Abstract
Bone morphogenetic proteins BMP2 and BMP6 play key roles in systemic iron homeostasis by regulating production of the iron hormone hepcidin. HFE also regulates hepcidin through a mechanism that intersects with the BMP-SMAD1/5/8 pathway. However, the relative roles of BMP2 compared with BMP6 and whether HFE regulates hepcidin through a BMP2-dependent mechanism remain uncertain. We therefore examined the iron phenotype of mice deficient for both Bmp2 and Bmp6 or both Bmp2 and Hfe compared with single knockout (KO) mice and littermate controls. Eight-week-old double endothelial Bmp6/Bmp2 KO mice exhibited a similar degree of hepcidin deficiency, serum iron overload, and tissue iron overload compared with single KO mice. Notably, dietary iron loading still induced liver SMAD5 phosphorylation and hepcidin in double Bmp6/endothelial Bmp2 KO mice, although no other BMP ligand mRNAs were increased in the livers of double KO mice, and only Bmp6 and Bmp2 mRNA were induced by dietary iron loading in wildtype mice. In contrast, double Hfe/endothelial Bmp2 KO mice exhibited reduced hepcidin and increased extrahepatic iron loading compared to single Hfe or endothelial Bmp2 KO mice. Liver phosphorylated SMAD5 and the SMAD1/5/8 target Id1 mRNA were also reduced in double Hfe/endothelial Bmp2 KO compared with single endothelial Bmp2 KO females. Finally, hepcidin and Id1 mRNA induction by homodimeric BMP2, homodimeric BMP6, and heterodimeric BMP2/6 were blunted in Hfe KO primary hepatocytes.
Conclusion
These data suggest that BMP2 and BMP6 work collaboratively to regulate hepcidin expression, that BMP2- and BMP6-independent SMAD1/5/8 signaling contributes a non-redundant role to hepcidin regulation by iron, and that HFE regulates hepcidin at least in part through a BMP2-independent, but SMAD1/5/8-dependent, mechanism.
Keywords: Iron, hepcidin, liver, bone morphogenetic protein, SMAD
Systemic iron homeostasis is regulated by the peptide hormone hepcidin and its receptor ferroportin, which control iron entry into circulation from dietary sources and body stores (1). Iron loading and inflammation stimulate the liver to produce and secrete hepcidin (2–4), which induces ferroportin degradation (5) to prevent iron overload and limit iron availability to pathogens. Iron deficiency and erythropoietic drive inhibit hepcidin production, thereby increasing iron availability for erythropoiesis (3). A failure of iron to appropriately upregulate hepcidin underlies the iron overload disorder hereditary hemochromatosis, whereas excessive hepcidin suppression by ineffective erythropoiesis contributes to the pathogenesis of iron loading anemias such as thalassemia (6, 7). A failure of iron deficiency anemia to appropriately suppress hepcidin underlies iron refractory iron deficiency anemia, whereas excessive hepcidin stimulation by chronic inflammation contributes to the pathogenesis of anemia of inflammation (7, 8).
The bone morphogenetic protein (BMP)-SMAD1/5/8 signaling pathway is a central mechanism for regulating hepcidin production in response to its main physiologic signals, including iron and erythropoietic drive (1, 9–11). Two BMP ligands have been identified to play critical roles in regulating hepcidin expression: BMP6 (12, 13) and BMP2 (14, 15). A key role for BMP6 was first suggested by a microarray analysis in mice demonstrating that liver Bmp6 mRNA expression was regulated by dietary iron concordantly with hepcidin (16). Global Bmp6 KO mice were subsequently found to have hemochromatosis, characterized by hepcidin deficiency, serum iron overload, tissue iron overload in the liver, heart, and pancreas, and reduced spleen iron (12, 13). Liver endothelial cells were then recognized as the key source for BMP6 with a hemochromatosis phenotype reported in conditional endothelial Bmp6 KO mice (17). In humans, heterozygous mutations in the BMP6 prodomain have also been associated with inappropriately low hepcidin and iron overload (18), although the functional impact of BMP6 prodomain mutations in humans is still controversial (19). A role for BMP2 was hypothesized when it was observed that the residual ability of dietary iron to induce hepcidin expression in Bmp6 KO mice was associated with an induction in liver SMAD1/5/8 signaling and was prevented by a neutralizing BMP2/4 antibody (14). A functional role for BMP2 was confirmed by the observation that endothelial Bmp2 KO mice developed hemochromatosis due to low hepcidin expression (14, 15). Moreover, dietary iron was found to regulate liver Bmp2 mRNA and circulating BMP2 protein levels concordantly with hepcidin (14, 20). Similar to Bmp6 KO mice, endothelial Bmp2 KO mice also exhibited a residual ability for iron to induce SMAD5 phosphorylation and hepcidin expression (20). An association between a common SNP in the BMP2 gene and serum ferritin has also been reported in humans with hemochromatosis due to mutations in HFE (21), the most common cause of hereditary hemochromatosis (22).
The precise molecular mechanisms by which HFE mutations cause hemochromatosis are still not fully understood. HFE is thought to regulate hepcidin production in response to iron by intersecting with BMP-SMAD1/5/8 signaling downstream or independent of BMP6 since liver Bmp6 mRNA was appropriately induced by iron overload, but liver SMAD1/5/8 signaling was inappropriately reduced relative to Bmp6 mRNA and liver iron levels in Hfe KO mice (23–25). Moreover, liver SMAD1/5/8 signaling was induced in parallel with hepcidin in mice overexpressing an Hfe transgene in the liver (26). Finally, a functional loss of HFE (due to ablation of the HFE localizing protein beta-2-microglobulin, B2M) further lowered liver SMAD1/5/8 signaling, worsened hepcidin deficiency, and worsened iron overload in Bmp6 KO mice, suggesting a BMP6-independent mechanism for HFE to intersect with the SMAD pathway to regulate hepcidin (27).
Here, we generated littermate mice with an endothelial KO of Bmp2 alone, an endothelial or global KO of Bmp6 alone, both Bmp2 and Bmp6, or neither to determine the relative roles of BMP2 and BMP6 in hepcidin regulation and systemic iron homeostasis. We also tested whether there is any residual pathway for iron-mediated hepcidin induction in mice with a combined global Bmp6 and endothelial Bmp2 KO. Finally, we generated littermate mice with an endothelial KO of Bmp2 alone, a global KO of Hfe alone, both, or neither to determine whether HFE regulates hepcidin through a BMP2-dependent pathway.
EXPERIMENTAL PROCEDURES
Animals
Animal protocols were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Littermate mice with a single endothelial KO of Bmp2 (Bmp2fl/fl;Bmp6wt/wt;Tek-Cre+), a single endothelial KO of Bmp6, (Bmp2wt/wt;Bmp6fl/fl;Tek-Cre+), a double endothelial KO of Bmp2 and Bmp6 (Bmp2fl/fl;Bmp6fl/fl;Tek-Cre+), and controls (Bmp2wt/wt;Bmp6wt/wt;Tek-Cre+) on a mixed C57BL/6, 129S6/SvEv, 129S4/SvJae background were generated by crossing Bmp2 KO males (Bmp2fl/fl;Tek-Cre+)(14) with endothelial Bmp6 floxed females (Bmp6fl/fl;Tek-Cre-)(17) as described in Figure S1A. Littermate single Bmp6 KO mice (Bmp6−/−;Bmp2fl/fl;Tek-Cre-) and double global Bmp6 and endothelial Bmp2 KO mice (Bmp6−/−;Bmp2fl/fl;Tek-Cre+) on a mixed C57BL/6, 129S6/SvEv, 129S4/SvJae background were generated by crossing Bmp2fl/fl;Tek-Cre+ males with global Bmp6 KO females (Bmp6−/−)(17) as described in Figure S1B. Littermate mice with a single endothelial KO of Bmp2 (Hfewt/wt;Bmp2fl/fl;Tek-Cre+), a single global KO of Hfe, (Hfe−/−;Bmp2fl/fl;Tek-Cre-), a double endothelial KO of Bmp2 and global KO of Hfe (Hfe−/−;Bmp2fl/fl;Tek-Cre+), and controls (Hfewt/wt;Bmp2fl/fl;Tek-Cre-) on a mixed C57BL/6, 129S4/SvJae background were generated by crossing Bmp2fl/fl;Tek-Cre+ males with Hfe KO females (Hfe−/−) (28) as described in Figure S1C. Mice were genotyped as previously described (14, 17, 28). All mice had ad libitum access to water and a house diet (Prolab 5P75 Isopro RMH 3000 containing 380 ppm iron) after weaning unless otherwise indicated.
