Abstract
Upstream open reading frames (uORFs) are tissue-specific cis-regulators of protein translation. Isolated reports have shown that variants that create or disrupt uORFs can cause disease. Here, in a systematic genome-wide study using 15,708 whole genome sequences, we show that variants that create new upstream start codons, and variants disrupting stop sites of existing uORFs, are under strong negative selection. This selection signal is significantly stronger for variants arising upstream of genes intolerant to loss-of-function variants. Furthermore, variants creating uORFs that overlap the coding sequence show signals of selection equivalent to coding missense variants. Finally, we identify specific genes where modification of uORFs likely represents an important disease mechanism, and report a novel uORF frameshift variant upstream of NF2 in neurofibromatosis. Our results highlight uORF-perturbing variants as an under-recognised functional class that contribute to penetrant human disease, and demonstrate the power of large-scale population sequencing data in studying non-coding variant classes.
Subject terms: Genome informatics, Genomics, Medical genomics, Genetics research
Upstream open reading frames (uORFs), located in 5’ untranslated regions, are regulators of downstream protein translation. Here, Whiffin et al. use the genomes of 15,708 individuals in the Genome Aggregation Database (gnomAD) to systematically assess the deleteriousness of variants creating or disrupting uORFs.
Introduction
Upstream open reading frames (uORFs) are ORFs encoded within the 5’ untranslated regions (5’UTRs) of protein coding genes. uORFs are found upstream of around half of all known genes1, and are important tissue-specific cis-regulators of translation. Active translation of a uORF typically reduces downstream protein levels by up to 80%1. There are strong signatures of negative selection acting on these elements, with fewer upstream start codons (uAUGs) present in the human genome than would be expected by chance1–3. In addition, the start codons of uORFs have been shown to be the most conserved sites in 5’UTRs1, supporting the importance of uORFs in the regulation of protein levels.
In humans, translation is initiated when the small ribosomal subunit, which scans from the 5’ end of the mRNA, recognises an AUG start codon4. The likelihood of an AUG initiating translation is dependent on local sequence context, and in particular the degree of similarity to the Kozak consensus sequence5,6. uORFs can inhibit translation through multiple mechanisms. For some genes, uORFs may be translated into a small peptide which can directly inhibit translation by interacting with and stalling the elongating ribosome at or near the uORF stop codon, creating a ‘roadblock’ for other scanning ribosomes7,8. It is also possible for this small peptide to have a distinct biological function9; however, in general uORFs do not show strong evidence for conservation of their amino acid sequence2,10. For other genes, translation from a uAUG appears to be sufficient to inhibit translation of the downstream protein, with the small uORF peptide only produced as a by-product.
Mechanisms of leaky scanning (whereby a scanning ribosome may bypass an uAUG), re-initiation (where the small ribosomal subunit remains bound to the mRNA and translation is re-initiated at the canonical AUG), and the existence of internal ribosome entry sites (from which the ribosome can start scanning part-way along the RNA), can all act to attenuate inhibition by uORFs, adding to the complexity of translational regulation10–12. Termination at a uORF stop codon can also trigger the nonsense-mediated decay pathway, further magnifying the inhibitory effects of uORFs11,13. To date, studies of translational regulation by individual uORFs have mainly been restricted to model organisms.
Recently, large scale studies have assessed the global translational repression ability of uORFs: in vertebrates, uORF-containing transcripts are globally less efficiently translated than mRNAs lacking uORFs, with this effect mediated by features of both sequence and structure2. Similarly, polysome profiling of 300,000 synthetic 5’UTRs identified uORFs and uAUGs as strongly repressive of translation, with the strength of repression dependent on the surrounding Kozak consensus sequence14.
Although 5’UTRs are typically not assessed for variation in either clinical or research settings, having been excluded from most exome capture target regions, there are several documented examples of variants that create or disrupt a uORF playing a role in human disease1,15–21. These studies have focused on single gene disorders or candidate gene lists, often when no causal variant was identified in the coding sequence. No study to date has characterised the baseline population incidence of these variants.
Here we describe a systematic genome-wide study of variants that create and disrupt human uORFs, and characterise the contribution of this class of variation to human genetic disease. We use the allele frequency spectrum of variants in 15,708 whole-genome sequenced individuals from the Genome Aggregation Database (gnomAD)22 to explore selection against variants that either create uAUGs or remove the stop codon of existing uORFs. Finally, we demonstrate that these variants make an under-recognised contribution to genetic disease.
Results
uAUG-creating variants are under strong negative selection
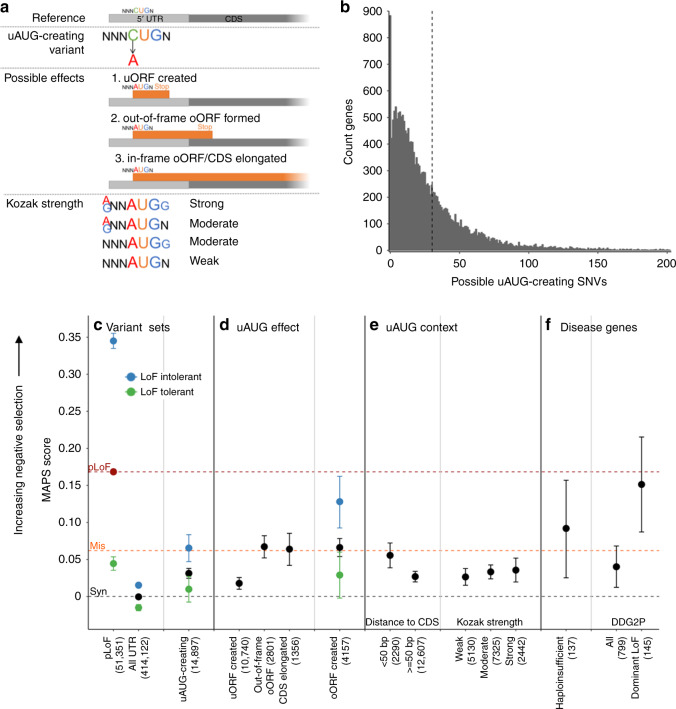
To estimate the deleteriousness of variants that create a novel AUG start codon upstream of the canonical coding sequence (CDS), we assessed the frequency spectrum of uAUG-creating variants observed in gnomAD (Fig. 1a). We identified all possible single nucleotide variants (SNVs) in the UTRs of 18,593 canonical gene transcripts (see Methods) that would create a new uAUG, yielding 562,196 possible SNVs, an average of 30.2 per gene (Fig. 1b). Of these, 15,239 (2.7%) were observed at least once in whole genome sequence data from 15,708 individuals in gnomAD (Supplementary Fig. 1a), upstream of 7697 distinct genes.
