Abstract
Purpose:
We conducted a meta-analysis to determine diagnostic performance of CT intravenous contrast extravasation (CE) as a sign of angiographic bleeding and need for angioembolization after pelvic fractures.
Materials and Methods
A systematic literature search combining the concepts of contrast extravasation, pelvic trauma, and CT yielded 206 potentially eligible studies. 23 studies provided accuracy data or sufficient descriptive data to allow 2×2 contingency table construction and provided 3855 patients for meta-analysis. Methodologic quality was assessed using the QUADAS-2 tool. Sensitivity and specificity were synthesized using bivariate mixed-effects logistic regression. Heterogeneity was assessed using the I2-statistic. Sources of heterogeneity explored included generation of scanner (64 row CT versus lower detector row) and use of multiphasic versus single phase scanning protocols.
Results
Overall sensitivity and specificity were 80% (95% CI: 66–90%, I2 = 92.65%) and 93% (CI: 90–96, I2 = 89.34%), respectively. Subgroup analysis showed pooled sensitivity and specificity of 94% and 89% for 64- row CT compared to 69% and 95% with older generation scanners. CE had pooled sensitivity and specificity of 95% and 92% with the use of multiphasic protocols, compared to 74% and 94% with single-phase protocols.
Conclusion
The pooled sensitivity and specificity of 64-row CT was 94 and 89%. 64 row CT improves sensitivity of CE, which was 69% using lower detector row scanners. High specificity (92%) can be maintained by incorporating multiphasic scan protocols.
Introduction
Approximately one in ten blunt trauma victims admitted to level 1 trauma referral centers sustain pelvic fractures (1). Bleeding pelvic fractures are an immediate life-threatening injury associated with significant mortality (2–7). Rapid hemorrhage control is associated with improved survival (2, 8), but surgical decision making remains challenging due to difficulty determining the bleeding source (2, 4, 9). Hemorrhage can arise from arterial injury, venous injury, or fractured bone ends (2, 5, 10). Angioembolization and external fixation are the most common treatment pathways for hemorrhage associated with pelvic fracture, with each therapy aimed at addressing different sources of bleeding (2, 3, 11). A central decision point critical to the timely and optimal deployment of appropriate resources and initial treatment strategies hinges on whether active arterial bleeding is present (2, 3, 12).
Early aggressive trans-catheter arterial embolization (TAE) is well established as an effective means of reducing transfusion requirement, complications, and mortality from arterial hemorrhage (2–4, 13–19), whereas low pressure bleeding from the rich pelvic venous plexus or fractured bone ends is best controlled through splinting, reduction of pelvic volume, and tamponade using external fixation (2, 3, 19, 20). Contrast enhanced CT is the cornerstone screening exam for evidence of arterial bleeding in patients with pelvic fractures who are sufficiently stable for transport to a trauma resuscitation unit CT scanner (19). In a recent epidemiologic study spanning 11 Level 1 trauma centers, CT was used in up to 85% of patients admitted in shock (2).
The Eastern Association for the Surgery of Trauma (EAST) practice management guidelines note that research has primarily focused on two imaging signs for determining need for TAE- CE and pelvic hematoma volume (19). The latter has been difficult to measure reliably or efficiently at the point of care using diameter-based or manual segmentation techniques (21–23). The EAST guidelines therefore emphasize CE as the most useful imaging predictor of the need for pelvic angiography and TAE in the clinical setting and recommend that patients with CE be considered for TAE regardless of hemodynamic status (19). A number of studies have assessed the accuracy of CE for predicting angiopositivity or hemostatic intervention with TAE (19), however published sensitivities and specificities are highly variable (11, 19, 24). Improvements in CT scanner technology should improve detection and characterization of bleeding, but there is speculation that improved image quality and temporal resolution with 64-detector row or higher CT scanners might also confound assessment due to increased detection of small self-limiting foci of CE (5, 9, 20, 24). Dynamic characterization of CE using multiphase image protocols is thought to improve diagnostic certainty in such cases, but there are no comparative effectiveness studies assessing the diagnostic performance benefit of multiphase over single phase protocols. Our objectives were to a) establish pooled accuracy metrics of CT for predicting angiopositivity and need for TAE despite the wide variability described in the literature, and b) determine whether diagnostic performance improves with the use of 64 detector row CT and multiphasic protocols (9, 22),
We hypothesized that 64- or higher MDCT scanners and multiphasic protocols would improve diagnostic performance. We also sought to provide more precise estimates of the diagnostic performance of CE for predicting major arterial injury (defined by positive angiographic findings and use of TAE for hemorrhage control). Several potentially confounding aspects of study design were investigated as sources of heterogeneity in the published results.
Materials and Methods
The protocol for this systematic review and meta-analysis was designed in consultation with an experienced biostatistician and research librarian using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (25), and QUADAS-2 background document (26). We determined the pooled diagnostic accuracy of contrast extravasation (CE) at admission trauma CT for correctly identifying arterial bleeding on subsequent angiography and need for TAE in patients who have sustained pelvic fractures. We also conducted sub-analyses based on whether 64-row CT was used and whether single- or multi-phase CT protocols were employed.
We initially conducted a comprehensive literature search that only included search strings that combined the concepts of contrast extravasation, pelvic trauma, and computed tomography, with appropriate synonyms. The following databases were queried: MEDLINE (PubMed), Embase (Elsevier), Scopus (Elsevier), and Cochrane Central Register of Controlled Trials (Wiley). A combination of text words and database-specific terminology (e.g., MeSH) were used. No date or language of publication restrictions were applied, and there were no limitations on age. All searches were completed on February 14, 2018. Titles and abstracts of all screened studies were uploaded to a bibliographic database for screening after filtering for duplicates (EndNote X8; Clarivate Analytics; Philadelphia, PA). Full details of search strategies are provided in [Online Appendix E1].
Study selection
Titles and abstracts were independently screened by two reviewers (a full-time trauma radiologist and a senior radiology resident). Discrepancies were arbitrated by an experienced interventional radiologist. Eligible entries had to constitute original research. All eligible manuscripts were accessible online or in print. For inclusion in the meta-analysis, studies had to report the following information: a) the number of patients with blunt pelvic ring disruptions/pelvic fractures, b) the number of patients that underwent CT as the index test, c) numbers of patients with CT scans positive or negative for pelvic intravenous contrast extravasation, d) numbers of patients with arterial bleeding on angiography, numbers of patients that underwent TAE, and number of patients with a negative reference test (conventional angiography negative or not performed). Manuscripts had to provide accuracy data or sufficient descriptive data to allow 2 × 2 contingency table construction. During screening, duplicate articles were removed. To complete the search, citation lists, and reviews were manually cross-checked for additional eligible studies. For publications with overlapping cohorts, only the study with the largest sample size was included.
Methodologic Quality Assessment
The two diagnostic radiology reviewers independently completed standard Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) questionnaires (Bristol University, Bristol, England) for each study to assess methodological quality (26). QUADAS-2 methodology has been widely used for meta-analyses and involves systematically assessing risk of bias in four domains (patient selection, reference standard, index test, and flow and timing), and applicability concerns in three domains (reference standard, index test, and patient selection). For each domain, bias and applicability are individually scored as low, unclear, or high. Disagreements between the two reviewers were arbitrated by the senior interventionalist reviewer.