For dietary iron experiments in Bmp6−/−;Bmp2fl/fl;Tek-Cre- and Bmp6−/−;Bmp2fl/fl;Tek-Cre+ mice, rodents received a low iron diet (2–6 ppm iron, Envigo #TD.80396) at weaning for 3 weeks to prevent the iron overload that develops on a standard rodent diet, and then either kept on the low iron diet for 1 more week or switched to the house diet. To examine the regulation of BMP ligands by dietary iron, heterozygous Bmp6wt/- mice (17) backcrossed for 10 generations to a C57BL/6N (Taconic) background were intercrossed to generate Bmp6−/− knockout and Bmp6wt/wt wildtype littermates. Dietary iron challenge experiments were also performed in wildtype (WT) offspring from heterozygous Hfewt/- mice that were originally on a C57BL/6J background (28) but have been breeding in our laboratory for many years, and WT C57BL/6J and C57BL/6NTac mice purchased from Jackson and Taconic respectively. Four- to five-week-old male and female mice were placed on a matched, purified low iron (Low Fe, 2–6 ppm iron, Envigo #TD.80396), iron sufficient (Control, 48 ppm iron, Envigo #TD.80394), or a high iron (High Fe, 2% carbonyl iron, Envigo #TD.08496) diet for 3 weeks.
RNA Isolation, Reverse Transcription, and qRT-PCR
Total liver RNA was isolated using QIAshredder and Purelink RNA mini kits (Invitrogen). First-strand cDNA was synthesized from 1 μg RNA using the High Capacity RNA-to-cDNA Kit (Applied Biosystems). PCR reactions were performed using the PowerUp SYBR Green Master Mix on the QuantStudio3 Real-Time PCR system (Applied Biosystems) using primers in Supplemental Table S1. Transcript levels were determined as previously described using the standard curve method (10).
Iron Analysis
Serum iron and unsaturated iron binding capacity were measured by colorimetric assay (Pointe Scientific) to calculate transferrin saturation according to manufacturer’s instructions. Tissue nonheme iron concentrations (in μg/g wet weight) were determined as described previously (10).
Immunoblot
Liver lysates were prepared and immunoblots performed as described previously (10) using rabbit anti-phosphorylated SMAD5 (pSMAD5; 1:500; ab92698; Abcam), rabbit anti-SMAD5 (1:1000; ab40771; Abcam), or mouse anti-actin (1:20,000 MAB1501; Millipore) antibodies. Immunoreactivity was visualized by chemiluminescence (SuperSignal West Pico, Thermo Scientific) and the G:box mini digital darkroom (Syngene). Quantification was performed using ImageJ 1.46v.
BMP2 ELISA
Serum BMP2 was measured using a BMP2 ELISA Kit (R&D Systems DBP200, lots P178778, P194447, P208993) in 1:1 dilution as previously described (20).
Statistics
Statistical significance was determined by 2-tailed Student’s t-test or one-way analysis of variance (ANOVA) with Tukey’s post hoc test for pairwise multiple comparisons using Prism 7 (GraphPad). Data with unequal variances were log transformed prior to statistical analysis. P < 0.05 was considered significant.
RESULTS
Double endothelial KO of Bmp2 and Bmp6 does not exacerbate hemochromatosis compared with single KO mice
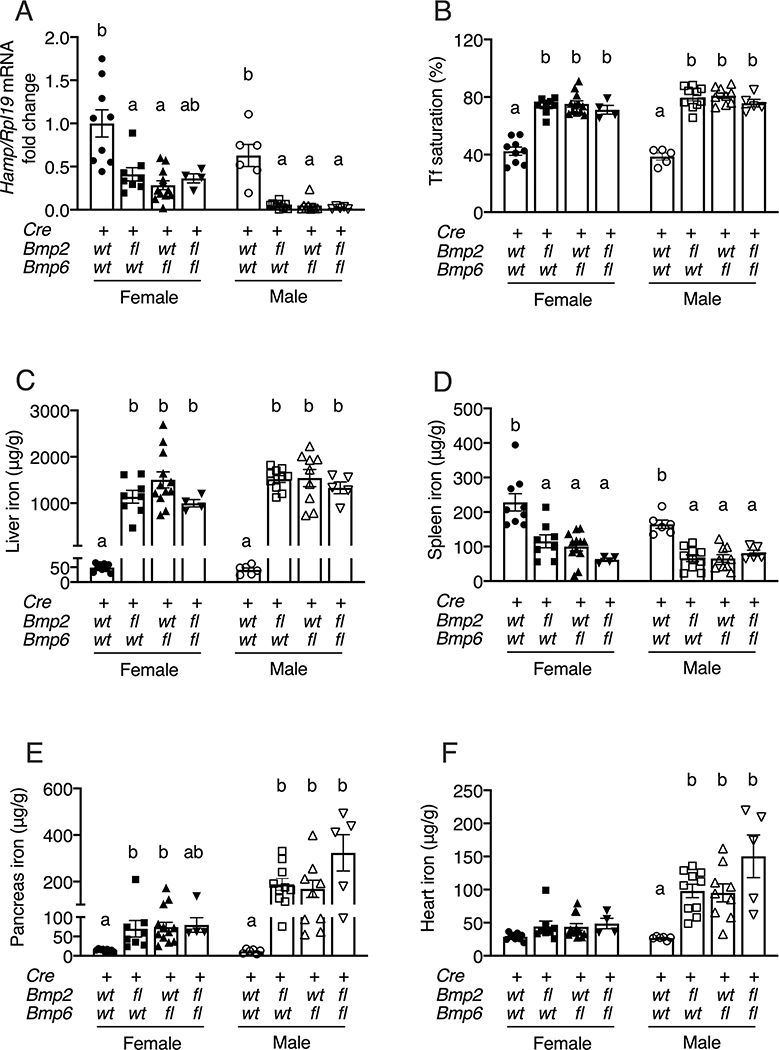
To determine the relative roles of BMP2 and BMP6 in hepcidin and iron homeostasis regulation, we generated littermate mice with a single endothelial KO of Bmp2 (Bmp2fl/fl;Bmp6wt/wt;Tek-Cre+), a single endothelial KO of Bmp6 (Bmp2wt/wt;Bmp6fl/fl;Tek-Cre+), a double endothelial KO of Bmp2 and Bmp6 (Bmp2fl/fl;Bmp6fl/fl;Tek-Cre+), and controls (Bmp2wt/wt;Bmp6wt/wt;Tek-Cre+), and analyzed their hepcidin levels and iron phenotype at 8 weeks of age. Hepcidin and iron parameters were analyzed separately in males and females due to previously established sex differences in mice (14, 17, 29). All KO mice exhibited reduced liver expression of hepcidin (Hamp) mRNA and the BMP-SMAD1/5/8 target transcript Id1 (Figure 1A, S2A–B), increased serum and liver iron (Figure 1B–C, S2C), and reduced spleen iron (Figure 1D) compared to control mice. Although the hepcidin reduction in female double endothelial Bmp2/Bmp6 KO mice did not quite reach statistical significance compared to controls by multivariable analysis, this was significantly lower relative to the higher degree of iron loading in these mice (Figure S2A). All male KO mice also exhibited increased extrahepatic iron loading in the pancreas and heart compared to controls, whereas female KO mice had minimal or no increases in extrahepatic iron that reached statistical significance only for pancreas in single KO mice (Figure 1E–F). In general, male KO mice appeared to have lower hepcidin and increased extrahepatic iron overload than female KO mice, consistent with prior reports in single Bmp6 and endothelial Bmp2 KO mice (14, 17, 29), although the current study was not powered to compare differences between sexes in addition to genotype. Notably, liver Hamp, liver Id1, serum iron, liver iron, and extrahepatic iron levels were not significantly different in sex-matched single endothelial Bmp6 compared with single endothelial Bmp2 KO mice. Moreover, none of these parameters was significantly different in double KO mice compared to sex-matched single KO mice (Figures 1, S2). Together, these results suggest that under baseline conditions, BMP2 and BMP6 predominantly work together to regulate hepcidin expression and iron homeostasis.
