Abstract
Background:
To systematically evaluate the effects of physical activity on physiological markers in breast cancer survivors.
Methods:
A systematic search of the PubMed, Wed of Science, Medline, CNKI and Wanfang Database was performed to identify eligible randomized controlled trials to explore physical activity on physiological markers in breast cancer survivors. STATA version 13.0 (Stata Corp LP, College Station, TX) was used for all statistical analyses.
Results:
A total of 11 articles with 941 cases were eligible in this meta-analysis. The results of the meta-analysis showed that physical activity could decrease the levels of insulin (SMD = −1.90, 95%CI: −3.2 to −0.60; I2 = 92.3%, P < .001), insulin-like growth factor 1 (IGF-I) (WMD = −4.67, 95%CI: −23.14 to 13.79; I2 = 96.2%, P < .001), insulin-like growth factor binding protein-3 (IGFBP-3) (WMD = −20.09, 95%CI: −47.15 to 6.97; I2 = 93.3%, P < .001). However, compared with the control group, there was not the significant change of insulin-like growth factor 2 (IGF-II), insulin-like growth factor binding protein-1 (IGFBP-1), leptin, adiponectin, glucose, C-reactive protein (CRP), Interleukin-6 (IL-6), Interleukin-10 (IL-10), and tumor necrosis factor alpha (TNF-ɑ) levels after the intervention.
Conclusions:
Physical activity could improve the insulin function that might be associated with decreasing the levels of IGF-I, IGFBP-3 and insulin in breast cancer survivors.
Keywords: breast cancer survivors, meta-analysis, physical activity, physiological markers
1. Introduction
Global cancer cases will present rapid growth, according to the American cancer society, as the growth and aging of global population, the new cancer cases increased quickly, the numbers of global cancer cases will reach 19.3 million from 2016 to 2025 and 24 million by 2035.[1] Exercise can offer a variety of psychological and physiological benefits, relieve the pressure and pain of patients in the process of treatment, relate to reducing the risk of cancer mortality. Studies have shown that exercise can improve the cancer-related fatigue and sleep symptoms, etc., and improve the survival quality of cancer patients effectively.[2] In addition, it can also induce the CD 16+ cells to shift doubly, increase the dissolving activity and improve the immune function of cancer patients. The biomarkers like insulin, insulin-like growth factor, IGF-binding protein, C-reactive protein, leptin, adiponectin, interleukin, etc played important roles in improving the body of cancer patients.[3] Moreover, the inflammatory factors including IL-6, IL-10, and TNF-ɑ were associated with breast cancer and suggested as potential mediators of physical activity on breast cancer.[4] Therefore, we used the method of evidence-based medicine to conduct a meta-analysis that focus on the concentration changes of insulin, IGF-I, IGFBP-1, IGFBP-3, leptin, adiponectin, glucose, CRP, IL-6, IL-10, and TNF-ɑ of cancer patients that exercise intervention caused.
2. Materials and methods
The ethical approval is not necessary in this study because it is a meta-analysis. This meta-analysis is strictly followed by the PRISMA scheme of evidence-based medicine.
2.1. Information retrieval
We searched the foreign databases such as PubMed, Wed of Science, Medline and Chinese databases such as Wanfang and CNKI. The English search terms: (breast cancer or breast carcinoma or breast neoplasm or breast tumor) and (exercise or physical activity) and (randomized controlled trial or RCT) and (insulin or inflammatory factor or inflammatory reaction or interleukin or interleukin 6 or IL-6 or IL-10 or TNF-a or leptin or adiponectin or glucose or insulin-like growth factor or IGF-binding protein or C-reactive protein or CRP). The Chinese search terms: (movement or activity) and (breast cancer) and (inflammation factors or inflammation or insulin or insulin-like growth factor or insulin or insulin-like growth factor binding protein or CRP or leptin or adiponectin or glucose or interleukin or interleukins) and (randomized or controlled or randomized controlled trials). The time limit for searching is from the search beginning to January 2017. Tracing the relevant references listed in the retrieved literature or review.
2.2. Inclusion and exclusion criteria
2.2.1. Study design
RCT, cross-over designed trail using the first stage data.
2.2.2. Object of study
The breast cancer patients diagnosed at any periods; the diagnosis is in accordance with “the China association of breast cancer diagnosis and treatment guidelines and standards (2015 edition) .”
2.2.3. Intervention
The experimental group carried out the exercise intervention, the control group did not or the normal measures.
2.2.4. Final indicator
The concentration of IL-6, IL-10, TNF-ɑ, leptin, adiponectin, glucose, insulin-like growth factor, IGF-binding protein, and C-reactive protein in plasma, which was unlimited measurement.
2.2.5. Exclusion criteria
-
1)
The repeated or poor-quality literatures;
-
2)
The literatures that the average and the standard deviation of the ending index cannot be calculated according to the experimental data.
2.3. Literatures screening
The searching results from different databases were imported into the Endnote X6 to rearrange. Screening the literatures using independently incorporated and excluded standards and mutual blindness by 2 researchers, first, reading the title and abstract of the literatures and weeding out the clearly not related literatures, then getting the literatures we may need preliminarily, downloading the full text and reading carefully to judge whether the literatures qualified. After screening, the results would be cross-checked, and we would communicate with the corresponding author when 2 judged results not inconsistent to discuss whether the document would be included.
