Abstract
The use of zebrafish (Danio rerio) in biomedical research has expanded at a tremendous rate over the last two decades. Along with increases in laboratories using this model, we are discovering new and important diseases. We review here the important pathogens and diseases based on some 20 years of research and findings from our diagnostic service at the NIH-funded Zebrafish International Resource Center. Descriptions of the present status of biosecurity programs, diagnostic and treatment approaches are included. The most common and important diseases and pathogens are two parasites, Pseudoloma neurophilia and Pseudocapillaria tomentosa, and mycobacteriosis caused by Mycobacterium chelonae, M. marinum and M. haemophilum. Less common but deadly diseases are caused by Edwardsiella ictaluri and Infectious Spleen and Kidney Necrosis Virus (ISKNV). Hepatic megalocytosis and egg associated inflammation and fibroplasia are common, apparently non-infectious, in zebrafish laboratories. Water quality diseases include supersaturation and nephrocalcinosis. Common neoplasms are spindle cell sarcomas, ultimobranchial tumors, spermatocytic seminomas, and a small cell carcinoma that is caused by a transmissible agent. Despite the clear biosecurity risk, researchers continue to use fish from pet stores, and here we document two novel coccidia associated with significant lesions in zebrafish from one of these stores.
Keywords: Danio rerio, fish diseases, biosecurity, treatment, diagnostics
Introduction
The benefit of zebrafish (Danio rerio), as part of an integrative approach to improving human health is being realized by the scientific community (Allen & Neely, 2010; Phillips & Westerfield, 2014). The NIH-funded Zebrafish International Resource Center (ZIRC) (https://zebrafish.org/home/guide.php) was established in the late 1990s as a genetic stock center for the maintenance and distribution of mutant and wild-type zebrafish lines (Grunwald & Eisen, 2002). As an important part of its mission to support the development of new zebrafish models, the Zebrafish Health Service within ZIRC was developed to provide expert diagnostic pathology services and consultation to the zebrafish research community. The Zebrafish Information Network (ZFIN), established around the same time as the ZIRC, provides online resources for data and information about the zebrafish genetics, publications, and laboratories. The ZFIN web site (http://zfin.org) now lists over 1,300 laboratories that employ zebrafish. Now concurrent with the expansion of the field, there are other stock centers, such as the European Zebrafish Resource Center (EZRC) (www.ezrc.kit.edu).
The field was initially led by investigations in developmental genetics in which experimental endpoints involved primarily embryos or larval fish (Eisen, 2020). Adult zebrafish are now used extensively as models throughout the biomedical research sector including areas as varied as infectious disease susceptibility and immune system function, aging, toxicology, oncology, and behavior (Aleström, Holter, & Nourizadeh-Lillabadi, 2006; Lin, Chiang, & Tsai, 2016; Naert & Vleminckx, 2018; Santoriello & Zon, 2012). The zebrafish is being used as a model for cancer (Liu & Leach, 2011; Yen, White, & Stemple, 2014), with studies involving transplantation of human tumors into immune compromised zebrafish (Taylor & Zon, 2009; Veinotte, Dellaire, & Berman, 2014; Yan et al., 2019).
Zebrafish are typically maintained at 28 °C in the laboratory, but zebrafish in their natural habitats, in India, often occur in much warmer waters (Spence et al. 2008). Hence, they can be adapted to higher temperatures. Cancer xenograft studies often include adapting the fish water temperatures of 35–37 °C, allowing for a more appropriate environment for the growth of tumors of human origin (Cabezas-Sainz et al., 2018; Moore & Langenau, 2016; Wertman, Veinotte, Dellaire, & Berman, 2016). Regarding infectious diseases, some 30 different bacterial pathogens have been studied using the zebrafish model (Meijer & Spaink, 2011; Torraca, Masud, Spaink, & Meijer, 2014; Torraca & Mostowy, 2018). The zebrafish is also being used as a model for viral and parasitic diseases, including those that are that infect humans (Crim & Riley, 2012; Gratacap & Wheeler, 2014). Holding zebrafish at elevated temperatures enables them to be infected with human pathogens (Brudal et al., 2014; Meijer, 2016; Stones et al., 2017; Tenor, Oehlers, Yang, Tobin, & Perfect, 2015). For example, we have demonstrated that zebrafish adapted to 37 °C can be experimentally infected with Toxoplasma gondii (Sanders et al., 2015); an important pathogen of humans and other mammals worldwide.
For the last two decades we have been investigating diseases in zebrafish in research facilities, with support from the NIH Office of Research Infrastructure Programs. This research also includes investigating modes of transmission and sources of pathogens, and methods for detection and control. Even subclinical diseases have the potential to impact research endpoints by causing non-protocol induced variations in live animal studies (Kent, Harper, & Wolf, 2012). Zebrafish that are used in research are maintained in scientific institutions using pathogen free water, but it is remarkable how prevalent pathogens are in these fish (Table 1). This is probably due in part to the fact that most researchers using the model are not trained in veterinary medicine or infectious diseases, and hence are not fully aware of the potential impact that underlying disease may have on their research (Kent et al., 2012). Moreover, in contrast to other aquaculture species, it has only been very recent that researchers obtain their stocks from suppliers that document the health and pathogen status of their fish. Indeed, many researchers still obtain zebrafish directly from ornamental fish retailers.
Table 1.
Important infectious diseases of zebrafish. Facilities: mean annual prevalence in facilitates based on data from 2006–2018 (752 cases involving 10,121 fish). Transmission: H=horizontal, V = vertical or maternal. ND = not determined as diagnosis for mycobacterial infections were usually at genus level.
| Pathogen or Disease | Facilities (%) | Pathology | Severity | Chronicity | Transmission | Impact |
|---|---|---|---|---|---|---|
| Pseudoloma | 45 | Neuritis, encephalitis, myositis, emaciation | Few within clinical disease | Chronic | V, H | Behavior, fecundity |
| Mycobacteriosis | 37 | Granulomas in various organs | Severe to subclinical: Depends on species | Subacute to chronic, Depends on species | V?, H | See below |
| M. chelonae | ND | Granulomas in coelomic organs | Often subclinical | Chronic | H | Cytokine expression |
| M. marinum | ND | Massive granulomatous inflammation in various organs | Severe | Subacute | H | High mortality, zoonotic |
| M. haemophilium | ND | Similar to M. marinum, with numerous bacteria in tissues | Severe | Chronic | H | High mortality over several months |
| Intestinal Carcinomas | 16 | Small cell carcinomas, many with epithelial hyperplasia & dysplasia | Moderate, | Chronic | H | Many with subclinical changes, compromise cancer and microbiome studies |
| Pseudocapillaria tomentosa | 10 | Emaciation. Severe enteritis | Severe | Chronic | H | Morbidity, microbiome alterations, promoter of intestinal cancer. Compromise diet, cancer and microbiome studies |
| Myxidium strasingeri | 22 | Minimal, parasite free in lumen of urinary tract | Minimal | Chronic | H, † | Probably minimal |
| Bacteria (non-acid fast) | 9 | Variable | Usually severe in a few fish | Acute | H | Unknown, fairly rare. |
| Edwardsiella ictaluri | < 1 | Severe, necrotizing, systemic | Severe | Acute | H | Very high mortality |
Likely involves an annelid alternate host.
With over 1,000 zebrafish research laboratories around the world, zebrafish are maintained in labs as small as a few tanks in an individual researcher’s laboratory to large multi-user core facilities with thousands of aquaria (Aleström et al., 2019; Hammer, 2020) The typical set up is comprised of multiple small aquaria on a recirculating system from commercial “racks” with about 30 tanks to large systems supporting hundreds of tanks. Regardless of size, the general design is recirculating effluent water through mechanical and biological filtration, followed by ultraviolet sterilization. Make-up water is usually provided by reverse osmosis water in which salts are added to achieve a conductivity of 250 to 1,700 microsiemens (Harper & Lawrence, 2011; Martins et al., 2016).
Here we present a review on the present status of zebrafish health, common diseases and pathogens, common diagnostic and biosecurity approaches, and methods used to control and prevent diseases in the zebrafish laboratory.
Biosecurity and Health Monitoring
The zebrafish research field was well established long before the implementation of routine diagnostics, health monitoring, sentinel programs, and other significant biosecurity measures. For example, Lawrence et al. (2012) reported that at that time only 5% of laboratories submitted fish for diagnostics. In 2012, ZIRC was the only major diagnostic laboratory available for zebrafish (https://zebrafish.org). Today, other commercial diagnostic laboratories offer zebrafish specific diagnostics (e.g. IDEXX – https://idexx.com). Diagnostic laboratories within universities, particularly veterinary diagnostic laboratories that include aquatics, are now also providing diagnostic services to zebrafish research programs. At the same time, institutional regulators are more frequently requiring health reports when zebrafish are imported into a research laboratory or core facility.