Figure 1: Combined endothelial Bmp6 and Bmp2 KO does not worsen hepcidin deficiency or iron overload compared to single KO mice.
Eight-week-old littermate female (closed symbols) and male (open symbols) control (Bmp2wt/wt;Bmp6wt/wt;Tek-Cre+), single endothelial Bmp2 KO (Bmp2fl/fl;Bmp6wt/wt;Tek-Cre+), single endothelial Bmp6 KO (Bmp2wt/wt;Bmp6fl/fl;Tek-Cre+), and double endothelial Bmp2 and Bmp6 KO (Bmp2fl/fl;Bmp6fl/fl;Tek-Cre+) mice were analyzed for (A) liver hepcidin (Hamp) relative to Rpl19 mRNA by qRT-PCR. The average of the control female mice was set to 1. (B) Serum transferrin saturation and tissue iron levels in the (C) liver, (D) spleen, (E) pancreas, and (F) heart were analyzed by colorimetric assay. For all graphs, individual data points are shown and bars represent mean ± SEM. Female and male mice were analyzed separately by one-way ANOVA with Tukey’s post-hoc test. Means without a common superscript differ significantly (P < 0.05).
BMP2 is not induced in Bmp6 KO mice despite iron overload
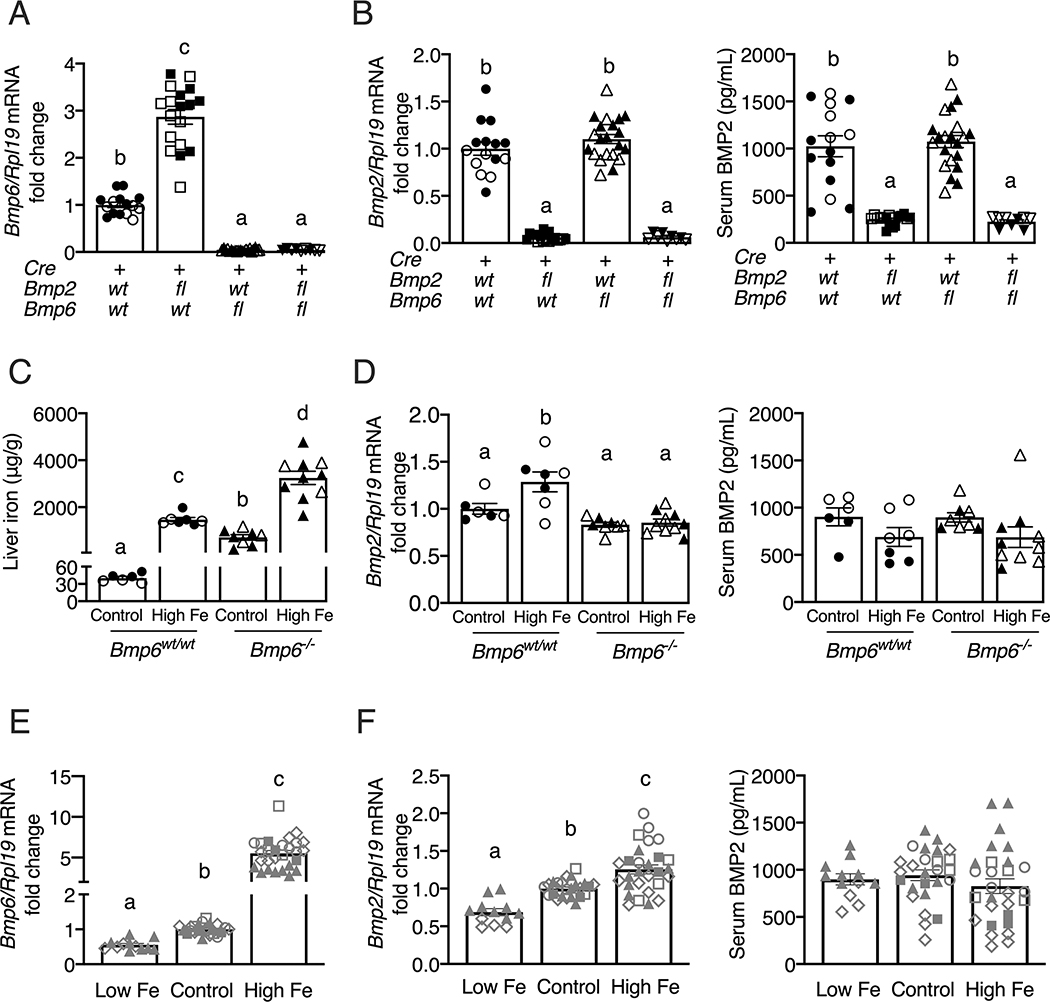
Total liver Bmp6 and Bmp2 mRNA levels were appropriately reduced by 92–96% in the respective single endothelial Bmp6, single endothelial Bmp2, and double endothelial Bmp2/Bmp6 KO mice (Figures 2A, 2B left). The reduction in liver Bmp2 mRNA also corresponded to a reduction in circulating BMP2 protein levels in single and double endothelial Bmp2 KO mice (Figure 2B right) as previously reported (20). Liver Bmp6 mRNA levels were induced in single endothelial Bmp2 KO mice (Figure 2A) as previously reported (14), presumably as a response to iron overload. However, although liver Bmp2 mRNA was induced by dietary iron loading in wildtype mice (Figure 2D, left), as previously reported (14, 20), liver Bmp2 mRNA was not increased in the single endothelial or global Bmp6 KO mice, either on a house or a high iron diet (Figures 2B left, 2D left) despite iron overload (Figures 1, 2C). Surprisingly, in contrast to our previous findings (20), dietary iron failed to regulate serum BMP2 protein levels in multiple cohorts of wildtype mice (Figures 2D right, 2F right), despite a consistent regulation of serum iron, liver iron, liver Hamp mRNA, liver Bmp6 mRNA, and liver Bmp2 mRNA that were similar to our prior findings (Figures 2C–F, S3) (14, 20). Serum BMP2 protein was also not changed in endothelial or global Bmp6 KO mice despite iron overload (Figure 2B right, 2D right). Thus, Bmp6 appears to be required for iron-mediated liver Bmp2 mRNA induction, although the impact of dietary iron on liver Bmp2 mRNA is quite modest compared with Bmp6, and dietary iron alone is not sufficient to regulate circulating BMP2 protein levels.
Figure 2: Liver Bmp6 mRNA is increased in endothelial Bmp2 KO mice, but Bmp2 mRNA and protein are not increased in endothelial Bmp6 KO mice.