Data extraction and processing
Using a standardized data extraction form, two reviewers extracted data on patient characteristics, study design, and features of the index and reference test. Areas of ambiguity were resolved in consensus. Data extracted included patient characteristics (total number of patients, age), study characteristics (inclusion and exclusion criteria, consecutive or non-consecutive patient selection or case-control design, retrospective versus prospective design), imaging characteristics (CT make and model; phases of acquisition- arterial, portal venous and delayed; and whether single or multiple phases were used), features of the index test (number of patients who underwent CT, time to CT from time of injury or admission, presence or absence of CT contrast extravasation), and features of the reference test (number of patients who underwent intervention, findings on angiography, whether angioembolization was performed; whether any other interventions were performed; timing between index and reference test, and whether all patients received the reference standard).
Statistical Analysis
2×2 tables of true positives, false positives, true negatives, and false negatives were used to determine or confirm reported sensitivity, specificity, and confidence intervals using the reference standard provided (angiopositivity or use of TAE). Forest plots were generated to show heterogeneity in sensitivity and specificity between studies, together with pooled results. Results were also plotted on a summary receiver operating characteristic curve. Variation across studies was explored using the inconsistency index (I2) and Cochran Q statistic for the parameters of interest (27, 28). For the Cochran Q statistic, p-values with a threshold below 0.05 were considered statistically significant. I2 values exceeding 50% were considered indicative of substantial heterogeneity. The possible presence of small study publication bias was assessed using a Deeks funnel plot asymmetry test, with p-values ≤ 0.1 indicative of significant bias (29).
Sensitivities and specificities were synthesized using mixed-effects bivariate logistic regression for the following parameters: whether 64-section CT or higher detector row scanners were explicitly used; whether multiphase protocols were employed; definition of a positive test (angiopositivity, versus performance of TAE); absolute prevalence of positive reference tests (threshold of 10%), and study size (threshold of 50 patients). Note that if scanner make and model was not provided, 64-section or higher MDCT was assumed not to have been used. All statistical analyses were performed using Stata/SE version 15 (Stata, College Station, TX).
Results
Study identification and selection
Our search initially yielded 205 unique references, of which 57 were deemed potentially relevant following review of titles and abstracts and were assessed for eligibility through detailed manual review of full text articles. 34 manuscripts that asked a different study question, had absent or incomplete accuracy data, or did not allow reconstruction of a 2×2 contingency table were eliminated. One article in Japanese was included after using online machine translation software (Google Translate; Google LLC, Mountainview, CA). One additional article was included following manual checking of references cited in the reviewed full texts, bringing the final tally of screened references to 206. 23 studies were ultimately included for further review and analysis. The screening and selection process is illustrated as a flowchart in Figure 1.
Figure 1.—
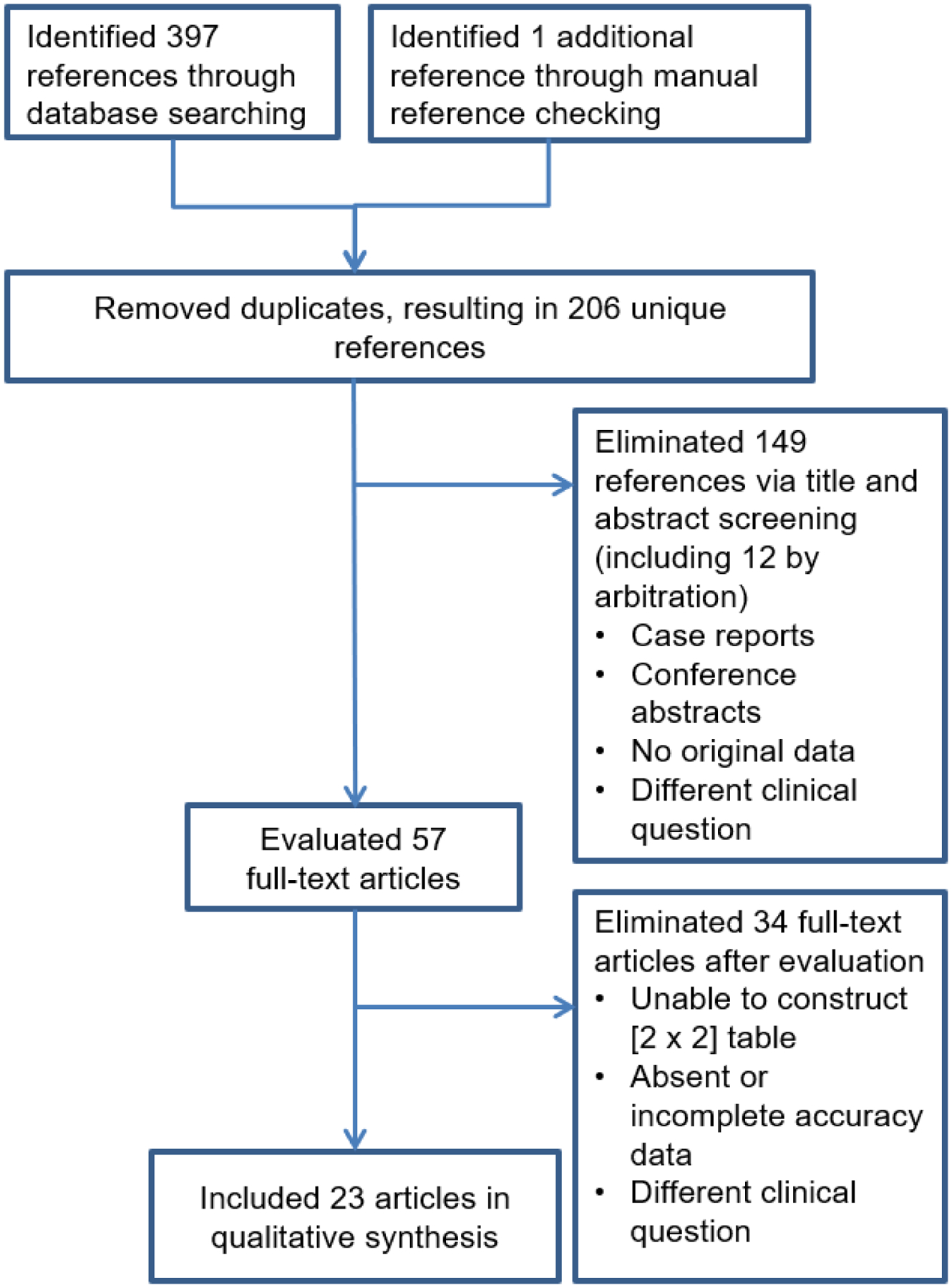
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) study selection flow diagram.
Data extraction and study characteristics
Study characteristics are shown in Table 1. The 23 studies included a total of 3855 patients with pelvic fractures that underwent CT (3–5, 9, 11, 20, 22, 24, 30–44). Year of publication ranged from 1996 to 2017. 21 of the 23 studies were carried out retrospectively and all studies were conducted at single centers. Seven of the 23 studies described the use of 64-section MDCT (20, 24, 30–32, 36, 38), and three of the seven reported the use of multiphasic CT protocols with at least arterial and portal venous phases (30, 31, 38). 16 studies either reported use of less than 64-section CT scanners (10 studies) (9, 11, 22, 37, 39–44) or did not report the manufacturer and model or number of detector rows (3–5, 24, 33, 34). One study that described use of 1–16 detector row scanners also employed a multiphasic scan protocol (9).
