Abstract
Hypoxia affects the physiology of cells and organisms; however, the mechanisms associated with hypoxia adaptation remain unknown in Tibetan chickens. In this study, we aimed to identify long noncoding RNAs (lncRNAs) involved in hypoxia adaptation in Tibetan chickens and Daheng broilers, to provide insights into the mechanisms underlying hypoxia induction. RNA sequencing results revealed that a total of 5504 lncRNAs and 16,779 microRNAs were differentially expressed in four Tibetan chickens and four Daheng broilers; 70 lncRNAs were up-regulated and 113 lncRNAs were down-regulated in the Tibetan chickens compared to the expression levels in the Daheng broilers. The differentially expressed lncRNAs (DElncRNAs) were enriched in the following Gene ontology terms: protein complex localization, small-molecule metabolic process, and RNA splicing. Kyoto Encyclopedia of Genes and Genomes analyses revealed that the DElncRNAs were mainly enriched in pathways that regulate cell junctions and intercellular spaces and oxygen or energy metabolism, mainly involved in hypoxic adaption. Moreover, a predicted ceRNA network with five DElncRNAs interacted with three miRNAs that acted on 42 pathways through 19 target genes. Quantitative real-time polymerase chain reaction was used to verify that the expression levels of ENSGALG00000008047, ENSGALG00000050044, and ENSGALG00000053982 were significantly lower in Tibetan chickens than in the Daheng broilers, consistent with the RNA sequencing results. We obtained lncRNA expression profiles for the heart tissue of Tibetan chickens for the first time and have provided novel data that may aid research on biological adaptation to hypoxic stress.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02272-8) contains supplementary material, which is available to authorized users.
Keywords: lncRNA sequencing, Tibetan chicken, Hypoxic adaptation
Introduction
Hypoxia is closely related to many human diseases, such as cardiovascular and cerebrovascular diseases (Foster et al. 2009), sleep apnea syndrome (Nanduri et al. 2015), cancer (Wilson and Hay 2011), ischemic injury (Collino et al. 2019), fracture (Muinos-Lopez et al. 2016), and altitude sickness (Coustet et al. 2015). Animals may experience hypoxia during strenuous exercise, at high altitudes and deep waters, and due to various pathological factors. Whole-body and partial-body hypoxia can trigger a series of adaptive reactions in the body and cells to maintain oxygen homeostasis. The body can adapt to short-term hypoxia through various mechanisms, such as defense response, compensatory response, and remedial response (Thakor et al. 2015; Shi et al. 2016). Hypoxia-inducible factors (HIF) are important central regulatory factors for detecting and adapting to the oxygen content of cells, and can transcriptionally activate genes that regulate oxygen homeostasis and metabolism (Choudhry and Harris 2018). Hypoxia adaptation and response are topics of great significance for clinical medicine, aerospace medicine, high altitude medicine, and sports medicine. For example, intermittent hypoxia adaption protects the myocardial injury through HIF regulation (Zhuang and Zhou 1999). Therefore, the molecular mechanism of hypoxia adaptation has always been a research hotspot.
Hypoxia adaptability is only found in species adapted through natural selection to high-altitude environments and special domesticated populations such as the Tibetan chickens and Tibetan pigs, which have lived in the Tibetan highlands for generations. However, the genetic mechanism of the hypoxia adaptation on native mammals (including humans) and birds in the plateau is still unclear.
Long noncoding RNA (lncRNA) are RNA strands that do not encode proteins and are generally longer than 200 nucleotides. LncRNA has been found to act in gene expression by different mechanisms, including genomic imprinting, transcription regulation, RNA splicing, translation regulation, and RNA interference (Yao et al. 2019). The core of the hypoxia adaptation mechanism is a series of complex reactions around hypoxia, among them lncRNA can regulate the expression of HIF and, conversely, HIF can also regulate the production of lncRNA (Choudhry and Harris 2018). According to the description by Choudhry and Harris, there are four types of interaction mechanisms between lncRNA and hypoxia, including direct and indirect paths. For example, lncRNA can directly bind to HIF-1α and activate its expression, affecting the development of oral carcinoma (Shih et al. 2017). Moreover, lncRNA OS-9 indirect inhibition in hypoxia abrogates by inducing HIF-1α interaction with proline hydroxylase domain protein that leads to HIF-1α degradation (Baek et al. 2005). Nevertheless, few studies have reported identifying specific expressions of non-coding RNAs in the context of hypoxia adaptation using high-throughput sequencing techniques. Therefore, the role of lncRNA in hypoxia adaptation remains largely unknown.
The Tibetan chicken is a breed native to Tibet which has been adapted to the low oxygen environment of the Tibetan plateaus. Under the same hypoxic conditions, the hatchability of Tibetan chickens was much higher than that of other chicken (Liu et al. 2009). This suggests that Tibetan chickens have a special mechanism for effectively capturing oxygen and maintaining tissue oxygen balance under hypoxic conditions. For this reason, the Tibetan chicken was chosen as an animal model for studying the mechanisms of hypoxic adaptation in this paper. The Daheng broiler, which is a chicken breed adapted to normoxic conditions, was used as a control.
