the last, each group was analyzed together with the control sample (15, 31). In order to deal with multiple comparisons, we set a primary statistical threshold of significance at p < .001 and cluster-extent based thresholding at 50 voxels (statistically significant clusters on the basis of the number of contiguous voxels), a cluster size recommended for uncorrected thresholds (15, 16, 32). Also, as in previous resting-state studies (12), we analyzed the connectivity of key interoceptive areas considering six regions of interest (ROIs): the bilateral insula, ACC, and somatosensory cortex (SSC). To this end, we extracted the mean time-courses of each ROI by averaging the blood-oxygen-level-dependent signal of all their voxels. The connectivity strength between these ROIs was defined with Pearson’s correlation coefficient; this yielded fifteen values representing the pairwise connections of these regions. To compare these measures between groups, we applied a Wilcoxon rank-sum test corrected with false discovery rate (FDR) to address the multiple comparisons problem. Also, to examine possible associations between FC differences and fatigue, we performed linear regression analyses between FC levels in the critical ROIs and fatigue.
Multivariate analysis: supervised learning models
We set three support vector machines (SVM) to perform a pairwise classification among all groups using both behavioral and neuroimaging data (see Supplementary material 1.2.3).
Results
Behavioral results: HBD task
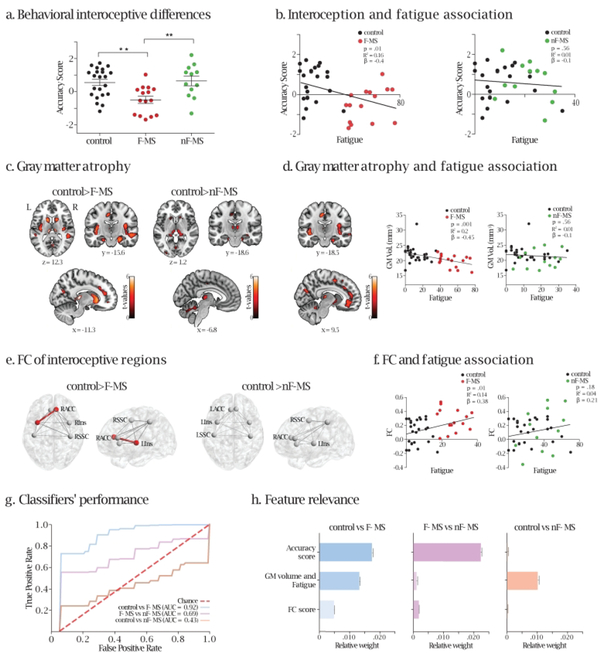
Results from the IC revealed decreased accuracy score in F-MS compared to controls and to nF-MS, while no significant differences were found between the latter two groups (one-way ANOVA: F = 7.69, df = 48, p < .001, post-hoc (Tukey): Controls vs F-MS: p < .001, Controls vs nF-MS: p = .77 and F-MS vs nF-MS: p < .001. Figure 1.a., Table 1). These results remained the same after controlling for executive functions (IFS, ANCOVA F (2,42) = 4.68, p =.014. Tukey’s HSD test: controls vs nF-MS: p = .94; controls vs F-MS: p = .005; F-MS vs nF-MS: p = .008), which discarded its influence in our findings. We also found a negative association between accuracy score and fatigue involving F-MS and control subjects (p=.01, R2=0.16, β=−0.4). By contrast, no significant association was found when considering nF-MS and control participants (p=.56, R2=0.01, β=−0.1) (Figure 1.b.). When considering F-MS and nF-MS patients, a negative association between accuracy score and fatigue was still present (p = .05, R2 = 0.14, β = −0.38, Supplementary Figure 1), although as expected it proved less strong than when considering controls and F-MS patients. No significant differences in interoceptive accuracy emerged among groups in the EC (Table 1), nor in the regression analysis (F-MS and controls: p =.3, R2 = 0.03, β = −0.17; nF-MS and controls: p =.23, R2 = 0.04, β = 0.2).