Table 1.—
Summary of studies included in the meta-analysis.
| Study Characteristics | Index Testing | Reference Standard | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study No. | Primary Author [Year] | No. patients meeting inclusion criteria | Mean Age | Study Design | CT manufacturer and model | 64-CT used? | CT phases | Definition of positive reference test | 2×2 table / accuracy provided or derived from raw data? | PR | SN | SP |
| 1 | Cerva [1996] | 30 | 42 | R | Siemens Hi-Q | < 64 | A | Active bleeding on angio | Derived from raw data | 63 | 84 | 100 |
| 2 | Stephen [1999] | 111 | 40 | R | GE High Speed Advantage | < 64 | PV | Active bleeding on angio requiring TAE | 2×2 table | 9 | 80 | 98 |
| 3 | Pereira [2000] | 290 | 39–52 | R | GE Hi-Speed/Picker PQ 6000 | < 64 | PV | Active bleeding on angio requiring TAE | 2×2 table, accuracy | 3 | 90 | 99 |
| 4 | Yao [2001] | 46 | 38 | P | GE HiSpeed, Picker PQ6000, Siemens Somatom Plus4 | < 64 | PV | Need for TAE or surgical intervention | Derived from raw data | 41 | 32 | 85 |
| 5 | Miller [2003] | 17 | 41 | R | ND | ND | ND | Active bleeding on angio | Accuracy | 29 | 60 | 92 |
| 6 | Nakajima [2003] | 67 | 45 | R | GE ProSeed Accell | <64 | ND | Active bleeding on angio | 2×2 table | 64 | 30 | 92 |
| 7 | Ryan [2004] | 479 | 40 | R | GE HiSpeed Advantage | <64 | PV | Active bleeding on angio requiring TAE | Derived from raw data | 2 | 88 | 100 |
| 8 | Brown [2005] | 37 | 49 | R | Picker (single detector row scanner) | < 64 | ND | Active bleeding on angio | Derived from raw data | 73 | 19 | 90 |
| 9 | Brasel [2007] | 604 | 40–47 | R | 1, 4, 8, and 16 detector row scanners | < 64 | PV, D | Active bleeding on angio | Accuracy | 3 | 90 | 96 |
| 10 | Anderson [2008] | 52 | 42 | R | GE Lightspeed Pro | 64 | A, PV, D | Arterial injury on angio | Derived from raw data | 13 | 100 | 84 |
| 11 | Kamaoui [2008] | 74 | 34 | R | Siemens Somatom Plus 4 | < 64 | A | Active bleeding on angio requiring TAE | Derived from raw data | 57 | 90 | 84 |
| 12 | Salim [2008] | 109 | 38 | P | ND | ND | ND | Arterial injury on angio | Derived from raw data | 58 | 14 | 91 |
| 13 | Mohseni [2011] | 127 | 38–49 | R | Toshiba Aquilion 64 | 64 | ND | Active bleeding on angio | Accuracy | 9 | 82 | 95 |
| 14 | Bozeman [2012] | 316 | 46 | R | ND | ND | Active bleeding on angio requiring TAE | Derived from raw data | 8 | 75 | 96 | |
| 15 | Tanizaki [2012] | 43 | 56–58 | R | ND | ND | ND | Active bleeding on angio | Accuracy | 67 | 69 | 93 |
| 16 | Anandakumar [2013] | 44 | ND | R | ND | ND | ND | Need for TAE | Derived from raw data | 25 | 100 | 88 |
| 17 | Brun [2014] | 95 | 33–38 | R | Philips Brilliance 40 and 64 | ≤ 64 | ND | Active bleeding on angio requiring TAE | Derived from raw data | 16 | 93 | 93 |
| 18 | Fu [2014] | 144 | ND | R | Toshiba Aquilion 64 | 64 | A, PV | Active bleeding on angio | 2×2 table, accuracy | 34 | 100 | 96 |
| 19 | Verbeek [2014] | 162 | 41 | R | Somatom Sensation 4 and 64 (after 2008) | ≤ 64 | ND | Need for pelvic hemorrhage control (defined as TAE, surgical ligation, and pelvic packing) | 2×2 table, accuracy | 24 | 82 | 71 |
| 20 | Kuo [2016] | 201 | 43 | R | ND | ND | ND | Arterial injury on angio | Derived from raw data | 35 | 61 | 97 |
| 21 | Juern [2017] | 497 | 44–48 | R | GE VCT Lightspeed 64 | 64 | PV | Active bleeding on angio | 2×2 table, accuracy | 3 | 100 | 88 |
| 22 | Lai [2017] | 66 | 44 | R | Toshiba Aquilion 64 | 64 | A, PV | Active bleeding on angio | Accuracy | 62 | 88 | 80 |
| 23 | Yoshikawa [2017] | 244 | 35–37 | R | Toshiba Aquilion 16 TSX-101A | < 64 | ND | Need for TAE | Accuracy | ND | 57 | 86 |
Abbreviations :R = retrospective, P = prospective; Pts = patients; PR = prevalence of positive reference tests; SN = sensitivity; SP = specificity; ND = not described; A = arterial, PV = portal venous, D = delayed
For the reference standard, 13 studies employed arterial injury on angiography as the positive reference test (3–5, 9, 20, 22, 31, 34, 36, 38, 40, 44) while 10 employed decision to perform TAE (8 studies) (11, 32, 33, 35, 37, 39, 42, 43). Two of the latter studies noted other hemostatic interventions to control arterial injury in addition to TAE (24, 41). Seven studies had prevalence of a positive reference test of ≤ 10% (9, 20, 35, 36, 39, 42, 43) and six studies included ≤ 50 patients (3, 22, 33, 34, 41, 44).
Quality Assessment
There were no major concerns regarding applicability and no studies were excluded from the analysis on the basis of quality assessment. The Deeks funnel plot did not demonstrate strong small study bias in the form of significant asymmetry in the data (p = 0.62) (figure 2). QUADAS-2 scores of risk of bias and concerns regarding applicability for each included study is shown in figure 3. Four of 23 studies had a high risk of bias attributable to patient selection, namely exclusion of patients with hypotension (3); poor quality CT scans or concomitant sources of bleeding outside of the pelvis (30); exclusion of patients with low energy falls (33); and inclusion of only patients with pelvic fractures deemed severe (40). In one study, case-control study design was considered to bias tabulation of the reference standard (33).