2.4. Data extraction
The 2 researchers extracted the data from the final included literature, which was designed in advance. The extracted content included the first author, the published age, the experimental area, the average age, the sample size, the experimental period, the interventions of the experimental group and the control group, and the average and standard deviation of concentration of the final indicator such as IL-6, IL-10, TNF-a, leptin, adiponectin, glucose, insulin-like growth factor, IGF-binding protein and C-reactive protein in the experimental group and the control group.
2.5. Evaluation of trials quality
Using the Cochrane collaboration's tool for assessing risk of bias to define the methodological quality of the included literatures.[5] This tool defined the methodological quality of the included literatures from 6 fields included selection bias, performance bias, detection bias, attrition bias, reporting bias, other bias. Every index included 3 options that are low risk of bias/ unclear risk of bias/high risk of bias. If the above quality standards are fully met, which remind us that the risk of partial reclination is the minimum and grade A; while one or more of these criteria is met, the likelihood of partial reclining is moderate and grade B; and the above criteria are not met at all, the migration risk is high and grade C.
2.6. Statistical processing
The outcomes of this study are continuous variables, if the data unit of the same variable is different, we chose the SMD (standardized mean difference) and 95%CI as the effect magnitude to calculate the amount of the combined effect, and the data unit of the same variable is same, we used the weighted mean difference (WMD) and 95%CI as the effect magnitude to calculate the amount of the combined effect. First, analyzing the characteristics of the clinical population and methods included in the study, if there is a clinical heterogeneity, only carried out the descriptive analysis, whereas the next stage is meta-analysis. Before merging of the meta-analysis, we should carry out the heterogeneity test by Q inspection (the range of I2 is 0%∼100%), if the I2 ≤ 50%, P > .1, showing that the heterogeneity between studies is small and can use a fixed effects model to do the meta-analysis; on the contrary, when the I2 > 50%, P < .1, indicating that there is an obvious heterogeneity between studies and can use a randomized effects model to do the meta-analysis. In addition, we should conduct the sensitivity analysis to explore the heterogeneity of the research when necessary. In order to control the impact of publication bias, the meta-analysis results were examined by Begg test and the Egger test. The STATA 13.0 software was used for statistical analysis. The test level was statistically significant by P < .05.
3. Results
3.1. The searching results and the basic characteristics of the included studies
Retrieving 1312 references from all databases, using the Endnote X6 to eliminate 237 repeated references, eliminating the 1029 uncorrelated references by reading the titles and abstracts, going back to 6 references, eliminating the 41 unqualified references by downloading and reading the full text of 52 possible qualified references, including 11 randomized controlled trials[6,7,8,9,10,11,12,13,14,15,16] eventually. The screening process as shown in Figure 1.
Figure 1.
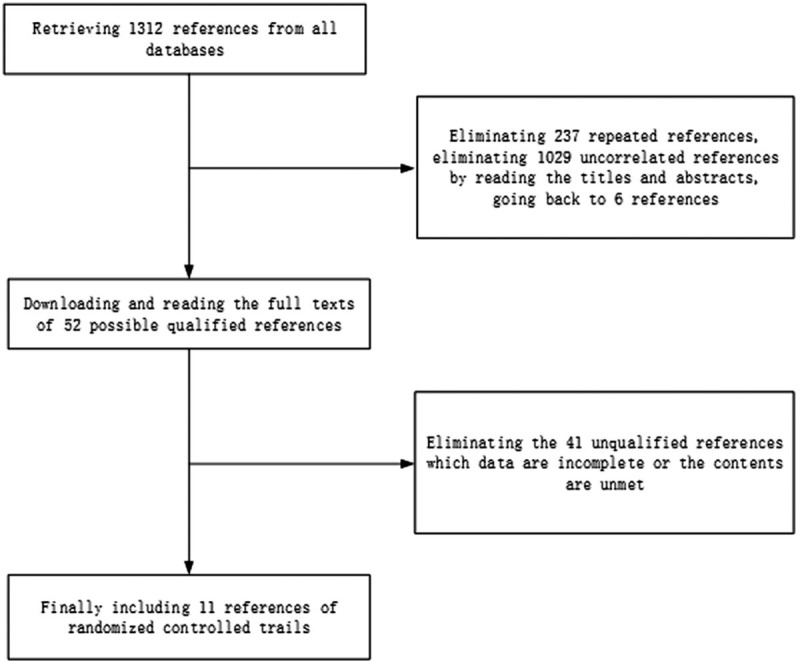
Flow chart of study selection.
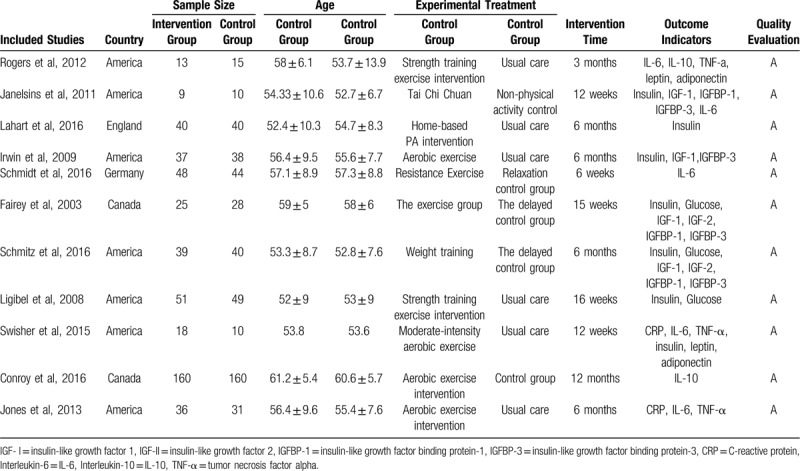
The included articles were all retrieved by the SCI, 7 were from the United States, 2 were from Canada, 1 was from the UK, and 1 was from Germany. There were 941 patients totally that 476 belonged to intervention groups and 465 belonged to control group, the average age was 56.3 ± 6.4. The duration of the intervention is from 6 weeks to 12 months. The quality level of the literature was grade A, all of which were low-grade bias.