Fig. 1.
uAUG-creating variants have strong signals of negative selection, suggesting they are deleterious. a Schematic of uAUG-creating variants, their possible effects and how the strength of the surrounding Kozak consensus is determined. b The number of possible uAUG-creating SNVs in each of 18,593 genes, truncated at 200 (159 genes have >200). In total we identified 562,196 possible uAUG-creating SNVs, an average of 30.2 per gene (dotted line), with 883 genes having none. c–f MAPS scores (a measure of negative selection) for different variant sets. The number of observed variants for each set is shown in brackets. MAPS for classes of protein-coding SNVs are shown as dotted lines for comparison (synonymous–grey, missense–orange, and predicted loss-of-function (pLoF)–red point and red dotted line). Errors bars were calculated using bootstrapping (see methods). c While overall UTR variants display a selection signature similar to synonymous variants, uAUG-creating variants have significantly higher MAPS (indicative of being more deleterious; permuted P < 1 × 10−4). Variants are further subdivided into those upstream of, or within genes tolerant (green dot) and intolerant (blue dot) to LoF22, with uAUG-creating variants upstream of LoF intolerant genes showing significantly stronger signals of selection than those upstream of LoF tolerant genes (permuted P = 1 × 10−4). pLoF variants are likewise stratified for comparison. d uAUG-creating variants that create an oORF or elongate the CDS show a significantly higher signal of selection than uORF-creating variants (P < 1 × 10−4; oORF created:out-of-frame oORF and CDS elongated combined). e The deleteriousness of uAUG-creating variants depends on the context into which they are created, with stronger selection against uAUG-creation close to the CDS, and with a stronger Kozak consensus sequence. f uAUG-creating variants are under strong negative selection upstream of genes manually curated as haploinsufficient26 and developmental disorder genes reported to act via a dominant LoF mechanism. Abbreviations: CDS coding sequence, uAUG upstream AUG, uORF upstream open reading frame, oORF overlapping open reading frame, MAPS mutability adjusted proportion of singletons, pLoF predicted loss-of-function, DDG2P Developmental Disease Gene to Phenotype
We compared the mutability adjusted proportion of singletons (MAPS) score, a measure of the strength of selection acting against a variant class23, for 14,897 observed high-quality autosomal uAUG-creating SNVs to other classes of coding and non-coding SNVs (see methods). As negative selection acts to prevent deleterious variants from increasing in frequency, damaging classes of variants have skewed frequency spectra, with a higher proportion appearing as singletons (i.e., observed only once in the gnomAD data set),23 reflected in a higher MAPS score. Whilst all observed UTR SNVs have an overall MAPS score almost identical to synonymous variants, uAUG-creating SNVs have a significantly higher MAPS score (permuted P < 1 × 10−4; Fig. 1c), indicating a considerable selective pressure acting to remove these from the population.
We next evaluated subsets of uAUG-creating variants predicted to have distinct functional consequences. In addition to creating distinct uORFs, uAUGs may result in overlapping ORFs (oORFs) where the absence of an in-frame stop codon within the UTR results in an ORF that reads into the coding sequence, either in-frame (elongating the CDS), or out-of-frame (Fig. 1a). uAUG-creating variants that form oORFs have a significantly higher MAPS score than uORF-creating variants (permuted P < 1 × 10−4), and equivalent to missense variants in coding regions (Fig. 1c; Supplementary Fig. 1a).
We also investigated the context of uAUG-creating variants and find that uAUGs created within 50 bp of the CDS have higher MAPS than those created further away (permuted P = 0.0042), although this may be driven by the higher propensity of these variants to form oORFs. We did not observe a significantly greater MAPS score for uAUG-creating variants arising on a background of a strong Kozak consensus, though we observe a trend in this direction (Fig. 1c).
Given that uAUGs are expected to dramatically decrease downstream protein levels, we hypothesised that uAUG-creating variants would behave similarly to pLoF variants and thus be more deleterious when arising upstream of genes intolerant to LoF variation. Indeed, we show a significantly higher MAPS score for uAUG-creating SNVs upstream of genes which are most intolerant to pLoF variants (top sextile of LOEUF score22; 3193 genes) when compared to those that are most tolerant (bottom sextile; permuted P < 1 × 10−4; Fig. 1c). To ensure that the observed increase in MAPS score upstream of pLoF intolerant genes is not purely because the UTRs of these genes are more highly conserved, we compared the conservation of potential uAUG sites with the remainder of the 5’UTR, across all sextiles of LOEUF score. Overall, a significantly higher proportion of possible uAUG-creating bases have phyloP scores >2 (10.3%), when compared to all other UTR bases (8.6%; Fisher’s P < 1 × 10−100), with the size of this effect increasing as the corresponding genes become more intolerant to pLoF variants (Supplementary Fig. 2).
Next, we calculated MAPS for uAUG-creating variants arising upstream of 1,659 genes known to cause developmental disorders (DD; confirmed or probable genes from the Developmental Disease Gene to Phenotype (DDG2P) database). While uAUG-creating variants upstream of all DD genes do not show a signal of selection above all observed uAUG-creating variants, the MAPS score is significantly inflated when limiting to 279 DD genes with a known dominant LoF mechanism (permuted P = 0.0012; Fig. 1c).
Variants that disrupt uORF stop codons are selected against
As uAUG-creating variants that form oORFs have a significantly higher MAPS score than those with an in-frame UTR stop codon, we hypothesised that variants that disrupt the stop site of existing uORFs should also be under selection (Fig. 2a). These stop-removing variants could either be SNVs that change the termination codon to one that codes for an amino acid, or frameshifting indels within the uORF sequence that cause the uORF to read through the normal stop codon. If there is no other in-frame stop codon before the CDS will result in an oORF.
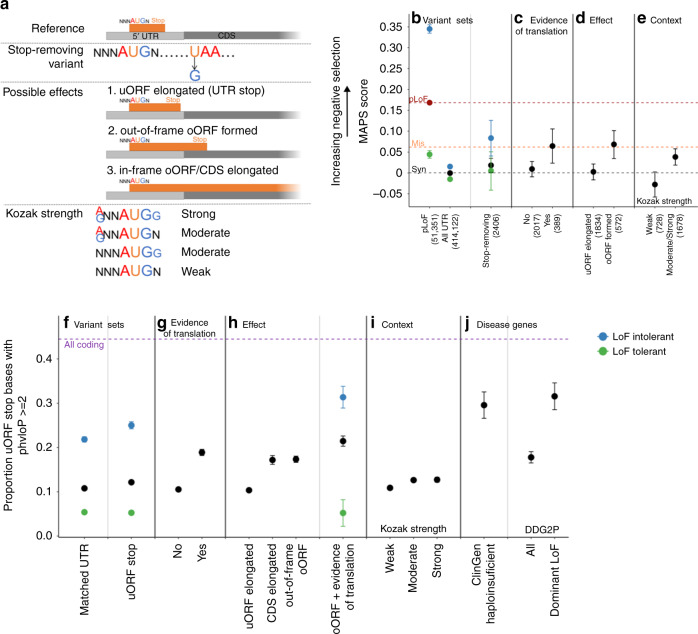
Fig. 2.
