Abstract
Neural tube defects (NTDs) are a broad class of congenital birth defects that result from the failure of neural tube closure during neurulation. Folic acid supplementation has been shown to prevent the occurrence of NTDs by as much as 70% in some human populations, and folate deficiency in a pregnant woman is associated with increased risk for having an NTD affected infant. Thus, folate transport-related genes and genes involved in the subsequent folate-mediated one carbon metabolic pathway have long been considered primary candidates to study the genetic etiology of human NTDs. Herein, we review the genes involved in folate transport and one carbon metabolism thus far identified as contributing variants that influence human NTD risk, and place these findings in the context of our evolving understanding of the complex genetic architecture underlying these defects
Keywords: Neural tube defects, one carbon metabolism, folate transporters, genomics
1. Introduction
Neural tube defects (NTDs) are a broad class of congenital malformations, including spina bifida and anencephaly, which are estimated to affect approximately 18.6 per 10,000 births globally1. While the etiologies of NTDs are multifactorial, encompassing both environmental and complex genetic components, it is well known that one carbon metabolism (OCM) is arguably the most critical modifier of risk associated with proper neural tube closure. OCM encompasses a range of single-carbon transfer reactions, mediated by the vitamin cofactor, folate, which are essential for various cellular processes including: proliferation, epigenetic regulation, metabolic homeostasis, and maintenance of cellular redox balance2. Knockout mouse models for various OCM genes result in NTD phenotypes; and in humans, improving maternal folate status through peri-conceptional folic acid supplementation or nutritional fortification with folic acid results in significantly decreased prevalence of NTDs in almost every practicing population3. Indeed, folic acid is the single greatest weapon in the arsenal of NTD prevention. Given the importance of folate and OCM in the biology of NTDs, genes involved in folate uptake, folate transport, and OCM have been considered prime candidates as genetic risk factors for these birth defects. While many variants of OCM-related genes have been identified in NTD genomic association studies and candidate gene analyses, we are far from truly understanding the roles of these variants in the genetic architecture underlying birth defects. Therefore, in this review we provide an overview of folate transport and one carbon metabolism and review the genes in this pathway thus far identified as contributing variants that increase human NTD risk. We then explain how these findings fit into our deepening understanding of the genetic components contributing to NTD etiology.
2. Folate transporters and NTDs
Humans, like all other animals, are not capable of biosynthesizing folates, and therefore must consume them in their diet2. Folates must then be distributed to critical tissues and transported into cells via folate transporters. We will discuss the role of known folate transporters and their association with NTDs in mice and in humans.
2.1. Folate Receptors
Folate Receptors (FRs) are cysteine rich glycosylphosphatidylinositol-anchored proteins that have a high binding affinity to folate and mediate the uptake of folate to cells via endocytosis-mediated internalization of receptor-folate complexes4. Folate is then released to the cytoplasm of cells from the acidified endosome followed by FR-mediated endocytosis with the help of the proton-coupled folate transporter (PCFT)5. It has also been proposed that FR1 may act as a nuclear transcription factor that regulates expression of pluripotency factors6,7. The expression of folate receptors is limited to the placenta, the neural tube, and the kidney during embryonic development, and the expression of FR1 is limited to the epithelial cells of the choroid plexus, lung, and renal tubular cells in adults. Three separate genes (FR1, 2, and 3) encode folate receptors in humans, whereas there are only two genes (Folr1 and Folr2) in the mouse. Folr1 deficiency in mice is associated with cranial neural tube defects along with heart defects, facial malformations, and early embryonic lethality by embryonic day E10, whereas Folr2 depletion in the mouse results in no significant phenotypic malformations8. The Folr1 nullizygous phenotypes can be rescued with maternal folate supplementation in the form of folinic acid8. A higher concentration of FR autoantibodies in maternal serum has been suggested to be a risk factor in human NTDs9 and a recent report implies the existence of a genetic association between FRs and myelomeningocele with the discovery of twelve novel variants in human cases10. Folate deficiency due to a brain specific loss-of-function mutation of FOLR1 in humans is associated with cerebral folate transport defects that cause brain malformations and several neurological disorders including epilepsy, which can be partly reversed by folinic acid treatment11.