Sources of Zebrafish
There are no commercial zebrafish suppliers for research similar to those for mice (e.g., Jackson Laboratory, Bar Harbor, ME and Charles River Laboratories, Boston, MA). The ZIRC provides well defined genetic lines and health reports to the research community (https://zebrafish.org), and their genetic stocks have been distributed among more than 100 laboratories in 28 countries. For example, in 2017, the ZIRC shipped over 230,000 fish to the research community. In Europe, EZRC has some 30,000 mutations available. The Sinnhuber Aquatic Resource Center (SARL) (https://ehsc.oregonstate.edu/sarl) at Oregon State University was developed over 10 years ago as a facility that was SPF for Pseudoloma neurophilia (Kent et al., 2011), and they provide wild type and some mutant lines that are SPF for this microsporidium and many other pathogens. In addition, ad hoc distribution of zebrafish amongst researchers is still a major source of fish. For example, in 2018, Children’s Hospital Boston sent over 35 shipments of live adult and larval zebrafish to laboratories in 20 states in the United States and five different countries (C. Lawrence, pers. comm. 24 Oct 2019).
The vast majority of laboratories that conduct research using mice use Specific Pathogen Free (SPF) animals from reputable suppliers, and fish used in aquaculture usually come from suppliers that provide comprehensive health histories. The only significant pathogens recorded at ZIRC have been M. chelonae and P. neurophilia (Murray, Varga, & Kent, 2016). Through a strict rederivation and quarantine program, the SARL facility has remained free of the microsporidium as well as all other major zebrafish pathogens except M. chelonae (Barton, Johnson, & Tanguay, 2016; Kent et al., 2011).
Pet store fish
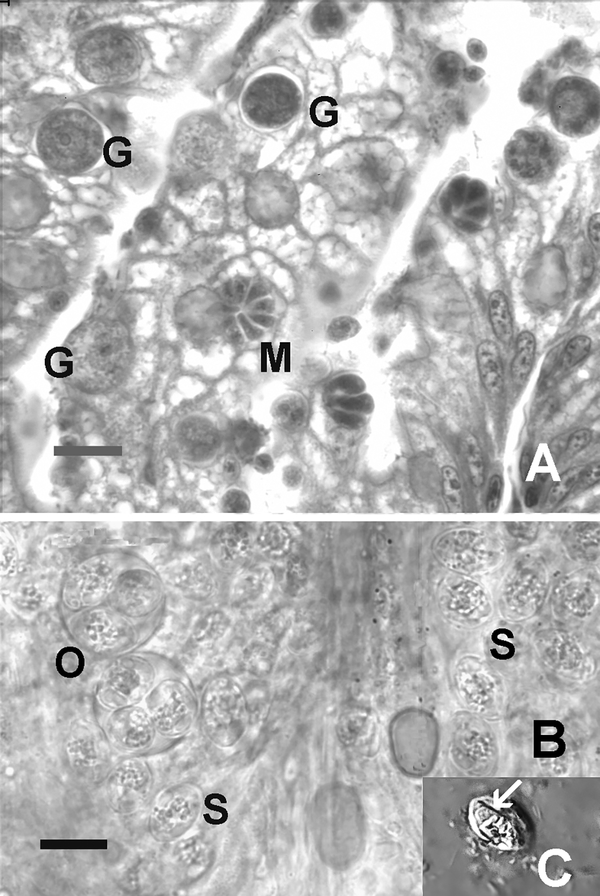
Many zebrafish researchers still acquire fish from laboratories with no or minimal health histories. Moreover, some still use pet stores as a source of their fish. For example, a review of 559 papers on behavioral studies with adult zebrafish showed the following regarding the source of the fish: 338 used named academic sources, 7 used scientific commercial sources, 214 obtained fish from wholesalers that supply fish to pet fish stores, 58 identified their source as local pet shops, and 123 did not cite their source. One of us (S. Spagnoli) is conducting an ongoing survey of zebrafish from pet stores and to date we are finding the typical pathogens found in research facilities (e.g., P. neurophilia and P. tomentosa), as well as, a higher prevalence of encysted trematode metacercariae. However, there are other important pathogens lurking in pet store fish that are deadly to zebrafish (e.g. Edwardsiella ictuluri and infectious spleen and kidney necrosis virus [ISKNV]) and there are undoubtedly many other microorganisms in the vast number of pet fish species that would be pathogenic to zebrafish. For example, with only examining approximately 100 zebrafish from pet stores, we discovered two novel coccidia, both associated with severe lesions in zebrafish (Figure 1). These pose a significant threat to zebrafish laboratories as oocysts are the source of the infection; this stage is very stable in the water environment and would likely be resistant to chlorine, as applied for disinfecting zebrafish embryos. Lastly, in recent years, researchers have been more frequently importing wild zebrafish from India. A few parasites have been documented in zebrafish from India (Smith et al., 2011) and certainly they carry many other undescribed microorganisms, some of which could be serious pathogens in captive zebrafish.
Figure 1.
Novel coccidian in zebrafish from a pet store. Bars = 10 μm A) Histological section of intestine showing various developmental stages in the epithelium. M = meront, G = gamont. Hematoxylin and eosin. B) Wet mount preparation (bright field) of coccidia in coelomic cavity. O = sporulated oocysts with four sporocysts. Numerous free sporocysts (S) are present. C) Sporocysts with suture (arrow), consistent with Goussia spp. Nomarksi phase interference.
Biosecurity Approaches
Similar to other forms of aquaculture, most large zebrafish facilities only introduce new lines of fish as “eggs only”, in which fish are bred in a quarantine facility, embryos disinfected with chlorine (Harper & Lawrence, 2011). Iodine, which is typically used in for disinfecting eggs with finfish aquaculture, is now beginning to be used instead of chlorine (Chang et al., 2015) or in conjunction with chlorine (Melancon et al., 2017). These sterilized embryos are then introduced into the new facilities. Simple mechanical rinsing of embryos was shown to eliminate detectable pathogens in embryos from parents that were infected with a variety of zebrafish pathogens (Crim et al., 2017). The ZIRC has a more involved process (Murray et al., 2016), in which adult fish are screened and most lines are cryopreserved. For mutant and transgenic lines, adult males are imported. After sufficient sperm samples have been cryopreserved, the males are euthanized, fixed, and processed into paraffin blocks. Thawed sperm is used in in vitro fertilization to fulfill customer orders. When sperm samples become depleted, the line must be regenerated at ZIRC and reared to an age when sperm samples can be reamplified. Before the line is regenerated, histological sections are cut from the paraffin blocks of all males contributing to sperm samples. Histopathology results are used to assign a disease status that determines whether the line can be reared in the main fish room or, if virulent pathogens are identified or suspected, in the quarantine room. For importation of wild-type lines, males and females must be imported and live spawned in the quarantine room. In this case, embryos are surface-sanitized with bleach and held in quarantine pending histopathology on all spawned adults. Diagnostic results on adults again determine relative risk of pathogen contamination of the progeny and whether progeny should be reared in the main fish room or the quarantine room. Wild-type lines are imported when they can be the only fish in the quarantine room, so the room can be disinfected before and after their arrival.
The SARL has an even more stringent protocol because, compared to ZIRC, it less frequently introduces new lines and is SPF for all the zebrafish pathogens except M. chelonae. See Kent et al. (2011) and Barton et al. (2016) for details of their protocol, but in brief new lines must pass through a 3 tiered system consisting of red, yellow, and green zones. Red zone: fish are imported from suppliers with health records and observed for clinical signs. Fish are spawned, surface sanitized with bleach and F1s are held in isolation in a separate rack (yellow zone). The appropriate number of fry are tested by PCR for P. neurophilia so that the pathogen would be detected at > 1% prevalence (with 95% probability of detection). If they test negative, fry are reared to maturity in the green zone, spawned and tested as above. The F2 generation, if they test negative, is moved into the main facility. As an added precaution, unlike most other zebrafish facilities, no live feeds are used at SARL. Unless necessary for the study, fish are retired between 1–1.5 years of age to mitigate increases in M. chelonae infections, as this infection increases with age (Kent et al., 2011). Both SARL and ZIRC also employ general biosecurity methods typically used in aquaculture, including one way policies for staff movement (i.e. not entering the main facility after working in quarantine), staff education on biosecurity, use of personal protection equipment, etc. Both facilities restrict movement of visitors and staff – e.g. at SARL they must wait 24 hours from last contact with aquatic animals to enter the facility, including the SARL quarantine.
Along with artificial diets, most facilities use live feeds, such as rotifers, paramecia, and brine shrimp. All of these may be vectors for pathogens that they ingest and concentrate (Peterson et al. 2013; Chang, Benedict & Whipps 2019). Mason et al. (2016) detected Mycobacterium spp. in rotifers in their laboratory, and we have detected Mycobacterium haemophilum in a rotifers destine for use as feed at ZIRC. The SARL uses artificial diets for the entire rearing cycle to avoid this biosecurity risk (Barton et al. 2016).
In spite of stringent biosecurity protocols, M. chelonae is detected in fish at both ZIRC and SARL (Whipps et al. 2008; Kent et al. 2011). This is an indication of the difficulty in eliminating bacterial species that are ubiquitous in the environment, persisting in fish tissue and biofilms. In both facilities, M. chelonae infections are typically subclinical, diagnosed post-mortem in asymptomatic fish. In addition, a low prevalence of vertical transmission of Mycobacterium spp. infection, which would evade surface-disinfection of embryos, has not been ruled out.