uORF stop codons are highly conserved and stop-removing variants show strong signals of negative selection. a Schematic of uORF stop-removing variants, their possible effects, and how the strength of the surrounding Kozak consensus is determined. b–e MAPS scores (a measure of negative selection) for different variant sets. The number of observed variants for each set is shown in brackets. MAPS for classes of protein-coding SNVs are shown as dotted lines for comparison (synonymous–black, missense–orange and predicted loss-of-function (pLoF)–red point and red dotted line). Confidence intervals were calculated using bootstrapping (see methods). b Stop-removing SNVs have a nominally higher MAPS score than all UTR SNVs (permuted P = 0.030). Variants are further subdivided into those upstream of, or within genes tolerant (green dot) and intolerant (blue dot) to LoF22, with pLoF variants likewise stratified for comparison. Stop-removing SNVs (c) with evidence of translation (in sorfs.org) and (d) that create an oORF have signals of selection equivalent to missense variants. e A significantly higher MAPS is calculated for stop-removing variants where the uORF start site has a strong/moderate Kozak consensus, compared to those with a weak Kozak (permuted P = 7 × 10−4). f–j Since MAPS is only calculated on observed variants, we also looked at the conservation of all possible uORF stop site bases, reporting the proportion of bases with phyloP scores >2. All coding bases are shown as a purple dotted line for comparison. f The stop sites of predicted uORFs are significantly more conserved than all UTR bases matched on gene and distance from the CDS (Fisher’s P = 1.8 × 10−17). uORF stop bases are most highly conserved when (g) the uORF has evidence of translation, (h) the variant results in an oORF, (i) the uORF start site has a strong/moderate Kozak consensus, and (j) upstream of curated haploinsufficient genes and developmental genes with a known dominant LoF disease mechanism. Error bars represent 95% binomial confidence intervals. CDS coding sequence, uORF upstream open reading frame, oORF overlapping open reading frame, MAPS mutability adjusted proportion of singletons, DDG2P Developmental Disease Gene to Phenotype
We identified all possible SNVs that would remove the stop codon of a predicted uORF (n = 169,206; see methods), and calculated the MAPS score for 2,406 such variants observed in gnomAD. Stop-removing SNVs have a nominally higher MAPS score than all UTR SNVs (permuted P = 0.030). This difference is greater when specifically considering stop-removing SNVs which are upstream of LoF intolerant genes (permuted P = 0.0012), result in an oORF (permuted P = 2 × 10−4), or where the uORF has either prior evidence of translation (documented in sorfs.org24; permuted P = 0.0049), or a strong/moderate Kozak consensus (permuted P = 7 × 10−4; Fig. 2b).
As the power of MAPS is limited by the small number of stop-removing variants in each category observed in gnomAD, we performed a complementary analysis investigating base level conservation at all uORF stop sites using PhyloP25. A significantly greater proportion of uORF stop site bases have PhyloP scores >2 (12.2%) compared to UTR bases matched by gene and distance from the CDS (10.8%; Fisher’s P = 1.8 × 10−17; Fig. 2c). This proportion is significantly higher where there is evidence supporting translation of the uORF (18.9%; Fisher’s P = 3.6 × 10−83) or when removing the stop would result in an oORF (either in-frame or out-of-frame; 17.2% and 17.4%, respectively; Fisher’s P = 3.0 × 10−25 and 2.6 × 10−47, respectively). Furthermore, a greater proportion of stop site bases have PhyloP scores >2 when the uORF start codon has a strong or moderate Kozak when compared to a weak Kozak consensus (12.7% vs 10.9%; Fisher’s P = 5.5 × 10−10; matched UTR bases Fisher’s P = 0.88; Fig. 2c).
The increased power of this analysis enables us to convincingly demonstrate that uORF stop sites upstream of (1) LoF intolerant genes, (2) genes manually curated as haploinsufficient26, and (3) developmental disorder genes with a dominant LoF mechanism, are all highly conserved. Stop sites upstream of genes in these groups have 21.9%, 29.6% and 31.6% of bases with PhyloP >2, respectively (Fisher’s P = 8.2 × 10−250, 4.7 × 10−43 and 1.4 × 10−52 compared to all stop site bases, respectively; Fig. 2c), suggesting that removing these stop sites is likely to be deleterious.
Specific genes are sensitive to uAUG-perturbing variants
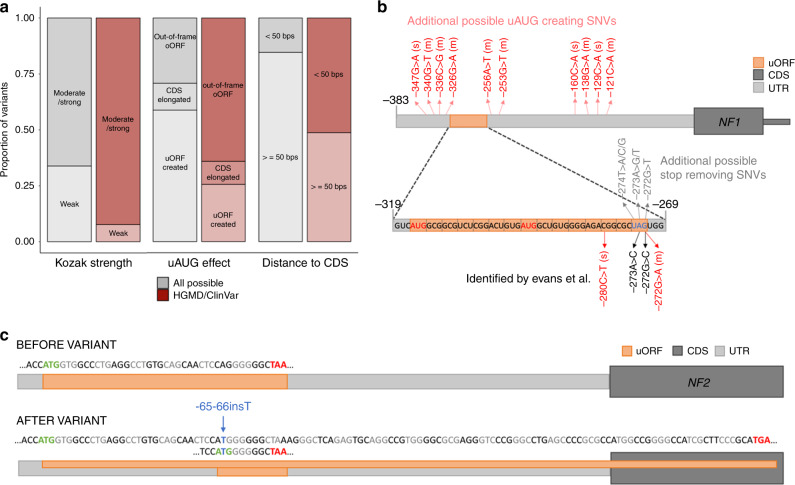
We searched the Human Gene Mutation Database (HGMD)27 and ClinVar28 for uORF-creating or uORF-disrupting variants, identifying 39 uAUG-creating and four stop-removing (likely) pathogenic/disease mutations in 37 different genes. All four stop-removing variants disrupt uORFs with uAUGs in a strong or moderate Kozak consensus and result in an oORF overlapping the CDS (Supplementary Table 2). Compared to all possible uAUG-creating variants in these 37 genes, the 39 reported disease-causing uAUG-creating variants (Supplementary Table 1) are significantly more likely to be created into a moderate or strong Kozak consensus (binomial P = 3.5 × 10−4), create an out-of-frame oORF (binomial P = 1.1 × 10−5), and be within 50 bp of the CDS (binomial P = 3.9 × 10−7; Fig. 3a). These results support the assertion that the variant classes identified by MAPS as under strongest negative selection are most likely to be disease causing.
Fig. 3.