2.2. Reduced folate carrier (SLC19A1)
Reduced Folate Carrier 1 (RFC1) is an anion antiporter mediating the intake of reduced folates at a neutral pH, such as 5-methyltetrahydrofolate (5-methyl-THF) or 5-formyltetrahydrofolate (5-formyl-THF); however, with relatively low affinity for folic acid12. RFC1 is ubiquitously expressed during early development and is expressed in human tissues including the brush border of the small and large intestine, the basolateral membrane of renal tubular epithelium, hepatocytes, choroid plexus, and retinal pigment epithelium. Thus, it has been considered a primary folate transporter in cells. Rfc1 gene inactivation in mice is embryonic lethal before E6.513. Maternal folic acid supplementation of heterozygous dams with low dosage extended embryo survival until E9.5-E10.5, although they presented with severe neural tube defects, while high-dose folic acid supplementation rescued Rfc1 null embryos until term (E18.5)13. A common human polymorphism of RFC1 (A80G) is associated with several diseases including neural tube defects14, and recently eight rare variants of RFC1, including one pathogenic variant, were found in myelomeningocele patients10. These findings suggest a close association between RFC genes and neural tube closure defects in both the human and mouse.
2.3. Proton coupled folate transporter (SLC46A1)
PCFT is an electrogenic folate transporter that is highly expressed in the duodenum and jejunum in humans15. It has the highest affinity for folate at pH5.5, and also has a high affinity for 5-methyl-THF and 5-formyl-THF under low pH conditions. PCFT mediates the folate absorption in the brush-border membrane of the small intestine, which has a low pH microenvironment. It is also a critical folate transporter in the central nervous system, as it mediates the folate absorption from blood to cerebrospinal fluid in the choroid plexus. Additionally, it has been reported that PCFT releases folates from acidic endosomes in cultured cells, suggesting a supportive role of PCFT in FR1 dependent folate transport5. Loss-of-function mutations in the PCFT gene in humans is associated with hereditary folate malabsorption (HFM) syndrome15, which causes systemic cerebral folate deficiency (CFD). This condition responds well to therapeutic intervention with chronic high dose folinic acid. Pcft null mutant mice show a lack of folate uptake in the intestines and low folate concentration and subsequent increased homocysteine levels in serum and several organs. Therefore, the Pcft knockout mouse may serve as a murine HFM model. Parental folate supplementation was shown to rescue folate deficiency-induced anemia in Pcft mutant mice and increased the survival length of Pcft KO mice16.
2.4. Mitochondrial Folate Transporter (SLC25A32)
Folates enter the mitochondria through the mitochondrial folate transporter (MFT), coded for by the gene SLC25A32. Inactivation of this gene in mice results in severe NTDs that can be partially rescued by maternal formate supplementation, but not 5-methyl-THF or other folate species17. Simulations have suggested that tetrahydrofolate (THF) is the predominant folate species transported into the mitochondria, and that transport of THF by MFT is dependent on π-cation interactions with residue Trp142, since site-specific mutagenesis of this residue in Chinese Hamster Ovary cells rendered MFT nonfunctional in terms of facilitating mitochondrial folate uptake18. One human NTD case has been identified with two heterozygous loss-of-function variants in SLC25A32, both with predicted disruption of the Trp142 residue17. Additionally, four singleton rare missense variants were identified in four separate NTD patients17.
3. One Carbon Metabolism and NTDs
3.1. Dihydrofolate Reductase (DHFR)
After uptake, folic acid, the form commonly delivered in peri-conceptional multivitamins and fortified diets, must then be reduced to THF before it can participate in one carbon reactions. This reduction occurs through the enzyme, dihydrofolate reductase (DHFR). Impairment of this enzyme, as observed by the action of its inhibitor, methotrexate, results in failure of cells to utilize folic acid for OCM2. A 19 base pair deletion in DHFR, previously reported to be associated with unmetabolized folic acid in serum and decreased red blood cell folate19, was found to correlate with increased risk for spina bifida birth outcomes20. This particular variant has been extensively studied in several populations where results have been variable, with some studies suggesting increased risk for NTDs20,21, and others suggesting no correlation or possibly even a protective effect depending on the population being studied22,23. The conflicting results surrounding association of this mutant with NTD risk suggests the contribution of this 19 base deletion may depend on the genetic background of the populations under study.