In this research, we aim to reveal the molecular mechanism of low oxygen adaptation in Tibetan chickens, and provide genetic resources for the breeding and industrialization of high-quality flavoured chicken, so as to realize the scientific utilization of Tibetan chicken germplasm resources. In this paper, high-throughput sequencing was used to screen differentially expressed genes and regulatory pathways in chicken embryonic heart tissues with different hypoxic adaptive phenotypes.
Materials and methods
Ethics statement
All chicken experiments were approved via the animal care and ethical committee of the Sichuan Animal Science Academy. Additionally, all experimental procedures and animal care performed in the present study were approved according to the recommendations of the Sichuan academy of animal science. All efforts were made to minimize suffering.
Experimental chickens
Four Tibetan chickens were obtained from high altitude areas with complex and geographical conditions and an average elevation of 4000 m, low atmospheric pressure, intense radiation, and low temperature. Four Daheng broilers, which is a local breed of Sichuan, with similar age and nutrient conditions were purchased from a low altitude area and served as the control group. The fertilized eggs of the Tibetan chickens and Daheng broilers were collected within seven days and hatched for 16 days under hypoxic conditions (13% O2). The heart tissues were carefully separated from the egg embryos, then quickly put into liquid nitrogen to be preserved. Each chicken breed replicated four times, thus eight chicken libraries were used in total.
Total RNA extraction, cDNA library construction, and sequencing
Total RNA was extracted from the embryonic heart tissues by TRIzol reagent (Invitrogen Life Technologies, Inc., Carlsbad, CA, USA) according to the manufacturer's instruction, and then immediately stored in a refrigerator at − 80 °C. The concentration and purity of total RNA were detected by gel electrophoresis and spectrophotometer (Nanodrop® ND-1000). High-quality RNA was used to constructed cDNA libraries after eliminating the ribosomal RNA using a Ribo-zero Gold rRNA Removal Kit (Illumina, San Diego, CA, USA). Library quality was evaluated by the Agilent Bioanalyzer 2100 system according to the manufacturer’s instructions. High-throughput lncRNA sequencing was performed on the Hiseq™ 2500 platform (Illumina, USA).
Differential expression and functional enrichment analysis
For lncRNA sequencing, the raw reads were qualified by FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The gene expression data in the eight samples were normalized by fragments per kilobase of exon per million fragments mapped (FPKM). The differential expression used the DESeq2.0 algorithm and screening criteria was . Target genes were predicted based on the differentially expressed lncRNA (DElncRNA).
Functional analysis of the predicted DElncRNA targets was performed by using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) and Gene Ontology (GO) Consortium platforms, and the significantly differentially term was defined as a threshold of FDR < 0.05. Pathway analyses of the predicted DElncRNA targets were performed using the Kyoto encyclopedia of genes and genomes (KEGG) based on the hypergeometric distribution. Significantly differentially pathway was defined at P < 0.05 or an enrichment score of > 1.5.
Analysis of the lncRNA-miRNA-mRNA-pathway network
To evaluate the relationship between lncRNA expression and co-expression regulation of mRNA, a lncRNA-miRNA-mRNA-pathway network was constructed. To construct the competing endogenous RNA (ceRNA) network, we selected five DElncRNAs involved in oxygen metabolism or hypoxic adaptation. Additionally, the predicted target genes of the five DElncRNAs were used for network construction. Each given lncRNA-mRNA pair was targeted by a common miRNA to construct the lncRNA-miRNA-mRNA ceRNA network. Finally, the pathway corresponding to the target gene was added to the ceRNA network diagram. The network was visualized using Cytoscape software (v.3.6.1).
Quantitative reverse transcription PCR (qRT-PCR) verification
We selected five significantly expressed DElncRNAs used in the ceRNA network to verify the reliability of sequencing the data by qRT-PCR. All primers were synthesized by Sangon Biotech (Shanghai, China) and are shown in Supplement Table 1. Briefly, the cDNA was synthesized using the RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific, Inc.), and then using FastStart Universal SYBR Green Master mix to amplify the cDNA with QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific, Inc., Madison, WI, USA) according to the manufacturer’s instructions. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) served as an internal reference gene, and gene expression was determined by the 2−ΔΔCq method.
Statistical analysis
All the experiments were repeated three times. The results are presented as the means ± standard deviation (SD). Comparisons were made using Student's t test (two-tailed) as indicated between two groups. GraphPad Prism 8.0 software (San Diego, CA, USA) was used for graphing. P values < 0.05 indicated significance.
Results
Overall view sequencing data
Statistics and quality evaluations were conducted on the sequencing data, and the results are shown in Supplement Table 2. The clean reads obtained from the eight samples ranged from 63,212,964 to 117,832,946, and the Q30 and GC percentages were 93.05–94.38, and 46.37–51.13, respectively.