The role of uAUG-creating and uORF stop-removing variants in disease. a The proportion of 39 uAUG variants observed in HGMD and ClinVar (red bars) that fit into different sub-categories compared to all possible uAUG-creating SNVs (grey bars) in the same genes (n = 1022). Compared to all possible uAUG-creating variants, uAUG-creating variants observed in HGMD/ClinVar were significantly more likely to be created into a moderate or strong Kozak consensus (binomial P = 3.5 × 10−4), create an out-of-frame oORF (binomial P = 1.1 × 10−5), and be within 50 bp of the CDS (binomial P = 3.9 × 10−7). b Schematic of the NF1 5’UTR (light grey) showing the location of an existing uORF (orange) and the location of variants previously identified in patients with neurofibromatosis29 in dark red (uAUG-creating) and black (stop-removing). uAUG-creating variants are annotated with the strength of the surrounding Kozak consensus in brackets (“s” for strong and “m” for moderate). All four published variants result in formation of an oORF out-of-frame with the CDS. Also annotated are the positions of all other possible uAUG-creating variants (light red; strong and moderate Kozak only), and stop-removing variants (grey) that would also create an out-of-frame oORF. c Schematic of the NF2 5’UTR (grey) showing the effects of the −65-66insT variant. The reference 5′UTR contains a uORF with a strong Kozak start site. Although the single-base insertion creates a novel uAUG which could be a new uORF start site, it also changes the frame of the existing uORF, so that it overlaps the CDS out-of-frame (forms an oORF). We predict this is the most likely mechanism of pathogenicity. CDS coding sequence, uORF upstream open reading frame, oORF overlapping open reading frame, HGMD the human gene mutation database
This analysis highlights disease genes where aberrant translational regulation through uORFs is an important disease mechanism. Previous analysis of the NF1 gene in 361 patients with neurofibromatosis type 1 identified four 5’UTR variants as putatively disease-causing29. These variants were found in six unrelated probands, all of whom were negative for coding variants in both NF1 and SPRED1. Three of the four variants either occurred de novo or were shown to segregate with disease in the family (Supplementary Fig. 3a). While uAUG creation was proposed as the mechanism behind two of these variants, we now show that the other two variants both disrupt the stop codon of an existing uORF, resulting in an oORF which is out-of-frame with the CDS. This existing uORF has two start sites, both with strong Kozak consensus, and has prior evidence of active translation24. In Fig. 3b, we show these four variants along with an additional six stop-removing and ten uAUG-creating variants that would be predicted to also cause neurofibromatosis type 1 through the same mechanism if observed. In addition to these sixteen SNVs, indels that create high-impact uAUGs (oORF creating with strong/moderate Kozak consensus) or that cause a frameshift within the sequence of the existing uORF, resulting in an oORF, would also be predicted to cause disease.
A second example is IRF6, where three uAUG-creating variants have been identified in seven patients with Van de Woude syndrome30,31. These variants all arise in the context of a strong or moderate Kozak consensus and result in an out-of-frame oORF. There are nine additional possible uAUG-creating variants that would be predicted to yield the same effect in IRF6 (Supplementary Fig. 4), suggesting it would be prudent to screen Van de Woude patients across all twelve sites.
Genes where perturbing uORFs is likely important in disease
To guide the research and clinical identification of uAUG-creating and stop-removing variants (referred to collectively as uORF-perturbing variants), we set about identifying genes where these variants are likely to be of high importance. Investigating 17,715 genes with annotated 5’UTRs and at least one possible uORF-perturbing variant, we first identified 4,986 genes where uORF-perturbing variants are unlikely to be deleterious: genes with existing oORFs (strong/moderate Kozak or evidence of translation), with predicted high-impact uORF-perturbing SNVs of appreciable frequency in gnomAD (>0.1%), with no possible high-impact uORF-perturbing SNVs, or that are tolerant to LoF (see methods; Supplementary Fig. 5a). Interestingly, these genes include 453 LoF intolerant (14.2% of most constrained LOEUF sextile) and 163 curated haploinsufficient or LoF disease genes (14.6%). Of the remaining 12,729 genes considered, 3191 (25.1%) are LoF-intolerant, known haploinsufficient or LoF disease genes and hence are genes where uORF-perturbing variants have a high likelihood of being deleterious (Fig. 4a). Despite only 18.0% of all classified genes falling into this high likelihood category (19.0% of all UTR bases when accounting for UTR length), 79% of uORF-perturbing variants in HGMD and ClinVar are found upstream of these genes (Fisher’s P = 1.6 × 10−9; Fig. 4b).
Fig. 4.
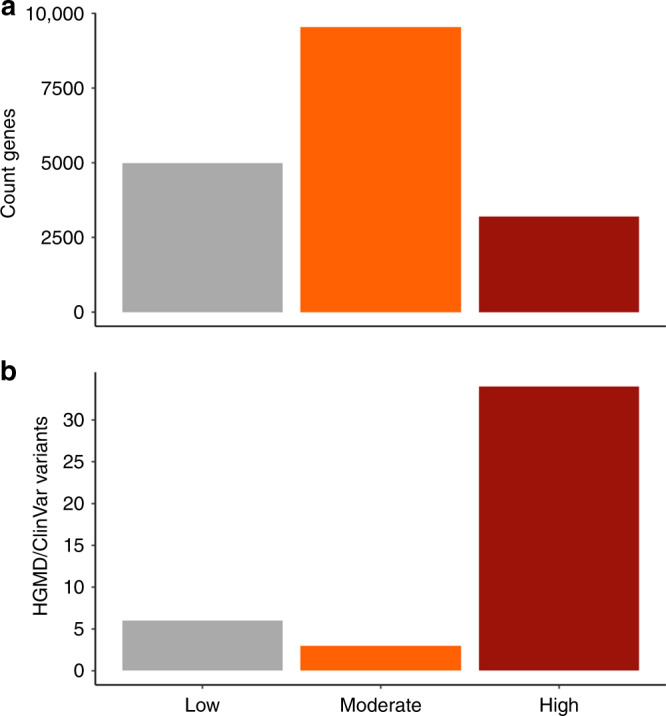
Identifying genes where uORF creating or disrupting variants are likely to have a role in disease. Genes were split into three distinct categories representing a ‘low’, ‘moderate’ and ‘high’ likelihood that uORF-perturbing variants are important. Low likelihood genes include those with existing oORFs, common (>0.1%) oORF creating variants in gnomAD or that are tolerant to LoF. Those in the high likelihood category are remaining genes that are LoF-intolerant or where haploinsufficient or LoF is a known disease mechanism (see methods). a The number of genes in each of the three categories. b The number of uAUG-creating and uORF stop-removing variants in HGMD upstream of genes in each category. Although only 19.2% of all classified genes fall into the high likelihood category (21.4% of all UTR bases when adjusting for UTR length), 83.7% of uORF-perturbing variants identified in HGMD and ClinVar are found upstream of these genes (Fisher’s P = 1.4 × 10−19)
There are 296 genes that have at least 10 possible high-impact uORF-perturbing SNVs, and for which LoF and/or haploinsufficiency is a known mechanism of human disease (either curated as haploinsufficient, curated as acting via a LoF mechanism in DDG2P or with ≥ 10 high-confidence pathogenic LoF variants documented in ClinVar), including both IRF6 and NF1. We predict these to be a fruitful set to search for additional disease-causing uORF-perturbing variants (Supplementary Data 1; Supplementary Fig. 5b). To aid in the identification of uORF-perturbing variants we have created plugin for the Ensembl Variant Effect Predictor (VEP)32 which annotates variants for predicted effects on translational regulation (available at https://github.com/ImperialCardioGenetics/uORFs).