(A-B) Eight-week-old littermate female (black closed symbols) and male (black open symbols) control (Bmp2wt/wt;Bmp6wt/wt;Tek-Cre+), single endothelial Bmp2 KO (Bmp2fl/fl;Bmp6wt/wt;Tek-Cre+), single endothelial Bmp6 KO (Bmp2wt/wt;Bmp6fl/fl;Tek-Cre+), and double endothelial Bmp2 and Bmp6 KO (Bmp2fl/fl;Bmp6fl/fl;Tek-Cre+) mice were analyzed for (A) liver Bmp6 and (B, left) Bmp2 relative to Rpl19 mRNA by qRT-PCR. The average of the control mice was set to 1. (B, right) Serum BMP2 protein levels were quantitated by ELISA. (C-D) Four-week-old female (black closed symbols) and male (black open symbols) global Bmp6−/− and littermate WT mice (Bmp6wt/wt) were fed a matched, purified iron sufficient (Control, 48 ppm iron) or high iron (High Fe, 2% carbonyl iron) diet for 3 weeks, followed by analysis of (C) liver iron, (D, left) liver Bmp2 relative to Rpl19 mRNA by qRT-PCR, and (D, right) serum BMP2 protein levels by ELISA. For qRT-PCR analysis, the average of the control Bmp6wt/wt mice was set to 1. (E-F) Four- to five-week-old WT C57BL/6 male and female mice from four independent cohorts (grey symbols: ○ male Hfewt/wt littermates; ◻◼ female and male Bmp6wt/wt littermates; ▲ Jackson female WT; ◇ Taconic male WT) were fed a matched, purified low iron (Low Fe, 2–6 ppm iron), iron sufficient (Control, 48 ppm iron), or high iron (High Fe, 2% carbonyl iron) diet for 3 weeks, followed by analysis of (E) liver Bmp6 and (F, left) Bmp2 relative to Rpl19 mRNA by qRT-PCR. The average of the control diet-fed mice was set to 1. (F, right) Serum BMP2 protein levels were quantified by ELISA. For all graphs, individual data points are shown and bars represent mean ± SEM. Results were analyzed by one-way ANOVA with Tukey’s post-hoc test. Means without a common superscript differ significantly (P < 0.05).
Liver SMAD5 phosphorylation and hepcidin expression are still inducible by iron in mice with a combined global Bmp6 and endothelial Bmp2 KO
We previously reported that chronic dietary iron loading still induced liver SMAD phosphorylation and hepcidin expression in single Bmp6 global KO mice and single Bmp2 endothelial KO mice, and that BMP neutralizing antibodies inhibited this effect in Bmp6 KO mice, suggesting a residual pathway for iron-mediated, BMP/SMAD-dependent hepcidin induction in the absence of BMP2 or BMP6 (14, 20). We therefore sought to determine whether there was any residual pathway for iron-mediated hepcidin expression in mice with a combined loss of Bmp2 and Bmp6. Although the endothelial KO mice provide the most balanced comparison of the relative effects of BMP2 and BMP6 (since embryonic lethality precludes utilizing global Bmp2 KO mice (30)), the 98% recombination efficiency of the Tek-Cre in liver endothelial cells results in low level residual liver BMP ligand expression in this model (17). To minimize this impact, we generated littermate mice with a single global Bmp6 KO (Bmp6−/−;Bmp2fl/fl;Tek-Cre-) or a combined global Bmp6 and endothelial Bmp2 KO (Bmp6−/−;Bmp2fl/fl;Tek-Cre+). Analogous to the results in endothelial conditional KO mice, double Bmp6−/−;Bmp2fl/fl;Tek-Cre+ mice exhibited similar liver Hamp mRNA, serum iron, liver iron, spleen iron, and extrahepatic iron levels in the heart and pancreas compared to single Bmp6−/−;Bmp2fl/fl;Tek-Cre- mice at 8 weeks of age (Figure S4).
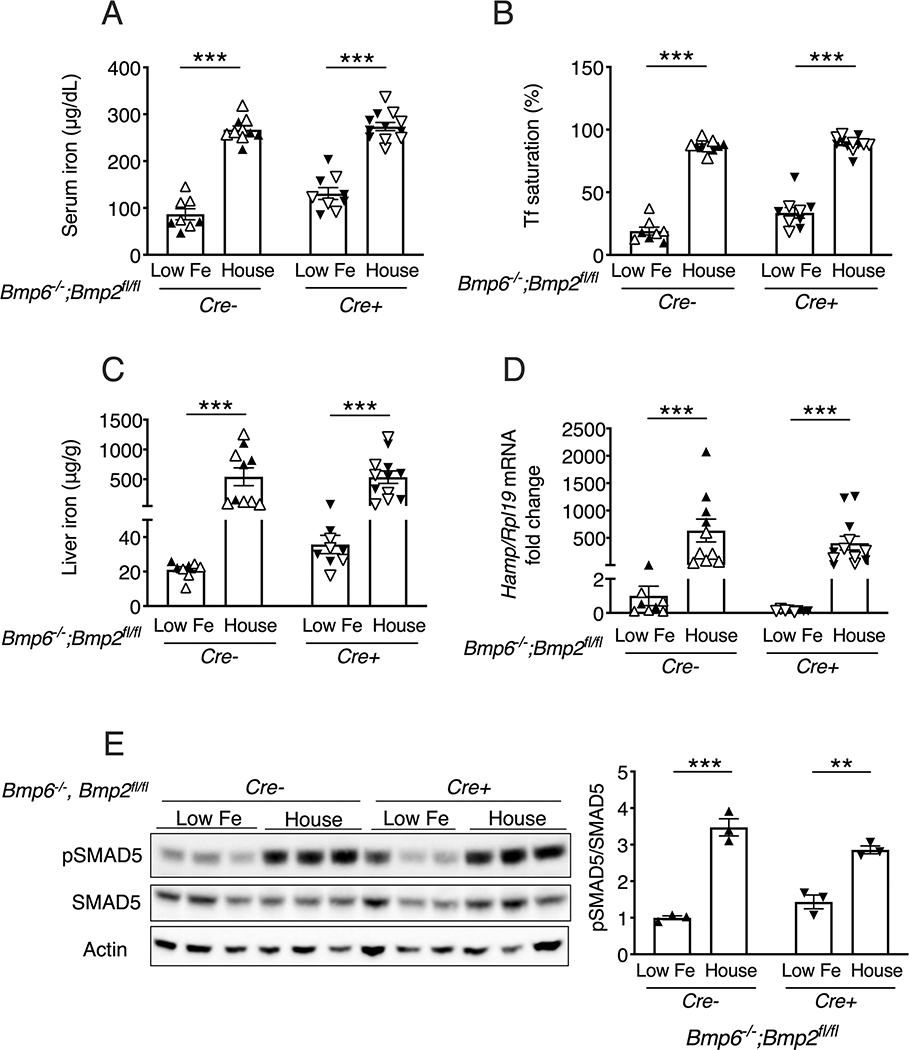
To test the response to iron loading, single Bmp6−/−;Bmp2fl/fl;Tek-Cre- and double Bmp6−/−;Bmp2fl/fl;Tek-Cre+ mice were weaned to a low iron diet (2–6 ppm) to prevent the iron overload induced by a house diet (14). After 3 weeks, mice were either kept on the low iron diet or switched to the house diet containing 380 ppm iron for 1 week. Serum iron and liver iron levels were robustly induced by the switch to the higher iron house diet in both genotypes (Figure 3A–C), along with a slight increase in pancreas iron (Figure S5). Similar to prior reports (14, 31), liver phosphorylated SMAD5 protein and Hamp mRNA were both significantly induced by the house diet in single Bmp6−/−;Bmp2fl/fl;Tek-Cre- mice (Figure 3D–E). Notably, the house diet also significantly induced liver phosphorylated SMAD5 protein and Hamp mRNA in double Bmp6−/−;Bmp2fl/fl;Tek-Cre+ mice (Figure 3D–E). Similar findings were seen in single Bmp6−/−;Bmp2fl/fl;Tek-Cre- and double Bmp6−/−;Bmp2fl/fl;Tek-Cre+ mice switched to a matched purified iron sufficient diet (48 ppm iron) where iron is the only dietary component changed compared to the low iron diet (Figure S6).
