Abstract
Background
Advances in modern spinal fusion techniques have allowed for less peri-operative morbidity and more rapid recovery from surgery. The addition of endoscopy to minimally invasive surgery (MIS) fusion techniques represents the latest progression of efforts to minimize the impact of surgical intervention.
Technique
MIS transforaminal lumbar interbody fusion (TLIF) is performed endoscopically through a sub-centimeter working portal. Patients undergo light conscious sedation and remain awake to facilitate feedback with the surgeon and enhance post-operative recovery.
Results
Previously reported results of the first 100 cases performed by the senior author at a single institution are summarized. This cohort has been characterized by brief post-operative length of stay, low complication profile, and marked improvement in patient-reported outcomes scores, with no cases of pseudarthrosis at 1-year follow up.
Conclusions
The latest technical considerations and adaptations of a novel technique for endoscopic MIS spinal fusion without general anesthesia are described. A refined surgical technique and anesthetic protocol are presented in detail with recommendations for the successful implementation and performance of the procedure.
Electronic supplementary material
The online version of this article (10.1007/s11420-020-09748-6) contains supplementary material, which is available to authorized users.
Keywords: fusion, spinal surgery, endoscopy, MIS, TLIF, technique
Introduction
Over many decades, innovations in spinal surgery techniques have evolved to better meet patients’ needs and modern healthcare systems’ demands. Rates of spinal surgery have increased steadily, as well, particularly in the growing elderly population [2, 9]. Lumbar fusion is regarded as one of the most painful and debilitating of surgical procedures [4], and patients increasingly opt to avoid the traditional open surgical approach in favor of minimally invasive surgery (MIS), desiring reduced pain and fewer complications.
Various MIS techniques have been developed, all of which aim to improve clinical outcomes, reduce morbidity, and limit post-operative pain. For lumbar fusion in particular, the MIS transforaminal lumbar interbody fusion (TLIF) has become a favorable option for the treatment of degenerative lumbar disease [8]. First described in 2003, the MIS TLIF employs a tubular retractor docked over the facet joint to facilitate total facetectomy and both ipsilateral and contralateral discectomy prior to placement of an interbody cage, followed by percutaneous pedicle screws with rod fixation [3].
As with the advent of MIS fusion techniques in the previous decade, the recent development of endoscopic TLIF techniques has further advanced this approach to lumbar fusion surgery. Although endoscopes were first used in spinal surgery in the 1980s, when Parviz Kambin employed them for percutaneous discectomies, endoscopic innovations in lumbar fusion have occurred more recently [1, 10]. Richard Fessler and colleagues established endoscopy as a safe and useful adjunct to MIS TLIF, resulting in equivalent outcomes and less peri-operative morbidity in a small series [6]. Description of Kambin’s triangle and subsequent cadaveric analyses have identified operative zones in which an endoscopic TLIF may be safely performed [5]. More recent data have validated the incorporation of endoscopic approaches for lumbar fusion to augment reductions in post-operative pain levels, opioid use, and length of hospital stay [12]. Therefore, the endoscopic TLIF may be a more appealing surgical option for patients resistant to undergoing even the traditional MIS TLIF, which still necessitates an open incision for muscular dissection to facilitate tube placement.
We have previously described initial results of our novel endoscopic MIS technique without the use of general anesthesia for one- and two-level TLIFs [11]. Here, we focus on details and improvements of surgical and anesthetic techniques and summarize the results from the first 100 treated patients to ascertain where further refinements in the technique can be achieved.
Technique
Anesthetic Technique
Conscious sedation is administered by our dedicated anesthesia team by way of a continuous infusion of propofol and ketamine. Initially, medications are titrated to achieve a light to moderate sedation level; spontaneous ventilation and purposeful response to verbal or noxious stimuli are maintained. No opioid medication or additional spinal, epidural, or general analgesic is used. Supplemental oxygen is provided via nasal cannula or face mask. As the patient is positioned prone without an advanced airway, the experience and comfort level of the anesthesia team are critical to this technique. Continuous patient monitoring and communication between surgeon and anesthesiologist allow for the safety and success of the procedure.
The appropriate level of conscious sedation confers several advantages. The surgeon is provided feedback via painful stimuli if there is any irritation of neural elements. The absence of general anesthetics enables a swift post-operative recovery, with relatively low incidence of amnesia, vertigo, nausea, dysphagia, or other adverse effects that may delay recovery, functional rehabilitation, and discharge.
Additional medications administered peri-operatively include pre-operative ondansetron and glycopyrrolate to limit intra-operative emesis. This has been a relatively recent addition to the regimen after two cases of intra-operative emesis that resulted in conversion to general endotracheal anesthesia (GETA). Oxymetazoline spray is also administered pre-operatively to avoid epistaxis, after this was the cause of one intra-operative conversion to GETA. Regarding analgesics, local liposomal bupivacaine is administered to subcutaneous tissue and paraspinal musculature during the procedure for pain control both intra- and post-operatively.