Figure 2.—
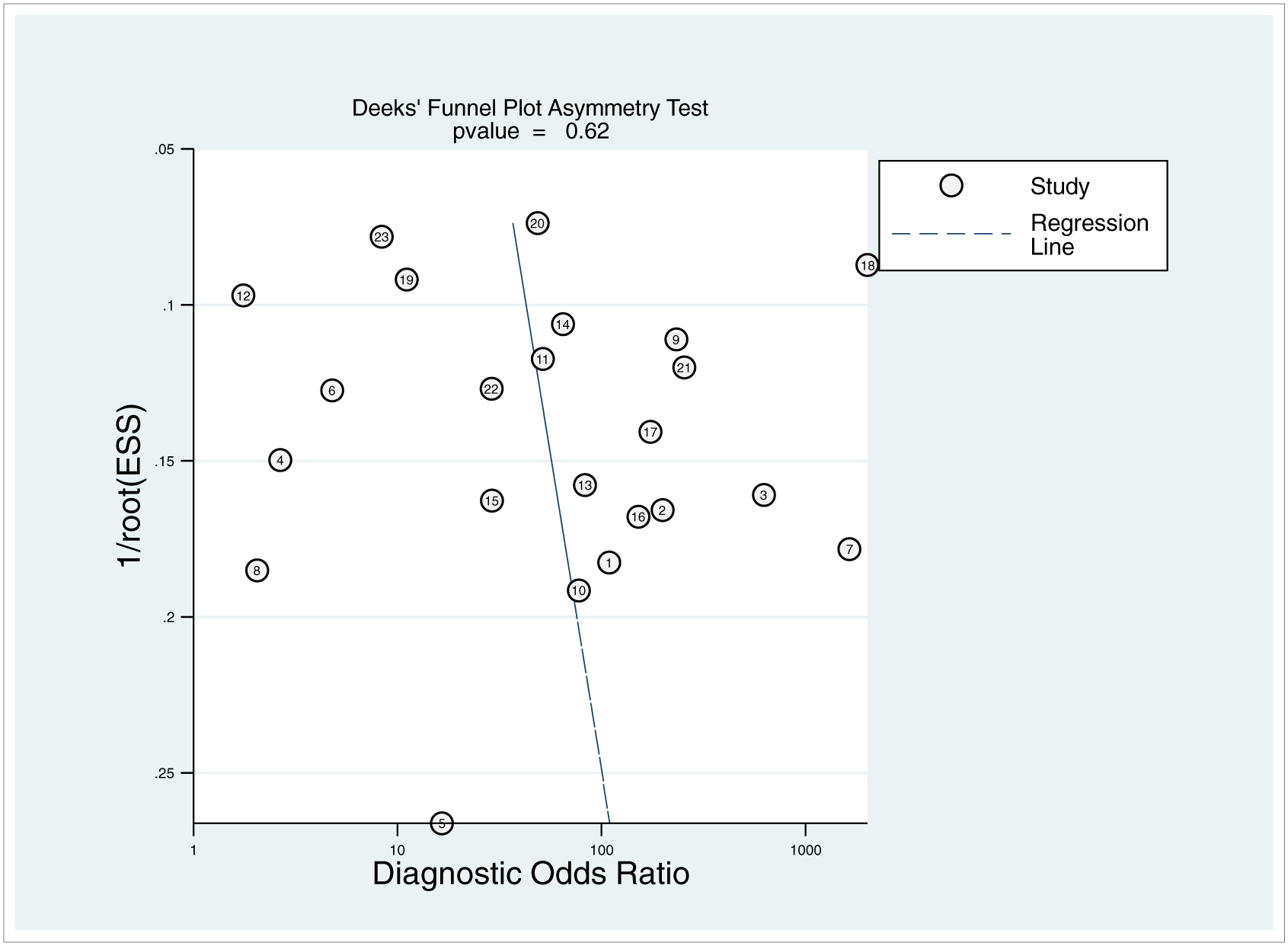
Deeks funnel plot shows no significant asymmetry in the data to suggest publication bias (p = 0.62). ESS = effective sample size. Circled numbers refer to study number.
Figure 3.—
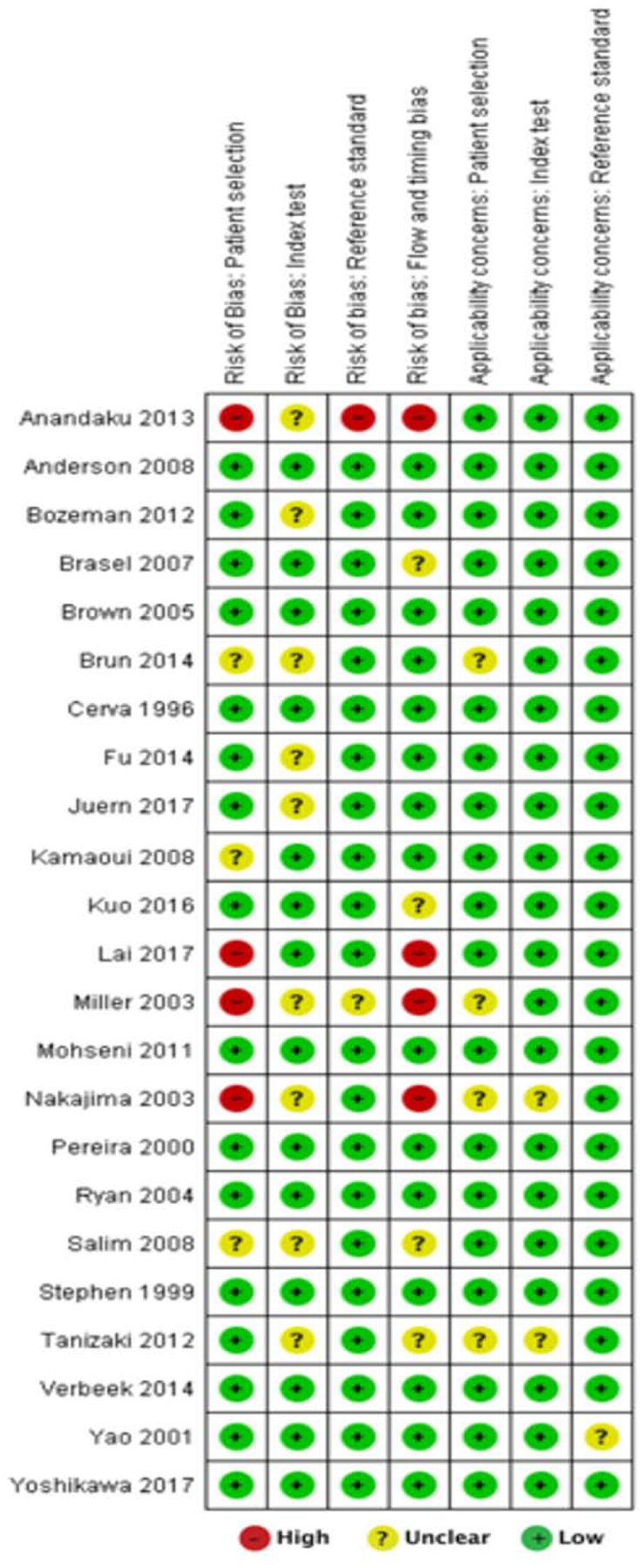
QUADAS-2 methodological assessment for bias and applicability for each study included in the meta-analysis.
Data analysis
The primary analysis showed that the pooled sensitivity and specificity of contrast extravasation on CT for predicting major arterial injury was 80% (95% CI: 66–90%) and 93% (95% CI: 90–96%) respectively (Table 2). The area under the hierarchical summary receiver operating characteristic curve (AUC) revealed overall excellent accuracy of 95% (95% CI: 93–97%) (Figure 4). Forest plots (Figure 5) indicate that heterogeneity (I2) was high, exceeding 50% for both sensitivity and specificity.