Figure 1.
a. Behavioral performance in the HBD task. The asterisks indicate decreased accuracy score (y-axis) in F-MS compared to controls and nF-MS. Higher scores indicate better performance. One-way ANOVA: F = 7.69, df = 48, p<.001, post-hoc (Tukey): Controls vs F-MS: p<.001, Controls vs nF-MS: p = .77 and F-MS vs nF-MS: p<.001. Figure 1.a., Table 1). b. Interoception and fatigue. Scatter plots of the association between accuracy score (y-axis) and fatigue (x-axis), when considering controls and F-MS (left inset), and controls and nF-MS (right inset). Higher scores on the y-axis indicate better interoceptive accuracy, while higher values on the xaxis represent elevated levels of fatigue. c. Gray matter atrophy. Left inset: Clusters of significant grey matter volume atrophy of the bilateral insula, the right ACC, the bilateral MCC, the thalamus, the caudate, and the putamen of F-MS patients compared to controls (see also Supplementary Table 2). Right inset: Clusters of significant grey matter volume atrophy of the left MCC, the bilateral thalamus, the caudate, and the putamen of nF-MS patients compared to controls (see also Supplementary Table 2). Uncorrected p-value < .001, extent threshold = 50 voxels. R = right; L = left. d. Association between fatigue and atrophy. An inverse regression emerged between grey matter volume and fatigue when considering F-MS and controls (left inset, uncorrected p-value < .001, extent threshold = 50 voxels) but not when considering nFMS and controls or F-MS and nF-MS (Supplementary Figure 3). Clusters of significant grey matter atrophy involve key interoceptive areas such as the insula, the ACC, and the MCC bilaterally, among other regions (Supplementary Table 2). Right inset: scatter plots of the association between grey matter volume of the areas of significant differences between controls and F-MS patients (y-axes) and fatigue (x-axes), for controls and F-MS (left, p=.001, R2=0.2, β = −0.45) and controls and nF-MS (right, p=.56, R2=0.01, β = −0.1). e. FC of interoceptive regions. ROI analysis including the insula, the ACC, and the SSC bilaterally showed increased connectivity between the right ACC and the left insula in F-MS compared to controls (left panel, p=.002, t=−3.1, pFDR=0.04). No difference in connectivity was found between controls and nF-MS patients (right side). f. Associations between FC and fatigue. Scatter plots of the association between FC (y-axes) and fatigue (x-axes) for controls and F-MS patients (left) and for controls and nF-MS patients (right). Higher scores on the y-axis indicate increased FC values, while higher values on the x-axis represent elevated levels of fatigue. g. Supervised learning models’ performance. Mean ROC curves of 200 iterations over a five-fold cross-validation for each SVM, where true positive rate (TPR or sensitivity) is presented along the y-axis and false positive rate (FPR or 1 – specificity) is displayed along the x-axis. The higher the area under the curve (AUC) score, the better the classifier’s performance. h. Feature relevance. Mean relative weight by feature for each SVM, obtained after computing their permutation importance in every iteration. Higher values indicate a greater contribution to classifier’s performance, while those close to zero are most likely to be non-informative (or irrelevant) since they do not improve differentiation between classes (or groups). RACC: right anterior cingulate cortex; LACC: left anterior cingulate cortex; RIns: right insula; LIns: left insula; RSSC: right somatosensory cortex; LSSC: left somatosensory cortex. GM: gray matter.
VBM results: Gray matter atrophy and its association with fatigue
Compared to controls, only F-MS patients showed decreased gray matter volume in interoceptive areas (bilateral insula, right ACC) –Figure 1.c., Supplementary Table 2. Also, compared to controls, both patient groups presented atrophy of the midcingulate cortex (MCC), although this was more pronounced in F-MS patients (bilateral in F-MS vs. left MCC in nF-MS). No differences in gray matter atrophy were present between nF-MS and F-MS (Supplementary Figure 2).