Figure 3: Dietary iron loading induces liver SMAD5 phosphorylation and hepcidin expression in double global Bmp6/endothelial Bmp2 KO mice.
Littermate female (closed symbols) and male (open symbols) single global Bmp6 KO (Bmp6−/−;Bmp2fl/fl;Tek-Cre-) and double global Bmp6 and endothelial Bmp2 KO (Bmp6−/−;Bmp2fl/fl;Tek-Cre+) mice were placed on a low iron diet (2–6 ppm iron) at weaning for 3 weeks to prevent the iron overload induced by the house diet. Mice were either maintained on the low iron diet (Low Fe) or switched to the house diet for 1 more week. Mice were analyzed for (A) serum iron, (B) serum transferrin saturation, and (C) liver iron by colorimetric assay. (D) Livers were analyzed for Hamp relative to Rpl19 mRNA by qRT-PCR. The average of the single Bmp6 KO mice on the low iron diet was set to 1. (E) Livers were analyzed for phosphorylated SMAD5 (pSMAD5) relative to total SMAD5 and Actin protein by immunoblot and chemiluminescence quantitation. For all graphs, individual data points are shown and bars represent mean ± SEM. ** P < 0.01, *** P < 0.001 relative to low iron diet mice of the same genotype by Student’s t-test.
The ability of dietary iron to induce SMAD5 phosphorylation and hepcidin in double Bmp6−/−;Bmp2fl/fl;Tek-Cre+ mice suggests that another BMP ligand may contribute to iron-mediated hepcidin induction. We therefore examined whether any other BMP ligand was increased in endothelial Bmp2 and/or Bmp6 KO mice or was regulated by dietary iron in WT mice. In contrast to liver Bmp2 and Bmp6 mRNA, which were consistently reduced by a low iron diet and increased by a high iron diet (Figure 2E–F), liver Bmp4, Bmp5, Bmp7, and Bmp9 mRNA were not concordantly regulated by dietary iron in WT mice (Figure S7, left). In fact, Bmp4, Bmp5, and Bmp9 were reduced by a high iron diet. Similarly, no BMPs were induced despite iron overload in single or double endothelial Bmp2 and/or Bmp6 KO mice (Figure S7, right).
Combined global Hfe and endothelial Bmp2 KO lowers hepcidin and worsens extrahepatic iron overload compared with single KO mice
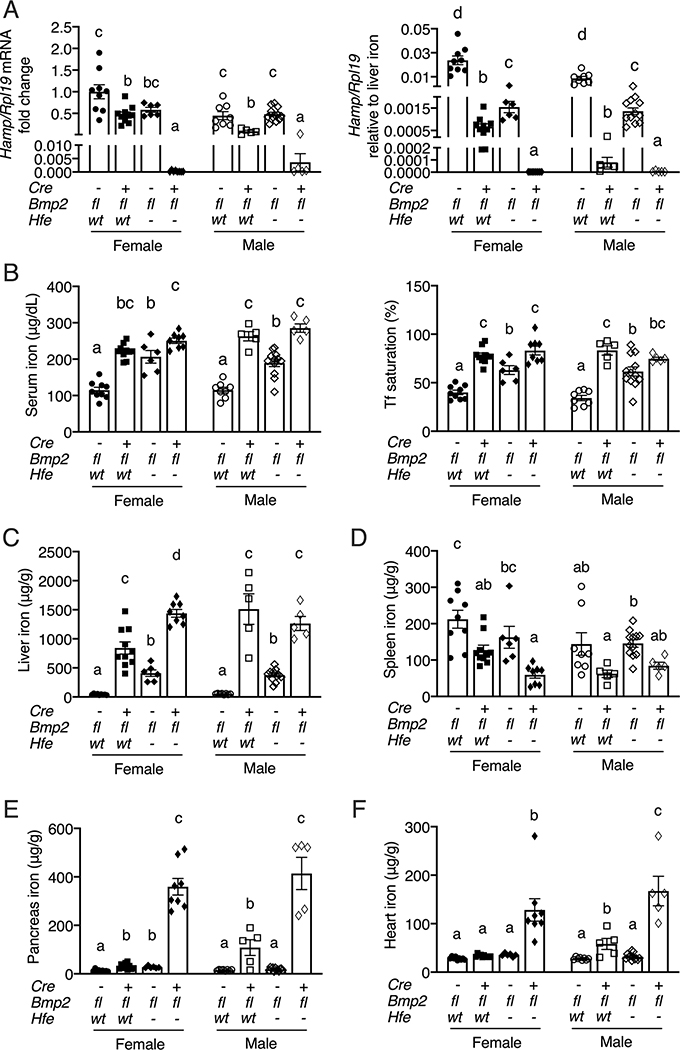
HFE was previously proposed to stimulate hepcidin through a pathway that intersects with the BMP-SMAD signaling cascade, but downstream or independent of BMP6 (23–25, 32, 33). To determine if HFE-mediated hepcidin regulation is dependent on BMP2, we generated littermate mice with a single endothelial KO of Bmp2 (Hfewt/wt;Bmp2fl/fl;Tek-Cre+), a single global KO of Hfe, (Hfe−/−;Bmp2fl/fl;Tek-Cre-), a double endothelial KO of Bmp2 and global KO of Hfe (Hfe−/−;Bmp2fl/fl;Tek-Cre+), and controls (Hfewt/wt;Bmp2fl/fl;Tek-Cre-), and analyzed their iron phenotype. Hamp mRNA levels were significantly reduced in single endothelial Bmp2 KO mice compared to controls, whereas Hamp mRNA was only reduced in Hfe KO mice when analyzed relative to liver iron content (Figure 4A). This was associated with significantly increased serum iron, transferrin saturation, and liver iron in all single KO mice (Figure 4B–C). Spleen iron was significantly reduced in endothelial Bmp2 KO females, although there was only a trend toward reduction in males and no significant change in female or male Hfe KO mice (Figure 4D). Male endothelial Bmp2 KO mice had significant extrahepatic iron loading in both pancreas and heart, whereas little or no extrahepatic iron loading was seen in Hfe KO males or single KO females of either genotype (Figures 4E–F). The overall hemochromatosis phenotype was worse in the single endothelial Bmp2 KO mice compared with Hfe KO mice as evidenced by significantly increased transferrin saturation and liver iron in both sexes (Figure 4B–C) and significantly lower hepcidin, lower spleen iron, and increased heart and pancreas iron in males (Figures 4A, D–F). Notably, Hamp mRNA levels were markedly lower in double Hfe/endothelial Bmp2 KO mice compared to controls (~1/5000 lower in females, ~1/120 in males) or single KO mice (~1/3000 lower in females, ~1/24–1/130 in males) (Figure 4A). Moreover, this was associated with significantly increased iron overload in double KO mice compared with controls and single KO mice, as evidenced by higher transferrin saturation and/or serum iron in double KO mice compared with controls or Hfe KO mice (Figure 4B), increased pancreas and heart iron in double KO mice compared with controls or single KO mice (Figure 4E–F), and increased liver iron in double KO compared with control or single KO females (Figure 4C). Liver Bmp2 mRNA and circulating BMP2 protein were appropriately reduced in the respective KOs (Figure S8). Similar to Bmp6 KO mice, no increase in liver Bmp2 mRNA or serum BMP2 protein was seen in Hfe KO mice despite iron overload (Figure S8). Together, these data demonstrate a worse hemochromatosis phenotype in double Hfe−/−;Bmp2fl/fl;Tek-Cre+ mice compared with single KO mice, suggesting that HFE can function independent of BMP2 in hepcidin and iron homeostasis regulation. Data from the single KO mice also suggest that BMP2 has a stronger impact on hepcidin and iron homeostasis regulation than HFE.