3.2. The results of meta-analysis
3.2.1. The level of Insulin
Seven studies analyzed the effects of exercise on the levels of insulin in the blood of breast cancer patients had statistical heterogeneity, and so we used the randomized effects model to do the merging analysis. The results showed that compared with the control group, exercise had lower levels of insulin in breast cancer patients (SMD = −1.90, 95%CI: −3.2 to −0.60; I2 = 92.3%, P < .001), as shown in Figure 2.
Figure 2.
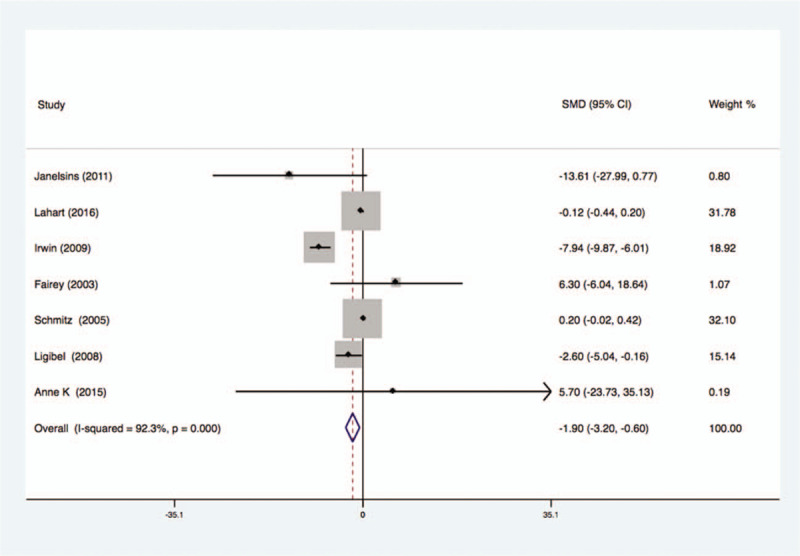
Forest plot of comparison for insulin.
3.2.2. The level of IGF-I
Four studies analyzed the effects of exercise on the levels of IGF-I in the blood of breast cancer patients had statistical heterogeneity, and so we used the randomized effects model to do the merging analysis, the results showed that the IGF-I of 2 groups had statistical significance (WMD = −4.67, 95%CI: −23.14 to 13.79; I2 = 96.2%, P < .001), as shown in Figure 3.
Figure 3.
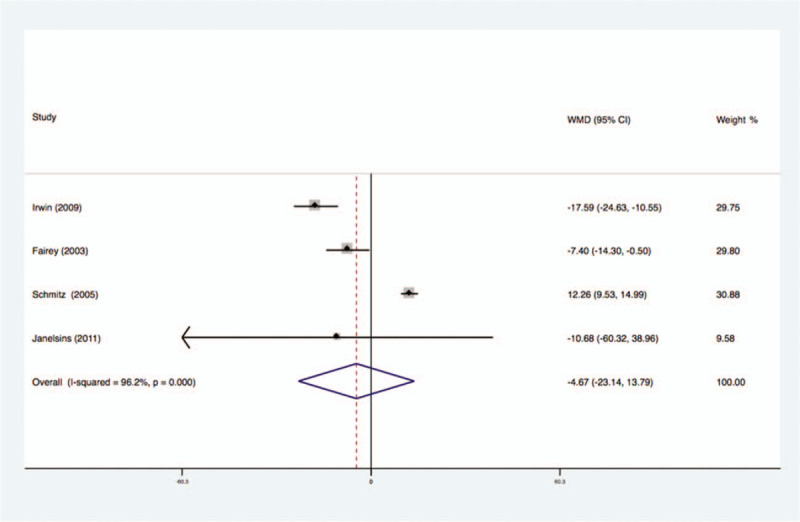
Forest plot of comparison for IGF-1.
3.2.3. The level of IGFBP-1
Three studies analyzed the effects of exercise on the levels of IGFBP-1 in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the IGFBP-1 of 2 groups had no statistical significance (WMD = −2.90, 95%CI: −3.90 to −1.90; I2 = 0%, P = .66), as shown in Figure 4.
Figure 4.
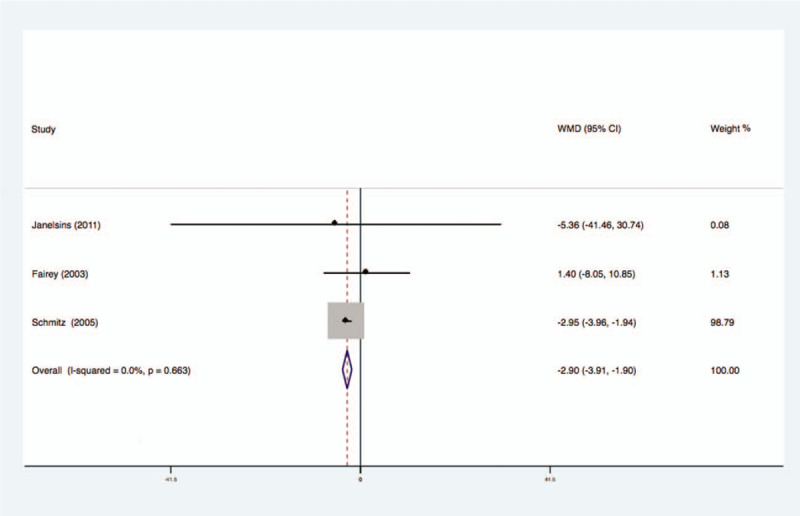
Forest plot of comparison for IGFBP-1.