Monitoring strategies
Recommendations and strategies for disease control in zebrafish laboratories have been developed and implemented in several laboratories and programs (Kent et al., 2009; Barton et al., 2016; Murray, Varga, & Kent, 2016; Borges et al., 2016; Collymore, Crim, & Lieggi, 2016; Martins et al., 2016; Mason et al., 2016; Mocho, 2016). These programs vary depending on the configuration of laboratories, pathogen status, and needs of investigators. In general, these programs include directed evaluation of moribund fish and inclusion of sentinel programs in which fish are maintained in tanks receiving water directly from effluent of tanks from the main facilities. In recirculating systems, fish will often be inadvertently transported into sumps, either by escaped adults or from fry that are washed out of the tanks and then grow up in sumps. These also provide a useful opportunistic source of sentinel fish. With large populations, it may be appropriate to also include evaluation of apparently healthy fish from large populations of fish within the main facility. Lastly, screening of incoming fish within quarantine strategies should also be included. Specific pathogens and diagnostic methods are discussed below. Whereas PCR methods are very sensitive and specific, we recommend including histopathology as part of a routine monitoring program as this provides a broad overview of the health of the animal without a priori selection of pathogens and is useful for detection of non-infectious diseases.
Overview of Diseases
Tables 1 and 2 summarize the common infectious, non-infectious, neoplastic, and idiopathic diseases of zebrafish, with prevalence and distribution based largely on findings of the ZIRC diagnostic service. This resource center was established in 2000 and since then we have examined over 15,000 fish, representing over 1,300 cases from approximately 370 research laboratories. The tables summarize these results from these diagnostic findings. They do not describe every disease, pathogen or lesion that been documented, but rather only those that we designate as “important” because they are either common or because they have been shown to be deadly to zebrafish in research laboratories. More detailed reviews of diseases in zebrafish can be found in special journal issues (ILAR Journal, Vol. 53, 2012; Zebrafish, Vol. 13, 2016). Moreover, a book “The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases and Research Applications” was recently published in early 2020. This is part of a book series of the American College Laboratory Animal Medicine, published by Elsevier, which includes in-depth chapters on infectious diseases, water quality and idiopathic diseases, neoplasia, and diagnostic methods,
Table 2.
Important and common noninfectious diseases, including neoplasms of zebrafish. Facilities: mean annual prevalence in facilitates based on data from 2006–2018 (752 cases involving 10,121 fish). ND = not determined.
| Lesion or Disease | Facilities (%) | Pathology | Clinical Signs | Causes | Impact |
|---|---|---|---|---|---|
| Gill lesions | 23 | Epithelial hypertrophy and hyperplasia. Fusion of secondary lamellae in severe cases | Respiratory distress, may only occur when stressed | Poor water quality (ammonia, nitrite, nitrates). Occasionally bacteria present, but generally secondary to poor water quality | Respiratory distress and mortality if severe. Ammonia toxicity associated with immune suppression, decreased growth, and mortality |
| Egg-associated inflammation | 47 | Degenerating egg material and inflammation in the ovary | Usually subclinical, distended abdomen, rarely external ulcers | Unknown, regular spawning recommended as possible prevention | Severe lesions likely reduce egg production and fecundity |
| Nephrocalcinosis | 21 | Dilation of renal ducts and tubules with calcium deposits | Subclinical | High dissolved CO2, low O2,↓ Mg, ↑ Se and As | Usually subclinical, diagnosed post-mortem. Impaired renal function and growth noted in other fish. |
| Spinal deformity | 18 | Various spinal abnormalities (malformation, dislocation, fracture, dysplasia, neoplasia, degeneration) | Kyphosis, lordosis, scoliosis, platyspondyly | Environmental, genetic, dietary, toxins, infectious agents | May alter swimming behavior |
| Operculum malformation | ND | Outward or inward curling and shortening of opercular flap(s) | Operculum fails to completely cover gill cavity and gills are visible | Unknown, postulate genetic, dietary, and environmental factors | May decrease growth rate and impair ability to cope with poor water quality and respiratory challenge |
| Supersaturation | ND | Gas emboli in tissues may be visible grossly or in histological sections | Highly variable; bubbles in tissues (esp. eyes and fins), exophthalmia, altered buoyancy, lethargy, ↑ respirations at bottom of tank, mortality | Exposure to supersaturated water | ↓ growth, secondary infections, mortality |
| Hepatic megalocytosis | 26 | Greatly enlarged hepatocyte nuclei and cytoplasmic elements. Multi-nucleate hepatocytes may be present | Subclinical | Xenobiotics, toxins, idiopathic | Effects on hepatic physiology unknown |
| Biliary and pancreatic duct proliferative lesions | 21 | Ductal hyperplasia, dysplasia, and neoplasia | Subclinical | Unknown, higher prevalence of bile duct hyperplasia in TL line | Unknown |
| Seminoma | 20 | 1)Benign spermatocytic or hyperplasia (most common) 2) Mostly earlier sperm developmental stages (oocytes may be present) 3) Dysgerminomas (aggressive and infiltrative) |
The most common type of seminoma is typically subclinical, but may result in abdominal distention | Possible hormonal influence. Have been associated with carcinogen exposure | Unknown, space occupying changes |
| Ultimobranchial tumors | 9 | Hyperplasia, adenoma, and adenocarcinoma | Subclinical | Unknown. Theoretically, electrolyte and osmotic imbalances could stimulate hyperplasia | Unknown |
| Chordoma | 2 | Tumors of notochord remnants typically occur in intestine, less commonly in axial skeleton | Subclinical | Unknown | Tumors in intestine could disrupt digestion. Spinal deformity possible with axial skeleton tumors. |
| Peripheral nerve sheath tumors | 8 | Soft tissue sarcoma; storiform pattern, bundles and streams of spindle cells | Varies with location of tumor; swelling, emaciation, exophthalmia, lethargy | Unknown | Impact may vary with location of tumor |
| Lymphosarcoma | 4 | Monomorphic, round, basophilic cells invading tissues, often in epithelial tissues (skin, mouth, and gills) | Emaciation, skin pallor, “lionhead”, edema, exophthalmia | Unknown. | General declining health |
| Thyroid tumors | 2† | Hyperplasia (simple, ectopic, and nodular), adenoma, and adenocarcinoma distinguished by cellular atypia, mitotic index, and aggressive growth | Red masses between ventral mandibles and in ectopic locations | Iodine deficiency can instigate hyperplastic growth | Thyroid hormone can affect sex ratios of progeny. Unknown if thyroid tumors skew hormone levels enough to have this effect. |
This number increases to 3% when considering additional facilities with reports of thyroid hyperplasia and suspect thyroid adenoma.
1. Pseudoloma neurophilia
This microsporidium is remarkably common in zebrafish facilities and has occurred in about half of zebrafish research facilities, based on the ZIRC diagnostic cases. The widespread distribution can be explained by the historical sharing of zebrafish between facilities and the infectious spore stage is resistant to levels of chlorine used to surface-sanitize embryos (Ferguson, Watral, Schwindt, & Kent, 2007). Moreover, the parasite is vertically transmitted within eggs (Sanders, Watral, Clarkson, & Kent, 2013). Infected fish may be emaciated, but most are clinically normal. As the species name denotes, it infects the central nervous system. Consistent with its location of infection, the parasite has been associated with behavior changes; hence the potential for non-protocol induced variation in behavior research (Spagnoli, Sanders, & Kent, 2017; Spagnoli, Xue, & Kent, 2015).
2. Mycobacteriosis
Mycobacterial infections are common in zebrafish (Table 1) where infections are usually diagnosed to the genus level using acid fast stains in tissue sections. Most infections are caused by M. chelonae (Whipps, Matthews, & Kent, 2008; Whipps & Kent, 2020). Infected fish often appear normal, even when multiple granulomas and swim bladder infections are present. Mycobacterium haemophilum and Mycobacterium marinum infections, while less common than M. chelonae, cause high mortalities (Whipps, Lieggi, & Wagner, 2012). The latter has also been reported to cause hand infections in staff working with zebrafish (Mason et al., 2016). Although both bacteria ultimately cause high mortality, the course of the infection is more rapid with M. marinum - i.e., with M. haemophilum, mortality may continue for months and ultimately result in large numbers of moribund or dead fish (Whipps et al., 2007)
3. Pseudocapillaria tomentosa
It may be surprising that this parasitic nematode is rather common in zebrafish (Kent & Sanders, 2020) (Table 1) considering the laboratory environment in which zebrafish are maintained. However, this could be explained by the lack of biosecurity in many facilities, including sourcing fish from pet stores, and the resistance of nematodes to chlorine (Martins, Watral, Rodrigues-Soares, & Kent, 2017). The parasite induces a chronic disease, often with prominent emaciation and high mortality. Histological examination of infected fish reveals profound chronic enteritis and occasionally coelomitis (Kent, Bishop-Stewart, Matthews, & Spitsbergen, 2002; Murray & Peterson, 2015). Epithelial hyperplasia and dysplasia is also a common finding, which extends as far as a field change past the site of infection. As with some other parasites that cause chronic inflammation, infections by P. tomentosa predisposes fish to intestinal neoplasia (Gaulke et al., 2019; Kent et al., 2002). Zebrafish are now being used extensively in microbiome research and we found profound differences in microbiome profiles in zebrafish with the infection (Gaulke et al., 2019).