Table 2.—
Pooled statistics of the diagnostic performance of CT contrast extravasation for major arterial injury
| Parameter | |
|---|---|
| Total no. of studies | 23 |
| Sensitivity (%) | 80 (66, 90) |
| I2 (%) | 92.65 (90.55, 94.75) |
| Specificity (%) | 93 (90, 96) |
| I2 (%) | 89.34 (85.94, 92.74) |
| I2 (%) | 83.59 (83.59, 91.78) |
| Diagnostic odds ratio | 59 (24, 146) |
| I2 (%) | 100.00 (100.00, 100.00) |
| Area under the curve | 0.95 (0.93, 0.97) |
Note: data are pooled estimates with 95% confidence intervals in parentheses.
Figure 4.—
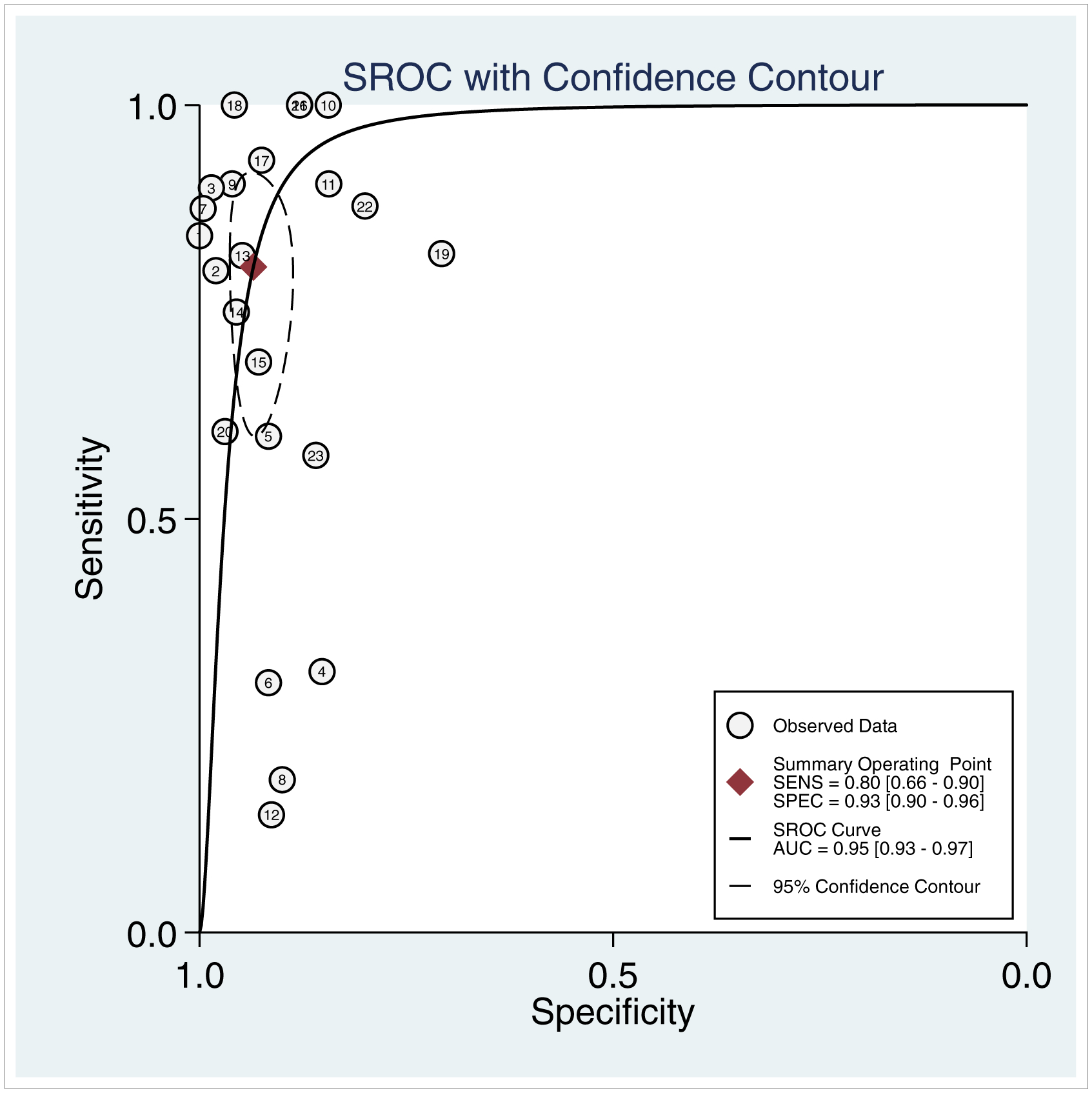
Hierarchical summary ROC (SROC) curve for the diagnostic performance of CT contrast extravasation for determining major arterial injury after pelvic fracture. The curve illustrates sensitivities achieved at different levels of specificity, with the diamond in the top left corner indicating optimal sensitivity (SENS) and specificity (SPEC) estimates. Values in brackets represent 95% confidence intervals and dashed circle represents the 95% confidence contour. Circled numbers refer to the study number.
Figure 5.—
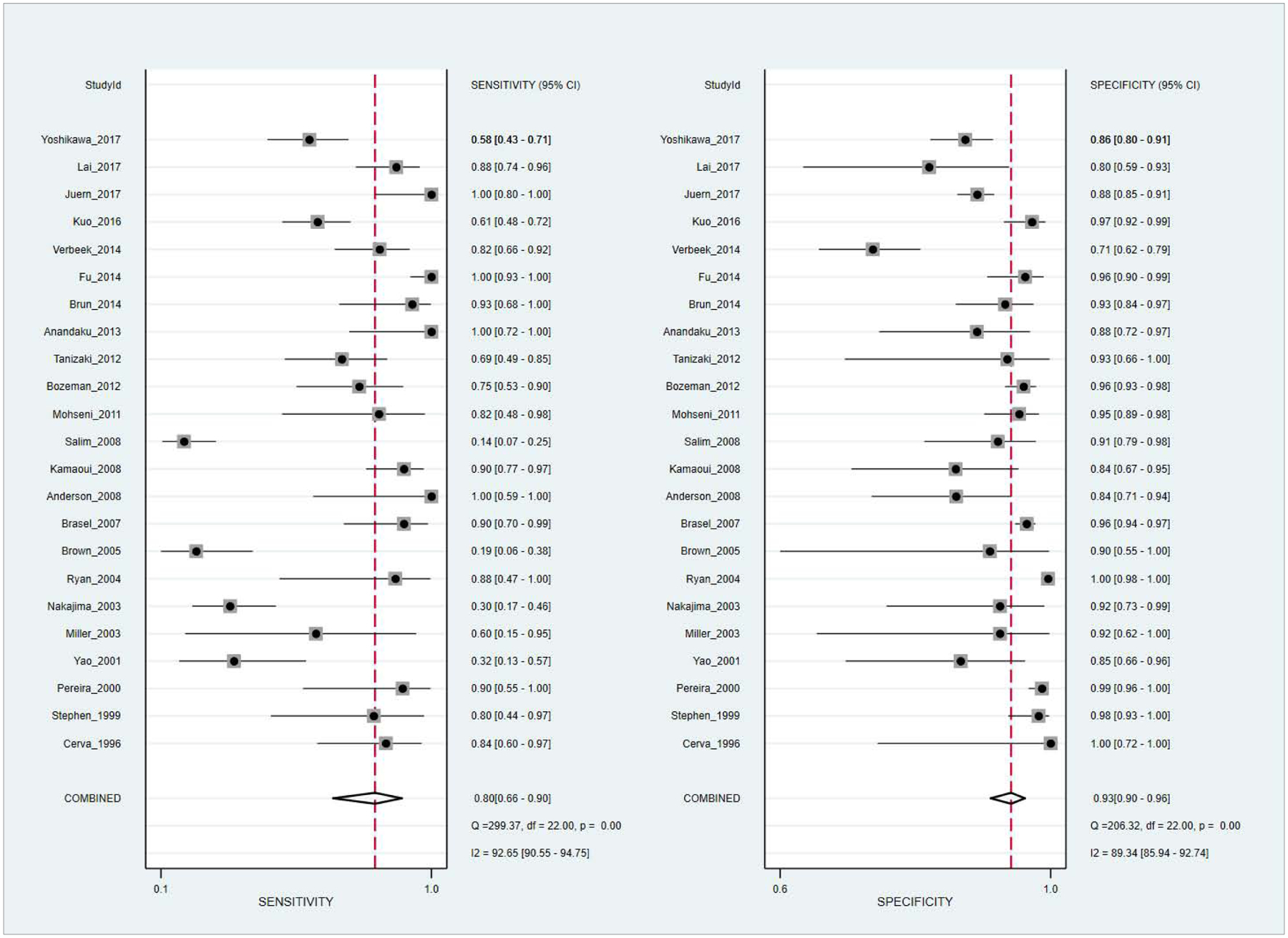
Forest plots show diagnostic performance estimates (sensitivity and specificity [dotted squares]) of CT contrast extravasation for major arterial injury after pelvic fractures with 95% confidence intervals (horizontal lines). Vertical dashed line and diamonds (bottom) represent pooled summary estimates of sensitivity and specificity. Heterogeneity (I2 and Cochran Q statistics) are shown (bottom right).