3.2.4. The level of IGFBP-3
Four studies analyzed the effects of exercise on the levels of IGFBP-3 in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the IGFBP-3 of 2 groups had statistical significance (WMD = −20.09, 95%CI: −47.15 to 6.97; I2 = 93.3%, P < .001), as shown in Figure 5.
Figure 5.
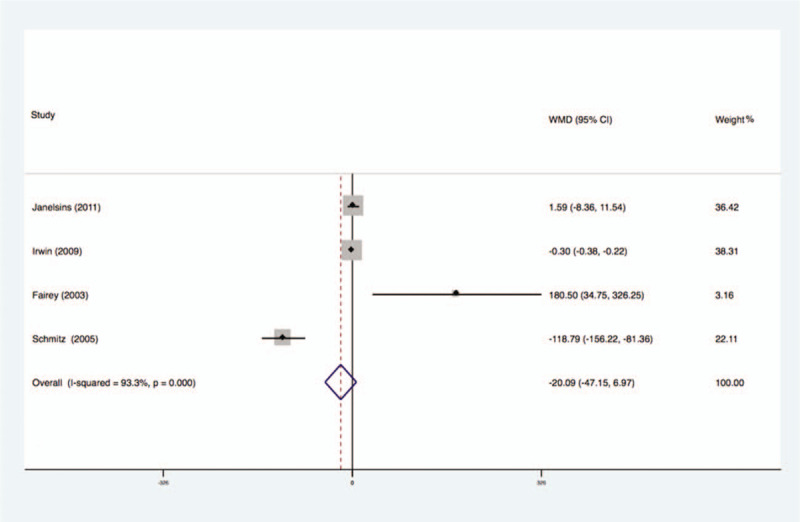
Forest plot of comparison for IGFBP-3.
3.2.5. The level of IGF-II
Two studies analyzed the effects of exercise on the levels of IGF-II in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the IGF-II of 2 groups had no statistical significance (WMD = −54.21, 95%CI: −61.41 to −47.00; I2 = 0%, P = .66), as shown in Figure 6.
Figure 6.
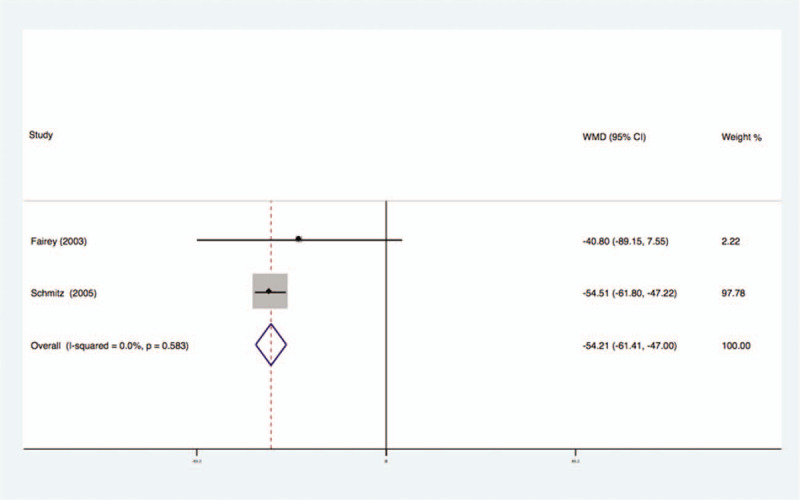
Forest plot of comparison for IGF-II.
3.2.6. The level of Leptin
Two studies analyzed the effects of exercise on the levels of Leptin in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the Leptin of 2 groups had no statistical significance (WMD = −7.69, 95%CI: −21.46 to 6.08; I2 = 0%, P = .87), as shown in Figure 7.
Figure 7.
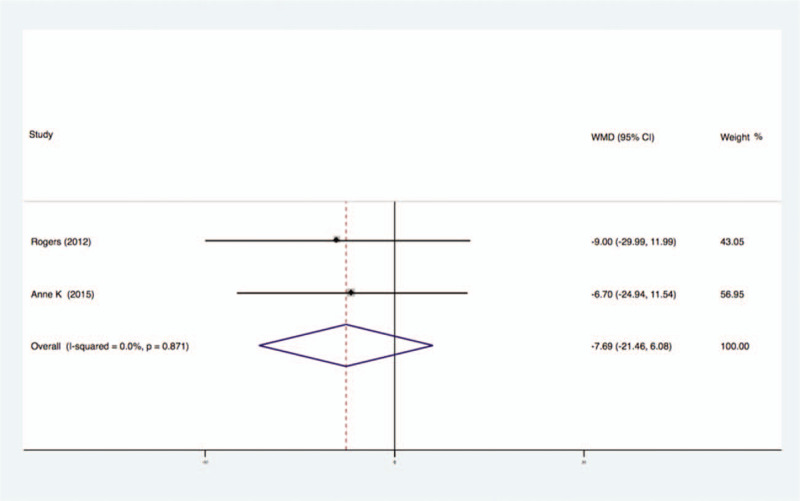
Forest plot of comparison for Leptin.