Antiparasitic drugs typically used for nematodes, such as macrocyclic lactones, are effective for treating the infection. Emamectin, the ivermectin analog used for treating sea lice, is effective as both an oral or bath treatment (Kent, Watral, Gaulke, & Sharpton, 2019). Eggs in the environment are killed by high levels of chlorine, iodine, UV, desiccation, and heat (Kent, Watral, Villegas, & Gaulke, 2019). Eggs of P. tomentosa do not survive freezing, neither by cryopreservation typically used for preserving zebrafish sperm nor by freezing without cryopreservant (Norris, Watral, & Kent, 2018).
4. Mycoplasma and Transmissible Intestinal Tumors
Whereas P. tomentosa is a promoter of intestinal cancers, the tumors often occur in facilities with no history of the nematode (Paquette et al., 2013). Indeed, our recent review of the ZIRC diagnostic case database revealed that the worm was present in only about one fourth of the facilities or cases in which intestinal neoplasia was diagnosed. Burns et al. (2018) showed that some intestinal carcinomas are naturally transmitted by either co-habitation with or exposure to effluent from affected fish. They also correlated neoplasms with the presence of a specific mycoplasma’s 16s rDNA sequence that was close to M. penetrans. We have been maintaining the disease in zebrafish in our laboratory using this method for the last 6 years in the absence of the worm and are presently at the 15th passage.
5. Edwardsiella ictaluri
Whereas opportunistic Gram-negative bacteria have been reported to cause disease in zebrafish, E. ictaluri is clearly a deadly pathogen in zebrafish (Hawke et al., 2013). Fortunately, the infection is usually seen in fish in quarantine and rarely has be transported into main research facilities. The isolates from zebrafish are genetically distinct from those infecting catfish and tilapia (Griffin et al., 2016). The bacterium has been reported in aquarium fishes such as the green knifefish (Eigemmania virescens) (Valenciennes, 1836) (Kent & Lyons, 1982), Danio devario (Hamiton-Buchanan, 1822) (Blazer, Shotts, & Waltman, 1985), and rosy barb (Puntius conchonius) (Humphrey, Lancaster, Gudkovs, & McDonald, 1986). The bacterium continues to cause outbreaks in barbs and danios, including zebrafish, in tropical fish farms. The bacterium is deadly to a variety of Danio spp. and has destroyed upwards of 20,000 to 40,000 fish. Even though antibiotic susceptibility showed sensitivity to a number of antibiotics in vitro, neither bath nor oral treatments were very effective, regardless of the therapy. There has been a vaccination program initiated with AquaTactics® against the bacterium, called ESZ, to reduce the infection in zebrafish in wholesale production (J. Hawke, Louisiana State University, Baton Rouge, LA, 27 August 2019).
6. Viral Diseases
Viral diseases frequently emerge in fish species concurrent to their development in aquaculture industries. Likewise, only recently have two naturally occurring viral infections been recognized to cause disease in zebrafish. Red-spotted grouper nervous necrosis virus (RGNNV), a Betanodavirus, generally called Viral Nervous Necrosis (VNN), was the first naturally-occurring viral infection reported for zebrafish (Binesh, 2013). More recently, Infectious Spleen and Kidney Necrosis Virus (ISKNV) was documented in a zebrafish facility in Spain (Bermúdez et al., 2018). The latter is of particular concern as it has broad host specificity, is very common in aquarium fishes (Rimmer et al., 2015), and is lethal to zebrafish (Crim & Riley, 2012).
7. Orphan Viruses and Opportunistic Bacteria
There are many “orphan” viruses that have been documented that are common in finfishes – i.e., viruses that replicate in fish and fish cell lines, but cause no or minor at most pathological changes. For example, in a review by Hetrick and Hedrick (1993) we see that of the 17 aquareoviruses that they list, only 4 cause disease. With molecular techniques, many non-pathogenic viruses or virus sequences have been found without culture. For example, countless endogenous retroviruses sequences that do not develop into pathogenic viruses have been found in many animals, including zebrafish (Crim, 2020). Shi et al. (2018) in one study alone expanded the list of fish RNA viruses from about 50 to 150 by obtaining sequences from fish. Pertaining to zebrafish, metagenomic analysis identified a novel picornavirus was found in the intestines of zebrafish from 23 out of 41 facilities examined (Altan et al., 2019), but the virus was not associated with specific lesions or disease.
Likewise, genera of bacteria with members that are pathogenic are often isolated from the intestines of live zebrafish, tissues of dead fish, or from their environments. This includes members of the genera Pseudomonas, Aeromonas, Vibrio, Mycoplasma and Mycobacterium (Gaulke et al., 2016, 2019; Legendre et al., 2016; Pullium, Dillehay, & Webb, 1999; Roeselers et al., 2011). These strains or species should not be considered pathogenic without other supporting evidence, such as connecting infections to lesions consistent with bacterial infections (Pullium et al., 1999). We often conduct microbiologic and histopathologic evaluations on the same individual fish, or at least the same fish from the population, to correlate putative pathogens with specific lesions (Kent et al., 2020). Many laboratories are now conducting research using zebrafish that are severely immunosuppressed by either mutations or gamma irradiation obliterating immune cells (Moore & Langenau, 2016). There are now immune-compromised lines equivalent to SCID mice and these fish have no functional B or T cells (Jung et al., 2016). Undoubtedly, these fish would be more susceptible to common opportunistic bacteria found in zebrafish systems.
8. Water Quality Problems
Zebrafish are subject to most of the same water quality concerns as other fishes reared in intensive recirculating systems, including gas super saturation, metal toxicities in new systems, ammonia, and nitrite toxicity in crowded systems or when biological filters fail (Murray, Lains, & Spagnoli, 2020) (Table 2). Gill epithelial hypertrophy and hyperplasia is not an uncommon finding in routine screening of sump or sentinel zebrafish. This lesion is typically associated with suboptimal water or environmental parameters, which may include elevated ammonia and nitrite. Occasionally mats of bacteria are associated with hyperplastic gill lesions, although this is also most often secondary to poor water quality and not a primary bacterial problem.
Gas super saturation is particularly problematic (Table 2) and is often insidious because we have seen several cases in which fish do not show obvious gas bubbles in the gills or skin. Adult zebrafish may hover at the bottom of a tank and respire rapidly in well-oxygenated water. Typically gas supersaturation is often described as occurring as a result of a leak on the suction side of a centrifugal pump or when cold water is pumped from underground and warmed under pressure. We have also seen gas bubble disease result from air being trapped in pressurized plumbing that feeds a rack that is out of use for some time period. We observed gas bubble disease when in a system without pumps. In this situation, high water usage dropped the water level in a sump and air became entrained at the surface of a gravity-fed pipe that dropped approximately 4 meters.
Another disease of water quality that is relatively common in laboratory zebrafish is nephrocalcinosis. Calcium deposits in renal tubules and ducts may occur secondary to high dissolved CO2. We see a range of nephrocalcinosis lesions in zebrafish by histology, most of which are diagnosed in clinically normal fish. The potential impact on growth and kidney function in zebrafish have not been reported. Calcium deposits in renal tubules and ducts may occur secondary to high dissolved CO2, and hence stocking density should be evaluated as crowding can increase CO2. However, the lesions may also reflect altered renal physiology that is secondary to systemic disease.
9. Idiopathic Diseases
In addition to neoplasms, a few idiopathic diseases or lesions are somewhat common (Matthews, 2009; Murray et al., 2020). Hepatic megalocytosis is frequently observed in fish of various ages, but is often not associated with clinical disease. At present the causes are unknown and could range from anthropogenic chemicals in new systems, diet, or toxins from natural organisms such as algae, cyanobacteria, and fungi. A natural source should be considered given that the latter two taxa produce toxins (microcystin and aflatoxin, respectively) that have been shown to cause this lesion in fish (Andersen et al., 1993; Hendricks, 1994). Egg retention and atresia, called Egg Associated Inflammation and Fibroplasia (EAIF) is common and here it is likely due to lapses in spawning in female fish as they typically spawn at least once a month. Hepatic megalocytosis and egg-associated inflammation are frequently identified in histological sections of clinically normal fish, and their unknown impact of organ physiology should be considered when interpreting research data where endpoints involve evaluation of liver and gonad anatomy and physiology.
Diagnostic Methods for Zebrafish
Histology
In the context of finfish aquaculture, study of the diseases of zebrafish in research facilities is relatively new. Once a particular species of fish is reared in captivity at a large scale, invariably novel pathogens and diseases are discovered. The same applies for zebrafish and hence at this time histopathology is usually the primary or first line diagnostic test. A major strength of histopathology is that it facilitates the documentation of changes in a variety of tissues and organs with no a priori assumptions relating to a specific disease. The small size of the fish allows for examination of essentially all organs on one slide using sagittal sections of a whole fish. In fact, essentially all the important diseases of laboratory zebrafish were first recognized in moribund fish within histological sections.