A novel uORF frameshift causes neurofibromatosisN type 2
We analysed targeted sequencing data from a cohort of 1134 unrelated individuals diagnosed with neurofibromatosis type 2, which is caused by LoF variants in one of these prioritised genes, NF2. We identified a single 5’UTR variant in two unrelated probands in this cohort (ENST00000338641:−66-65insT; GRCh37:chr22:29999922A >AT) that segregates with disease in three additional affected relatives across the two families (Supplementary Fig. 3b; Supplementary Table 3). This variant could act through two distinct uORF-disrupting mechanisms. While the insertion does create a new uAUG (in the context of a moderate Kozak consensus) an in-frame stop codon after only three codons would suggest only a weak effect on CDS translation. However, the NF2 UTR contains an existing uORF with prior evidence of translation24 and a strong Kozak consensus. The observed insertion changes the frame of this existing uORF, causing it to bypass the downstream stop codon and create an out-of-frame oORF (Fig. 3c). This oORF is predicted to lower translation of NF2, consistent with the known LoF disease mechanism, however, functional follow-up is required to confirm this hypothesis.
Discussion
We used data from 15,708 whole human genomes to explore the global impact of variants that create or perturb uORFs in 5’UTRs, which can lead to altered translation of the downstream protein. We show that creating a new uORF and hence initiating translation from an uAUG is an important regulatory mechanism. Our data suggest that the major underlying mechanism of translational repression by uORFs is likely to be through competitive translation, since it is unlikely that novel peptides produced by uAUG-creating variants will be functional, and the most deleterious types of uAUG-creating and stop-removing variants are those that form oORFs.
Selective pressure on strongly translated uORFs has maintained features that promote re-initiation and prevent constitutive translational repression. Specifically, existing uORFs are selected to be short, further from the CDS, and to lack strong Kozak sequences2. This is in agreement with our results, which show a strongly skewed frequency spectrum for observed variants predicted to strongly inhibit translation, and an over-representation of these deleterious variants in disease cases.
We have defined a new category of variants, high-impact uORF-perturbing variants, a subset of which are likely to act as LoF by severely impacting translation. This class contains 145,398 possible SNVs (110,357 uAUG-creating and 35,041 stop-removing) across the genome, which are predicted to form oORFs from an uAUG with a strong or moderate Kozak consensus, or with prior evidence of translation. Of these, 3213 (2.2%) are observed in the whole genome sequence data from gnomAD. In addition, uAUG-creating insertions and deletions or frameshifts that transform existing uORFs into oORFs would also be predicted to have a high impact.
Whilst uORF-perturbing variants resulting in constitutive translational repression are likely to have LoF effects, the complex mechanisms of translational regulation including leaky scanning, re-initiation and the existence of internal ribosome entry sites makes it difficult to confidently predict the functional consequences of individual variants. Even variants predicted to be of high-impact may only result in partial LoF, reducing power to identify significant signals of selection. Confident interpretation of variants for a role in disease will require functional studies to assess the downstream impact of these variants on protein levels and/or additional genetic evidence, such as de novo occurrence or segregation with disease. It will also be interesting to study the impact of uORF-perturbing variants causing partial LoF on coding variant penetrance and their role in common disease phenotypes.
Even at a sample size of 15,708 individuals, we had limited power to observe uORF-perturbing variants, given their very small genomic footprint. Despite this, we identified specific genes such as NF1, NF2 and IRF6, where uORF perturbation appears to be an important disease mechanism. In anticipation of future studies with much larger cohorts of WGS cases, we have identified a set of genes where there is a high likelihood that this mechanism will contribute to disease. This will also be useful for rare disease diagnosis, where even if WGS is undertaken this class of pathogenic variation is likely not evaluated and under-diagnosed.
In this work, we used variant frequencies in a large population dataset to study the global impact of a specific class of non-coding variants with a predicted functional effect. Previous studies using non-coding constraint have focused on entire regulatory regions33 or concentrated exclusively on splicing34,35. These and other studies36 have concluded that signals of constraint and selection are likely confined to individual bases33 and diluted out when studying larger regions. Our results support this assertion; as the signal of negative selection associated with all UTR variants is not discernible from synonymous variants. We show the power of grouping individual non-coding bases by functional effect to identify subsets of variants with strong signals of selection.
Methods
Ethics statement
We have complied with all relevant ethical regulations. This study was overseen by the Broad Institute’s Office of Research Subject Protection and the Partners Human Research Committee, and was given a determination of Not Human Subjects Research. Informed consent was obtained from all participants.
Study dataset
We used the 15,708 whole genome sequenced individuals from version 2.1.1 of the Genome Aggregation Database (gnomAD), which is fully described in our companion paper22. These data were downloaded from https://gnomad.broadinstitute.org/downloads and queried using Hail version 0.2 (https://hail.is).
Definition of 5’UTRs
The start and end positions and sequence of the 5’UTRs of all protein-coding genes were downloaded from Ensembl biomart (Human genes GRCh37.p13) and filtered to only include canonical transcripts. Genes with no annotated 5’UTR on the canonical transcript were removed.
Identification and classification of uAUG-creating variants
Reading through each UTR from start to end (5’ to 3’), we identified all instances where a SNV would create an ATG. We recorded the positions of all possible stop codons (TAA, TGA and TAG) and annotated each uAUG-creating variant with whether or not there was an in-frame stop codon within the UTR. To annotate the strength of the Kozak consensus into which the uAUG was formed we assessed the positions at −3 and +3 relative to the A of the AUG, known to be the most important bases for dictating strength of translation. If both the −3 base was either A or G and the +3 was G, Kozak was annotated as ‘Strong’, if either of these conditions was true, Kozak was deemed to be ‘Moderate’ and if neither was the case ‘Weak’. uAUG-creating variants were also annotated with the distance to, and the frame relative to the coding sequence (CDS).
Identification and classification of stop-removing variants
Existing uORFs were defined as the combination of an ATG and in-frame stop codon (TAA, TGA or TAG) within a UTR. Each predicted uORF was annotated with the positions of all alternative downstream in-frame stop codons within the UTR and with the frame relative to the coding sequence. The Kozak strength of each uORF was defined as outlined above for uAUG-creating variants. Where multiple uAUGs converge on the same stop codon, the uORF is annotated with the strongest Kozak consensus. To identify uORFs with prior evidence of translation we downloaded all human small open reading frames (sORFs) from sorfs.org, a public repository of sORFs identified in humans, mice and fruit flies using ribosome profiling24. Predicted uORFs were marked as having prior evidence if the annotated stop codon matched an entry from sorfs.org.
Stop-removing variants were identified as SNVs that would change the base of a stop codon to any sequence that would not retain the stop (i.e., did not create another of TAA, TGA or TAG).