Surgical Technique
Patients are positioned prone on a Jackson table with the abdomen free to reduce intra-abdominal and central venous pressure. The arms are extended in the “superman” position. Kambin’s triangle, the anatomical space comprising the traversing nerve root, exiting nerve root, and superior aspect of the caudal vertebra at a given level, is approached with a spinal needle and nitinol wire. This is completed on the side of the more significant pathology at the target level under fluoroscopic guidance (Fig. 1). Successive cannulated dilators allow for the introduction of an 8-mm working cannula through which both the endoscope and instruments may be simultaneously passed (Fig. 2). This allows for an entirely uniportal technique. The endoscope used has a 6.3-mm outer diameter, a 3.7-mm working channel, and a 30° viewing angle; it is initially inserted through this cannula for visualization of the disc space and of the traversing and exiting nerve roots.
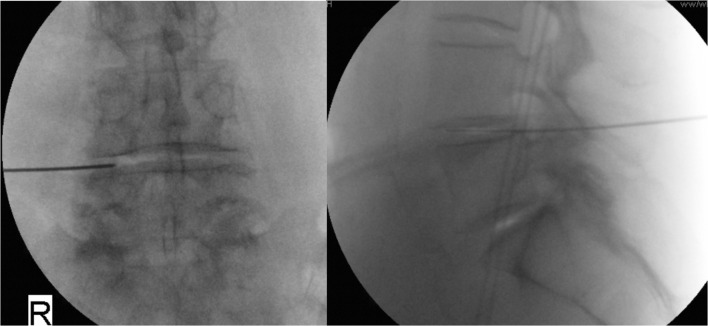
Fig. 1.
Fluoroscopic images confirming intra-distal placement of a nitinol wire via a transforaminal approach at the target level.
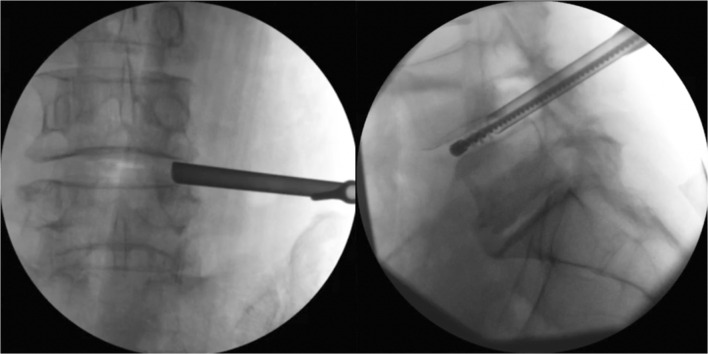
Fig. 2.
Fluoroscopic images showing the 8-mm working portal placed transforaminally (left) and the use of the portal for endplate preparation via stainless-steel brush (right).
Nerve roots are decompressed using pathology-specific endoscopic instruments, including pituitary rongeurs, curettes, micro-osteotomes, high-speed drills, and bipolar electrocautery. The disc space is similarly cleared of disc material, and adjacent endplates are prepared for bony fusion (Fig. 3). A high-speed drill equipped with a stainless-steel brush provides effective removal of residual disc material and cartilaginous endplate. A silicone balloon catheter filled with radiopaque medium allows for fluoroscopic assessment of the full extent of the discectomy and defines the location of the residual cartilaginous endplate (Fig. 4). Further endplate preparation may then be carried out, if necessary. This portion of the technique has been modified since initial adoption, after two early cases of post-operative interbody cage migration.
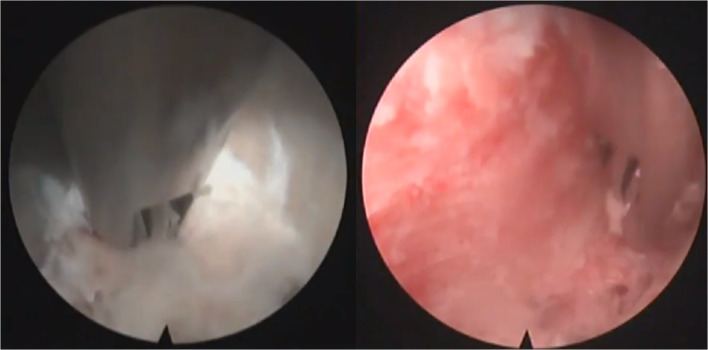
Fig. 3.
Endoscopic visualization of initial discectomy (left) and final endplate preparation (right).