3.2. Mitochondrial One Carbon Metabolism
Once reduced to the THF form, folates can then be loaded with carbon units derived from carbon donors such as serine, glycine, dimethylglycine, and sarcosine with serine being the predominant contributor in vivo. Those carbon units are then partitioned out of the folate cycle to various functions of OCM such as purine, thymidine, and methionine synthesis. The folate cycle itself, is physically compartmentalized between the cytosol, mitochondria, and nucleus24. Conventionally, it is understood that the majority of carbon units enter the cycle in the mitochondrial compartment, and through folate-mediated one carbon transfers, are increasingly oxidized to produce the single-carbon ion, formate24. The mitochondria-derived formate is then exported to the cytosol where it serves as the predominant carbon pool for cytosolic OCM24. The specific role of mitochondrial OCM is of interest to identifying NTD risk and prevention, since mouse knockout models of certain mitochondrial OCM genes result in defects that are not folate responsive and are instead rescued by formate supplementation. As mentioned earlier, while folic acid is the primary line of defense in NTD prevention, a baseline rate of 5 per 10,000 live births have proven to be unresponsive to folate25. Therefore, variants of mitochondrial OCM genes may contribute to these unpreventable cases, thus increasing NTD risk regardless of maternal folate status.
In the mitochondria, THF can be loaded with carbon units donated from serine via the activity of SHMT2 (the mitochondrial serine hydroxymethyltransferase), from glycine via the Glycine Cleavage System, and dimethylglycine or sarcosine by DMGDH (dimethylglycine dehydrogenase) and SARDH (sarcosine dehydrogenase) respectively. While few variants in SHMT2 and DMGDH have been identified in human patients, at least three SARDH polymorphisms (rs573904, rs2797840, and rs2873817) have been identified as nominally associated with NTD risk26,27. But of all mitochondrial gene products that contribute carbon units to the folate cycle, the Glycine Cleavage System is the most well studied and has the most identified human variants associated with NTDs. During glycine cleavage, the enzyme GLDC (glycine decarboxylase) transfers a carbon unit from glycine to the enzyme, AMT (amino-methyl transferase), which in turn transfers the carbon unit to THF. Knockout of either gene encoding these enzymes results in partially penetrant NTDs in mice28,29, which, in Gldc mutants, could be rescued by formate28. Furthermore, variants in AMT and GLDC that may contribute to human NTD risk have been identified in several studies28,29,30.
After carbon units are loaded onto THF, they are oxidized to formate by the actions of mitochondrial enzymes MTHFD2/2L (methylene tetrahydrofolate dehydrogenase2/2L) and MTHFD1L. MTHFD2 and MTHFD2L are bifunctional, performing both dehydrogenation of 5,10-methylene-THF to 5,10-methyneyl-THF and subsequent hydrolysis of that product to 10-formyl-THF. While mitochondrial 10-formyl-THF may have multiple fates, including synthesis of formyl-methionine for mitochondrial gene translation, MTHFD1L specifically converts the formyl group to a free formate ion, regenerating THF in the process. While no variants in MTHFD2 or MTHFD2L have been associated with NTD risk, a common insertion/deletion polymorphism in MTHFD1L (rs3832406) was found to be loosely associated with NTDs in a human study31. Furthermore, knockout of this gene in mice results in NTD phenotypes similar to null Slc25a32 phenotypes, with Mthfd1l knockouts being similarly unresponsive to folate supplementation and rescuable by formate32.
3.3. Cytosolic One Carbon Metabolism
Once carbon units are exported to the cytosol in the form of mitochondrially-derived formate, and they re-enter the folate cycle through the activity of MTHFD1. MTHFD1 is trifunctional, performing all three reactions carried out by the combined efforts of MTHFD2/2L and MTHFD1L in mitochondria, although typically in the reverse direction given a favorable flux of carbon units entering the cytosol as formate. A meta-analysis of nine studies spanning 4,300 NTD cases suggested that one particular polymorphism of MTHFD1 (rs2236225) increased the likelihood of neural tube defects by 15–30%33.
Cytosolic 10-formyl-THF may contribute its carbon unit to purine synthesis via enzymes coded for by the genes GART and ATIC, both functioning at different steps in purine metabolism. The GART polymorphism, rs2070388, was determined to have an increased odds ratio of 1.89–1.96 in NTD cases compared to controls in an Irish study34.