Calculating MAPS
For each set of variants we computed the mutability adjusted proportion of singletons, or MAPS. The basis of this approach has previously been described23. Briefly, for each substitution, accounting for 1 base of surrounding context (e.g., ACG ->ATG), we calculated the proportion of all possible variants (−3.9885 < GERP < 2.6607, 15 × < gnomAD coverage < 60 × ) that are observed in intergenic/intronic autosomal regions in a downsampled set of 1000 gnomAD whole-genomes. For C > T changes at CpG sites, variant proportions are calculated separately for three distinct bins of methylation. These proportions are then scaled so that the weighted genome-wide average is the human per-base, per-generation mutation rate (1.2e−8). The creation of these context-dependent mutation rates is described in more detail in our companion paper22.
To determine the transformation between these mutation rates and the expected proportion of singletons, for each substitution and context (and methylation bin for CpGs), we regress the mutation rates against the observed proportion of singletons for synonymous variants. We use synonymous as a relatively neutral class of variants which should not be subject to any biases being investigating in UTRs, but that are distinct from bases used to define the model.
For a given list of possible variants, annotated with gnomAD allele counts using Hail (https://hail.is), we take only those that are observed in gnomAD and annotate each with the transformed mutation rate given the variant context (which now corresponds to the expected chance this site will be a singleton), and sum these values across the entire variant list to give an expected number of singletons. Variants are excluded if they are outliers on coverage in gnomAD (15× <coverage <60×), were found on the X or Y chromosome, or were filtered out of the gnomAD whole genomes.
Finally, this expected number of singletons is compared to the number of sites that are observed as singletons in gnomAD, to estimate MAPS.
| 1 |
Confidence intervals were calculated using bootstrapping. For a list of n observed variants, n variant sites are sampled at random with replacement and used to calculate MAPS. This is repeated over 10,000 permutations before the 5th and 95th percentiles of the resulting MAPS distribution are taken as confidence intervals.
P-values we calculated using the same bootstrapping approach but for each permutation MAPS was calculated for each of the two variant sets of interest, A and B. The P-value was defined as the proportion of permutations where MAPS of B was less than MAPS of A.
| 2 |
For coding variants, MAPS was calculated using the predicted impact on the canonical transcript.
Using PhyloP to assess base-level conservation
Per-base vertebrate PhyloP scores were extracted from the Combined Annotation Dependent Depletion (CADD) version v1.4 GRCh37 release files and used to annotate lists of all possible coding, UTR and uORF stop bases. To remove biases due to gene context and distance from the coding sequence, we created a set of matched UTR bases which comprised the 3 bases immediately upstream and downstream of the stop. Conserved bases were defined as those with PhyloP > = 2. We also checked for a significant difference between the entire distribution of scores using a Wilcoxon rank sum test for all stop-removing compared to matched UTR bases (P = 8.1 × 10−9).
Identifying disease gene lists
Developmental disease genes were downloaded from The Developmental Disorders Genotype-Phenotype Database (DDG2P) on the 6th October 2018. We included only genes categorised as ‘confirmed’ or ‘probable’. Genes with a known dominant LoF mechanism were identified using the ‘allelic requirement’ and ‘mutation consequence’ annotations.
Genes intolerant and tolerant to LoF variants were identified using data from Karczewski et al. 201922. Genes were ordered by their loss-of-function observed/expected upper bound fraction (LOEUF) scores and the top and bottom sextiles were categorised as tolerant and intolerant, respectively.
We downloaded data from The Clinical Genome Resource (ClinGen) Dosage Sensitivity Map on 21st January 2019 (https://www.ncbi.nlm.nih.gov/projects/dbvar/clingen/). Genes manually curated as haploinsufficient were defined as those with a score of 3 (sufficient evidence). In addition, we added genes curated as severe or moderately haploinsufficient by the MacArthur lab (https://github.com/macarthur-lab/gene_lists/tree/master/lists).
Searching for uORF-perturbing variants in HGMD and ClinVar
Lists of all possible uAUG-creating and stop-removing SNVs were intersected with all DM variants from HGMD pro release 2018.1 and all ClinVar Pathogenic or Likely Pathogenic variants from the August 2018 release (clinvar_20180805.vcf). In addition, we created a list of all possible 1–5 bp deletions that would create an uAUG, annotated as described for SNVs above, and also searched for these variants. We did not investigate small insertions or deletion >5 bps due to the inhibitory number of possible variants.
Sub-classifying genes
uAUG-creating variants were classified as ‘high-impact’ if they are formed into a high or moderate Kozak consensus and if they either form an oORF or result in transcript elongation. Stop removing variants were similarly classified as ‘high-impact’ if the original uORF start site has a strong or moderate Kozak and/or the uORF is documented in sorfs.org and the variants results in a oORF or a transcript elongation.
Genes were divided into nine categories according to the following logic.
Class 0–genes with no annotated 5’UTR on the canonical transcript.
Class 1–genes with no possible uAUG-creating or stop-removing SNVs identified.
Class 2–remaining genes with no possible SNVs of predicted high-impact.
Class 3–remaining genes where the UTR has a high-confidence oORF (strong/moderate Kozak or documented in sorfs.orf) indicating creating a second would be of low-impact.
Class 4–remaining genes where one or more identified high-impact SNVs have AF > 0.1% in gnomAD (genomes AC >15).
Class 5–remaining genes that are tolerant to LoF variants.
Class 8–remaining genes curated as haploinsufficient by ClinGen or the MacArthur lab, curated as acting via a loss-of-function mechanism in DDG2P or with > = 10 high-confidence Pathogenic LoF variants in ClinVar (known LoF disease genes).
Class 7–remaining genes intolerant to LoF variants or with > = 2 high-confidence Pathogenic LoF variants in ClinVar.
Class 6–all genes not classified into any other class.
The nine gene classes were grouped into three categories corresponding to low (classes 2, 3, 4 and 5), moderate (class 6) and high (classes 7 and 8) likelihood that high-impact uORF-perturbing variants would have a deleterious effect.