The grey matter volume of key interoceptive areas (bilateral insula, ACC, MCC) was negatively associated with fatigue, but only when considering controls/F-MS patients. No such association was observed in controls/nF-MS patients (Figure 1.d. and statistical details in Supplementary Table 2.c.), nor in F-MS/nF-MS patients (Supplementary Figure 3).
Functional Connectivity results
FC analyses revealed increased connectivity between the right ACC and the left insula in F-MS compared to controls (p = .002, t = −3.1, pFDR = 0.04, Figure 1.e, Supplementary Table 3). No connectivity differences were observed between controls and nF-MS or between F-MS/nF-MS patients.
F-MS patients and controls had a positive association between fatigue and FC of the right ACC/left insula (p = .01, R2 = 0.14, β = 0.38, Figure 1.f). No association was found when considering controls and nF-MS patients (p = .18, R2 = 0.04, β = 0.21) or when considering F-MS/nF-MS patients (p = .13, R2 = 0.08, β = 0.29).
Multivariate analysis
A SVM including controls and F-MS patients achieved outstanding classification performance (AUC = 0.92 ± 0.12), and another one performed on F-MS and nF-MS patients (AUC = 0.69 ± 0.29) was just below the threshold for acceptable results (AUC > 0.7). Conversely, a control vs. nF-MS model (AUC=0.43 ± 0.25) suggested no discrimination between groups. Feature relevance analysis (Figure 1.h) revealed their relative weights: in the classification model considering F-MS and controls, all the three features (behavioral, structural, and functional) presented a large contribution, with the behavioral score providing the highest contribution; in the one encompassing F-MS and nF-MS, the behavioral score was the one that contributed the most; and finally in the model that considers controls and nF-MS only the gray matter volume provides critical information for the discrimination between both groups.
Discussion
In line with a relevant hypothesis (2, 3, 6, 12, 33), we showed that behavioral, structural, and functional interoceptive alterations are a hallmark of F-MS patients. Our study sheds new light on the neurocognitive foundations of fatigue and their link with neurovisceral alterations.
HBD outcomes showed that only F-MS patients presented behavioral interoceptive disturbances that were associated with fatigue levels. This was exclusive to the IC, as opposed to the EC, demonstrating that the patients’ deficit was specifically interoceptive rather than secondary to motor responses or general attentional disturbances. Furthermore, there were no differences between F-MS and nF-MS in disability and disease progression (Table 1, EDSS and MSSS) or in executive functioning (Table 1, IFS), which could affect performance in the IC given the need to coordinate disparate (interoceptive, attentionals and motoric) processes. Moreover, differences in IC accuracy among groups were still present after covarying by IFS scores. Our findings thus provide a novel cardiac interoceptive pathway associated with fatigue, showing that behavioral interoceptive alterations are specific to F-MS patients.
Compatibly, our VBM analysis revealed that, compared to controls, only F-MS patients showed decreased gray matter volume in the bilateral insula and in the right ACC. Moreover, MCC atrophy, though present in both patient groups, was more pronounced in F-MS patients. Importantly, in this sample, the lower the volume of such interoceptive areas, the higher the reported fatigue. This specific association may explain previous reports showing that (a) interoceptive regions are more atrophied in MS (8, 12), and (b) atrophy in these areas (including the right ACC and right MCC) correlates with fatigue levels in the disease (34).
Also, we found that greater connectivity between the right ACC and the left insula in F-MS patients compared to controls, was positively associated with their levels of fatigue. Although previous reports have repeatedly evinced abnormal FC patterns involving the anterior and posterior cingulate (35–37) as well as the somatosensory cortices (37), alongside disruptions in relevant anatomical pathways such as the cingulate bundle (14); current data is heterogeneous, including both hypo-and hyper-connectivity patterns. While resting-state connectivity may be reduced when structural damage becomes prevalent (11), hyper-connectivity patterns could reflect compensatory mechanisms in initial disease stages (10, 12, 33, 38). Our results pave the way for further FC studies aimed to elucidate these patterns as a function of disease progression.