Subgroup analyses were performed to explore sources of heterogeneity related to use of 64-row CT, multiphasic protocols, as well as study characteristics including use of angiopositivity versus TAE as the reference test, sample size, and prevalence of the positive reference test. Subgroup analyses (Table 3) showed that studies that reported use of 64-row MDCT had significantly higher combined sensitivities compared to studies that did not, with non-overlapping 95% confidence intervals (94% (86–97%) versus 69% (51–82%)). There was a trend toward lower specificity with the use of 64-section MDCT (89% (81–93%) versus 95% (92–97%)), however combined specificity was high when multiphasic protocols were employed (92% (84–97%)).
Table 3.—
Pooled statistics for sensitivity and specificity of CT contrast extravasation for major arterial injury (subgroup analyses).
| Group | No. of studies | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Overall | 23 | 80 (66, 90) | 93 (90, 96) |
| CT detector rows | |||
| 64 | 7 | 94 (86, 97)† | 89 (81, 93) |
| <64 | 16 | 69 (51, 82)† | 95 (92, 97) |
| Scanning protocol | |||
| Multiphasic | 4 | 95 (83, 99)† | 92 (84, 97) |
| Single phase | 19 | 74 (57, 85)† | 94 (90, 96) |
| Reference standard | |||
| Angiopositivity | 13 | 80 (53, 93) | 93 (90, 95) |
| TAE or other intervention | 10 | 82 (68, 91) | 94 (87, 97) |
| Prevalence of positives | |||
| ≥ 10% | 16 | 76 (54, 89) | 90 (85, 93)† |
| < 10% | 7 | 86 (75, 92) | 97 (94, 99)† |
| Study size | |||
| n > 50 | 17 | 84 (69, 93) | 94 (90, 96) |
| n ≤ 50 | 6 | 64 (33, 87) | 90 (82, 94) |
Note: data are pooled estimates with 95% confidence intervals in parentheses.
indicates statistically significant differences between the two subgroups (p < 0.05).
There were no significant effects on diagnostic performance related to the choice of angiopositivity versus decision to perform TAE as the reference standard. Studies with lower prevalence of major arterial injury or larger sample size had significantly higher pooled specificities (P < 0.001).
Discussion
Pelvic fracture-related hemorrhage is life-threatening but potentially reversible with timely and appropriately chosen intervention (2, 45). TAE is the most common definitive method of hemorrhage control in patients with arterial sources of bleeding, while external fixation is used to control low pressure venous or bone bleeding through splinting of sharp bone ends, reduction of pelvic volume, and tamponade (2). In current practice, the great majority of patients with pelvic fractures undergo CT on admission. CE on computed tomography should be both a sensitive and specific sign of subsequent angiographic bleeding to ensure appropriate triage to angiography or external fixation (19). False negative CT exams could cause delay to definitive therapy with angioembolization, while false positive CT exams may result in unnecessary activation of the angiography suite, an invasive procedure associated with potential complications, and potential delay in controlling venous sources of hemorrhage through external fixation. An increasing number of patients with pelvic fractures and hemodynamic instability are undergoing admission contrast-enhanced trauma CT (2). In this context, there are several possible physiologic causes of false negative exams that can reduce sensitivity and adversely affect the utility of CE as a sign for ruling out arterial injury requiring hemostatic intervention. These include transient arterial spasm, hypotension, thrombosis, and tamponade (46–48).
In the overall analysis, pooled specificity of CE was high (93% (90–96%)), while sensitivity was limited (80% (66–90%)). Pooled sensitivity of CE was significantly higher in studies that described use of 64-row CT (94% (86–97%) versus 69% (51–82%)). There was a trend toward lower specificity with the use of 64-section MDCT (89% (81–93%)) than without (95% (92–97%)), with marginally overlapping confidence intervals. There appears to be a trade-off wherein increased sensitivity of higher detector row scanners, at some threshold, leads to increased detection of small, self-limiting, clinically insignificant arterial bleeding.
CT is a static exam. However, multiphase protocols provide at least one other time point for detecting potentially transient hemorrhage. Multiphasic protocols also improve characterization of bleeding by allowing assessment of dynamic changes in size of blush between phases (38). Increasing size and decreasing density of contrast blush confirms active bleeding from an arterial source (38), while blush that doesn’t appear to change in size between phases may represent a pseudoaneurysm or focus of self-limiting arterial bleed. In this way, improved characterization of the bleeding source using multiphasic scanning can potentially mitigate against the diagnostic uncertainty caused by increased detection of small self-limiting foci of CE with newer scanner technology. Multiphasic protocols maintained both high sensitivity (95% (83–99%)) and specificity (92% (84–97%)). The findings of our meta-analysis suggest that multiphasic protocols should be routinely employed with 64-detector row or higher CT scanners.
There were no major effects on diagnostic performance based on whether angiopositivity or decision to perform angioembolization were used as the reference test. We did find that study size and absolute prevalence of positive reference tests were important sources of heterogeneity.
Our study had several limitations, including the relatively small number of included studies. Furthermore, other imaging predictors, such as presence of large pelvic hematomas and unstable patterns of high-energy pelvic ring disruption are less well studied but known independent predictors of angiopositivity and decision to perform TAE (4, 11, 21, 22, 33, 35, 46). It is unclear to what extent additional features such as the pelvic fracture pattern and hematoma volume may have contributed to heterogeneity, however, these additional features can help resolve instances where CE may be falsely positive or negative. For example, the probability that absence of CE is due to vasospasm or hypotension increases with the size of pelvic hematoma and and the degree of pelvic instability (46). Future studies should evaluate diagnostic performance of multiple concurrent CT imaging features given the inherently multifactorial nature of these injuries. Automated or semi-automated quantitative visualization tools may ultimately result in more granular and objective probabilistic personalized decision support and outcome prediction (23, 46, 49–52). It remains unclear why so few studies on this topic have incorporated 64-row or higher MDCT and multiphasic imaging. Similar observations regarding the paucity of studies using 64-row CT (a technology that has been commercially available since 2004) (12) have been made for disparate traumatic injuries such as penetrating wounds involving the diaphragm (53). The imaging of trauma remains an understudied area, and the acquisition of CT scanners at trauma centers likely lags considerably behind the current state of the art.