Identification of the DElncRNA
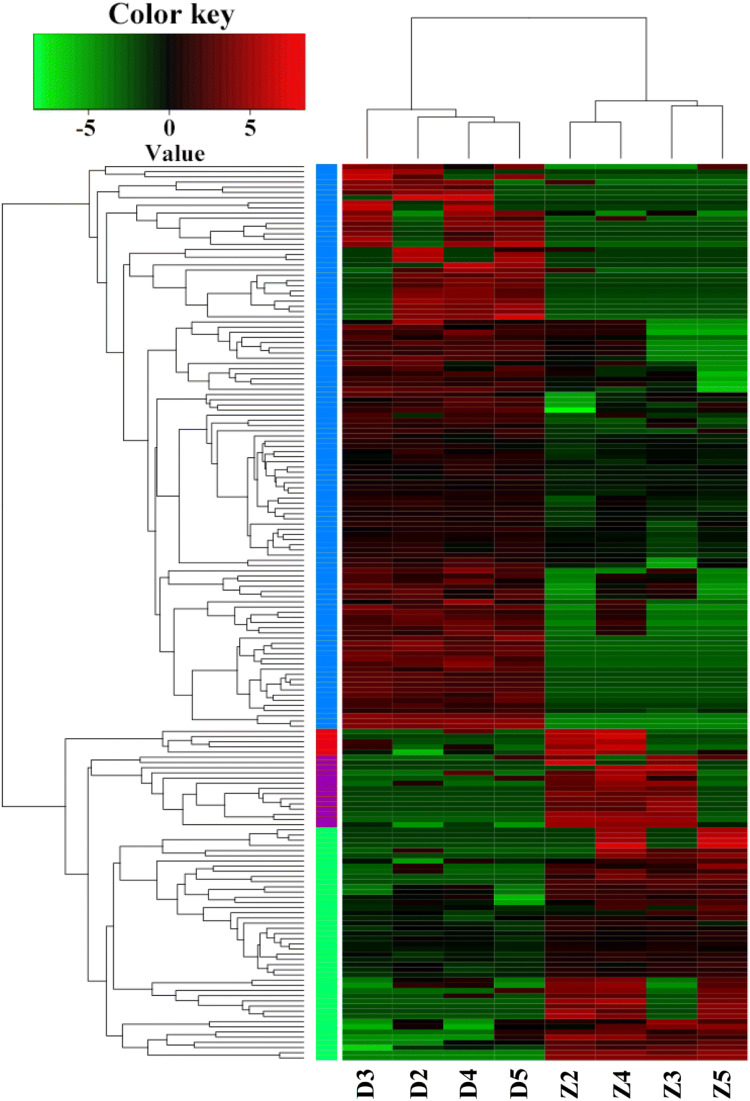
In total, we identified 5504 lncRNAs and 16,779 mRNAs (Table 1) and their expression profiles in the Tibetan chickens and Daheng broilers. After FPKM standardization, differentially expressed genes were identified by using the DESeq2.0 algorithm. As shown in Table 1, we identified 183 DElncRNAs, with 70 of them being up-regulated and 113 down-regulated in Tibetan chickens compared to the expression in Daheng broilers. The cluster heatmap showed samples from the same variety source grouped into a single category, indicating a high degree of confidence in repeated operations (Fig. 1). Moreover, were identified 890 mRNAs with 466 of them being up-regulated and 424 down-regulated in Tibetan chickens compared to the expression in Daheng broilers (Table 1).
Table 1.
The number of long noncoding RNAs (lncRNA) and mRNAs in the sequencing results
| Genes | Total | DE | Up | Down |
|---|---|---|---|---|
| lncRNAs | 5504 | 183 | 70 | 113 |
| mRNAs | 16,779 | 890 | 466 | 424 |
DE differential expression, Z vs D Tibetan chickens compared with Daheng broilers, respectively
Fig. 1.
Cluster heatmaps of the differentially expressed lncRNAs (DElncRNAs). The cluster heatmap shows samples from Tibetan chickens (Z) and Daheng broilers (D) grouped each into a single category (red: upregulated in Tibetan chickens, green: downregulated in Tibetan chickens)
DElncRNA targets for GO analysis
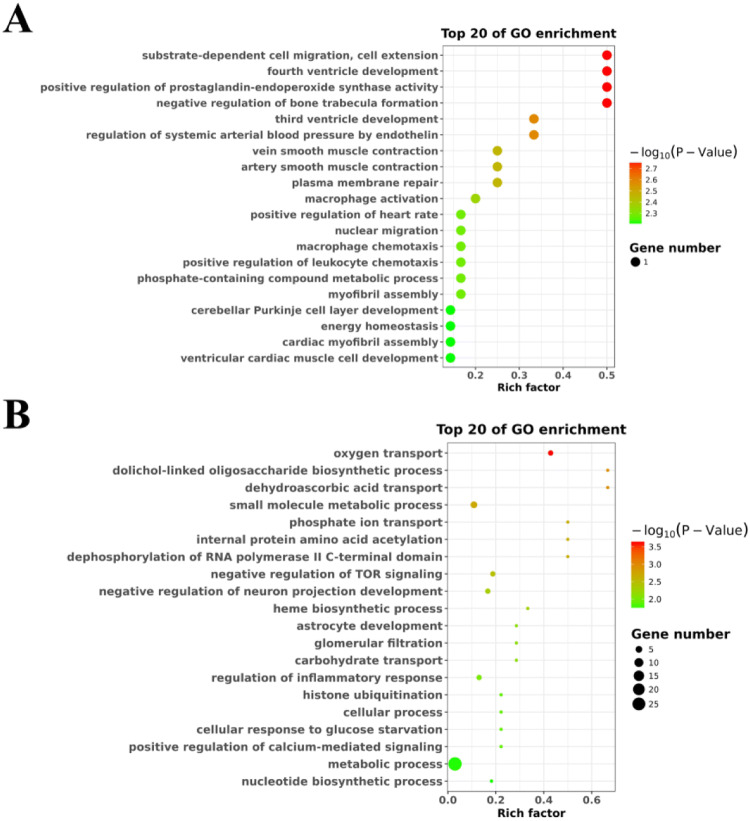
To explore the role of DElncRNAs in the hypoxia adaptability of Tibetan chickens, we functionally annotated all the DElncRNAs through GO and KEGG database. As shown in Fig. 2, down-regulated DElncRNAs in the Tibetan chicken were significantly enriched in GO terms associated with protein complex localization, small-molecule metabolic processes, and RNA splicing (Fig. 2a), whereas the DElncRNAs up-regulated in the Tibetan chicken were significantly enriched in the prostaglandin biosynthetic process (Fig. 2b).