As expected, both groups of patients presented gray matter atrophy relative to the control group; however, the involvement of gray matter areas of interoceptive regions was exclusive to patients with fatigue. The absence of structural and FC differences between both patient samples was also expected. Given that MS is a very complex disease with high heterogeneity in terms of atrophy and functional connectivity alterations (17, 18), and since our patient groups differed only in fatigue symptoms (without differences in disability, disease progression, years of disease evolution, or cognitive skills), minimal or null differences were anticipated in the neuroanatomical comparisons between F-MS and nF-MS. In addition, the absence of differences at this level is supported by the fact that gray matter atrophy and FC of interoceptive areas are not as solid (or directly related) markers of interoception as the HBD measure (a task specially designed to tap this ability). In addition, neuroanatomical differences, for instance at the functional level, could also be masked by compensatory mechanisms. Therefore, the comparison of each patient group against the control group allowed revealing more subtle differences that could otherwise be overlooked.
Finally, our classification analysis combining behavioral, neuroanatomical, and FC features yielded robust classification rates between controls and F-MS (AUC = 0.92) and proper classification rates between F-MS and nF-MS patientes (AUC = 0.69). According to our previous findings, the accuracy score was the most important feature for these two classification models, while neuroanatomical and FC variables contributed to a lesser extent. This further supports the role of the behavioral index as a robust proxy for interoception in comparison with the other neurophisiological features. On the other hand, the classification performance was near chance for the model between controls and the nF-MS; this was expected given the absence of behavioral or functional differences between them. In this sense, despite the low classification rate, the characteristic that contributed the most was the degree of cerebral atrophy. In summary, the machine learning results support the relevance of the behavioral measure to discriminate F-MS patients from healthy controls and from patientes without fatigue, highlighting the possibility of establishing affordable and scalable interoceptive tools as objective proxies of fatigue in MS. Notwithstanding, more studies are necessary to test the robustness of these observations.
Our work has some limitations and questions for further research. First, we used a modest sample size, but similar to previous research on similar topics (12, 15, 36). Second, our assessment of fatigue was based on self-report scales. Even though this is a standard and considerably consistent practice (14, 29), future works should cover other dimensions of fatigue such as fatigability–objective cognitive or motor decline during task performance. (33). Third, more studies are needed to test the role of different interoceptive dimensions in fatigue, such as interoceptive sensitivity and awareness (6, 15). Fourth, regarding the resting-state fMRI preprocessing pipeline, we have used an approach similar to previous works (6, 42, 43, 45, 46) in which brain global signal was not considered as a nuisance variable given that its application may remove both neural and non-neural signals and artificially introduce anti-correlation in connectivity results (38–40). However, future works could replicate the present findings under different resting-state fMRI preprocessing pipelines. In addition, future assessment should also tackle whether the interoceptive alterations depend on the disease stage and the incidence of inflammatory processes (2).
Briefly, our study provides novel support for recent models of fatigue as a case of interoceptive disruptions in MS (2, 3, 6, 33). Behavioral measures of interoceptive processing may represent objective, affordable, and scalable complements to current clinical assessments of fatigue in MS. Our findings could have important therapeutic implications (33), as mindfulness and other forms of interoceptive training may impact the patients’ subjective experience of fatigue. In this sense, our results might represent an empirical step towards a new translational research agenda on the issue.
Supplementary Material
Acknowledgements
We would like to thank all the subjects that participated of the study. This study was supported by grants from CONICET, CONICYT/FONDECYT Regular [grant number 1170010], FONDAP [grant number 15150012], INECO Foundation, by the Inter-American Development Bank (IDB), by PICT [grant number 2017-1818, 2017 1820] and by the National Institute On Aging of the National Institutes of Health under Award Number R01AG057234 (to IA).