Figure 4: Combined global Hfe and endothelial Bmp2 KO worsens hepcidin deficiency and iron overload compared to single KO mice.
Eight-week-old littermate female (closed symbols) and male (open symbols) control (Hfewt/wt;Bmp2fl/fl;Tek-Cre-), single endothelial Bmp2 KO (Hfewt/wt;Bmp2fl/fl;Tek-Cre+), single global Hfe KO (Hfe−/−;Bmp2fl/fl;Tek-Cre-), and double endothelial Bmp2 and global Hfe KO (Hfe−/−;Bmp2fl/fl;Tek-Cre+) mice were analyzed for (A) liver Hamp relative to Rpl19 mRNA by qRT-PCR. The average of the control female mice was set to 1. Hamp/Rpl19 mRNA was divided by liver iron content to normalize for the degree of iron overload in the right panel. (B) Serum iron, serum transferrin saturation and tissue iron levels in the (C) liver, (D) spleen, (E) pancreas, and (F) heart were analyzed by colorimetric assay. For all graphs, individual data points are shown and bars represent mean ± SEM. Female and male mice were analyzed separately by one-way ANOVA with Tukey’s post-hoc test. Means without a common superscript differ significantly (P < 0.05).
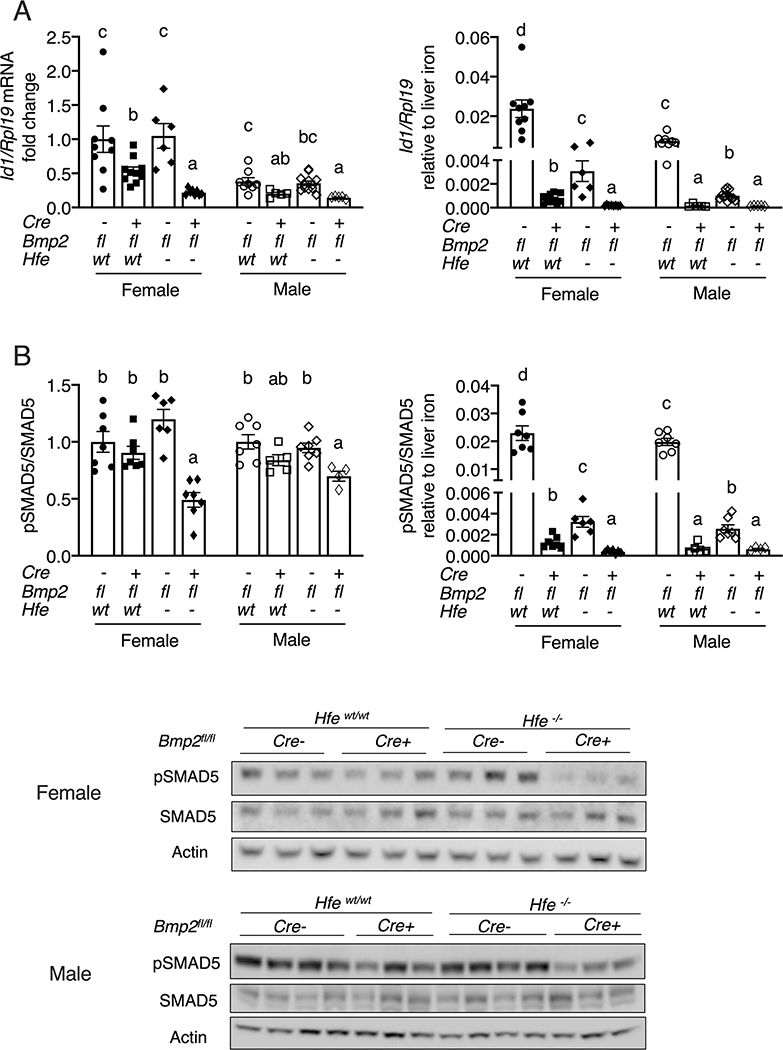
Interestingly, liver SMAD5 phosphorylation and Id1 mRNA levels were significantly lower in female double Hfe/endothelial Bmp2 KO mice compared with single KO mice, with similar trends in males (Figure 5). Although SMAD5 phosphorylation was not significantly lower in either single KO strain compared with controls, and Id1 mRNA was not lower in Hfe KO mice compared with controls, both were inappropriately lower relative to the higher body iron burden of these mice (Figure 5) as previously reported (23). Induction of Hamp and Id1 mRNA by BMP6 homodimer, BMP2 homodimer, and BMP2/6 heterodimer were all blunted in Hfe−/− primary hepatocytes compared with WT hepatocytes (Figure S9). These data are consistent with a functional role for HFE in inducing hepcidin by enhancing the SMAD1/5/8 signaling response to BMP ligands, and suggest that the BMP2-independent function is still SMAD1/5/8 dependent.
Figure 5: Combined global Hfe and endothelial Bmp2 KO reduces liver SMAD5 phosphorylation and Id1 mRNA compared to single KO mice.
Eight-week-old littermate female (closed symbols) and male (open symbols) control (Hfewt/wt;Bmp2fl/fl;Tek-Cre-), single endothelial Bmp2 KO (Hfewt/wt;Bmp2fl/fl;Tek-Cre+), single global Hfe KO (Hfe−/−;Bmp2fl/fl;Tek-Cre-), and double endothelial Bmp2 and global Hfe KO (Hfe−/−;Bmp2fl/fl;Tek-Cre+) mice were analyzed for (A) Id1 relative to Rpl19 mRNA by qRT-PCR and (B) phosphorylated SMAD5 (pSMAD5) relative to total SMAD5 and Actin protein by immunoblot and chemiluminescence quantitation. The average of the control female mice was set to 1. Id1/Rpl19 mRNA and pSMAD5/SMAD5 were divided by liver iron content to normalize for the degree of iron overload in the right-sided panels. For all graphs, individual data points are shown and bars represent mean ± SEM. Female and male results were analyzed separately by one-way ANOVA with Tukey’s post-hoc test. Means without a common superscript differ significantly (P < 0.05). One representative immunoblot each for female and male mice are shown.
DISCUSSION
BMP2 and BMP6 have key roles in regulating hepcidin expression and systemic iron balance since mice with an endothelial KO of either of these genes exhibit hemochromatosis. However, their relative roles have remained uncertain. Here, we show that littermate mice with a single endothelial KO of Bmp2 or Bmp6 alone had similar levels of hepcidin deficiency, serum iron loading and liver iron loading. Surprisingly, a combined KO of endothelial Bmp2 and Bmp6 did not significantly worsen the hemochromatosis phenotype of single KO mice. Similarly, a KO of endothelial Bmp2 did not significantly worsen the hemochromatosis phenotype of global Bmp6 KO mice. These data suggest that under iron adequate or overload conditions, BMP2 and BMP6 mainly work together to regulate hepcidin expression and systemic iron homeostasis and that neither endogenously expressed BMP2 or BMP6 appreciably compensates for the loss of the other.
The results in double global or endothelial Bmp6/endothelial Bmp2 KO mice are in sharp contrast to double Hfe/endothelial Bmp2 KO mice, where there was a robust exacerbation of hepcidin deficiency and extrahepatic iron overload compared with single Hfe or endothelial Bmp2 KO mice. Single endothelial Bmp2 KO mice also exhibited a stronger overall hemochromatosis phenotype compared with single Hfe KO mice, particularly in males. Additionally, multiple prior studies have demonstrated a clear exacerbating impact on hepcidin deficiency and extrahepatic iron overload in mice with a double KO of Bmp6 and/or other hemochromatosis genes including Hfe or its chaperone B2m, Tfr2 (encoding transferrin receptor 2), and Hjv (encoding hemojuvelin) compared to the respective single KO mice on a standard diet (25, 27, 34). These findings demonstrate the feasibility of detecting an exacerbating hemochromatosis phenotype in mice with a global Bmp6 KO or endothelial Bmp2 KO on a standard rodent diet. Moreover, these results suggest that whereas BMP2 and BMP6 predominantly function together in hepcidin regulation, BMP2 and HFE can function independently. Previous publications also support an at least partially independent role for BMP6 and HFE, TFR2, and HJV (27, 34).