Fig. 2.
Gene Ontology (GO) enrichment based on the target genes of differentially expressed lncRNAs (DElncRNAs) between Tibetan chickens and Daheng broilers. a The top 20 GO enrichment annotated by down-regulated target genes in heart embryonic tissue of Tibetan chickens. b The top 4 GO enrichment annotated by up-regulated target genes in Tibetan chickens. The left side represents the GO term, the right represents enrichment, and the size of the solid circle indicates the number of genes. The change of bubbles from green to red means that the degree of gene function is increased, and the large bubbles indicate that the number of genes is enriched
DElncRNA targets for KEGG pathway analysis
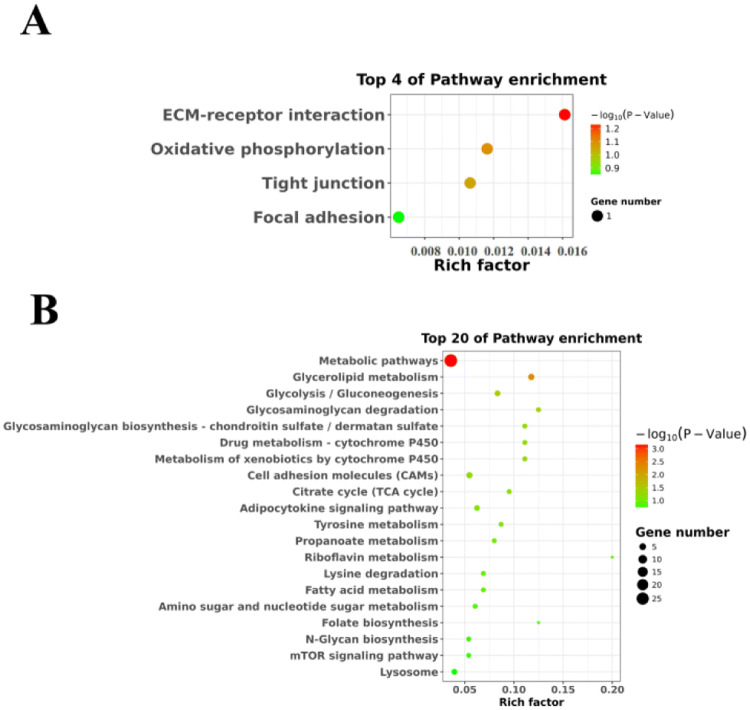
Similarly, the up-regulated and down-regulated DElncRNA enrichment pathways were not similar for the two groups. Apart from the metabolic pathway and the Notch signaling pathway, the down-regulated DElncRNAs in the Tibetan chicken were mainly concentrated in energy-related pathways, such as insulin signaling pathway, glycolysis/gluconeogenesis, fatty acid metabolism, and amino sugar and nucleotide sugar metabolism (Fig. 3a). In addition, the up-regulated DElncRNAs in the Tibetan chicken were mainly enriched in the focal adhesion pathway (Fig. 3b). Notably, these DElncRNAs are involved in many pathways of intercellular connectivity, such as glycosaminoglycan degradation, focal adhesion, cell adhesion molecules (CAMs), gap junction, tight junction, and adherens junction. These suggest that Tibetan chickens may have adapted to the hypoxic environment by regulating their basic energy metabolism or cell junction during the long-term hypoxic domestication.