For adult fish, most diagnostic samples are preserved in either 10% buffered formalin or Dietrich’s solution and then fish are decalcified before processing. In our experience, we prefer the Dietrich’s solution for optimal cellular detail, but 10% formalin is satisfactory. Larval fish are often examined by histology and here other fixatives are often employed, such as paraformaldehyde (Copper et al., 2018). Immunohistochemistry has been utilized in zebrafish diagnostics (MacDonald, 1999; Meritet, Spagnoli, Fischer, & Löhr, 2019; Paquette et al., 2015). However, most antibodies were created for mammal antigens and thus results should be interpreted with caution. One advantage of whole fish sections is each section provides positive and negative control tissues for specific antibodies in study.
Molecular Detection Methods
The rapid growth of the zebrafish model can be attributed primarily to the availability of a variety of tools enabling the detection, characterization, and modification of molecules of DNA. It is therefore fitting that the key tools used to assess the health of zebrafish today would include the detection of pathogen DNA by PCR. Because the development of PCR in the mid-1980s, detection techniques based on this method have become a mainstay in the diagnostic laboratory. There are several reasons for this: increased sensitivity, the ability to quickly obtain results, amenability to high throughput sampling, and the ability to multiplex. In most diagnostic laboratories, cost is also a factor and PCR based methods tend to be the most cost-effective compared to more complex methods such as microbial culture and histology, which are relatively low-throughput, costly, and require interpretation of results by highly trained personnel.
The first PCR tests for zebrafish pathogens used conventional methods and included tests for Pseudoloma neurophilia (Whipps & Kent, 2006). These types of assays have been largely supplanted by the kinetic or quantitative PCR (qPCR) based methods and some veterinary diagnostic laboratories even offer specific PCR tests for zebrafish to the research community, including the Oregon Veterinary Diagnostic Laboratory (https://vetmed.oregonstate.edu/diagnostic) and IDEXX (https://www.idexxbioanalytics.com/zebrafish-health-monitoring). Digital droplet PCR (ddPCR) has shown to be a powerful tool for detecting DNA in a variety of water sources and we are adapting this platform for zebrafish pathogens. The two main benefits of ddPCR are that precise quantification of DNA in samples is not based on a standard curve and that it is independent of amplification efficiency. In addition, the partitioning of a PCR into tens of thousands of individual reactions dilutes the effects of inhibitors on the reaction (Demeke & Dobnik, 2018). This method is particularly suitable for environmental detection of DNA due to the highly variable concentrations of inhibitors found in these samples.
Disease Diagnostics vs Pathogen Screening
The assessment of environmental samples as a nonlethal proxy for screening for the presence of validated and potential pathogens in zebrafish colonies is attractive for a number of reasons; many laboratories have several distinct populations that came from multiple sources and small fish numbers comprised of unique and valuable lines or mutants. Lethal sampling of very small populations of fish to screen for pathogens that may be presence at low prevalence is often untenable in these situations. There are already several reports describing detection of most of the important zebrafish pathogens from environmental samples using PCR based methods (Crim et al., 2017; Mason et al., 2016; Mocho, 2016; Sanders et al., 2011; Whipps et al., 2007, 2008, 2012). Given the life cycle and its distinct eggs, P. tomentosa can be detected by microscopy in fish feces (Murray & Peterson, 2015) and filter sludge (Mocho, 2016).
As zebrafish facilities are highly controlled systems (e.g. source water is well-characterized) one would expect that the environmental sample matrix would be fairly consistent. However, water quality varies dramatically from facility to facility and even within tanks housed on the same system. Amount of water flow, stocking density, and feed components are just a few of the variables that can impact the quality of a water sample used for molecular detection of pathogens. The volume of water to sample and the method of sampling is also a very important factor with screening for pathogens in water.
Interpreting results from environmental screening can be fairly simple: if a recognized virulent zebrafish pathogen is present in any detectable amount, it is most likely present in fish in the facility. However, interpretation of results become complicated with common opportunistic pathogens present, including M. chelonae and others of the numerous environmental Mycobacterium species and Gram-negative bacteria (except E. ictaluri).
Susceptibility to disease by environmental bacteria is highly variable among fish species, and thus evidence of pathogenicity of a particular bacterium in one fish species does not necessarily translate to pathogenicity in zebrafish. Aeromonas spp., Pseudomonas spp., Vibrio spp. and enteric Gram-negative species have been reported from zebrafish facilities, from both fish and the environment (Borges et al., 2016; Marancik, Collins, Afema, & Lawrence, 2019).
Chandrarathna et al. (2018) described lesions consistent with bacterial septicemias in zebrafish infected with certain strains of Aeromonas hydrophilia and Aeromonas veronii. Many of these bacteria are ubiquitous in aquatic environments so connecting their presence to disease in a zebrafish colony requires further investigation, particularly in the absence of clinical signs and histological changes. False negative culture results may occur with inaccurate sampling of focal infections or with slow-growing and difficult to culture bacteria (such as Mycobacterium haemophilum and E. ictaluri). False positive cultures may occur with skin or gut contamination of samples, which is common with small fish, and in mixed bacterial infections where the easily cultured bacteria are not responsible for the observed fish morbidity and mortality. Hence, causality of an infectious disease requires: a) the presence of an infectious agent and b) evidence of pathological changes associated with that infectious agent in the host. Importantly, the potential contamination of external surfaces by an organism can make results difficult to interpret. Chandrarathna et al. (2018) was able to induce mortality with various isolates of Aeromonas spp. by exposing fish by intraperitoneal injection. In contrast, we failed to induce disease by injecting zebrafish with bacteria identified as Pleisomonas shigelloides and Burkholderia cepacia from moribund zebrafish, as well as, Aeromonas hydrophila from a moribund koi carp, Cyprinus carpio (Linnaeus, 1758) (unpublished data).
The use of qPCR-based assays to detect the presence of specific DNA in environmental samples (eDNA) has been used in natural systems for several years to quantify pathogens (Gomes et al., 2017; Peters et al., 2018) and locate endangered or introduced invasive fish species in rivers and lakes (Deiner et al., 2017; Valentini et al., 2016). Hence, identification of a specific pathogen can be achieved by screening fish by a sensitive, organism-specific PCR based assay, but these assays only detect the presence of DNA and not viable organisms. Given the concerns above, the detection of pathogens in a tank, biofilm, or entire aquatic life support system involves a number of variables that significantly impact the sensitivity and overall predictive value of the results obtained. The sampling, processing of samples collected, overall detection assay design, and interpretation of results require careful consideration and empirical data describing the environmental disposition for many of the pathogen and potential opportunistic pathogens of interest is often lacking. More broadly, health monitoring involves more than pathogen identification and the ability to identify microbes in the environment is only one component of an array of tools we use to evaluate overall fish health.
Histology with Microbiology
We often perform bacterial culture or PCR analysis and histopathology on the same fish to connect novel microorganisms with disease. This is particularly useful because of the risk of bacterial contamination of internal structures. Histology also provides useful information to discern if the bacteria actually represent an infection and is associated with a pathological change. For Gram-negative septicemia and aerocystitis (swim bladder infections), we usually use the dorsal approach to perform aseptic cultures of the kidney and swim bladder. For mycobacteria, we recommend removing a portion of the spleen and liver. These can then be examined by culture, acid-fast imprints, or preserved in ethanol or frozen for PCR analysis. The remaining carcasses are fixed in Dietrich’s fixative and processed for histological evaluation. We have found that both of the methods to obtain samples for bacteriology do not significantly compromise histological evaluations on the same fish.
Treatment
Compared to food fish aquaculture, there have been few treatment regimens developed for zebrafish. Various anthelmintics have been incorporated into the diet to treat P. tomentosa and we recently showed that bath exposure of emamectin benzoate delivered as a bath treatment was quite effective (Kent et al., 2019). Isolates of E. ictaluri have been found susceptible to Romet-30®, oxytetracycline, florfenicol, and enrofloxacin (Hawke et al., 2013), but most of these have yet to be tested in vivo. Recent in vivo studies showed that enrofloxacin was effective for treating infections in zebrafish (J. Hawke, personal communication, 13 July 2019). The Whipps laboratory (SUNY College of Environmental Science & Forestry, Syracuse, New York) is developing antibiotics, such as tigecycline and clarithromycin, for treating mycobacteriosis in zebrafish (Chang, Doerr, & Whipps, 2017). These may be useful in treating small populations of compromised or precious mutant or transgenic lines. The benefits of treatment will have to be weighed against the risk of developing antibiotic resistance and long-term exposure of lab personnel to zoonotic pathogens during treatment. Fumagillin is commonly used for treating microsporidiosis in fishes (Kent, Shaw, & Sanders, 2014), but this has yet to be tested with P. neurophilia.
Conclusion
Although zebrafish are afflicted by some unique diseases and pathogens, overall the types of infectious and non-infectious diseases are consistent with those found in other freshwater fishes reared in intensive, recirculating aquaculture. As study of diseases of zebrafish has only been significantly pursued for the last 20 years, it is likely that we will continue to identify more important diseases. Also, the continued use of pet stores as a source of research fish and the expansion of studies using wild or immune compromised zebrafish will continue to provide putatively novel diseases and causative agents in zebrafish research laboratories.
Acknowledgment
This research was funded in part by the NIH ORIP R24OD010998 to M.L.K. The Zebrafish International Resource Center, which includes both research and the diagnostic laboratory, is funded by NIH ORIP (award P40OD01 1021).