Cytosolic 5,10-methylene-THF could contribute carbon units to thymidine synthesis via thymidylate synthase (TYMS), to glycine and serine metabolism via the cytosolic serine hydroxymethyltransferase (SHMT1), or it could be reduced to 5-methyl-THF by the enzyme MTHFR (methylene tetrahydrofolate reductase) to feed carbon units into the methionine cycle. When examining single nucleotide polymorphisms associated with NTD risk under conditions of low maternal folate status, SHMT1 variant, rs12939757, was associated with increased NTD risk in the infant34. Interestingly, that same study found some maternal variants of TYMS and MTHFR, as well one infant variant of TYMS to be slightly protective against NTDs35. That study also observed three variants of MTHFD1 (rs2236224, rs2236225, and rs11627387) to increase NTD risk35. Of all OCM genes associated with human NTD risk, MTHFR mutant C677T is probably the most well-studied variant, being associated with a two to four-fold increase in NTD risk36. Other MTHFR variants, such as the A1298C mutant, have also been identified to slightly increase NTD risk36.
3.4. The Methionine Cycle
Carbon units from 5-methyl-THF can be used to synthesize methionine from homocysteine through the methionine synthase enzyme (coded for by MTR) in conjunction with its cofactor, vitamin B12. Methionine synthase reductase (coded by MTRR) is also required to activate the methionine synthase enzyme. A maternal study demonstrated enhanced risk for mothers harboring the rs1808349 MTRR variant, as well MTHFD1 rs2236225, MTHFR rs1801133, and RFC1 rs105122637. While studies have yielded conflicting results on the association of certain MTR variants on NTD risk, one meta-analysis looking at the common A66G mutant did not find increased NTD risk across 8 studies38.
While methionine can be utilized in many aspects of amino acid metabolism, in the methionine cycle it is converted to S-adenosylmethionine (SAM) by methionine adenosyl transferase 1a (MAT1A). Carbon units from SAM are then unloaded by various methyl transferases for methylation of various substrates, including DNA, lipids, and proteins. The product of these demethylations of SAM is S-adenosylhomocysteine, which is converted back to homocysteine to regenerate methionine. Alternatively, homocysteine can be utilized for cysteine metabolism via the transsulfuration pathway to produce the critical antioxidant, glutathione. Increased homocysteine levels are a common risk factor for NTDs39, likely because elevated homocysteine is a biomarker for impaired OCM.
4. Conclusion
Given the known importance of folate and OCM in neural tube closure, it is hardly surprising that variants associated with NTD risk have been found in several OCM related genes. However, it is important to consider that no OCM gene has been singularly identified as causative in any NTD case, and variants in OCM genes are not necessarily found in NTDs at a higher rate than variants in other pathways, such as the planar cell polarity pathway. In many studies, OCM genes were specifically targeted for sequencing and association analysis based on the already established understanding that OCM is a critical component of neural tube development. While more untargeted approaches do uncover novel variants that may be associated with NTD risk, the rare and diverse nature of these birth defects prevent genetic association studies of NTD cases from gaining sufficient statistical power to properly assess risk of any particular variant. In some cases, meta-analyses have been performed to try to overcome this lack of power33. While some common variants are consistently linked to NTDs across multiple studies, such as MTHFR C677T and MTHFD1 rs2236225, the association of other variants with NTDs is often inconclusive. At best, many of these studies are limited to only identifying candidate genes and variants that do not definitively translate into an informative understanding of the genetic architecture underlying NTDs.
All evidence collected over the last 30 years indicates that the genetic component of NTD pathology is best described by the omnigenic model of inheritance, whereby the genetic architecture of complex traits is spread across the genome40, with the risk incurred by variants of “core” genes being determined by the interactions of those variants with other variants in all other genes. This hypothesis is supported by evidence that accumulation of singleton loss-of-function variants in any one individual may be more strongly determinative of NTD risk than any one variant in any particular gene41. Therefore, while OCM genes clearly represent a core mechanistic component of neural tube development, and damaging variants in this pathway may confer increased risk for human NTDs, deepening our understanding of the genetic etiology of NTDs will require understanding how these variants interact with an individual’s global genomic landscape. As such, developing future NTD-prevention strategies will undoubtedly require a precision medicine approach.