Fig. 3.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment based on differentially expressed lncRNAs (DElncRNAs) target genes between Tibetan chickens and Daheng broilers. a The top 20 KEGG pathway enrichment annotated by down-regulated target genes in heart embryonic tissue of Tibetan chickens. b The top 20 KEGG pathway enrichment annotated by up-regulated target genes in heart embryonic tissue of Tibetan chickens. The size of the solid circle indicates the number of genes. The change of bubbles from green to red means that the degree of gene function is increased, and the large bubbles indicate that the number of genes is enriched
DElncRNA-miRNA-mRNA-pathway network
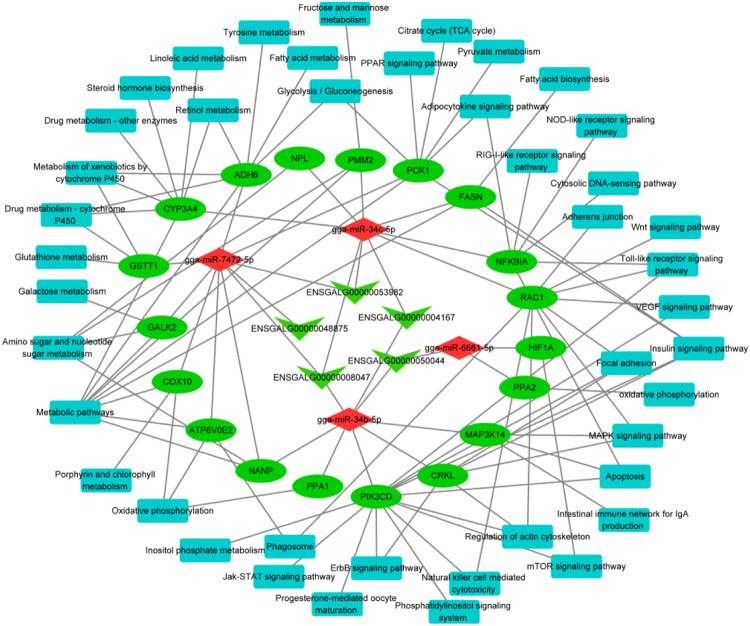
Many studies have confirmed that lncRNA functions through ceRNA mechanisms, hence, we performed a ceRNA predictive analysis on the DElncRNA. Considering that the pathway annotated by target genes can help us understand the role of DElncRNA in the hypoxia adaptability of Tibetan chickens, we added the annotated pathways as new elements into the ceRNA network to construct a DElncRNA-miRNA-mRNA-pathway network. As shown in Fig. 4, ENSGALG00000053982, ENSGALG00000008047, and ENSGALG00000004167 interacted with gga-miR-34b-5p at the same time. Besides, ENSGALG00000053982, ENSGALG00000008047, and ENSGALG00000048875 interacted with gga-miR-7472-5p. Moreover, the downregulation of ENSGALG00000050044 targeted gga-miR-6661-5p and may regulate pyrophosphatase (inorganic) 2 (PPA2) via oxidative phosphorylation, and gga-miR-6661-5p targeted the hypoxia-inducible factor 1 alpha (HIF1A) via mTOR signaling pathway in Tibetan chickens. All the five DElncRNAs that interacted with the four miRNAs acted on 42 pathways through 19 target genes. Notably, these pathways were related to oxygen or energy metabolism, such as oxidative phosphorylation, citrate cycle (TCA cycle), metabolism of xenobiotics by cytochrome P450, and insulin signaling pathway. Besides, DElncRNAs may also function through the immune stress pathway to adapt to hypoxic conditions, such as the natural killer cell-mediated cytotoxicity pathway (Mu et al. 2019).
Fig. 4.
The competing endogenous RNA (ceRNA) network of the lncRNA-miRNA-mRNA-pathway constructed with 5 selected long noncoding RNAs (lncRNAs) and the top 4 predicted microRNAs (miRNAs). Green arrows indicate lncRNA, diamond indicates miRNA, ellipse indicates mRNA, and rectangle indicates pathway
Verification by qRT-PCR
To verify the RNA-seq results, we selected five key lncRNAs with high differential expression multiples and high abundance in the embryonic heart tissues of Tibetan chickens. The qRT-PCR results are shown in Fig. 5. The expressions of ENSGALG00000008047 (P < 0.05), ENSGALG00000050044 (P < 0.001), and ENSGALG00000053982 (P < 0.001) were significantly lower in the embryonic heart tissues of the Tibetan chickens compared to those in the heart tissues of the Daheng broilers. Furthermore, ENSGALG00000004167 and ENSGALG00000048875 expressions were non-significant in Tibetan chickens.
Fig. 5.
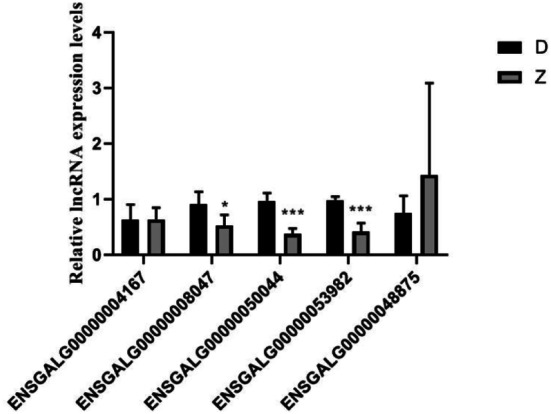
Validation of candidate long noncoding RNAs (lncRNAs) expression in 4 pairs of Tibetan chickens (Z) and Daheng broilers (D) heart embryonic tissues by quantitative real-time polymerase chain reaction (qRT-PCR). Gene expression of lncRNA was normalized to glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) levels in three biological replicates. The comparison was performed using the t test. (*P < 0.05, ***P < 0.001)
Discussion
Maintaining oxygen balance is vital for animals. Exposure of animals to hypoxic conditions can trigger a set of adverse physiological reactions, including strokes, myocardial ischemia, and tumor growth and metastasis (Michiels 2004). To better understand the effects caused by hypoxia in humans, we used the Tibetan chicken, which is a bird completely adapted to hypoxic environments, as an experimental model to explore the specific expression pattern of lncRNA in hypoxic adaptation, aiming to provide ideas for future hypoxic adaptation engineering.