3.2.7. The level of Adiponectin
Two studies analyzed the effects of exercise on the levels of Adiponectin in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the Adiponectin of 2 groups had no statistical significance (WMD = 0.21, 95%CI: −3.05 to 3.46; I2 = 0%, P = .80), as shown in Figure 8.
Figure 8.
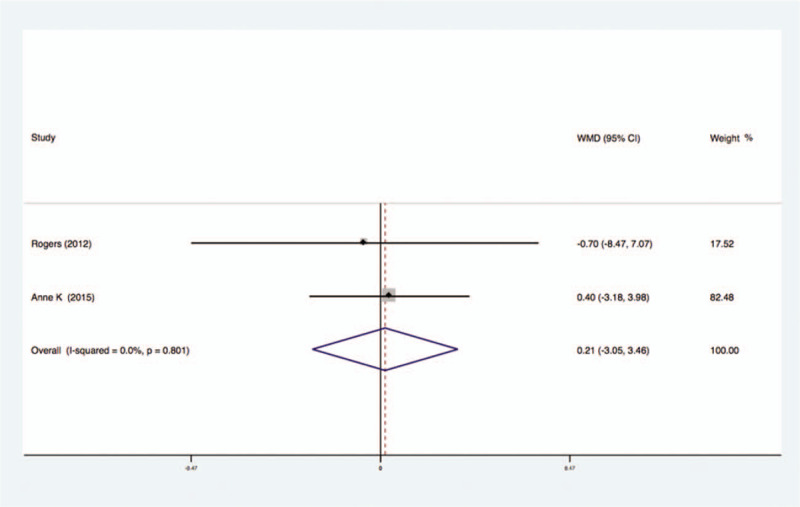
Forest plot of comparison for Adiponectin.
3.2.8. The level of glucose
Three studies analyzed the effects of exercise on the levels of glucose in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the glucose of 2 groups had no statistical significance (WMD = −0.02, 95%CI: −0.40 to 0.36; I2 = 0%, P = .47), as shown in Figure 9.
Figure 9.
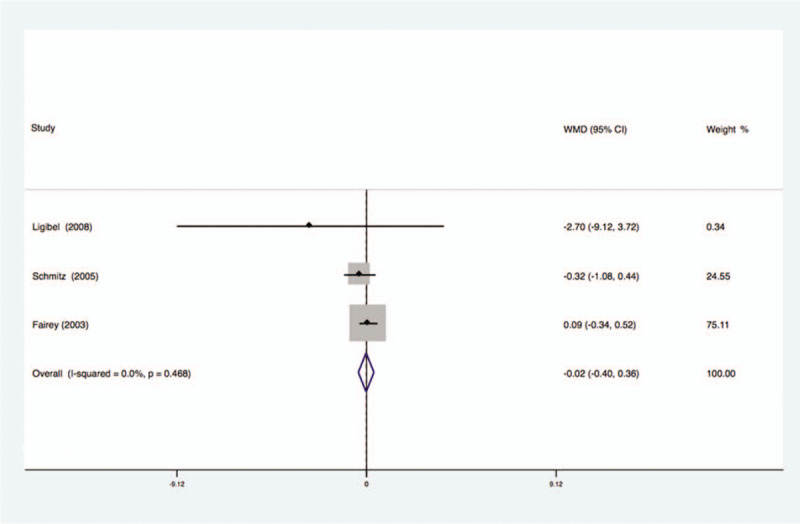
Forest plot of comparison for Glucose.
3.2.9. The level of CRP
Two studies analyzed the effects of exercise on the levels of CRP in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the CRP of 2 groups had no statistical significance (WMD = 0.06, 95%CI: −0.59 to 0.71; I2 = 0%, P = .54), as shown in Figure 10.
Figure 10.
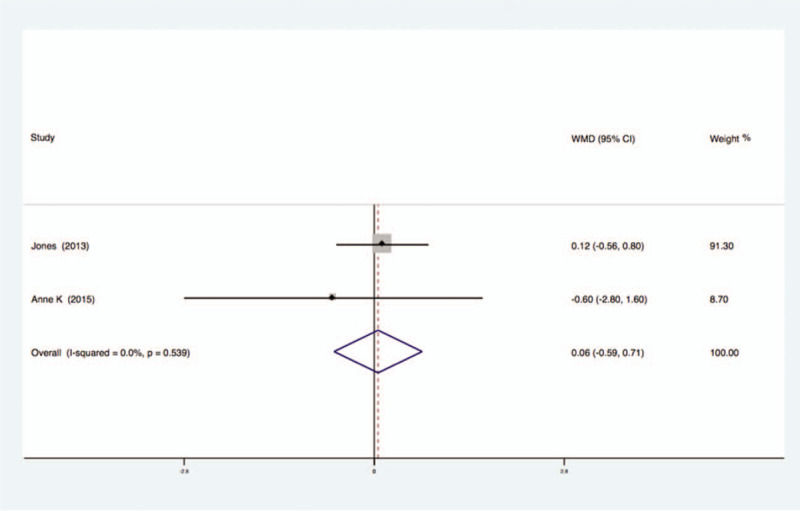
Forest plot of comparison for CRP.
3.2.10. The level of IL-6
Five studies analyzed the effects of exercise on the levels of IL-6 in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the IL-6 of 2 groups had no statistical significance (WMD = −0.21, 95%CI: −0.46 to 0.04; I2 = 0%, P = .67), as shown in Figure 11.
Figure 11.