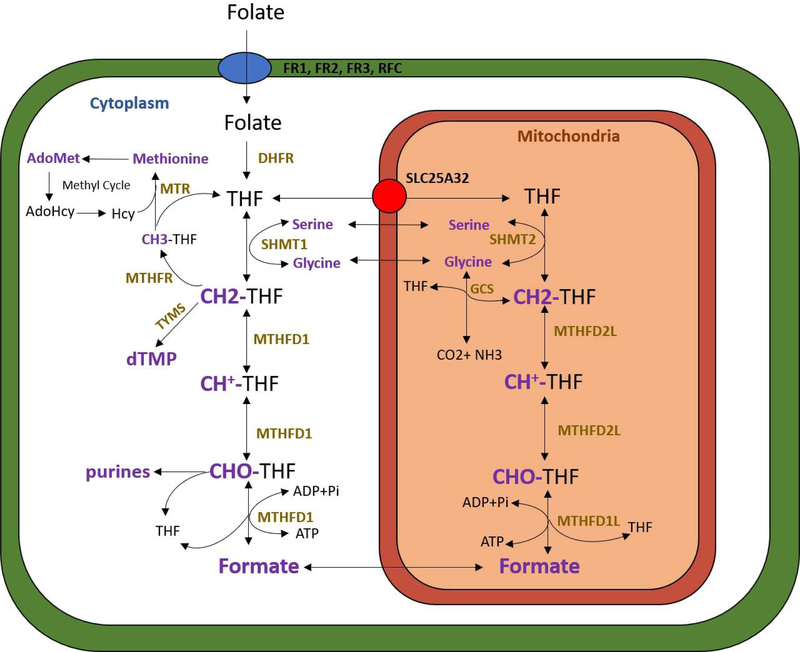
Figure 1. Overview of folate one carbon metabolism.
Transport of folates into the cytosol and mitochondria are depicted by the blue and red circles respectively. Compartmentalization of one carbon metabolism between the cytosol and mitochondria is demonstrated, with key enzymes participating in one carbon reactions displayed in gold font. The Glycine Cleavage System (GCS) includes the enzymes AMT and GLDC. Carbon units, carbon donors, and carbon acceptors are displayed in purple font. CH3-THF = 5-methyl-THF, CH2-THF = 5,10-methylene-THF, CH+-THF = 5,10-methenyl-THF, CHO-THF = 10-formyl-THF. *This figure was modified from our collaborators’ in Momb et al. 201332.
Table 1.
The phenotypes of folate transporters knock out mice.
| Mouse gene | mouse phenotypes | References |
|---|---|---|
| Folr1 | embryonic lethal at E10.0 cranial neural tube defects (E9.5) normal | Piedrahita et al. 19998 |
| Folr2 | normal | Piedrahita et al., 19998 |
| Rfc (Slc19a1) | embryonic lethal at E6.5 cranial neural tube defects with EIIA-Cre (E9.5), Wnt1-Cre (E10.5) and TTR-Cre (E9.5) | Gelineau-van Waes et al., 200813
Toriyama et al., 201742 |
| Pcft (Slc46a1) | microcytic normochromic anemia/ pancytopenia systemic folate deficiency | Salojin et al., 201116 |
| Mft (Slc25a32) | exencephaly, craniorachischisis (E12.5) | Kim et al., 201817 |
Highlights.
We provide an overview of folate transport and one carbon metabolism, reviewing the genes in this pathway thus far identified as contributing variants to human NTD risk.
The rare and diverse nature of NTDs coupled with their complex, multifactorial etiology present a continuing challenge to elucidating their underlying genetic architecture.
Developing future prevention strategies will likely require a precision medicine approach by understanding how these variants influence NTD risk within an omnigenic context.
Acknowledgments
Funding
The authors graciously acknowledge the generous support from NICHD grants HD081216, HD083809, HD067244, HD093758 and HD095520. In addition, Dr. Finnell is a member of the T32 ES027801 Training in Precision Environmental Health training grant funding by the National Institute of Environmental Health Sciences. He also receives financial support from the endowment of the William T. Butler, M.D., Distinguished Chair at Baylor College of Medicine.