Footnotes
Competing interests
The authors report no competing interests.
References
- 1.Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nature reviews Neurology. 2017;13(11):662–75. Epub 2017/10/14. [DOI] [PubMed] [Google Scholar]
- 2.Hanken K, Eling P, Hildebrandt H. The representation of inflammatory signals in the brain - a model for subjective fatigue in multiple sclerosis. Frontiers in neurology. 2014;5:264 Epub 2015/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMorris T, Barwood M, Corbett J. Central fatigue theory and endurance exercise: Toward an interoceptive model. Neuroscience and biobehavioral reviews. 2018;93:93–107. Epub 2018/04/03. [DOI] [PubMed] [Google Scholar]
- 4.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews Neuroscience. 2002;3(8):655–66. Epub 2002/08/03. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends in neurosciences. 2014;37(1):39–46. Epub 2013/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quadt L, Critchley HD, Garfinkel SN. The neurobiology of interoception in health and disease. Annals of the New York Academy of Sciences. 2018. Epub 2018/07/06. [DOI] [PubMed] [Google Scholar]
- 7.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biological psychiatry. 2009;66(5):415–22. Epub 2009/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lansley J, Mataix-Cols D, Grau M, Radua J, Sastre-Garriga J. Localized grey matter atrophy in multiple sclerosis: a meta-analysis of voxel-based morphometry studies and associations with functional disability. Neuroscience and biobehavioral reviews. 2013;37(5):819–30. Epub 2013/03/23. [DOI] [PubMed] [Google Scholar]
- 9.Faivre A, Rico A, Zaaraoui W, Crespy L, Reuter F, Wybrecht D, et al. Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult Scler. 2012;18(9):1251–8. Epub 2012/02/07. [DOI] [PubMed] [Google Scholar]
- 10.Rocca MA, Valsasina P, Martinelli V, Misci P, Falini A, Comi G, et al. Large-scale neuronal network dysfunction in relapsing-remitting multiple sclerosis. Neurology. 2012;79(14):1449–57. Epub 2012/09/08. [DOI] [PubMed] [Google Scholar]
- 11.Roosendaal SD, Schoonheim MM, Hulst HE, Sanz-Arigita EJ, Smith SM, Geurts JJ, et al. Resting state networks change in clinically isolated syndrome. Brain : a journal of neurology. 2010;133(Pt 6):1612–21. Epub 2010/04/02. [DOI] [PubMed] [Google Scholar]
- 12.Salamone PC, Esteves S, Sinay VJ, Garcia-Cordero I, Abrevaya S, Couto B, et al. Altered neural signatures of interoception in multiple sclerosis. Human brain mapping. 2018;39(12):4743–54. Epub 2018/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreasen AK, Jakobsen J, Soerensen L, Andersen H, Petersen T, Bjarkam CR, et al. Regional brain atrophy in primary fatigued patients with multiple sclerosis. NeuroImage. 2010;50(2):608–15. Epub 2010/01/12. [DOI] [PubMed] [Google Scholar]
- 14.Pardini M, Bonzano L, Bergamino M, Bommarito G, Feraco P, Murugavel A, et al. Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Mult Scler. 2015;21(4):442–7. Epub 2014/08/26. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Cordero I, Sedeno L, de la Fuente L, Slachevsky A, Forno G, Klein F, et al. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2016;371(1708). Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoris A, Abrevaya S, Esteves S, Salamone P, Lori N, Martorell M, et al. Multilevel convergence of interoceptive impairments in hypertension: New evidence of disrupted body-brain interactions. Human brain mapping. 2018;39(4):1563–81. Epub 2017/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Annals of neurology. 2000;47(6):707–17. Epub 2000/06/14. [DOI] [PubMed] [Google Scholar]