In this study, comparing with the expression patterns in the Daheng broilers, we identified 70 up-regulated and 113 down-regulated DElncRNAs in Tibetan chickens. On the one hand, we found that some DElncRNAs were enriched in pathways of intercellular connectivity, such as glycosaminoglycan degradation, focal adhesion, cell adhesion molecules (CAMs), gap junction, tight junction, and adherens junction. The glycosaminoglycan is one of the main components of the extracellular matrix. A study found that in the course of an acute lung injury, accompanied by short-term acute hypoxia, the content, synthesis and distribution of glycosaminoglycans significantly changed (Papakonstantinou et al. 2000). Another study showed that bovine pulmonary artery endothelial cells cultured under hypoxic conditions accumulated significantly less proteoglycan in the medium than cells cultured under normal conditions (Humphries et al. 1986). These results suggest that glycosaminoglycans are substantially involved in the pulmonary hypoxic environment response. Focal adhesion form part of the cell-to-extracellular matrix connections and play a key role in cell proliferation, migration, signaling, and adhesion (Cox et al. 2006). Under hypoxic conditions, targeted inhibition of adhesion kinase can reduce cardiac fibrosis and protect cardiac remodeling (Zhang et al. 2017). Similarly, there is a high expression of cell adhesion molecules in coronary artery disease patients and is associated with the apnea–hypopnea index, which affects patient prognosis (El-Solh et al. 2002). Accordingly, we speculate that these up-regulated DElncRNAs may provide greater possibilities for oxygen circulation in animals by regulating cell junctions and intercellular spaces, thereby facilitating the adaptation to hypoxic environments. On the other hand, we found that other DElncRNAs were enriched in pathways connected to energy metabolism, such as insulin signaling pathway, amino sugar and nucleotide sugar metabolism, glycolysis/gluconeogenesis, and fatty acid metabolism. Animals cannot survive without oxygen, which is required for energy metabolism. One study found that hypoxia inhibits adipogenesis, and interferes with fatty acid metabolism and insulin signaling (Ye 2009). In addition, the regulation of mitochondrial respiration to increase glycolytic activity and compensate for insufficient energy supply under hypoxic conditions is also a manifestation of animals' adaptation to hypoxic environments (Iyer et al. 1998). Thus, we speculate that these down-regulated DElncRNAs may contribute to the hypoxic adaptation of Tibetan chickens by regulating their energy metabolism.
Increasing evidence has shown that lncRNAs can regulate target gene expression via functioning as a ceRNA for miRNA (Yang et al. 2019). In our study, we screened five DElncRNAs (ENSGALG00000053982, ENSGALG00000008047, ENSGALG00000004167, ENSGALG00000048875, and ENSGALG00000050044), which may be a sponge to three miRNAs (miR-34b-5p, miR-7472-5p, and miR-6661-5p). The miR-34a, miR-34b, and miR-34c make up the miR-34 family, and the sequences between the three differ by only two or three bases, so these miR-34 have partially similar target genes and functions (He et al. 2007). The downregulation of miR-34b-5p inhibits the accumulation of reactive oxygen species and alleviates intestinal ischemia and hypoxic injury (Wang et al. 2016), and its inhibition attenuates inflammation and apoptosis in acute injury mouse model (Xie et al. 2018). Eltzschig and Carmeliet (2011) reported that oxygen-sensing mechanisms and hypoxia signaling are potential therapeutic targets for the treatment of inflammatory diseases, which outlines the link between hypoxia and inflammation in such diseases. Besides, miR-34c-5p directly regulates soluble guanylyl cyclase β1 expression during hypoxia (Xu et al. 2012). Our results indicated that miR-34b may be an important link for DElncRNA to regulate the adaptation of Tibetan chickens to hypoxia. Also, there is evidence that the miR-34 family are important components of the p53 network, which inhibits hypoxia-inducible factor-stimulated transcription (Blagosklonny et al. 1998). Regrettably, no literature related to miR-7472-5p and miR-6661-5p was found, so we analyzed their target genes. One of the target genes for miR-7472-5p was HIF1A, which was annotated into the mTOR signaling pathway. HIF1 mediates the expression of pyruvate dehydrogenase kinase, a metabolic conversion substance necessary for cells to adapt to hypoxia (Kim et al. 2006). Research found that inhibiting miR-544 expression can regulate mTOR signaling pathways, thereby disrupting hypoxic adaptability of tumor cells (Haga et al. 2015). It is also possible that miR-7472-5p has a hypoxic regulation mechanism similar to that of miR-544. The miR-6661-5p target PPA2 was annotated into the oxidative phosphorylation pathway. PPA has a specific production, degradation, and transportation mechanisms in the cell, and abnormal PPA2 directly mediates some disease manifestations (Terkeltaub 2001). Studies have shown that low-level expression of PPA results in decreased cell viability in response to hypoxia (Mustroph et al. 2005). Oxidative phosphorylation is a hypoxia-dependent product. Tumors usually maintain a mixture of aerobic glycolysis and oxidative phosphorylation bioenergy, and oxidative phosphorylation activates HIF, triggering a series of cellular regulatory mechanisms (Plecita-Hlavata et al. 2015). Therefore, the above evidence indicates that miR-6661-5p may be a small molecule that directly regulates oxygen metabolism and assists Tibetan chickens in adapting to a hypoxic environment.