Abbreviations
- NTD
Neural Tube Defect
- OCM
One Carbon Metabolism
- FR
Folate Receptor
- PCFT
Proton-Coupled Folate Transporter
- RFC
Reduced Folate Carrier
- HFM
Hereditary Folate malabsorption
- CFD
Cerebral Folate Deficiency THF: Tetrahydrofolate
- DHFR
Dihydrofolate Reductase
- SHMT1, 2
Serine Hydroxymethyltransferase 1, 2
- SARDH
Sarcosine Dehydrogenase
- DMGDH
Dimethylglycine dehydrogenase
- GLDC
Glycine Decarboxylase
- AMT
Amino-methyl transferase
- MTHFD1, 1L, 2, 2L
Methylene Tetrahydrofolate Dehydrogenase 1, 1L, 2, 2L
- TYMS
Thymidylate Synthase
- MTHFR
Methylene Tetrahydrofolate Reductase
- MTR
Methionine Synthase
- MTRR
Methionine Synthase Reductase
- MAT1A
Methionine Adenosyl Transferase 1A
- SAM
S-Adenosylmethionine
Footnotes
Conflict of Interest
None declared.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Blencowe H, Kancherla V, Moorthie S, Darlison MW, and Modell B. (2018) Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis, Ann NY Acad Sci 1441(1): 31–46. [DOI] [PubMed] [Google Scholar]
- [2].Ducker GS, and Rabinowitz JD (2017) One-carbon metabolism in health and disease, Cell Metabolism 25, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wallingford J, Niswander LA, Shaw GM, and Finnell RH (2013) The continuing challenge of understanding, preventing, and treating neural tube defects, Science 339, 1222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen C, Ke J, Zhou XE, Yi W, Brunzelle JS, Li J, Yong EL, Xu HE, and Melcher K. (2013) Structural basis for molecular recognition of folic acid by folate receptors, Nature 500, 486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao R, Min SH, Wang Y, Campanella E, Low PS, and Goldman ID (2009) A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis, J Biol Chem 284, 4267–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boshnjaku V, Shim KW, Tsurubuchi T, Ichi S, Szany EV, Xi G, Mania-Farnell B, McLone DG, Tomita T, and Mayanil CS (2012) Nuclear localization of folate receptor alpha: a new role as a transcription factor, Sci Rep 2, 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mohanty V, Shah A, Allender E, Siddiqui MR, Monick S, Ichi S, Mania-Farnell B, Mclone DG, Tomita T, and Mayanil CS (2016) Folate receptor alpha upregulates Oct4, Sox2, and Klf4 and downregulates miR-138 and miR-let-7 in cranial neural crest cells, Stem Cells 34, 2721–2732. [DOI] [PubMed] [Google Scholar]
- [8].Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RG, and Finnell RH (1999) Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development, Nat Genet 23, 228–232. [DOI] [PubMed] [Google Scholar]
- [9].Rothenberg SP, da Costa MP, Sequeira JM, Cracco J, Roberts JL, Weedon J, and Quadros EV (2004) Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect, N Engl J Med 350, 134–142. [DOI] [PubMed] [Google Scholar]
- [10].Findley TO, Tenpenny JC, O’Byrne MR, Morrison AC, Hixson JE, Northrup H, and Au KS (2017) Mutations in folate transporter genes and risk for human myelomeningocele, Am J Med Genet A 173, 2973–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, Dechent P, Wevers R, Grosso S, and Gartner J. (2009) Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism, Am J Hum Genet 85, 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang CH, Sirotnak FM, and Dembo M. (1984) Interaction between anions and the reduced folate/methotrexate transport system in L1210 cell plasma membrane vesicles: directional symmetry and anion specificity for differential mobility of loaded and unloaded carrier, J Membr Biol 79, 285–292. [DOI] [PubMed] [Google Scholar]
- [13].Gelineau-van Waes J, Heller S, Bauer LK, Wilberding J, Maddox JR, Aleman F, Rosenquist TH, and Finnell RH (2008) Embryonic development in the reduced folate carrier knockout mouse is modulated by maternal folate supplementation, Birth Defects Res A Clin Mol Teratol 82, 494–507. [DOI] [PubMed] [Google Scholar]