Footnotes
Data Availability Statement The authors elect not to share data due to the confidential nature of the ZIRC health service client data. There is a summary table, which includes some of the numbers indirectly used to derive the tables for this paper, available on the ZIRC Health Services website at [https://zebrafish.org/wiki/_media/health/submission/submissions_summary_2006-2018.pdf].
References
- Aleström P, D’Angelo L, Midtlyng PJ, Schorderet DF, Schulte-Merker S, Sohm F, & Warner S (2019). Zebrafish: Housing and husbandry recommendations. Laboratory animals, 0, 1–12. doi: 10.1177/0023677219869037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleström P, Holter JL, & Nourizadeh-Lillabadi R (2006). Zebrafish in functional genomics and aquatic biomedicine. Trends in biotechnology, 24, 15–21. doi: 10.1016/j.tibtech.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Allen JP, & Neely MN (2010). Trolling for the ideal model host: zebrafish take the bait. Future microbiology, 5, 563–569. doi: 10.2217/fmb.10.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan E, Kubiski SV, Boros A, Reuter G, Sadeghi M, Deng X, Creighton EK, Crim MJ, & Delwart E (2019). A highly divergent picornavirus infecting the gut epithelia of zebrafish (Danio rerio) in research institutions worldwide. Zebrafish, 16, 291–299. doi: 10.1089/zeb.2018.1710 [DOI] [PubMed] [Google Scholar]
- Andersen RJ, Luu HA, Chen DZ, Holmes CF, Kent ML, Le Blanc M, Taylor FJ, &Williams DE (1993). Chemical and biological evidence links microcystins to salmon “netpen liver disease” Toxicon, 31: 1315–1323. doi: 10.1016/0041-0101(93)90404-7 [DOI] [PubMed] [Google Scholar]
- Barton CL, Johnson EW, & Tanguay RL (2016). Facility design and health management program at the Sinnhuber Aquatic Research Laboratory. Zebrafish, 13, S39–S43. doi: 10.1089/zeb.2015.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez R, Losada AP, de Azevedo AM, Guerra-Varela J, Pérez-Fernández D, Sánchez L, Padrós F, Nowak B, & Quiroga MI (2018). First description of a natural infection with spleen and kidney necrosis virus in zebrafish. Journal of fish diseases, 41, 1283–1294.doi: 10.1111/jfd.12822 [DOI] [PubMed] [Google Scholar]
- Binesh CP (2013). Mortality due to viral nervous necrosis in zebrafish Danio rerio and goldfish Carassius auratus. Diseases of aquatic organisms, 104, 257–260. doi: 10.3354/dao02605 [DOI] [PubMed] [Google Scholar]
- Blazer VS, Shotts EB, Waltman WD (1985). Pathology associated with Edwardsiella ictaluri in catfish, Ictalurus punctatus Rafinesque, and Danio devario (Hamilton-Buchanan, 1822). Journal of fish biology, 27, 167–175. doi: 10.1111/j.1095-8649.1985.tb04018. [DOI] [Google Scholar]
- Borges AC, Pereira N, Franco M, Vale L, Pereira M, Cunha MV, … Rebelo M (2016). Implementation of a zebrafish health program in a research facility: a 4-year retrospective study. Zebrafish, 13, S115–S126. doi: 10.1089/zeb.2015.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudal E, Ulanova LS, O Lampe E, Rishovd AL, Griffiths G, & Winther-Larsen HC (2014).Establishment of three Francisella infections in zebrafish embryos at different temperatures. Infection and immunity, 82, 2180–2194. doi: 10.1128/IAI.00077-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AR, Watral V, Sichel S, Spagnoli S, Banse AV, Mittge E, … Kent ML (2018). Transmission of a common intestinal neoplasm in zebrafish by cohabitation. Journal of fish diseases, 41, 569–579. doi: 10.1111/jfd.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas-Sainz P, Guerra-Varela J, Carreira MJ, Mariscal J, Roel M, Rubiolo JA, Sánchez L (2018). Improving zebrafish embryo xenotransplantation conditions by increasing incubation temperature and establishing a proliferation index with ZFtool. BMC cancer, 18, 3. doi: 10.1186/s12885-017-3919-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrarathna H, Nikapitiya C, Dananjaya S, Wijerathne C, Wimalasena S, Kwun H, Jung, Heo G, Lee J, & De Zoysa M (2018). Outcome of co-infection with opportunistic and multidrug resistant Aeromonas hydrophila and A. veronii in zebrafish: identification, characterization, pathogenicity and immune responses. Fish & shellfish immunology, 80, 573–581. doi: 10.1016/j.fsi.2018.06.049 [DOI] [PubMed] [Google Scholar]
- Chang CT, Benedict S, Whipps CM (2019). Transmission of Mycobacterium chelonae and Mycobacterium marinum in laboratory zebrafish through live feeds. Journal of fish diseases. doi: 10.1111/jfd.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CT, Colicino EG, DiPaola EJ, Al-Hasnawi H, Jabbar, & Whipps CM (2015). Evaluating the effectiveness of common disinfectants at preventing the propagation of Mycobacterium spp. isolated from zebrafish. Comparative biochemistry and physiology, 178, 45–50. doi: 10.1016/j.cbpc.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CT, Doerr KM, & Whipps CM (2017). Antibiotic treatment of zebrafish mycobacteriosis: tolerance and efficacy of treatments with tigecycline and clarithromycin. Journal of fish diseases, 40, 1473–1485. doi: 10.1111/jfd.12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collymore C, Crim MJ, & Lieggi C (2016). Recommendations for health monitoring and reporting for zebrafish research facilities. Zebrafish, 13, S138–S148. doi: 10.1089/zeb.2015.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copper JE, Budgeon LR, Foutz CA, van Rossum DB, Vanselow DJ, Hubley MJ, Cheng KC (2018). Comparative analysis of fixation and embedding techniques foroptimized histological preparation of zebrafish. Comparative biochemistry and physiology, Part C: Toxicology & pharmacology, 208, 38–46. doi: 10.1016/j.cbpc.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crim MJ (2020). Viral diseases In Cartner S, Eisen J, Farmer S, Guillemin K, Kent ML, & Sanders GS (Eds.), The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases and Research Applications (pp. 509–526). London: Elsevier. [Google Scholar]
- Crim MJ, Lawrence C, Livingston RS, Rakitin A, Hurley SJ, & Riley LK (2017). Comparison of antemortem and environmental samples for zebrafish health monitoring and quarantine. Journal of the American Association for Laboratory Animal Science, 56, 412–424. [PMC free article] [PubMed] [Google Scholar]
- Crim MJ, & Riley LK (2012). Viral diseases in zebrafish: what is known and unknown. ILAR journal, 53, 135–143. doi: 10.1093/ilar.53.2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner K, Bik HM, Mächler E, Seymour M, Lacoursière‐Roussel A, Altermatt F,… & Pfrender ME (2017). Environmental DNA metabarcoding: Transforming how we survey animal and plant communities. Molecular ecology, 26, 5872–5895. doi: 10.1111/mec.14350 [DOI] [PubMed] [Google Scholar]
- Demeke T, & Dobnik D (2018). Critical assessment of digital PCR for the detection and quantification of genetically modified organisms. Analytical and Bioanalytical Chemistry, 410, 4039–4050. doi: 10.1007/s00216-018-1010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS (2020). History of zebrafish research In Cartner S, Eisen J, Farmer S, Guillemin K, Kent ML, & Sanders GS. (Eds.), The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases and Research Applications (pp. 3–14). London: Elsevier. [Google Scholar]
- Ferguson JA, Watral V, Schwindt AR, & Kent ML (2007). Spores of two fish microsporidia (Pseudoloma neurophilia and Glugea anomala) are highly resistant to chlorine. Diseases of aquatic organisms, 76, 205–214. [DOI] [PubMed] [Google Scholar]
- Gaulke CA, Barton CL, Proffitt S, Tanguay RL, & Sharpton TJ (2016). Triclosan exposure is associated with rapid restructuring of the microbiome in adult zebrafish. PLOS one, 11, e0154632. doi: 10.1371/journal.pone.0154632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulke CA, Martins ML, Watral VG, Humphreys IR, Spagnoli ST, Kent ML, & Sharpton TJ (2019). A longitudinal assessment of host-microbe-parasite interactions resolves the zebrafish gut microbiome’s link to Pseudocapillaria tomentosa infection and pathology. Microbiome, 7, 10. doi: 10.1186/s40168-019-0622-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes GB, Hutson KS, Domingos JA, Chung C, Hayward S, Miller TL, & Jerry DR (2017). Use of environmental DNA (eDNA) and water quality data to predict protozoan parasites outbreaks in fish farms. Aquaculture, 479, 467–473. doi: 10.1016/j.aquaculture.2017.06.021 [DOI] [Google Scholar]
- Gratacap RL, & Wheeler RT (2014). Utilization of zebrafish for intravital study of eukaryotic pathogen-host interactions. Developmental and comparative immunology, 46, 108–115. doi: 10.1016/j.dci.2014.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MJ, Reichley SR, Greenway TE, Quiniou SM, Ware C, Gao DX, Gaunt PS, Yanong RP, Pouder DB, Hawke JP, & Soto E (2016). Comparison of Edwardsiella ictaluri isolates from different hosts and geographic origins. Journal of fish diseases, 39, 947–969. doi: 10.1111/jfd.12431 [DOI] [PubMed] [Google Scholar]