In conclusion, the lncRNA expression profiles of heart tissue from Tibetan chickens and Daheng broilers were obtained for the first time, and 183 DElncRNAs (70 up-regulated and 113 down-regulated) were identified from the two species. The DElncRNAs were enriched in GO terms associated with protein complex localization, small-molecule metabolic processes, and RNA splicing. The KEGG pathway analysis showed that DElncRNAs were mainly enriched in pathways that regulate cell junctions and intercellular spaces and oxygen or energy metabolism, with all of them being involved in hypoxic adaption. Moreover, the ceRNA network analysis suggested that DElncRNAs can regulate target gene expression by functioning as a ceRNA for miRNA contributing to hypoxic adaption in Tibetan chickens. Collectively, this study provides novel evidence that may help advance future research on biological adaptation to hypoxic stress.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization and Funding acquisition: CY, XJ. Data curation: ZZ, MQ, HD, QL, CY, WG. Formal analysis: ZZ, MQ, HP, BX, XX. Experimental studies: ZZ, MQ, HD, QL, CY, WG, HP, BX, XX, XS, LY, CH, JC. Software: ZZ, XS, LY, CH, JC. Writing—original draft & review & editing: All authors. All authors read and approved the final manuscript.
Funding
This work was supported by the Science Fund for Distinguished Young Scholars of Sichuan Province (No. 2019JDJQ0021); The National modern agricultural technology system construction of China (No. CARS-41-G04); The Province Key Technologies R & D Program of Livestock and Poultry Breeding Programs of Sichuan Province (No. 2016NYZ0043).
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethics approval and consent to participate
All chickens experimental were approved via the animal care and ethical committee of Sichuan Animal Science Academy. All experimental procedures and animal care performed in the present study were approved according to the recommendations of Sichuan academy of animal science. All efforts were made to minimize suffering.
Contributor Information
Chaowu Yang, Email: cwyang@foxmail.com.
Xiaosong Jiang, Email: xsjiang2017@163.com.
References
- Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B, Pearson M, Chan DA, Giaccia AJ, Semenza GL. OS-9 interacts with hypoxia-inducible factor 1alpha and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1alpha. Mol Cell. 2005;17(4):503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, An WG, Romanova LY, Trepel J, Fojo T, Neckers L. p53 inhibits hypoxia-inducible factor-stimulated transcription. J Biol Chem. 1998;273(20):11995–11998. doi: 10.1074/jbc.273.20.11995. [DOI] [PubMed] [Google Scholar]
- Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab. 2018;27(2):281–298. doi: 10.1016/j.cmet.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Collino F, Lopes JA, Correa S, Abdelhay E, Takiya CM, Wendt CHC, de Miranda KR, Vieyra A, Lindoso RS. Adipose-derived mesenchymal stromal cells under hypoxia: changes in extracellular vesicles secretion and improvement of renal recovery after ischemic injury. Cell Physiol Biochem. 2019;52(6):1463–1483. doi: 10.33594/000000102. [DOI] [PubMed] [Google Scholar]
- Coustet B, Lhuissier FJ, Vincent R, Richalet JP. Electrocardiographic changes during exercise in acute hypoxia and susceptibility to severe high-altitude illnesses. Circulation. 2015;131(9):786–794. doi: 10.1161/CIRCULATIONAHA.114.013144. [DOI] [PubMed] [Google Scholar]
- Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem. 2006;99(1):35–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121(5):1541–1547. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi: 10.1056/nejmra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, Brugniaux JV, Pialoux V, Duggan CT, Hanly PJ, Ahmed SB, Poulin MJ. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. J Physiol. 2009;587(Pt 13):3287–3299. doi: 10.1113/jphysiol.2009.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga CL, Velagapudi SP, Strivelli JR, Yang WY, Disney MD, Phinney DG. Small molecule inhibition of miR-544 biogenesis disrupts adaptive responses to hypoxia by modulating ATM-mTOR signaling. ACS Chem Biol. 2015;10(10):2267–2276. doi: 10.1021/acschembio.5b00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries DE, Lee SL, Fanburg BL, Silbert JE. Effects of hypoxia and hyperoxia on proteoglycan production by bovine pulmonary artery endothelial cells. J Cell Physiol. 1986;126(2):249–253. doi: 10.1002/jcp.1041260214. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12(2):149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhang LF, Song ML, Bao HG, Zhao CJ, Li N. Highly efficient dissociation of oxygen from hemoglobin in Tibetan chicken embryos compared with lowland chicken embryos incubated in hypoxia. Poult Sci. 2009;88(12):2689–2694. doi: 10.3382/ps.2009-00311. [DOI] [PubMed] [Google Scholar]
- Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164(6):1875–1882. doi: 10.1016/S0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Li W, Wu B, Chen J, Chen X. Transcriptome analysis reveals new insights into immune response to hypoxia challenge of large yellow croaker (Larimichthys crocea) Fish Shellfish Immunol. 2019;98:738–747. doi: 10.1016/j.fsi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- Muinos-Lopez E, Ripalda-Cemborain P, Lopez-Martinez T, Gonzalez-Gil AB, Lamo-Espinosa JM, Valenti A, Mortlock DP, Valenti JR, Prosper F, Granero-Molto F. Hypoxia and reactive oxygen species homeostasis in mesenchymal progenitor cells define a molecular mechanism for fracture nonunion. Stem Cells. 2016;34(9):2342–2353. doi: 10.1002/stem.2399. [DOI] [PubMed] [Google Scholar]
- Mustroph A, Albrecht G, Hajirezaei M, Grimm B, Biemelt S. Low levels of pyrophosphate in transgenic potato plants expressing E. coli pyrophosphatase lead to decreased vitality under oxygen deficiency. Ann Bot. 2005;96(4):717–726. doi: 10.1093/aob/mci223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Peng YJ, Yuan G, Kumar GK, Prabhakar NR. Hypoxia-inducible factors and hypertension: lessons from sleep apnea syndrome. J Mol Med (Berl) 2015;93(5):473–480. doi: 10.1007/s00109-015-1274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstantinou E, Karakiulakis G, Tamm M, Perruchoud AP, Roth M. Hypoxia modifies the effect of PDGF on glycosaminoglycan synthesis by primary human lung cells. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L825–834. doi: 10.1152/ajplung.2000.279.5.L825. [DOI] [PubMed] [Google Scholar]
- Plecita-Hlavata L, Jezek J, Jezek P. Aglycemia keeps mitochondrial oxidative phosphorylation under hypoxic conditions in HepG2 cells. J Bioenerg Biomembr. 2015;47(6):467–476. doi: 10.1007/s10863-015-9628-6. [DOI] [PubMed] [Google Scholar]
- Shi Q, Liu X, Wang N, Zheng X, Fu J, Zheng J. Nitric oxide from brain microvascular endothelial cells may initiate the compensatory response to mild hypoxia of astrocytes in a hypoxia-inducible factor-1alpha dependent manner. Am J Transl Res. 2016;8(11):4735–4749. [PMC free article] [PubMed] [Google Scholar]
- Shih JW, Chiang WF, Wu ATH, Wu MH, Wang LY, Yu YL, Hung YW, Wang WC, Chu CY, Hung CL, Changou CA, Yen Y, Kung HJ. Long noncoding RNA LncHIFCAR/MIR31HG is a HIF-1alpha co-activator driving oral cancer progression. Nat Commun. 2017;8:15874. doi: 10.1038/ncomms15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281(1):C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Allison BJ, Niu Y, Botting KJ, Seron-Ferre M, Herrera EA, Giussani DA. Melatonin modulates the fetal cardiovascular defense response to acute hypoxia. J Pineal Res. 2015;59(1):80–90. doi: 10.1111/jpi.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Yao J, Li Z, Zu G, Feng D, Shan W, Li Y, Hu Y, Zhao Y, Tian X. miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antioxid Redox Signal. 2016;24(17):961–973. doi: 10.1089/ars.2015.6492. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Xie W, Lu Q, Wang K, Lu J, Gu X, Zhu D, Liu F, Guo Z. miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin. J Cell Physiol. 2018;233(9):6615–6631. doi: 10.1002/jcp.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wang S, Liu J, Dou D, Liu L, Chen Z, Ye L, Liu H, He Q, Raj JU, Gao Y. Hypoxia induces downregulation of soluble guanylyl cyclase beta1 by miR-34c-5p. J Cell Sci. 2012;125(Pt 24):6117–6126. doi: 10.1242/jcs.113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Qiu Q, Qian X, Yi J, Jiao Y, Yu M, Li X, Li J, Mi C, Zhang J, Lu B, Chen E, Liu P, Lu Y. Long noncoding RNA LCAT1 functions as a ceRNA to regulate RAC1 function by sponging miR-4715-5p in lung cancer. Mol Cancer. 2019;18(1):171. doi: 10.1186/s12943-019-1107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21(5):542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes. 2009;33(1):54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fan G, Zhao H, Wang Z, Li F, Zhang P, Zhang J, Wang X, Wang W. Targeted inhibition of focal adhesion kinase attenuates cardiac fibrosis and preserves heart function in adverse cardiac remodeling. Sci Rep. 2017;7:43146. doi: 10.1038/srep43146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Zhou Z. Protective effects of intermittent hypoxic adaptation on myocardium and its mechanisms. Biol Signals Recept. 1999;8(4–5):316–322. doi: 10.1159/000014602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.