A limitation of our study is that we focused on mice receiving a standard rodent diet at 8 weeks of age. It is therefore possible that more subtle differences between double and/or single endothelial Bmp2 and/or Bmp6 KO mice might be uncovered in younger or older mice, under different iron loading or deficiency conditions, or in response to other treatments. Indeed, there was a slight tendency for increased extrahepatic iron overload in double endothelial or global Bmp6/Bmp2 KO mice compared with sex-matched single KO mice (Fig. 1E–F, S4E–F). There was also a tendency for lower hepcidin expression and higher serum and liver iron levels in mice with a combined global Bmp6/endothelial Bmp2 KO compared with single Bmp6 KO mice maintained on a low iron diet (Figure 3). We also previously reported that when maintained on a low iron diet and then challenged with acute oral iron gavage that increased serum iron without increasing liver iron, endothelial Bmp2 KO mice had a modest residual ability to induce hepcidin, whereas endothelial or global Bmp6 KO mice failed to induce hepcidin (20). This prior study was limited by the use of mice with different mixed background strains, which may have contributed to the apparent differences reported (20). A potential complicating feature of the Bmp2 and Bmp6 endothelial KO models is the small residual BMP ligand expression due to the 98% efficiency of Tek-Cre-mediated recombination (14, 17), an unavoidable limitation due to embryonic lethality of global Bmp2 KO mice (30). However, total liver Bmp6 and Bmp2 mRNA levels were markedly reduced by ≥92–95% in the corresponding Tek-Cre mice, leading to a >60–95% reduction in total liver and circulating BMP2 protein levels (14, 17, 20). Moreover, we previously reported that the phenotype of endothelial Bmp6 KO mice was only slightly less severe than global Bmp6 KO mice (17), and in the current study, we obtained similar results in double endothelial Bmp6/Bmp2 KO mice and double global Bmp6/endothelial Bmp2 KO mice. Importantly, the robust worsening of hepcidin deficiency and extrahepatic iron overload in the double Hfe/endothelial Bmp2 KO mice compared with single Hfe or endothelial Bmp2 KO mice demonstrate the feasibility of detecting an exacerbating hemochromatosis phenotype in mice with an endothelial ablation of Bmp2, even if it is not a full knockout. Taken together, our findings in the double KO mice demonstrate clear distinctions in the relative roles of BMP2 and BMP6, which predominantly work together in hepcidin regulation, and BMP2 and HFE, which can function independently; however, we cannot rule out a more subtle ability of BMP2 and/or BMP6 to work independently from the other to regulate hepcidin under some conditions.
Data in the double Bmp6/endothelial Bmp2 KO mice reveal the existence of another mechanism for iron-mediated hepcidin induction in the absence of both BMP6 and BMP2. We previously hypothesized that BMP2 accounts for the residual ability of iron to induce hepcidin in single Bmp6 knockout mice since a neutralizing BMP2/4 antibody inhibited hepcidin induction by iron in Bmp6 KO mice and endothelial Bmp2 KO mice exhibit hemochromatosis (14). However, we previously found that the neutralizing BMP2/4 antibody was not specific, but inhibited all BMPs aside from BMP9 (14). Moreover, in the current study, the additional deletion of endothelial Bmp2 in Bmp6 KO mice did not impact the ability of iron to induce hepcidin compared to single Bmp6 KO mice (Figure 3D). Although this is not a full Bmp2 KO, endothelial Bmp2 KO removes the majority of liver Bmp2 mRNA, liver BMP2 protein, and circulating BMP2 protein as discussed above, which is sufficient to cause hepcidin deficiency and iron overload on its own, and exacerbate hepcidin deficiency and hemochromatosis in Hfe KO mice. The lack of any impact of endothelial Bmp2 KO on iron-mediated hepcidin induction in Bmp6 KO mice makes it unlikely that the low level of residual BMP2 expression in the double KO mice accounts for the robust residual iron-mediated hepcidin induction. The most likely mechanism involves another BMP ligand or ligands given the strong SMAD pathway activation by iron in the double Bmp6/endothelial Bmp2 KO mice and the complete inhibition of iron-mediated hepcidin induction in Bmp6 KO mice by the pan-neutralizing BMP2/4 antibody (14). In contrast to BMP2 and BMP6, no other BMP ligand mRNAs were increased by dietary iron loading in wildtype mouse livers. We also did not find increased BMP ligand mRNAs in the livers of iron overloaded endothelial Bmp2 and/or Bmp6 KO mice. This does not preclude a role for these other BMPs in hepcidin and iron homeostasis regulation since BMP ligand expression and/or activity can be regulated at many other levels. Future studies will be needed to identify which other BMP ligands(s) contribute to hepcidin and iron homeostasis regulation and how their expression or activity is modified by iron.
Interestingly, combined Hfe and endothelial Bmp2 ablation reduced liver SMAD5 phosphorylation and Id1 mRNA expression compared with endothelial Bmp2 ablation alone. Although liver SMAD5 phosphorylation and Id1 mRNA levels in Hfe KO mice were not significantly different from controls, these were inappropriately low relative to the degree of iron overload as previously reported (23), since chronic dietary iron loading normally induces liver SMAD1/5/8 phosphorylation and Id1 mRNA expression in wildtype mice (9, 16). A reduction in liver phosphorylated SMAD5 expression has also been reported for mice with a double KO of Bmp6 and the HFE-localizing protein B2m compared with single Bmp6 KO mice (27). These data support the hypothesis that HFE regulates hepcidin by intersecting with the SMAD pathway, even in the absence of BMP2 or BMP6, potentially by modulating signaling by another BMP ligand. Prior studies using in vitro overexpression systems have suggested that this functional interaction might involve a physical interaction between HFE and the BMP type I receptor ALK3 (35) and/or the BMP co-receptor hemojuvelin/HJV (36). These models await further validation in vivo. Notably, although HFE has some ability to regulate hepcidin independent of BMP2 and BMP6, it remains possible that HFE also contributes to hepcidin regulation by BMP2 and BMP6 when they are available. Indeed, hepcidin induction by homodimeric BMP6, homodimeric BMP2, and heterodimeric BMP2/6 was impaired in Hfe−/− primary hepatocytes compared to wildtype hepatocytes, similar to a prior report for BMP6 (23).
Although liver Bmp2 mRNA failed be appropriately induced despite iron overload in both Bmp6 and Hfe KO mice, it is unlikely that this plays a major role in the pathogenesis of hemochromatosis in these mice. Bmp2 mRNA upregulation by dietary iron is quite modest (~30%) compared with Bmp6 (~5-fold). Additionally, although we previously found that serum BMP2 protein was upregulated in mice fed a high iron diet (20), the high iron diet failed to upregulate serum BMP2 protein in multiple cohorts of wildtype mice in the current study despite consistent increases in serum iron, liver iron, liver Hamp, Bmp6, and Bmp2 mRNA. Although the reason for the different findings in these studies is unclear, the current study demonstrates that changes in dietary iron alone are not sufficient to regulate serum BMP2 levels. Importantly, prior quantitative copy number analysis showed that Bmp2 mRNA is much more highly expressed than Bmp6 in liver endothelial cells both under control and high iron conditions, at least in mice (14). Thus, if BMP2 and BMP6 predominantly work together, as suggested by the findings in the double endothelial Bmp6 and Bmp2 KO compared with single KO mice, BMP6 expression would be the limiting factor, and a modest change in Bmp2 mRNA expression would be unlikely to have major functional significance.
