Abstract
Background
In the past decade, lateral lumbar interbody fusion (LLIF) has gained in popularity. A proposed advantage is the achievement of indirect neural decompression. However, evidence of the effectiveness of LLIF in neural decompression in lumbar degenerative conditions remains unclear.
Questions/Purposes
We sought to extrapolate clinical and radiological results and consequently the potential benefits and limitations of LLIF in indirect neural decompression in degenerative lumbar diseases.
Methods
We conducted a systematic review of the literature in English using the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and checklist. Scores on the Oswestry Disability Index (ODI) and visual analog scale (VAS) for back and leg pain were extracted, as were data on the following radiological measurements: disc height (DH), foraminal height (FH), foraminal area (FA), central canal area (CA).
Results
In the 42 articles included, data on 2445 patients (3779 levels treated) with a mean follow-up of 14.8 ± 5.9 months were analyzed. Mean improvements in VAS back, VAS leg, and ODI scale scores were 4.1 ± 2.5, 3.9 ± 2.2, and 21.9 ± 7.2, respectively. Post-operative DH, FH, FA, and CA measurements increased by 68.6%, 21.9%, 37.7%, and 29.3%, respectively.
Conclusion
Clinical results indicate LLIF as an efficient technique in indirect neural decompression. Analysis of radiological data demonstrates the effectiveness of symmetrical foraminal decompression. Data regarding indirect decompression of central canal and lateral recess are inconclusive and contradictory. Bony stenosis appears as an absolute contraindication. The role of facet joint degeneration is unclear. This systematic review provides a reference for surgeons to define the potential and limitations of LLIF in indirect neural elements decompression.
Electronic supplementary material
The online version of this article (10.1007/s11420-019-09734-7) contains supplementary material, which is available to authorized users.
Keywords: lateral lumbar interbody fusion, indirect neural decompression, lumbar stenosis, outcomes, extreme lateral interbody fusion, lumbar degenerative diseases
Introduction
In the USA, the volume of lumbar fusion increased by 62.3% between 2004 and 2015, and a 177% increase was registered in total hospital costs over a 12-year period, exceeding $10 billion in 2015 [34].
In the last decade, lateral lumbar interbody fusion (LLIF) has gained in popularity. The technique was described by Ozgur et al. in 2006 and represents a minimally invasive surgical procedure on the lumbar disc space via a retroperitoneal transpsoas approach [42]. The following advantages of LLIF have been reported: (1) indirect neural decompression and solid bone fusion with a large interbody cage that rests on both lateral sides of the vertebral end-plate epiphyseal ring, minimizing incidence of cage subsidence, nerve root lesions, post-operative radiculitis, and durotomies, and (2) a better restoration of sagittal and coronal patient profile compared with posterior techniques. The mechanism of indirect decompression relies on the recovery of disc height with consequent increase of foraminal height and stretching of the ligamentum flavum and posterior longitudinal ligament with restoration of central canal area [1, 5, 7, 9, 13, 15, 21, 32, 36, 37, 39, 45, 55, 56]. However, evidence of the effectiveness, advantages, and limitations of LLIF in neural decompression in different lumbar degenerative conditions remains unclear, perhaps negatively affecting the patient-selection process. The aim of this study was to systematically review the literature in order to extrapolate clinical and radiological results and, consequently, the potential benefits and limitations of LLIF in indirect neural decompression in lumbar degenerative diseases.
Materials and Methods
A systematic review of the literature was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). We searched Medline through PubMed, Google Scholar, Embase, and the Cochrane Central Register of Controlled Trials. The following key words and MeSH terms were used in the search: “lateral lumbar interbody fusion,” “extreme lateral interbody fusion,” “LLIF,” “XLIF,” “ELIF,” and “indirect decompression.”
The inclusion criteria were retrospective or prospective studies, including randomized controlled trials, non-randomized trials, cohort studies, case–control studies, and case series providing clinical and radiological results of LLIF in indirect neural decompression in lumbar degenerative diseases. Studies in English without any restriction on publication date were also included. All non-English articles, cadaveric studies, case reports, literature reviews, technical notes, and editorial letters were excluded.
One reviewer applied the foregoing criteria to select potentially relevant studies. Articles were initially identified based on titles and abstracts; the full-text version of the relevant trials was then obtained and evaluated. References of the identified articles were checked in order not to leave any relevant article unexplored.