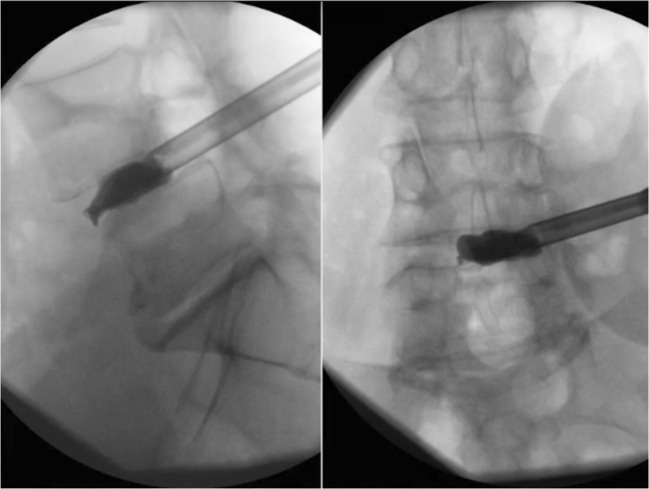
Fig. 4.
Radiopaque balloon catheter inflation in the interbody space allows for radiographic evaluation of discectomy and endplate preparation.
After adequate endplate preparation has been confirmed, 2.1 mg of recombinant human bone morphogenetic protein–2 is placed into the anterior disc space. Pre-treatment with radiopaque medium allows for fluoroscopic confirmation of placement in the desired location. A 22- or 25-mm OptiMesh (Spineology, St. Paul, MN, USA) expandable bone-graft containment mesh is then positioned in the disc space and filled with pre-machined allograft in situ. Appropriate placement and expansion allow for re-establishment of disc space height, additional indirect neural element decompression, and correction of any concomitant spondylolisthesis. It is worth noting that use of the endoscope precludes the need for any formal surgical approach, preserving all paraspinal musculature and bony architecture typically sacrificed for disc space access. It also allows for direct visualization of the foramen disc space, which facilitates accurate and thorough nerve root decompression and endplate preparation.
Pedicle screws are subsequently placed percutaneously. The paraspinal musculature within each of the four planned screw tracts are first injected with 20 mL of liposomal bupivacaine diluted 1:2 to 40 mL total volume. Anteroposterior fluoroscopic guidance allows for placement of trephine needles in the appropriate position and trajectory through these tracts. A guidewire is placed into each trephine needle, allowing for the placement of a cannulated awl and then a tap into the respective pedicles. Six or 7 mm pedicle screws are then placed; bilateral connecting rods are inserted subfascially; and set screws are placed to secure the construct. A total of five incisions are then closed with subcuticular sutures.
Results
Critical analysis of the first 100 such procedures performed by the senior author at a single institution demonstrate overall positive results with regard to clinical outcome, complication rate, and overall reduction in peri-operative morbidity. These results were previously reported [7], and we summarize them here. Consideration of awake endoscopic MIS TLIF was based on criteria including diagnosis of degenerative disc disease with grade I or II spondylolisthesis, as well as evidence of symptomatic spinal stenosis or focal nerve impingement at the same level. The average age of this cohort was 66 years. Of the 100 patients, 84 underwent procedures on a single level, with an average operative time of 84.5 ± 21.7 min, and average blood loss of 65.4 ± 76.6 mL; 16 patients underwent two-level fusions, with an average operative time of 128.1 ± 48.6 min, and average blood loss of 74.7 ± 33.6 mL; 77% of fusions were at L4–L5. Average length of stay was 1.4 ± 1 days.
Four cases in this series were converted to GETA intra-operatively due to emesis (2), epistaxis (1), and severe anxiety (1). After collective decision making among the surgical and anesthesia teams, all cases were completed in the same operative event after successful conversion to GETA. These episodes have prompted relatively simple adjustments to our peri-operative medication protocol that have eliminated subsequent incidence. Regardless, careful pre-operative discussion with the patient as well as our dedicated and vigilant anesthesia team regarding the potential risk of conversion to GETA remains a mainstay of our practice.
At a minimum of 1-year follow up, there have been no cases of hardware failure or pseudarthrosis, with all patients demonstrating contiguous, radiopaque interbody arthrodesis, with no evidence of motion at the involved segment on anteroposterior, lateral, flexion, and extension radiographs. Four patients died due to causes unrelated to the described surgical intervention. Oswestry Disability Index (ODI) data was available for 82% of surviving patients. Average post-operative ODI (17.2 ± 16.9) was significantly improved from pre-operative measures (25.6 ± 15.3; p = 0.000001). Complications included interbody cage migration (2), vertebral osteomyelitis (1), and endplate fracture (1). Three of these complications occurred within the first 50 performed cases.