- [14].De Marco P, Calevo MG, Moroni A, Merello E, Raso A, Finnell RH, Zhu H, Andreussi L, Cama A, and Capra V. (2003) Reduced folate carrier polymorphism (80A-->G) and neural tube defects, Eur J Hum Genet 11, 245–252. [DOI] [PubMed] [Google Scholar]
- [15].Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, and Goldman ID (2006) Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption, Cell 127, 917–928. [DOI] [PubMed] [Google Scholar]
- [16].Salojin KV, Cabrera RM, Sun W, Chang WC, Lin C, Duncan L, Platt KA, Read R, Vogel P, Liu Q, Finnell RH, and Oravecz T. (2011) A mouse model of hereditary folate malabsorption: deletion of the PCFT gene leads to systemic folate deficiency, Blood 117, 4895–4904. [DOI] [PubMed] [Google Scholar]
- [17].Kim J, Lei Y, Guo J, Kim SE, Wlodarczyk BJ, Cabrera RM, Lin YL, Nilsson TK, Zhang T, Ren A, Wang L, Yuan Z, Zheng YF, Wang HY, and Finnell RH (2018) Formate rescues neural tube defects caused by mutations in SLC25A32, PNAS 115, 46904695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lawrence SA, Hackett JC, and Moran RG (2011) Tetrahydrofolate recognition by the mitochondrial folate transporter, Journal of Biological Chemistry 286, 31480–31489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kalmbach RD, Choumenkovitch SF, Troen AP, Jacques PF, D’Agostino R, and Selhub J. (2008) A 19-base pair deletion polymorphism in dihydrofolate reductase is associated with increased unmetabolized folic acid in plasma and decreased red blood cell folate, J Nutr 138(12): 2323–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johnson WG, Stenroos ES, Spychala JR, Chatkupt S, Ming SX, and Buyske S. (2004) New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): a risk factor for spina bifida acting in mothers during pregnancy?, Am J Med Genet 124A, 339–345. [DOI] [PubMed] [Google Scholar]
- [21].Prasoona KR, Sunitha T, Srinadh B, Muni Kamari T, and Jyothy A. (2018) Maternal association and influence of DHFR 19 bp deletion variant predisposes foetus to anencephaly susceptibility: a family-based triad study, Biomarkers 23, 640–646. [DOI] [PubMed] [Google Scholar]
- [22].van der Linden IJ, Nguyen U, Heil SG, Franke B, Vloet S, Gellekink H, Heijer M, and Bloom HJ (2007) Variation and expression of dihydrofolate reductase (DHFR) in relation to spina bifida, Mol Genet Metab 91, 98–103. [DOI] [PubMed] [Google Scholar]
- [23].Parle-McDermott A, Pangilinan F, Mills JL, Kirke PN, Gibney ER, Troendle J, O’Leary VB, Molloy AM, Conley M, Scott JM, and Brody LC (2007) The 19-bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR) may decrease rather than increase risk for spina bifida in the Irish population, Am J Med Genet 143A, 1174–1180. [DOI] [PubMed] [Google Scholar]
- [24].Tibbetts AS, and Appling DR (2010) Compartmentalization of mammalian folate-mediated one-carbon metabolism, Annu Rev Nutr 30, 57–81. [DOI] [PubMed] [Google Scholar]
- [25].Heseker HB, Mason JB, Selhub J, Rosenberg IH, and Jacques PF (2009) Not all cases of neural tube-defect can be prevented by increasing the uptake of folic acid, British Journal of Nutrition 102, 173–180. [DOI] [PubMed] [Google Scholar]
- [26].Piao W, Guo J, Bao Y, Wang F, Zhang T, Huo J, and Zhang K. (2016) Analysis of polymorphisms of genes associated with folate-mediated one-carbon metabolism and neural tube defects in Chinese Han Population, Birth Defecst Research A: Clinical and Molecular Teratology 106, 232–239. [DOI] [PubMed] [Google Scholar]
- [27].Franke B, Vermeulen SHHM, Steegers-Theunissen RPM, Coenen MJ, Schijvenaars MMVAP, Scheffer H, den Heijer M, and Blom HJ (2009) An association study of 45 folate related genes in spina bifida: involvment of Cubilin (CUBN) and tRNA Aspartic Methyltransferase 1 (TRDMT1), Birth Defecs Research Part A 85, 216–226. [DOI] [PubMed] [Google Scholar]