- Grunwald DJ, & Eisen JS (2002). Headwaters of the zebrafish—emergence of a new model vertebrate. Nature reviews genetics, 3, 717. [DOI] [PubMed] [Google Scholar]
- Hammer HS (2020). Recirculating aquaculture systems (ras) for zebrafish culture In Cartner S, Eisen J, Farmer S, Guillemin K, Kent ML, & Sanders GS (Eds.), The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases and Research Applications (pp. 337–356). London: Elsevier. [Google Scholar]
- Harper C, & Lawrence C (2011). The Laboratory Zebrafish. Boca Raton, FL: CRC Press. [Google Scholar]
- Hawke JP, Kent M, Rogge M, Baumgartner W, Wiles J, Shelley J, … Peterson TS (2013). Edwardsiellosis caused by Edwardsiella ictaluri in laboratory populations of zebrafish Danio rerio. Journal of Aquatic Animal Health, 25, 171–183. doi: 10.1080/08997659.2013.782226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JD (1994) Carcinogenicity of afiatoxins in nonmammalian organisms In: Eaton DL & Groopman JD (Eds.), The toxicology of afiatoxins: human health, veterinary,and agricultural significance (pp. 103–106). Cambridge, MA: Academic Press. [Google Scholar]
- Hetrick FM & Hedrick RP (1993). New viruses described in finfish from 1988–1992. Annual review of fish diseases, 3, 187–207. doi: 10.1016/0959-8030(93)90034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Lancaster C, Gudkovs N, & McDonald W (1986). Exotic bacterial pathogens Edwardsiella tarda and Edwardsiella ictaluri from imported ornamental fish Betta splendens and Puntius conchonius, respectively: isolation and quarantine significance. Australian veterinary journal, 63, 369–71. doi: 10.1111/j.1751-0813.1986.tb02900 [DOI] [PubMed] [Google Scholar]
- Jung IH, Chung YY, Jung DE, Kim YJ, Kim D, Kim KS, & Park SW (2016). Impaired lymphocytes development and xenotransplantation of gastrointestinal tumor cells in prkdc-null SCID zebrafish model. Neoplasia, 18, 468–479. doi: 10.1016/j.neo.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK, Matthews JL, Spitsbergen JM (2002). Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comparative medicine, 52, 354–358. [PubMed] [Google Scholar]
- Kent ML, Buchner C, Watral VG, Sanders JL, Ladu J, Peterson TS, & Tanguay RL (2011).Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Diseases of aquatic organisms, 95, 73–79. doi: 10.3354/dao02333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sánchez-Morgado JM, Whipps CM (2009). Recommendations for control of pathogens and infectious diseases in fish research facilities. Comparative Biochemistry and Physiology, Part C: Toxicology & pharmacology, 149, 240–248. doi: 10.1016/j.cbpc.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Harper C, & Wolf JC (2012). Documented and potential research impacts of subclinical diseases in zebrafish. ILAR journal, 53, 126–134. doi: 10.1093/ilar.53.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML & Lyons JM (1982). Edwardsiella ictaluri in the green knife fish, Eigemannia virescens. Fish health news, 11, ii. [Google Scholar]
- Kent ML, Murray KN, Fisher K, Löhr C, Mulrooney D, & Sanders JL (2020). Special procedures for zebrafish diagnostics In Cartner S, Eisen J, Farmer S, Guillemin K, Kent ML, & Sanders GS (Eds.), The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases and Research Applications (pp. 547–558). London: Elsevier. [Google Scholar]
- Kent ML, & Sanders JL (2020). Important parasites in research facilities In Cartner S, Eisen J, Farmer S, Guillemin K,, Kent ML, & Sanders GS (Eds.), The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases and Research Applications (pp. 479–494). London: Elsevier. [Google Scholar]
- Kent ML, Shaw RW, & Sanders J (2014). Fish Microsporidia In Weiss LM & Becnel JJ (Eds.), In Microsporidia: Pathogens of opportunity (pp. 493–520). Hoboken, NJ: Wiley Enterprise. [Google Scholar]
- Kent ML, Watral V, Gaulke C, & Sharpton TJ (2019). Further evaluation of the efficacy of emamectin benzoate for treating Pseudocapillaria tomentosa (Dujardin 1843) in zebrafish Danio rerio (Hamilton 1822). Journal of fish diseases, 42, 1351–1357. doi: 10.1111/jfd.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Watral V, Villegas EN, & Gaulke C (2019). Viability of Pseudocapillaria tomentosa eggs exposed to heat, ultraviolet light, iodine, chlorine and desiccation.Zebrafish, 16, 460–468. doi: 10.1089/zeb.2019.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C, Ennis DG, Harper C, Kent ML, Murray K, & Sanders GE (2012). The challenges of implementing pathogen control strategies for fishes used in biomedical research. Comparative biochemistry and physiology, Part C: Toxicology & pharmacology, 155, 160–166. doi: 10.1016/j.cbpc.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Guillet B, Leguay E, Meunier E, Labrut S, Keck N,…, & Sohm F (2016).RESAMA: a network for monitoring health and husbandry practices in aquatic research facilities. Zebrafish, 13, S-56–S-65. doi: 10.1089/zeb.2015.1199 [DOI] [PubMed] [Google Scholar]
- Lin CY, Chiang CY, & Tsai HJ (2016). Zebrafish and Medaka: new model organisms for modern biomedical research. Journal of biomedical science, 23, 19. doi: 10.1186/s12929-016-0236-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, & Leach SD (2011). Zebrafish models for cancer. Annual review of pathology, 6, 71–93. doi: 10.1146/annurev-pathol-011110-130330 [DOI] [PubMed] [Google Scholar]
- MacDonald R (1999). Zebrafish immunohistochemistry In: Guille M (Ed.), Molecular methods In developmental biology: Xenopus and Zebrafish (pp. 77–88). Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Marancik D, Collins J, Afema J, & Lawrence C (2019). Exploring the advantages and limitations of sampling methods commonly used in research facilities for zebrafish health inspections. Laboratory animals, 0, 1–13. doi: 10.1177/0023677219864616 [DOI] [PubMed] [Google Scholar]
- Martins S, Monteiro JF, Vito M, Weintraub D, Almeida J, & Certal AC (2016). Toward an integrated zebrafish health management program supporting cancer and neuroscience research. Zebrafish, 13, S-47–S-55. doi: 10.1089/zeb.2015.1198 [DOI] [PubMed] [Google Scholar]
- Martins S, Monteiro JF, Vito M, Weintraub D, Almeida J, & Certal AC (2016). Toward an integrated zebrafish health management program supporting cancer and neuroscience research. Zebrafish, 13, S-47–S-55. doi: 10.1089/zeb.2015.1198 [DOI] [PubMed] [Google Scholar]
- Martins ML, Watral V, Rodrigues‐Soares JP, & Kent ML, Montgomery R, McFadden M,… Peirce J (2017). A method for collecting eggs of Pseudocapillaria tomentosa (Nematoda: Capillariidae) from zebrafish Danio rerioand efficacy of heat and chlorine for killing the nematode’s eggs. Journal of fish diseases, 40, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T, Snell K, Mittge E, Melancon E (2016).Strategies to Mitigate a Mycobacterium marinum Outbreak in a zebrafish research facility. Zebrafish, 13, S77–S87. doi: 10.1089/zeb.2015.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JL (2009). Common diseases of laboratory zebrafish In Detrich HW III, Zon LI, & Westerfield M (Eds.), Essential zebrafish methods: Genetics and genomics, 321–346. Cambridge, MA: Academic Press. [Google Scholar]
- Meijer AH (2016). Protection and pathology in TB: learning from the zebrafish model.Seminars in immunopathology, 38, 261–273. doi: 10.1007/s00281-015-0522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AH, & Spaink HP (2011). Host-pathogen interactions made transparent with the zebrafish model. Current drug targets, 12, 1000–1017. doi: 10.2174/138945011795677809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon E, Gomez De La, Torre Canny S, Sichel S, Kelly M, Wiles TJ, Rawls JF…& Guillemin K (2017). Best practices for germ-free derivation and gnotobiotic zebrafish husbandry. Methods in cell biology, 138, 61–100. doi: 10.1016/bs.mcb.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meritet DM, Spagnoli ST, Fischer KA, & Löhr CV (2019). Evaluating the effects of various decalcification protocols on immunohistochemical staining in zebrafish (Danio rerio). Zebrafish, 16, 280–290. doi: 10.1089/zeb.2018.1697 [DOI] [PubMed] [Google Scholar]
- Mocho JP (2016). Three-Dimensional Screen: A Comprehensive Approach to the Health Monitoring of Zebrafish. Zebrafish, 13, S132–S137. doi: 10.1089/zeb.2015.1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JC, & Langenau DM (2016). Allograft cancer cell transplantation in zebrafish In: Langenau DM (Ed.), Cancer and zebrafish: Advances in experimental medicine and biology (pp. 265–287). New York City, NY: Springer. [DOI] [PubMed] [Google Scholar]
- Murray KN, Lains D, & Spagnoli ST (2020). Water quality and idiopathic diseases of laboratory zebrafish In Cartner S, Eisen J, Farmer S, Guillemin K, Kent ML, & Sanders GS (Eds.), The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases and Research Applications (pp. 463–478). London: Elsevier. [Google Scholar]