Conclusion
The sensitivity of CE for angiographic evidence of bleeding and need for TAE is significantly improved with the use of 64-detector row scanners (94%). High specificity (92%) can be maintained by incorporating multiphasic scan protocols. Missed or delayed diagnosis of arterial injury and false positives resulting in unnecessary angiography will still occur with some frequency. Additional predictors of bleeding including pelvic hematoma and fracture severity should be taken into account.
Supplementary Material
Sources of Funding:
1. RSNA Research Scholar Grant (#RSCH1605) (PI: David Dreizin, MD)
2. NIH K08 EB027141-01A1 (PI: David Dreizin, MD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David Dreizin, Department of Diagnostic Radiology and Nuclear Medicine, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Yuanyuan Liang, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD.
James Dent, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD.
Nabeel Akhter, Department of Diagnostic Radiology and Nuclear Medicine, Vascular and interventional radiology, University of Maryland School of Medicine.
Daniel Mascarenhas, Orthopedic surgery, Rutgers Robert Wood Johnson Medical School.
Thomas M Scalea, Francis X Kelly Distinguished Professor in Trauma Surgery, Physician in Chief, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine.
References
- 1.Coccolini F, Stahel PF, Montori G, et al. Pelvic trauma: WSES classification and guidelines. World Journal of Emergency Surgery. 2017;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: results of an American Association for the Surgery of Trauma multi-institutional trial. Journal of Trauma and Acute Care Surgery. 2016;80(5):717–25. [DOI] [PubMed] [Google Scholar]
- 3.Miller PR, Moore PS, Mansell E, Meredith JW, Chang MC. External fixation or arteriogram in bleeding pelvic fracture: initial therapy guided by markers of arterial hemorrhage. Journal of Trauma and Acute Care Surgery. 2003;54(3):437–43. [DOI] [PubMed] [Google Scholar]
- 4.Salim A, Teixeira PG, DuBose J, et al. Predictors of positive angiography in pelvic fractures: a prospective study. Journal of the American College of Surgeons. 2008;207(5):656–62. [DOI] [PubMed] [Google Scholar]
- 5.Kuo L-W, Yang S-J, Fu C-Y, Liao C-H, Wang S-Y, Wu S-C. Relative hypotension increases the probability of the need for angioembolisation in pelvic fracture patients without contrast extravasation on computed tomography scan. Injury. 2016;47(1):37–42. [DOI] [PubMed] [Google Scholar]
- 6.Dalal SA, Burgess AR, Siegel JH, et al. Pelvic fracture in multiple trauma: classification by mechanism is key to pattern of organ injury, resuscitative requirements, and outcome. The Journal of trauma. 1989;29(7):981–1000; discussion −2. [PubMed] [Google Scholar]
- 7.Parreira JG, Coimbra R, Rasslan S, Oliveira A, Fregoneze M, Mercadante M. The role of associated injuries on outcome of blunt trauma patients sustaining pelvic fractures. Injury. 2000;31(9):677–82. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz DA, Medina M, Cotton BA, et al. Are we delivering two standards of care for pelvic trauma? Availability of angioembolization after hours and on weekends increases time to therapeutic intervention. Journal of Trauma and Acute Care Surgery. 2014;76(1):134–9. [DOI] [PubMed] [Google Scholar]
- 9.Brasel KJ, Pham K, Yang H, Christensen R, Weigelt JA. Significance of contrast extravasation in patients with pelvic fracture. Journal of Trauma and Acute Care Surgery. 2007;62(5):1149–52. [DOI] [PubMed] [Google Scholar]
- 10.Huittinen V-M, Slätis P. Postmortem angiography and dissection of the hypogastric artery in pelvic fractures. Surgery. 1973;73(3):454–62. [PubMed] [Google Scholar]
- 11.Yoshikawa S, Shiraishi A, Kishino M, et al. Predictive ability and interobserver reliability of computed tomography findings for angioembolization in patients with pelvic fracture. Journal of Trauma and Acute Care Surgery. 2018;84(2):319–24. [DOI] [PubMed] [Google Scholar]
- 12.Dreizin D, Munera F. Blunt polytrauma: evaluation with 64-section whole-body CT angiography. Radiographics. 2012;32(3):609–31. [DOI] [PubMed] [Google Scholar]
- 13.Agolini SF, Shah K, Jaffe J, Newcomb J, Rhodes M, Reed JF. Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. Journal of Trauma and Acute Care Surgery. 1997;43(3):395–9. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Menachem Y, Coldwell DM, Young J, Burgess AR. Hemorrhage associated with pelvic fractures: causes, diagnosis, and emergent management. AJR American journal of roentgenology. 1991;157(5):1005–14. [DOI] [PubMed] [Google Scholar]
- 15.Panetta T, Sclafani S, Goldstein A, Phillips T, Shaftan G. Percutaneous transcatheter embolization for massive bleeding from pelvic fractures. The Journal of trauma. 1985;25(11):1021–9. [PubMed] [Google Scholar]
- 16.Margolies MN, Ring EJ, Waltman AC, Kerr WS Jr, Baum S. Arteriography in the management of hemorrhage from pelvic fractures. New England Journal of Medicine. 1972;287(7):317–21. [DOI] [PubMed] [Google Scholar]
- 17.Velmahos GC, Toutouzas KG, Vassiliu P, et al. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. Journal of Trauma and Acute Care Surgery. 2002;53(2):303–8. [DOI] [PubMed] [Google Scholar]
- 18.Papakostidis C, Kanakaris N, Dimitriou R, Giannoudis PV. The role of arterial embolization in controlling pelvic fracture haemorrhage: a systematic review of the literature. European Journal of Radiology. 2012;81(5):897–904. [DOI] [PubMed] [Google Scholar]
- 19.Cullinane DC, Schiller HJ, Zielinski MD, et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture—update and systematic review. Journal of Trauma and Acute Care Surgery. 2011;71(6):1850–68. [DOI] [PubMed] [Google Scholar]
- 20.Juern JS, Milia D, Codner P, et al. Clinical significance of computed tomography contrast extravasation in blunt trauma patients with a pelvic fracture. Journal of Trauma and Acute Care Surgery. 2017;82(1):138–40. [DOI] [PubMed] [Google Scholar]
- 21.Blackmore CC, Jurkovich GJ, Linnau KF, Cummings P, Hoffer EK, Rivara FP. Assessment of volume of hemorrhage and outcome from pelvic fracture. Archives of surgery. 2003;138(5):504–9. [DOI] [PubMed] [Google Scholar]
- 22.Brown CV, Kasotakis G, Wilcox A, Rhee P, Salim A, Demetriades D. Does pelvic hematoma on admission computed tomography predict active bleeding at angiography for pelvic fracture? The American Surgeon. 2005;71(9):759–62. [PubMed] [Google Scholar]