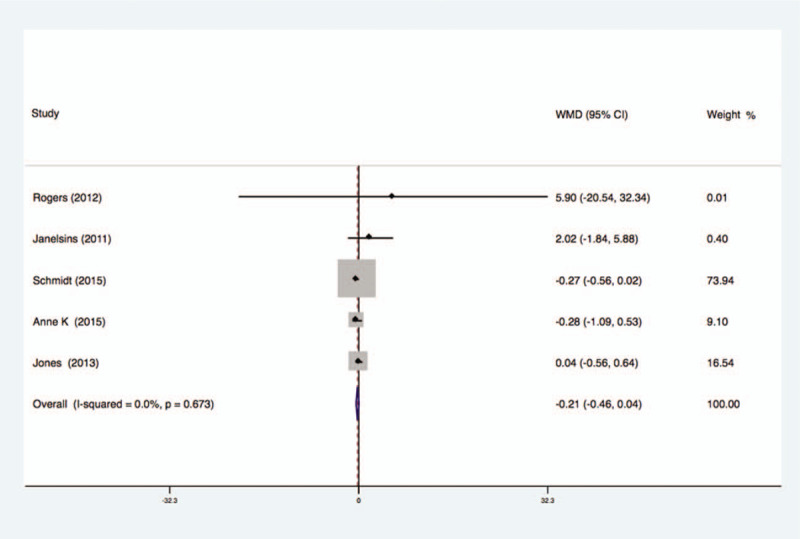
Forest plot of comparison for IL-6.
3.2.11. The level of IL-10
Two studies analyzed the effects of exercise on the levels of IL-10 in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the IL-10 of 2 groups had no statistical significance (WMD = −0.10, 95%CI: −0.31 to 0.11; I2 = 0%, P = .82), as shown in Figure 12.
Figure 12.
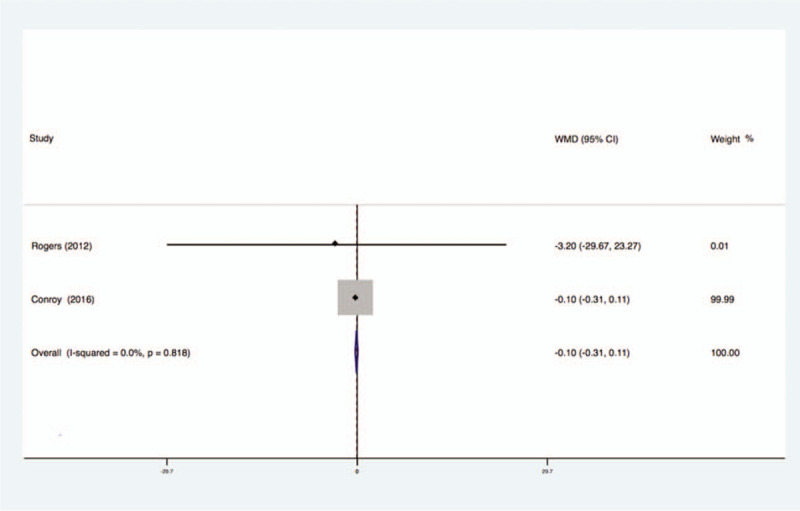
Forest plot of comparison for IL-10.
3.2.12. The level of TNF-a
Three studies analyzed the effects of exercise on the levels of TNF-a in the blood of breast cancer patients had no statistical heterogeneity, and so we used the fixed effects model to do the merging analysis, the results showed that the TNF-a of 2 groups had no statistical significance (WMD = −0.15, 95%CI: −0.44 to 0.14; I2 = 12%, P = .32), as shown in Figure 13.
Figure 13.
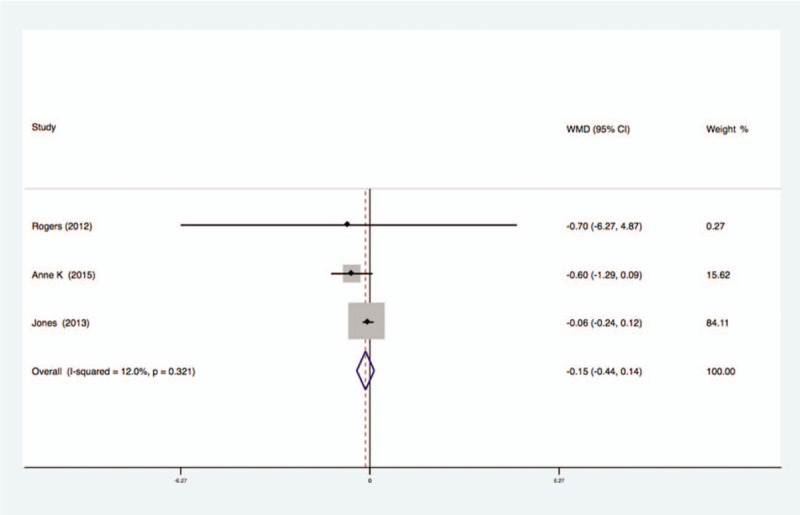
Forest plot of comparison for TNF-α.
3.3. The sensitivity and publication bias analysis
Due to the quantitative limitations of the literature, we were only able to analyze the sensitivity of meta-analysis of that the effects of exercise on the levels of insulin in the blood of breast cancer patients, the results showed that the end is stable and reliable as shown in Figure 14. In addition, the Begg test and the Egger test showed that there was no risk of publication bias (P > .1), and because of fewer than 10 references, we did not have a funnel analysis (Table 1).