Sequencing of individuals with neurofibromatosis type 2
A cohort of 1134 unrelated individuals with neurofibromatosis type 2 were recruited to the Centre for Genomic Medicine at St Mary’s Hospital, Manchester. All individuals fulfilled both Manchester and NIH criteria for diagnosis. Ethical approval for use of these samples and anonymised associated clinical data was obtained from the North West Greater Manchester Central Research Ethics Committee (reference 10/H1008/74). Informed consent was obtained from all participants. All patients were sequenced across the NF2 gene. Two individuals were identified to carry a single 5’UTR variant (ENST00000338641:-66-65insT; GRCh37:chr22:29999922A >AT). Both carriers were confirmed to have no variants in SMARCB1 or LZTRA1 and no coding variants in NF2. The -66-65insT variant segregated with disease in 3 affected siblings in one family and in affected parent and child in another (Supplementary Fig. 3b). Phenotypic data for all family members can be found in Supplementary Table 3.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
N.W. is supported by a Rosetrees and Stoneygate Imperial College Research Fellowship. This work was supported by the Wellcome Trust (107469/Z/15/Z), the Medical Research Council (UK), the NIHR Biomedical Research Unit in Cardiovascular Disease at Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, the NIHR Imperial College Biomedical Research Centre, the Fondation Leducq (11 CVD-01), a Health Innovation Challenge Fund award from the Wellcome Trust and Department of Health, UK (HICF-R6–373), and by NIDDK U54DK105566 and NIGMS R01GM104371. D.G.E. and M.J.S. are funded by the NIHR Biomedical research centre Manchester (IS-BRC-1215-20007). L.C.F. is supported by the Swiss National Science Foundation (Advanced Postdoc.Mobility 177853). N.Q. is supported by the Imperial College Academic Health Science Centre. The results published here are in part based upon data: (1) generated by The Cancer Genome Atlas managed by the NCI and NHGRI (accession: phs000178.v10.p8). Information about TCGA can be found at http://cancergenome.nih.gov, (2) generated by the Genotype-Tissue Expression Project (GTEx) managed by the NIH Common Fund and NHGRI (accession: phs000424.v7.p2), (3) generated by the Exome Sequencing Project, managed by NHLBI, (4) generated by the Alzheimer’s Disease Sequencing Project (ADSP), managed by the NIA and NHGRI (accession: phs000572.v7.p4). The views expressed in this work are those of the authors and not necessarily those of any of the funders. We would like to thank Dr. Christopher M Yates for his statistical advice.
Author contributions
N.W., D.G.M. and J.S.W designed the study and supervised the work. N.W., K.J.K., X.Z. and S.C. conducted analyses. M.J.S, D.G.E, gnomAD PG and gnomAD C provided data for the study. A.M.R., N.M.Q., S.S., O.R., J.A., A.H.O.-L., L.C.F., S.A.C. and P.J.R.B. provided critical feedback on all analyses and figures. The paper was written by N.W., D.G.M. and J.S.W. with comments provided by all other authors.
Data availability
All possible uAUG-creating and stop-removing SNVs for canonical Gencode transcripts along with likelihood classifications for all genes are available for download at https://github.com/ImperialCardioGenetics/uORFs.
Code availability
To aid in the identification of uORF-perturbing variants we have created a VEP plugin which annotates variants for predicted effects on translational regulation. This script, along with those used to analyse the data and create figures for the manuscript, is freely available at https://github.com/ImperialCardioGenetics/uORFs.
Competing interests
D.G.M. is a founder with equity in Goldfinch Bio. The remaining authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Douglas Stewart and other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Daniel G. MacArthur, James S. Ware.
A full list of consortium members appears at the end of the paper.
Contributor Information
Nicola Whiffin, Email: n.whiffin@imperial.ac.uk.
Genome Aggregation Database Production Team:
Irina M. Armean, Eric Banks, Louis Bergelson, Kristian Cibulskis, Ryan L. Collins, Kristen M. Connolly, Miguel Covarrubias, Beryl Cummings, Mark J. Daly, Stacey Donnelly, Yossi Farjoun, Steven Ferriera, Stacey Gabriel, Laura D. Gauthier, Jeff Gentry, Namrata Gupta, Thibault Jeandet, Diane Kaplan, Kristen M. Laricchia, Christopher Llanwarne, Eric V. Minikel, Ruchi Munshi, Benjamin M. Neale, Sam Novod, Nikelle Petrillo, Timothy Poterba, David Roazen, Valentin Ruano-Rubio, Andrea Saltzman, Kaitlin E. Samocha, Molly Schleicher, Cotton Seed, Matthew Solomonson, Jose Soto, Grace Tiao, Kathleen Tibbetts, Charlotte Tolonen, Christopher Vittal, Gordon Wade, Arcturus Wang, Qingbo Wang, Nicholas A. Watts, and Ben Weisburd
Genome Aggregation Database Consortium:
Carlos A. Aguilar Salinas, Tariq Ahmad, Christine M. Albert, Diego Ardissino, Gil Atzmon, John Barnard, Laurent Beaugerie, Emelia J. Benjamin, Michael Boehnke, Lori L. Bonnycastle, Erwin P. Bottinger, Donald W. Bowden, Matthew J. Bown, John C. Chambers, Juliana C. Chan, Daniel Chasman, Judy Cho, Mina K. Chung, Bruce Cohen, Adolfo Correa, Dana Dabelea, Mark J. Daly, Dawood Darbar, Ravindranath Duggirala, Josée Dupuis, Patrick T. Ellinor, Roberto Elosua, Jeanette Erdmann, Tõnu Esko, Martti Färkkilä, Jose Florez, Andre Franke, Gad Getz, Benjamin Glaser, Stephen J. Glatt, David Goldstein, Clicerio Gonzalez, Leif Groop, Christopher Haiman, Craig Hanis, Matthew Harms, Mikko Hiltunen, Matti M. Holi, Christina M. Hultman, Mikko Kallela, Jaakko Kaprio, Sekar Kathiresan, Bong-Jo Kim, Young Jin Kim, George Kirov, Jaspal Kooner, Seppo Koskinen, Harlan M. Krumholz, Subra Kugathasan, Soo Heon Kwak, Markku Laakso, Terho Lehtimäki, Ruth J. F. Loos, Steven A. Lubitz, Ronald C. W. Ma, Jaume Marrugat, Kari M. Mattila, Steven McCarroll, Mark I. McCarthy, Dermot McGovern, Ruth McPherson, James B. Meigs, Olle Melander, Andres Metspalu, Benjamin M. Neale, Peter M. Nilsson, Michael C. O’Donovan, Dost Ongur, Lorena Orozco, Michael J. Owen, Colin N. A. Palmer, Aarno Palotie, Kyong Soo Park, Carlos Pato, Ann E. Pulver, Nazneen Rahman, Anne M. Remes, John D. Rioux, Samuli Ripatti, Dan M. Roden, Danish Saleheen, Veikko Salomaa, Nilesh J. Samani, Jeremiah Scharf, Heribert Schunkert, Moore B. Shoemaker, Pamela Sklar, Hilkka Soininen, Harry Sokol, Tim Spector, Patrick F. Sullivan, Jaana Suvisaari, E. Shyong Tai, Yik Ying Teo, Tuomi Tiinamaija, Ming Tsuang, Dan Turner, Teresa Tusie-Luna, Erkki Vartiainen, Hugh Watkins, Rinse K. Weersma, Maija Wessman, James G. Wilson, and Ramnik J. Xavier
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10717-9.