- 18.Tahedl M, Levine SM, Greenlee MW, Weissert R, Schwarzbach JV. Functional Connectivity in Multiple Sclerosis: Recent Findings and Future Directions. Frontiers in neurology. 2018;9:828 Epub 2018/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):523–8. Epub 2002/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bushara KO, Hanakawa T, Immisch I, Toma K, Kansaku K, Hallett M. Neural correlates of cross-modal binding. Nature neuroscience. 2003;6(2):190–5. Epub 2002/12/24. [DOI] [PubMed] [Google Scholar]
- 21.Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain : a journal of neurology. 2008;131(Pt 5):1311–22. Epub 2008/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and cognitive neuroscience reviews. 2004;3(2):71–100. Epub 2004/11/13. [DOI] [PubMed] [Google Scholar]
- 23.Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(1):256–9. Epub 1990/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevinc G, Gurvit H, Spreng RN. Salience network engagement with the detection of morally laden information. Social cognitive and affective neuroscience. 2017;12(7):1118–27. Epub 2017/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011;69(2):292–302. Epub 2011/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52. Epub 1983/11/01. [DOI] [PubMed] [Google Scholar]
- 27.Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005;64(7):1144–51. Epub 2005/04/13. [DOI] [PubMed] [Google Scholar]
- 28.Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain : a journal of neurology. 2009;132(Pt 5):1299–309. Epub 2009/04/02. [DOI] [PubMed] [Google Scholar]
- 29.Flachenecker P, Kumpfel T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler. 2002;8(6):523–6. Epub 2002/12/12. [DOI] [PubMed] [Google Scholar]
- 30.Thomson EE, Kristan WB. Quantifying stimulus discriminability: a comparison of information theory and ideal observer analysis. Neural computation. 2005;17(4):741–78. Epub 2005/04/15. [DOI] [PubMed] [Google Scholar]
- 31.Melloni M, Billeke P, Baez S, Hesse E, de la Fuente L, Forno G, et al. Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain : a journal of neurology. 2016;139(11):3022–40. Epub 2016/09/30. [DOI] [PubMed] [Google Scholar]
- 32.de la Fuente A, Sedeno L, Vignaga SS, Ellmann C, Sonzogni S, Belluscio L, et al. Multimodal neurocognitive markers of interoceptive tuning in smoked cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2019;44(8):1425–34. Epub 2019/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manjaly ZM, Harrison NA, Critchley HD, Do CT, Stefanics G, Wenderoth N, et al. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. 2019. Epub 2019/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocca MA, Parisi L, Pagani E, Copetti M, Rodegher M, Colombo B, et al. Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology. 2014;273(2):511–20. Epub 2014/06/14. [DOI] [PubMed] [Google Scholar]
- 35.Bisecco A, Nardo FD, Docimo R, Caiazzo G, d’Ambrosio A, Bonavita S, et al. Fatigue in multiple sclerosis: The contribution of resting-state functional connectivity reorganization. Mult Scler. 2018;24(13):1696–705. Epub 2017/09/16. [DOI] [PubMed] [Google Scholar]
- 36.Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, et al. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. NeuroImage. 2002;15(3):559–67. Epub 2002/02/19. [DOI] [PubMed] [Google Scholar]
- 37.Hidalgo de la Cruz M, d’Ambrosio A, Valsasina P, Pagani E, Colombo B, Rodegher M, et al. Abnormal functional connectivity of thalamic sub-regions contributes to fatigue in multiple sclerosis. Mult Scler. 2018;24(9):1183–95. Epub 2017/06/29. [DOI] [PubMed] [Google Scholar]
- 38.Filippi M, Agosta F, Spinelli EG, Rocca MA. Imaging resting state brain function in multiple sclerosis. Journal of neurology. 2013;260(7):1709–13. Epub 2012/10/12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.