Figure 14.
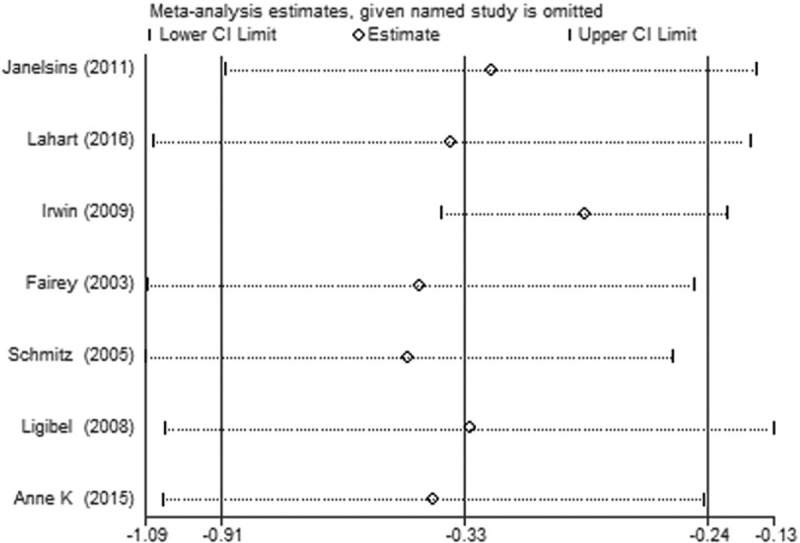
Sensitivity analysis of the meta-analysis.
Table 1.
The basic characteristics of included studies.
4. Discussion
4.1. The quality analysis of the literature included in the study
All 11 studies included in this meta-analysis were conducted with high quality randomized controlled trials. The detailed description of the allocation concealment, blind, intentional analysis and loss of the interview was given. Therefore, the evidence was higher.
4.2. Exercise affects the biochemical index of breast cancer patients
Despite the inconsistences of past evidences, the recent observational studies have shown that breast cancer patients who have an active body have a better prognosis than sedentary patients. According to the study of American nurses, the female breast cancer patients who have an active body have a better prognosis and lower risk of death than sedentary women.[16,17] Although the mechanism of how exercise improve the prognosis of patients with breast cancer have not studied completely, but a lot of research have confirmed that breast cancer patients with high weight are related with higher recurrence rate and mortality notably.[18] The study of Goodwin found that compared with the patients with the lowest quartile of insulin levels, the patients with the highest quartile of insulin levels have increased times risk of cancer recurrence and 3 times the risk of death, and high levels of IGFBP-3 can predict the distant metastasis of breast cancer in postmenopausal women. The people who do not exercise regularly and have obesity usually have higher insulin levels and insulin sensitivity.[18] The studies have shown that insulin and cytokines may be a critical media for weight gain. Our study found that exercise can reduce the levels of IGF-I (WMD = −4.67, 95%CI: −23.14 to 13.79), IGFBP-3 (WMD = −20.09, 95%CI: −47.15 to 6.97) and Insulin (SMD = −1.90, 95%CI: −3.2 to −0.60) effectively. However, our study did not find that exercise can alter the level of IGF-II, IGFBP-1, leptin, adiponectin, glucose, CRP, IL-6, IL-10, and TNF-a. Therefore, exercise may reduce the insulin levels by reducing the levels of IGF-I and IGFBP-3, which can block the proliferation of abnormal cells and reducing relapse and death.
In breast cancer, only 5% to 10% patients with breast cancer attributed to genetic inheritance. The physical activity is one of prevention efforts that focused on the modifiable risk factors of breast cancer.[19] The growth of breast cancer cells could be promoted by increased IGF-I which produced by polyploid adipose stem cells.[20] In the further study, IGF-I was proved that it could modulates biological responses via inducing the increasing of DDR1 collagen receptor, which provided the important target for breast cancer.[21] IGF-axis including IGF-I and IGFBP-3 were associated with metastasis development of breast cancer. The previous study demonstrated that the ratios of IGF-I/IGFBP3 were increased in the postmenopausal women with breast cancer in Taiwanese.[22] Not only IGF-I, but also IGFBP-3 have been demonstrated that they were related with the prognosis of breast cancer.[23] In the patients with breast cancer, physical activity could improve the expression levels of IGF's, which the molecular mechanism of physical activity on the tumor microenvironment.[24] Moreover, the IGFBP3 expression was upregulated by physical activity in breast cancer, which implied that the role of physical activity on the breast cancer were associated with the IGF-I/IGFBP3 axis.[25]
4.3. The limitation
Even if this meta-analysis was conducted via PRISMA approach of evidence-based medicine, which made rigorous literature inclusion and exclusion criteria, but there are no guidelines on how to exercise for cancer patients. Therefore, there are differences in the duration and intervention in different studies, and the sample is smaller, which may affect the stability of the results. However, the included studies were all high quality randomized controlled trials, which increased the reliability of the study. Although we do not restrict the language of publication, the research and study group we have included is Caucasian, which may influence our conclusions in other populations. Although our analysis showed that there is no publication bias, we have included only published randomized controlled trials, and because of no unpublished randomized controlled trial and limited literatures, we could not draw the all funnel diagrams, so the potential publication bias could not be ruled out.
5. Conclusion
Exercise can reduce the levels of Insulin, IGF-I and IGFBP-3 in blood of breast cancer patients significantly, but cannot alter the levels of IGF-II, IGFBP-1, leptin, adiponectin, glucose, CRP, IL-6, IL-10, and TNF-a significantly. Because of there is no related study that exercise affects the level of biochemical index in Asian crowd, so it is necessary to do such large multicenter randomized controlled trials to explore the differences that exercise affects the biochemical index in different races.