- [28].Narisawa A, Komatsuzaki S, Kikuchi A, Niihori T, Aoki Y, Fujiwara K, Tanemura M, Hata A, Suzuki Y, Relton CL, Grinham J, Leung KY, Partridge D, Robinson A, Stone V, Gustavsson P, Stanier P, Coop AJ, Greene ND, Tominaga T, Matsubara Y, and Kure S. (2012) Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans, Human Molecular Genetics, 21, 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pai YJ, Leung KY, Savery D, Hutchin T, Prunty H, Heales S, Brosnan ME, Brosnan JT, Copp AJ, and Greene ND (2015) Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice, Nature Commincations, 4, 6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shah RH, Northrup H, Hixson JE, Morrison AC, and Ku KS (2016) Genetic association of the glycine cleavage system genes and myelomeningocele, Birth Defects Research Part A: Clinical and Molecular Teratology, 106, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Parle-McDermott A, Pangilinan F, O’Brien KK, Mills JL, Magee AM, Troendle J, Sutton M, Scott JM, Kirke PN, Molloy AM, and Brody LC (2009) A common variant in MTHFD1L is associated with neural tube defects and mRNA splicing efficiency, Human Mutations, 30, 1650–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Momb J, Lewandoswki JP, Bryant JD, Fitch R, Surman DR, Vokes SA, and Appling DR (2013) Deletion of Mthfd1l causes embyronic lethality and neural tube and craniofacial defects in mice, PNAS 110, 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jiang J, Zhang Y, Wei L, Sun Z, and Liu Z. (2014) Association between MTHFD1 G1958A polymorphism and neural tube defects susceptibility: a meta-analysis, PLoS ONE 9, e101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pangilinan F, Molloy AM, Mill JL, Troendle JF, Parle-McDermott A, Signore C, O’Leary VB, Chines P, Seay JM, Geiler-Samerotte K, Mitchell A, VanderMeer JE, Krebs KM, Sanchez A, Comman-Homonoff J, Stone N, Conley M, Kirke PN, Shane B, Scott JM, and Brody LC (2012) Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects, BMC Medical Genetics 13, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Etheredge A, Finnell RH, Carmichael SL, Lammer EJ, Zhu H, Mitchell LE, and Shaw GM (2012) Maternal and infant gene-folate interactions and the risk of nueral tube defects, American Journal of Medical Genetics 158A, 2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yaliwal LV, and Desai RM (2012) Methylenetetrahydrofolate reductase mutations, a genetic cause for familial recurrent neural tube defects, Indian Journal of Human Genetic 18, 122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cai C, Fang Y, Shu J, Zhao L, Zhang R, Cao L, Wang Y, Zhi X, Cui H, Shi O, and Liu W. (2019) Association of neural tube defects with maternal alterations and genetic polymorphisms in one-carbon metabolic pathway, Italian Journal of Pediatrics 45, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang T, Lou J, Zhong R, Wu J, Zou L, Sun Y, Lu X, Liu L, Miao X, and Xiong G. (2013) Genetic variants in the folate pathway and the risk of neural tube defects: a meta-analysis of the published literature, PLoS ONE 8, e59570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Steegers-Theunissen RP, Boers GH, Trijbels FJ, Finkelstein JD, Blom HJ, Thomas CM, Borm GF, Wouters MG and Eskes TK. (1994) Maternal hyperhomocysteinemia: a risk factor for neural-tube defects? Metabolism 43, 1475–1480. [DOI] [PubMed] [Google Scholar]
- [40].Boyle EA, Li YI, and Pritchard JK (2017) An expanded view of complex traits: from polygenic to omnigenic, Cell 169, 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen Z, Lei Y, Zheng Y, Aguiar-Pulido V, Ross ME, Peng R, Jin L, Zhang T, Finnell RH, and Wang H. (2018) Threshhold for neural tube defect risk by accumulated singleton loss-of-function variants, Cell Research 0, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Toriyama M, Toriyama M, Wallingford JB, and Finnell RH (2017) Folate-dependent methylation of septins governs ciliogenesis during neural tube closure, FASEB J 31, 3622–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]