Discussion
In the experience of the senior author at a single institution, awake endoscopic TLIF provides several distinct advantages over traditional MIS and open techniques for single-level fusion. When compared with traditional MIS TLIF performed by the same surgeon, endoscopic TLIF had significantly shorter operative time, shorter post-operative length of stay, lower rates of nonroutine discharge, lower rates of complications, and reduced overall cost of acute hospitalization in a small series [13]. The reduced length of stay has been largely influenced by the mitigation in post-operative pain that this procedure offers. Along with the relative elimination of muscular dissection required by this approach, patients are subsequently able to embark on a more rapid return to functional status and comfort level permissive of discharge.
Over the course of the first 100 cases performed and beyond, several key adaptations have been made to target enhanced outcomes and patient experience. As described, our pre-operative medication protocol now includes ondansetron and glycopyrrolate, plus oxymetazoline spray, to reduce the incidence of intra-operative emesis and epistaxis, respectively. These additions were prompted by several early incidents of requisite conversion from sedation to GETA. Routine fluoroscopic evaluation of the targeted disc space to ensure adequate endplate preparation prior to fusion is now carried out after an early case of post-operative cage migration. The timing of local liposomal bupivacaine administration has been fine-tuned to just prior to percutaneous pedicle screw placement after ongoing critical monitoring of pain control in patients post-operatively. Of note, the majority of all described complications occurred in the early stages of implementation of this technique, likely indicating a learning curve.
Regular and rigorous evaluation of results and communication among all members of the dedicated surgical and anesthesia teams has been a mainstay of this technique, which continues to evolve and improve. Ongoing study at our institution will characterize outcomes in a larger sample size as the use of this technique continues, with the hope of describing the feasibility of its widespread application and subsequent implications for outcomes and cost.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
Compliance with Ethical Standards
Conflict of Interest
Alexander J. Butler, MD, and G. Damian Brusko, BS, declare that they have no conflicts of interest. Michael Y. Wang, MD, reports royalties from DePuy-Synthes Spine, Inc., Children’s Hospital of Los Angeles, Springer Publishing, and Quality Medical Publishing; personal fees as a consultant from DePuy-Synthes Spine, Inc., JoiMax USA, K2M, and Aesculap Spine; advisory board membership for Vallum; stock in Spinicity and Innovative Surgical Devices; and grants from the US Department of Defense.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
Informed consent was waived from all patients for being included in this study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Butler AJ, Alam M, Wiley K, Ghasem A, Rush AJ, III, Wang JC. Endoscopic lumbar surgery: the state of the art in 2019. Neurospine. 2019;16(1):15–23. doi: 10.14245/ns.1938040.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 2003;28(15 Suppl):S26–S35. doi: 10.1097/01.BRS.0000076895.52418.5E. [DOI] [PubMed] [Google Scholar]
- 4.Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- 5.Hardenbrook M, Lombardo S, Wilson MC, Telfeian AE. The anatomic rationale for transforaminal endoscopic interbody fusion: a cadaveric analysis. Neurosurg Focus. 2016;40(2):E12. doi: 10.3171/2015.10.FOCUS15389. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs RE, Podichetty VK, Santiago P, et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3(2):98–105. doi: 10.3171/spi.2005.3.2.0098. [DOI] [PubMed] [Google Scholar]
- 7.Kolcun JPG, Brusko GD, Basil GW, Epstein R, Wang MY. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurg Focus. 2019;46(4):E14. doi: 10.3171/2018.12.FOCUS18701. [DOI] [PubMed] [Google Scholar]
- 8.Qin R, Liu B, Zhou P, et al. Minimally invasive versus traditional open transforaminal lumbar interbody fusion for the treatment of single-level spondylolisthesis grades 1 and 2: a systematic review and meta-analysis. World Neurosurg. 2019;122:180–189. doi: 10.1016/j.wneu.2018.10.202. [DOI] [PubMed] [Google Scholar]
- 9.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37(1):67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 10.Telfeian AE, Veeravagu A, Oyelese AA, Gokaslan ZL. A brief history of endoscopic spine surgery. Neurosurg Focus. 2016;40(2):E2. doi: 10.3171/2015.11.FOCUS15429. [DOI] [PubMed] [Google Scholar]
- 11.Wang MY, Grossman J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurg Focus. 2016;40(2):E13. doi: 10.3171/2015.11.FOCUS15435. [DOI] [PubMed] [Google Scholar]
- 12.Wang MY, Chang PY, Grossman J. Development of an Enhanced Recovery After Surgery (ERAS) approach for lumbar spinal fusion. J Neurosurg Spine. 2017;26(4):411–418. doi: 10.3171/2016.9.SPINE16375. [DOI] [PubMed] [Google Scholar]
- 13.Wang Michael Y, Chang Hsuan Kan, Grossman Jay. Reduced Acute Care Costs With the ERAS® Minimally Invasive Transforaminal Lumbar Interbody Fusion Compared With Conventional Minimally Invasive Transforaminal Lumbar Interbody Fusion. Neurosurgery. 2017;83(4):827–834. doi: 10.1093/neuros/nyx400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)