Author contributions
This study was designed by Lv X and Xu QY, performed by Kang XY, Yu Z and Han SF. The data was analyzed by Kang XY, Lv X and Zhu YF. This paper was written by Xu QY and reviewed by Kang XY.
Conceptualization: Qun-Ying Xu, Xin Lv.
Data curation: Qun-Ying Xu, Shu-Fang Han, Xin Lv.
Formal analysis: Xin-Yao Kang, Ze Yu, Shu-Fang Han.
Investigation: Yu-Fang Zhu, Xin Lv.
Methodology: Xin-Yao Kang, Shu-Fang Han, Yu-Fang Zhu.
Software: Ze Yu.
Writing – review & editing: Xin-Yao Kang.
Footnotes
Abbreviations: IGF- I = insulin-like growth factor 1, IGF-II = insulin-like growth factor 2, IGFBP-1= insulin-like growth factor binding protein-1, IGFBP-3 = insulin-like growth factor binding protein-3, CRP = C-reactive protein, Interleukin-6 = IL-6, Interleukin-10 = IL-10, TNF-ɑ = tumor necrosis factor alpha, SMD = standardized mean difference, WMD = weighted mean difference.
How to cite this article: Kang XY, Xu QY, Yu Z, Han SF, Zhu YF, Lv X. The effects of physical activity on physiological markers in breast cancer survivors: a meta-analysis. Medicine. 2020;99:20(e20231).
The authors have no conflicts of interest to disclose.
References
- [1].Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2017;67:100–21. [DOI] [PubMed] [Google Scholar]
- [2].Tang F, Wang J, Tang Z, et al. Quality of life and its association with physical activity among different types of cancer survivors. PLoS One 2016;11:e0164971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. Bmj 2012;344:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neilson HK, Friedenreich CM, Brockton NT, et al. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev 2009;18:11–27. [DOI] [PubMed] [Google Scholar]
- [5].Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
- [6].Fairey AS, Courneya KS, Field CJ, et al. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2003;12:721–7. [PubMed] [Google Scholar]
- [7].Schmitz KH, Ahmed RL, Hannan PJ, et al. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev 2005;14:1672–80. [DOI] [PubMed] [Google Scholar]
- [8].Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol 2008;26:907–12. [DOI] [PubMed] [Google Scholar]
- [9].Swisher AK, Abraham J, Bonner D, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer 2015;23:2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jones SB, Thomas GA, Hesselsweet SD, et al. Effect of exercise on markers of inflammation in breast cancer survivors: the Yale exercise and survivorship study. Cancer Prev Res (Phila) 2013;6:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Conroy SM, Courneya KS, Brenner DR, et al. Impact of aerobic exercise on levels of IL-4 and IL-10: results from two randomized intervention trials. Cancer Med 2016;5:2385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lahart IM, Metsios GS, Nevill AM, et al. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. BMC Cancer 2016;16:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale exercise and survivorship study. Cancer Epidemiol Biomarkers Prev 2009;18:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther 2013;12:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schmidt ME, Meynkohn A, Habermann N, et al. Resistance exercise and inflammation in breast cancer patients undergoing adjuvant radiation therapy: mediation analysis from a randomized, controlled intervention trial. Int J Radiat Oncol Biol Phys 2016;94:329–37. [DOI] [PubMed] [Google Scholar]
- [16].Rice MS, Eliassen AH, Hankinson SE, et al. Breast cancer research in the nurses’ health studies: exposures across the life course. Am J Public Health 2016;106:1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ammitzboll G, Sogaard K, Karlsen RV, et al. Physical activity and survival in breast cancer. Eur J Cancer 2016;66:67–74. [DOI] [PubMed] [Google Scholar]
- [18].Nelson SH, Marinac CR, Patterson RE, et al. Impact of very low physical activity, BMI, and comorbidities on mortality among breast cancer survivors. Breast Cancer Res Treat 2016;155:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Neil-Sztramko SE, Boyle T, Milosevic E, et al. Does obesity modify the relationship between physical activity and breast cancer risk? Breast Cancer Res Treat 2017;166:367–81. [DOI] [PubMed] [Google Scholar]
- [20].Fajka-Boja R, Marton A, Toth A, et al. Increased insulin-like growth factor 1 production by polyploid adipose stem cells promotes growth of breast cancer cells. BMC Cancer 2018;18:872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mata R, Palladino C, Nicolosi ML, et al. IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget 2016;7:7683–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee SC, Tsai SM, Hou MF, et al. Increased Igf-I/Igfbp-3 ratios in postmenopausal taiwanese with breast cancer, irrespective of Er and Pr Statuses and Her2 expression in a case-control study. J Clin Lab Anal 2016;30:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Raval A, Trivedi S. Breast cancer: role of IGF-1 and IGFBP-3 expression in prognostication. Indian J Exp Biol 2016;54:619–29. [PubMed] [Google Scholar]
- [24].Meneses-Echavez JF, Jimenez EG, Rio-Valle JS, et al. The insulin-like growth factor system is modulated by exercise in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer 2016;16:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Torres-Mejia G, Angeles-Llerenas A, Ortega-Olvera C, et al. Moderate-intensity physical activity ameliorates the breast cancer risk in diabetic women. Diabetes Care 2012;35:2500–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