- 23.Dreizin D, Bodanapally UK, Neerchal N, Tirada N, Patlas M, Herskovits E. Volumetric analysis of pelvic hematomas after blunt trauma using semi-automated seeded region growing segmentation: a method validation study. Abdominal Radiology. 2016;41(11):2203–8. [DOI] [PubMed] [Google Scholar]
- 24.Verbeek DO, Zijlstra IA, van der Leij C, Ponsen KJ, van Delden OM, Goslings JC. Management of pelvic ring fracture patients with a pelvic “blush” on early computed tomography. Journal of Trauma and Acute Care Surgery. 2014;76(2):374–9. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529–36. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of clinical epidemiology. 2005;58(9):882–93. [DOI] [PubMed] [Google Scholar]
- 30.Lai Y-C, Wu C-H, Chen H-W, et al. Predictors of active arterial hemorrhage on angiography in pelvic fracture patients. Japanese Journal of Radiology. 2018;36(3):223–30. [DOI] [PubMed] [Google Scholar]
- 31.Fu C-Y, Wang S-Y, Liao C-H, et al. Computed tomography angiography provides limited benefit in the evaluation of patients with pelvic fractures. The American journal of emergency medicine. 2014;32(10):1220–4. [DOI] [PubMed] [Google Scholar]
- 32.Brun J, Guillot S, Bouzat P, et al. Detecting active pelvic arterial haemorrhage on admission following serious pelvic fracture in multiple trauma patients. Injury. 2014;45(1):101–6. [DOI] [PubMed] [Google Scholar]
- 33.Anandakumar V, Hussein FK, Varuun B, Zhu R. Predictive parameters for angiography and embolization in the bleeding pelvic fracture. Journal of clinical orthopaedics and trauma. 2013;4(2):70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanizaki S, Maeda S, Hayashi H, et al. Early embolization without external fixation in pelvic trauma. The American journal of emergency medicine. 2012;30(2):342–6. [DOI] [PubMed] [Google Scholar]
- 35.Bozeman MC, Cannon RM, Trombold JM, et al. Use of computed tomography findings and contrast extravasation in predicting the need for embolization with pelvic fractures. The American Surgeon. 2012;78(8):825–30. [PubMed] [Google Scholar]
- 36.Mohseni S, Talving P, Kobayashi L, et al. The diagnostic accuracy of 64-slice computed tomography in detecting clinically significant arterial bleeding after pelvic fractures. The American Surgeon. 2011;77(9):1176–82. [DOI] [PubMed] [Google Scholar]
- 37.Kamaoui I, Courbiere M, Floccard B, Monneuse O, Allaouchiche B, Pilleul F. Pelvic trauma: impact of iodinated contrast material extravasation at MDCT on patient management. Journal de radiologie. 2008;89(11 Pt 1):1729–34. [DOI] [PubMed] [Google Scholar]
- 38.Anderson SW, Soto JA, Lucey BC, Burke PA, Hirsch EF, Rhea JT. Blunt trauma: feasibility and clinical utility of pelvic CT angiography performed with 64–detector row CT. Radiology. 2008;246(2):410–9. [DOI] [PubMed] [Google Scholar]
- 39.Ryan MF, Hamilton PA, Chu P, Hanaghan J. Active extravasation of arterial contrast agent on post-traumatic abdominal computed tomography. Canadian Association of Radiologists Journal. 2004;55(3):160. [PubMed] [Google Scholar]
- 40.Nakajima M, Kuramoto K, Shimada E. Application of transcatheter arterial embolization in patients with severe pelvic fractures. Analysis by helical contrast-enhanced CT and Young-Burgess sub-classification. Toho Igakkai Zasshi. 2003;50(1):44–52. [Google Scholar]
- 41.Yao DC, Jeffrey RB Jr, Mirvis SE, et al. Using contrast-enhanced helical CT to visualize arterial extravasation after blunt abdominal trauma: incidence and organ distribution. American Journal of Roentgenology. 2002;178(1):17–20. [DOI] [PubMed] [Google Scholar]
- 42.Pereira SJ, O’brien DP, Luchette FA, et al. Dynamic helical computed tomography scan accurately detects hemorrhage in patients with pelvic fracture. Surgery. 2000;128(4):678–85. [DOI] [PubMed] [Google Scholar]
- 43.Stephen DJG, Kreder HJ, Day AC, et al. Early Detection of Arterial Bleeding in Acute Pelvic Trauma. Journal of Trauma and Acute Care Surgery. 1999;47(4):638. [DOI] [PubMed] [Google Scholar]
- 44.Cerva D Jr, Mirvis S, Shanmuganathan K, Kelly I, Pais S. Detection of bleeding in patients with major pelvic fractures: value of contrast-enhanced CT. AJR American journal of roentgenology. 1996;166(1):131–5. [DOI] [PubMed] [Google Scholar]
- 45.Stahel PF, Burlew CC, Moore EE. Current trends in the management of hemodynamically unstable pelvic ring injuries. Current opinion in critical care. 2017;23(6):511–9. [DOI] [PubMed] [Google Scholar]
- 46.Dreizin D, Bodanapally U, Boscak A, et al. CT prediction model for major arterial injury after blunt pelvic ring disruption. Radiology. 2018;287(3):1061–9. [DOI] [PubMed] [Google Scholar]
- 47.Raniga SB, Mittal AK, Bernstein M, Skalski MR, Al-Hadidi AM. Multidetector CT in Vascular Injuries Resulting from Pelvic Fractures: A Primer for Diagnostic Radiologists. RadioGraphics. 2019;39(7):2111–29. [DOI] [PubMed] [Google Scholar]
- 48.Dreizin D Commentary on “Multidetector CT in Vascular Injuries Resulting from Pelvic Fractures”. RadioGraphics. 2019;39(7):2130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dreizin D, Zhou Y, Zhang Y, Tirada N, Yuille AL. Performance of a Deep Learning Algorithm for Automated Segmentation and Quantification of Traumatic Pelvic Hematomas on CT. Journal of digital imaging. 2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Dreizin D, Li Y, Zhang Z, Wang Y, Yuille A. Multi-scale Attentional Network for Multi-focal Segmentation of Active Bleed After Pelvic Fractures. Machine Learning in Medical Imaging: 10th International Workshop, MICCAI MLMI 2019 Proceedings: Springer Nature, 2019; p. 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Battey TW, Dreizin D, Bodanapally UK, et al. A comparison of segmented abdominopelvic fluid volumes with conventional CT signs of abdominal compartment syndrome in a trauma population. Abdominal Radiology. 2019:1–8. [DOI] [PubMed] [Google Scholar]
- 52.Dreizin D, Bodanapally U, Mascarenhas D, et al. Quantitative MDCT assessment of binder effects after pelvic ring disruptions using segmented pelvic haematoma volumes and multiplanar caliper measurements. European radiology. 2018;28(9):3953–62. [DOI] [PubMed] [Google Scholar]
- 53.McDonald AA, Robinson BR, Alarcon L, et al. Evaluation and management of traumatic diaphragmatic injuries: A Practice Management Guideline from the Eastern Association for the Surgery of Trauma. Journal of Trauma and Acute Care Surgery. 2018;85(1):198–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