An unresolved question is why BMP2 and BMP6 predominantly function together to regulate hepcidin. An intriguing possibility is that BMP2 and BMP6 must act as heterodimeric proteins for optimal function. Indeed, BMPs function as dimeric proteins and there are some biologic contexts where heterodimeric BMP proteins are required for function whereas homodimeric proteins are not able to compensate (37, 38). However, exogenously added homodimeric BMP2 and BMP6 ligand can stimulate hepcidin expression both in vitro and in vivo (12, 39). Future studies will be needed to understand why endogenous BMP6 and BMP2 fail to appreciably compensate for the loss of the other in endothelial Bmp2 and Bmp6 KO mice respectively, and whether endogenous heterodimeric BMP2/6 ligand has an important function role in hepcidin regulation and systemic iron homeostasis.
In summary, our results demonstrate that BMP2 and BMP6 predominantly work together to regulate hepcidin expression and that HFE regulates hepcidin at least in part through a BMP2-independent, but SMAD-dependent, pathway. Our results also support the existence of additional BMP ligand(s) that play a role in iron-mediated hepcidin regulation to maintain systemic iron homeostasis. These findings provide new insights into how iron regulates hepcidin production and how this process is impaired in hereditary hemochromatosis.
Supplementary Material
Acknowledgments
Financial Support
This work was supported by NIH grant R01-DK087727 and a Massachusetts General Hospital Research Scholars Award to JLB. CW is supported in part by a Cooley’s Anemia Foundation Research Fellowship.
List of Abbreviations
- BMP
Bone morphogenetic protein
- KO
knockout
- B2M
beta 2 microglobulin
- WT
wildtype
- pSMAD5
phosphorylated SMAD5
- ANOVA
analysis of variance
- Hamp
hepcidin mRNA
- HJV
hemojuvelin
- Tf
transferrin
- TFR2
transferrin receptor 2
Footnotes
Conflict of Interest Disclosures
JLB has ownership interest in Ferrumax Pharmaceuticals and has received consulting fees from Keryx Biopharmaceuticals and Disc Medicine. All other authors have nothing to declare.
REFERENCES
- 1.Wang CY, Babitt JL. Liver iron sensing and body iron homeostasis. Blood 2019;133:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 2001;276:7811–7819. [DOI] [PubMed] [Google Scholar]
- 3.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 2002;110:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090–2093. [DOI] [PubMed] [Google Scholar]
- 6.Rivella S Iron metabolism under conditions of ineffective erythropoiesis in β-thalassemia. Blood. 2019;133:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camaschella C Iron deficiency. Blood. 2019;133:30–39. [DOI] [PubMed] [Google Scholar]
- 9.Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, Babitt JL. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology 2011;54:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CY, Core AB, Canali S, Zumbrennen-Bullough KB, Ozer S, Umans L, Zwijsen A, et al. Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice. Blood 2017;130:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arezes J, Foy N, McHugh K, Sawant A, Quinkert D, Terraube V, Brinth A, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood 2018;132:1473–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andriopoulos B Jr., Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet 2009;41:482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet 2009;41:478–481. [DOI] [PubMed] [Google Scholar]
- 14.Canali S, Wang CY, Zumbrennen-Bullough KB, Bayer A, Babitt JL. Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6. Am J Hematol 2017;92:1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch PS, Olsavszky V, Ulbrich F, Sticht C, Demory A, Leibing T, Henzler T, et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood 2017;129:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood 2008;112:1503–1509. [DOI] [PubMed] [Google Scholar]
- 17.Canali S, Zumbrennen-Bullough KB, Core AB, Wang CY, Nairz M, Bouley R, Swirski FK, et al. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood 2017;129:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daher R, Kannengiesser C, Houamel D, Lefebvre T, Bardou-Jacquet E, Ducrot N, de Kerguenec C, et al. Heterozygous Mutations in BMP6 Pro-peptide Lead to Inappropriate Hepcidin Synthesis and Moderate Iron Overload in Humans. Gastroenterology 2016;150:672–683 e674. [DOI] [PubMed] [Google Scholar]
- 19.McDonald CJ, Rishi G, Wallace DF, Subramaniam VN. Genetic Variants in the BMP6 Pro-Peptide May Not Cause Iron Loading and Should Be Interpreted With Caution. Gastroenterology 2016;151:770–771. [DOI] [PubMed] [Google Scholar]
- 20.Wang CY, Canali S, Bayer A, Dev S, Agarwal A, Babitt JL. Iron, erythropoietin, and inflammation regulate hepcidin in Bmp2-deficient mice, but serum iron fails to induce hepcidin in Bmp6-deficient mice. Am J Hematol 2019;94:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milet J, Dehais V, Bourgain C, Jouanolle AM, Mosser A, Perrin M, Morcet J, et al. Common variants in the BMP2, BMP4, and HJV genes of the hepcidin regulation pathway modulate HFE hemochromatosis penetrance. Am J Hum Genet 2007;81:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 1996;13:399–408. [DOI] [PubMed] [Google Scholar]
- 23.Corradini E, Garuti C, Montosi G, Ventura P, Andriopoulos B Jr., Lin HY, Pietrangelo A, et al. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology 2009;137:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kautz L, Meynard D, Besson-Fournier C, Darnaud V, Al Saati T, Coppin H, Roth MP. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood 2009;114:2515–2520. [DOI] [PubMed] [Google Scholar]
- 25.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology 2009;50:1992–2000. [DOI] [PubMed] [Google Scholar]
- 26.Corradini E, Schmidt PJ, Meynard D, Garuti C, Montosi G, Chen S, Vukicevic S, et al. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology 2010;139:1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latour C, Besson-Fournier C, Meynard D, Silvestri L, Gourbeyre O, Aguilar-Martinez P, Schmidt PJ, et al. Differing impact of the deletion of hemochromatosis-associated molecules HFE and transferrin receptor-2 on the iron phenotype of mice lacking bone morphogenetic protein 6 or hemojuvelin. Hepatology 2016;63:126–137. [DOI] [PubMed] [Google Scholar]
- 28.Levy JE, Montross LK, Cohen DE, Fleming MD, Andrews NC. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood 1999;94:9–11. [PubMed] [Google Scholar]
- 29.Latour C, Kautz L, Besson-Fournier C, Island ML, Canonne-Hergaux F, Loreal O, Ganz T, et al. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 2014;59:683–694. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 1996;122:2977–2986. [DOI] [PubMed] [Google Scholar]
- 31.Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, Ginzburg Y, Roth MP, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology 2011;53:1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolondi G, Garuti C, Corradini E, Zoller H, Vogel W, Finkenstedt A, Babitt JL, et al. Altered hepatic BMP signaling pathway in human HFE hemochromatosis. Blood Cells Mol Dis 2010;45:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan JD, Ryan E, Fabre A, Lawless MW, Crowe J. Defective bone morphogenic protein signaling underlies hepcidin deficiency in HFE hereditary hemochromatosis. Hepatology 2010;52:1266–1273. [DOI] [PubMed] [Google Scholar]
- 34.Latour C, Besson-Fournier C, Gourbeyre O, Meynard D, Roth MP, Coppin H. Deletion of BMP6 worsens the phenotype of HJV-deficient mice and attenuates hepcidin levels reached after LPS challenge. Blood 2017;130:2339–2343. [DOI] [PubMed] [Google Scholar]
- 35.Wu XG, Wang Y, Wu Q, Cheng WH, Liu W, Zhao Y, Mayeur C, et al. HFE interacts with the BMP type I receptor ALK3 to regulate hepcidin expression. Blood 2014;124:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol 2012;57:1052–1060. [DOI] [PubMed] [Google Scholar]
- 37.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 2009;11:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isaacs MJ, Kawakami Y, Allendorph GP, Yoon BH, Izpisua Belmonte JC, Choe S. Bone morphogenetic protein-2 and −6 heterodimer illustrates the nature of ligand-receptor assembly. Mol Endocrinol 2010;24:1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 2006;38:531–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.