- Murray KN, & Peterson TS (2015). Pathology in practice. P tomentosa infection in zebrafish. Journal of the American veterinary medical association, 246, 201–203. 10.2460/javma.246.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KN, Varga ZM, & Kent ML (2016). Biosecurity and health monitoring at the zebrafish international resource center. Zebrafish, 13, S30–S38. doi: 10.1089/zeb.2015.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert T, & Vleminckx K (2018). CRISPR/Cas9 disease models in zebrafish and Xenopus: the genetic renaissance of fish and frogs. Drug Discovery Today: Technologies, 28, 41–52. [DOI] [PubMed] [Google Scholar]
- Norris LJ, Watral V, & Kent ML (2018). Survival of bacterial and parasitic pathogens from zebrafish (Danio rerio) after cryopreservation and thawing. Zebrafish, 15, 188–201. doi: 10.1089/zeb.2017.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette CE, Kent ML, Buchner C, Tanguay RL, Guillemin K, Mason TJ, & Peterson TS (2013). A retrospective study of the prevalence and classification of intestinal neoplasia in zebrafish (Danio rerio). Zebrafish, 10, 228–236. doi: 10.1089/zeb.2012.0828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette CE, Kent ML, Peterson TS, Wang R, Dashwood RH, & Löhr CV (2015). Immunohistochemical characterization of intestinal neoplasia in zebrafish Danio rerio indicates epithelial origin. Diseases of aquatic organisms, 116, 191–197. doi: 10.3354/dao02924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters L, Spatharis S, Dario MA, Dwyer T, Roca I, Kintner A, … Praebel K (2018). Environmental DNA: A New Low-Cost Monitoring Tool for Pathogens in Salmonid Aquaculture. Frontiers in Microbiology, 9, 3009. doi: 10.3389/fmicb.2018.03009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TS, Ferguson JA, Watral VG, Mutoji KN, Ennis DG, & Kent ML (2013). Paramecium caudatum enhances transmission and infectivity of Mycobacterium marinum and M. chelonae in zebrafish Danio rerio. Diseases of aquatic organisms, 106, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JB, & Westerfield M (2014). Zebrafish models in translational research: Tipping the scales toward advancements in human health. Disease models & mechanisms, 7, 739 743. doi: 10.1242/dmm.015545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullium JK, Dillehay DL, & Webb S (1999). High mortality in zebrafish (Danio rerio). Contemporary topics in laboratory animal science, 38, 80–83. [PubMed] [Google Scholar]
- Rimmer AE, Becker JA, Tweedie A, Lintermans M, Landos M, Stephens F, & Whittington RJ (2015). Detection of dwarf gourami iridovirus (Infectious Spleen and Kidney Necrosis Virus) in populations of ornamental fish prior to and after importation into Australia, with the first evidence of infection in domestically farmed platy (Xiphophorus maculatus). Preventive veterinary medicine, 122, 181–194. doi: 10.1016/j.prevetmed.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, & Rawls JF (2011). Evidence for a core gut microbiota in the zebrafish. The ISME journal, 5, 1595–1608. doi: 10.1038/ismej.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, & Kent ML (2011). Development of a sensitive assay for the detection of Pseudoloma neurophilia in laboratory populations of the zebrafish Danio rerio.Diseases of aquatic organisms, 96, 145–156. doi: 10.3354/dao02375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Watral V, Clarkson K, & Kent ML (2013). Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the zebrafish, Danio rerio. PloS one, 8, e76064. doi: 10.1371/journal.pone.0076064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Zhou Y, Moulton HM, Moulton ZX, McLeod R, Dubey JP, … Kent ML (2015). The zebrafish, Danio rerio, as a model for Toxoplasma gondii: an initial description of infection in fish. Journal of fish diseases, 38, 675–679. doi: 10.1111/jfd.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoriello C and Zon LI, (2012). Hooked! Modeling human disease in zebrafish. The Journal of clinical investigation, 122, 2337–2343. doi: 10.1172/JCI60434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Lin XD, Chen X, Tian JH, Chen LJ, Li K, Wang W, Eden JS, Shen JJ, Liu L, Holmes EC, & Zhang YZ (2018). The evolutionary history of vertebrate RNA viruses. Nature, 556, 197–202. doi: 10.1038/s41586-018-0012-7 [DOI] [PubMed] [Google Scholar]
- Smith C, Ondračková M, Spence R, Adams S, Betts DS, & Mallon E (2011). Pathogen mediated selection for MHC variability in wild zebrafish. Evolutionary ecology research, 13, 589–605. [Google Scholar]
- Spagnoli S, Sanders J, & Kent ML (2017). The common neural parasite Pseudoloma neurophilia causes altered shoaling behaviour in adult laboratory zebrafish (Danio rerio) and its implications for neurobehavioural research. Journal of fish diseases, 40, 443–446. doi: 10.1111/jfd.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli S, Xue L & Kent ML (2015). The common neural parasite Pseudoloma neurophilia is associated with altered startle response habituation in adult zebrafish (Danio rerio): Implications for the zebrafish as a model organism. Behavioural brain research, 291, 351–360. doi: 10.1016/j.bbr.2015.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, & Smith C (2008). The behaviour and ecology of the zebrafish, Danio rerio. Biological reviews of the cambridge philosophical society, 83, 13–34. 10.1111/j.1469-185X.2007.00030 [DOI] [PubMed] [Google Scholar]
- Stones DH, Fehr A, Thompson L, Rocha J, Perez-Soto N, Madhavan V, … Krachler AM (2017). Zebrafish (Danio rerio) as a Vertebrate Model Host To Study Colonization, Pathogenesis, and Transmission of Foodborne Escherichia coli O157. mSphere, 2, e00365–17. doi: 10.1128/mSphereDirect.00365-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, & Zon LI (2009). Zebrafish tumor assays: The state of transplantation. Zebrafish, 6, 339–346. doi: 10.1089/zeb.2009.0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenor JL, Oehlers SH, Yang JL, Tobin DM, & Perfect JR (2015). Live imaging of host parasite interactions in a zebrafish infection model reveals cryptococcal determinants of virulence and central nervous system invasion. mBio, 6, e01425–15. doi: 10.1128/mBio.01425-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torraca V, Masud S, Spaink HP, & Meijer AH (2014). Macrophage-pathogen interactions in infectious diseases: new therapeutic insights from the zebrafish host model. Disease models & mechanisms, 7, 785–797. doi: 10.1242/dmm.015594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torraca V, & Mostowy S (2018). Zebrafish Infection: From Pathogenesis to Cell Biology. Trends in cell biology, 28, 143–156. doi: 10.1016/j.tcb.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini A, Taberlet P, Miaud C, Civade R, Herder J, Thomsen PF,… & Gaboriaud C (2016). Next‐generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Molecular ecology, 25, 929–942. doi: 10.1111/mec.13428 [DOI] [PubMed] [Google Scholar]
- Veinotte CJ, Dellaire G, & Berman JN (2014). Hooking the big one: The potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Disease models & mechanisms, 7, 745–754. doi: 10.1242/dmm.015784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman J, Veinotte CJ, Dellaire G, & Berman JN (2016). The zebrafish xenograft platform: evolution of a novel cancer model and preclinical screening tool. Cancer and Zebrafish, 9, 289–314. doi: 10.1007/978-3-319-30654-4_13 [DOI] [PubMed] [Google Scholar]
- Whipps CM, Dougan ST., Kent ML. 2007. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiology Letters, 270, 21–26. [DOI] [PubMed] [Google Scholar]
- Whipps CM, & Kent ML (2006). Polymerase chain reaction detection of Pseudoloma neurophilia, a common microsporidian of zebrafish (Danio rerio) reared in research laboratories. Journal of the American Association for Laboratory Animal Science, 45, 36–39. [PMC free article] [PubMed] [Google Scholar]
- Whipps CW, & Kent ML (2020). Bacterial and fungal diseases In Cartner S, Eisen JS, Guillemin K, Kent ML, & Sanders GS (Eds.), The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases and Research Applications (pp. 495–508). London: Elsevier. [Google Scholar]
- Whipps CM, Lieggi C, & Wagner R (2012). Mycobacteriosis in zebrafish colonies. ILAR journal, 53, 95–105. doi: 10.1093/ilar.53.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps CM, Matthews JL, & Kent ML (2008). Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish Danio rerio. Diseases of aquatic organisms, 82, 45–54. doi: 10.3354/dao01967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Brunson DC, Tang Q, Do D, Iftimia NA, Moore JC,… & Hong X (2019). Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell, 177(7), 1903–1914. doi: 10.1016/j.cell.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J, White RM, & Stemple DL (2014). Zebrafish models of cancer: Progress and future challenges. Current opinion in genetics & development, 24(100), 38–45. doi: 10.1016/j.gde.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]