References
- 1.Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc. Natl Acad. Sci. U. S. A. 2009;106:7507–7512. doi: 10.1073/pnas.0810916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnstone TG, Bazzini AA, Giraldez AJ. Upstream ORFs are prevalent translational repressors in vertebrates. EMBO J. 2016;35:706–723. doi: 10.15252/embj.201592759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iacono M, Mignone F, Pesole G. uAUG and uORFs in human and rodent 5’untranslated mRNAs. Gene. 2005;349:97–105. doi: 10.1016/j.gene.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299:1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozak M. An analysis of 5’-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noderer WL, et al. Quantitative analysis of mammalian translation initiation sites by FACS‐seq. Mol. Syst. Biol. 2014;10:748. doi: 10.15252/msb.20145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinnebusch AG, Ivanov IP, Sonenberg N. Translational control by 5’-untranslated regions of eukaryotic mRNAs. Science. 2016;352:1413–1416. doi: 10.1126/science.aad9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jousse C, et al. Inhibition of CHOP translation by a peptide encoded by an open reading frame localized in the chop 5′ UTR. Nucleic Acids Res. 2001;29:4341–4351. doi: 10.1093/nar/29.21.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji Z, Song R, Regev A, Struhl K. Many lncRNAs, 5’UTRs, and pseudogenes are and some are likely to express functional proteins. Elife. 2015;4:e08890. doi: 10.7554/eLife.08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couso J-P, Patraquim P. Classification and function of small open reading frames. Nat. Rev. Mol. Cell Biol. 2017;18:575–589. doi: 10.1038/nrm.2017.58. [DOI] [PubMed] [Google Scholar]
- 11.Wethmar K. The regulatory potential of upstream open reading frames in eukaryotic gene expression. Wiley Interdiscip. Rev. RNA. 2014;5:765–778. doi: 10.1002/wrna.1245. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Rothnagel JA. 5′‐Untranslated regions with multiple upstream AUG codons can support low‐level translation via leaky scanning and reinitiation. Nucleic Acids Res. 2004;32:1382–1391. doi: 10.1093/nar/gkh305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chugunova A, Navalayeu T, Dontsova O, Sergiev P. Mining for small translated ORFs. J. Proteome Res. 2018;17:1–11. doi: 10.1021/acs.jproteome.7b00707. [DOI] [PubMed] [Google Scholar]
- 14.Sample, P. J. et al. Human 5′ UTR design and variant effect prediction from a massively parallel translation assay. Preprint at https://www.biorxiv.org/content/10.1101/310375v1 (2018). [DOI] [PMC free article] [PubMed]
- 15.Schulz J, et al. Loss-of-function uORF mutations in human malignancies. Sci. Rep. 2018;8:2395. doi: 10.1038/s41598-018-19201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, et al. Mutation of the CDKN2A 5’ UTR creates an aberrant initiation codon and predisposes to melanoma. Nat. Genet. 1999;21:128–132. doi: 10.1038/5082. [DOI] [PubMed] [Google Scholar]
- 17.Wen Y, et al. Loss-of-function mutations of an inhibitory upstream ORF in the human hairless transcript cause Marie Unna hereditary hypotrichosis. Nat. Genet. 2009;41:228–233. doi: 10.1038/ng.276. [DOI] [PubMed] [Google Scholar]
- 18.Occhi G, et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLoS Genet. 2013;9:e1003350. doi: 10.1371/journal.pgen.1003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbosa C, Peixeiro I, Romão L. Gene expression regulation by upstream open reading frames and human disease. PLoS Genet. 2013;9:e1003529. doi: 10.1371/journal.pgen.1003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthes T, et al. Severe hemochromatosis in a Portuguese family associated with a new mutation in the 5’-UTR of the HAMP gene. Blood. 2004;104:2181–2183. doi: 10.1182/blood-2004-01-0332. [DOI] [PubMed] [Google Scholar]
- 21.von Bohlen AE, et al. A mutation creating an upstream initiation codon in the SOX9 5’ UTR causes acampomelic campomelic dysplasia. Mol. Genet Genom. Med. 2017;5:261–268. doi: 10.1002/mgg3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karczewski, K. J. et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. Preprint at https://www.biorxiv.org/content/10.1101/531210v2 (2019).
- 23.Lek M, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olexiouk, V., Crappé, J. & Verbruggen, S. sORFs. org: a repository of small ORFs identified by ribosome profiling. Nucleic Acids44, D324–D329 (2015). [DOI] [PMC free article] [PubMed]
- 25.Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010;20:110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riggs ER, et al. Copy number variant discrepancy resolution using the ClinGen dosage sensitivity map results in updated clinical interpretations in ClinVar. Hum. Mutat. 2018;39:1650–1659. doi: 10.1002/humu.23610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenson PD, et al. The Human Gene Mutation Database: providing a comprehensive central mutation database for molecular diagnostics and personalised genomics. Hum. Genom. 2009;4:69. doi: 10.1186/1479-7364-4-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landrum MJ, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42:D980–D985. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans DG, et al. Comprehensive RNA analysis of the NF1 gene in classically affected NF1 affected individuals meeting NIH criteria has high sensitivity and mutation negative testing is reassuring in isolated cases with pigmentary features only. EBioMedicine. 2016;7:212–220. doi: 10.1016/j.ebiom.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lima RLLF, et al. Prevalence and nonrandom distribution of exonic mutations in interferon regulatory factor 6 in 307 families with Van der Woude syndrome and 37 families with popliteal pterygium syndrome. Genet. Med. 2009;11:241–247. doi: 10.1097/GIM.0b013e318197a49a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo S, et al. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat. Genet. 2002;32:285–289. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaren W, et al. The ensembl variant effect predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Short PJ, et al. De novo mutations in regulatory elements in neurodevelopmental disorders. Nature. 2018;555:611–616. doi: 10.1038/nature25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, et al. Base-specific mutational intolerance near splice sites clarifies the role of nonessential splice nucleotides. Genome Res. 2018;28:968–974. doi: 10.1101/gr.231902.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord J, et al. Pathogenicity and selective constraint on variation near splice sites. Genome Res. 2019;29:159–170. doi: 10.1101/gr.238444.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An Joon-Yong, Lin Kevin, Zhu Lingxue, Werling Donna M., Dong Shan, Brand Harrison, Wang Harold Z., Zhao Xuefang, Schwartz Grace B., Collins Ryan L., Currall Benjamin B., Dastmalchi Claudia, Dea Jeanselle, Duhn Clif, Gilson Michael C., Klei Lambertus, Liang Lindsay, Markenscoff-Papadimitriou Eirene, Pochareddy Sirisha, Ahituv Nadav, Buxbaum Joseph D., Coon Hilary, Daly Mark J., Kim Young Shin, Marth Gabor T., Neale Benjamin M., Quinlan Aaron R., Rubenstein John L., Sestan Nenad, State Matthew W., Willsey A. Jeremy, Talkowski Michael E., Devlin Bernie, Roeder Kathryn, Sanders Stephan J. Genome-wide de novo risk score implicates promoter variation in autism spectrum disorder. Science. 2018;362(6420):eaat6576. doi: 10.1126/science.aat6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All possible uAUG-creating and stop-removing SNVs for canonical Gencode transcripts along with likelihood classifications for all genes are available for download at https://github.com/ImperialCardioGenetics/uORFs.
To aid in the identification of uORF-perturbing variants we have created a VEP plugin which annotates variants for predicted effects on translational regulation. This script, along with those used to analyse the data and create figures for the manuscript, is freely available at https://github.com/ImperialCardioGenetics/uORFs.