The following data, when available, were extracted from the articles: number of patients, number of treated levels, mean age of the population (years), body mass index (BMI), mean follow-up (years), indications for surgery, data on surgical strategy (stand-alone LLIF, LLIF plus lateral instrumentation, LLIF plus posterior instrumentation), cage height and width, cage geometry (parallel or lordotic), sagittal cage position in disc space, radiological parameters (disc height (DH), foraminal height (FH), foraminal area (FA), central canal area (CA), clinical results (Oswestry Disability Index [ODI] and visual analog scale [VAS] of leg and back pain), and failed indirect decompression needing surgical revision. In the analysis of foramen height and area, a distinction between left and right side was performed, when possible. The studies that did not declare a specific datum were excluded by the global evaluation of that parameter.
The level of evidence of the studies was assigned based on the 2011 Oxford Centre for Evidence-based Medicine Levels of Evidence [35].
Statistical Analysis
Categorical variables were expressed as the number of cases or percentage. Continuous variables were reported as mean ± standard deviation (SD). The heterogeneity in population demographic features was assessed using the Pearson χ2 and analysis of variance (ANOVA) tests. Correlations between variables were assessed with the coefficient regression test. All analyses were performed with Stata Software, version 14.2 (StataCorp, College Station, TX, USA).
Results
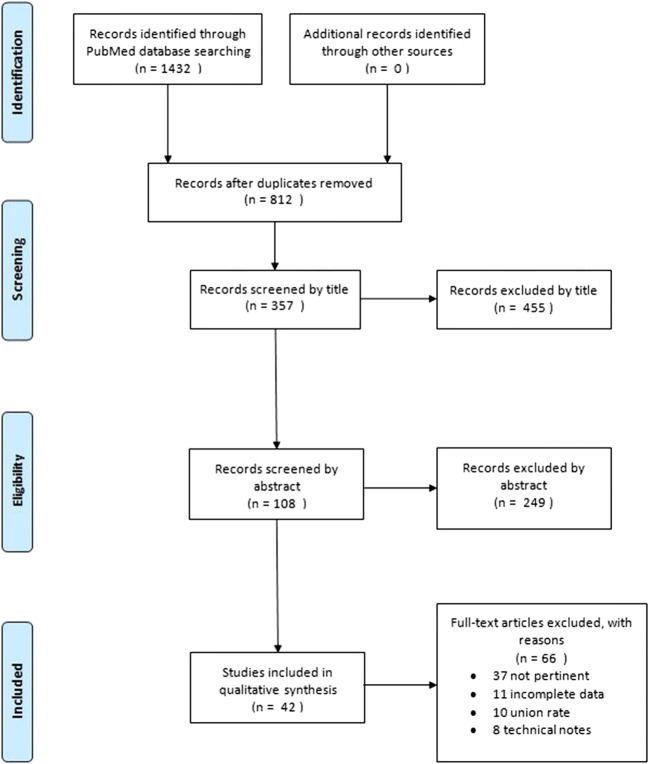
A total of 42 articles were eventually included in our systematic review [1, 3–5, 7–9, 11, 13–15, 17, 19–25, 27–30, 32, 33, 36–41, 43–46, 49, 51, 52, 55, 56, 59, 60]. Two studies were level II evidence [19, 52], seven were level III evidence [11, 24, 32, 46, 49, 56, 59], and the remaining were level IV evidence (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the search and inclusion strategy.
Pooled data available in the included studies indicated 2445 patients (mean age, 64.2 ± 3.4 years; mean BMI, 28.4 ± 2.1 kg/m2) underwent LLIF. The population analyzed appeared homogeneous for available demographic data: male/female ratio (Pearson’s χ2 test, p > 0.05) and average age (ANOVA test, p > 0.05). The mean follow-up time was 14.8 ± 5.9 months. A total of 3779 intervertebral disc spaces were operated on; surgical data were available only for 2639 levels. The most frequently treated levels were L4–L5 (43.3%), L3–L4 (31.4%), and L2–L3 (17.5%). Almost all of the interventions were performed in the lumbar region, with the exception of 41 surgeries involving thoracic discs. The main surgical indications were spondylolisthesis, degenerative disc disease, adult degenerative scoliosis, central canal stenosis, adjacent segment disease, post-laminectomy syndrome, disc hernia, and foraminal stenosis. Table 1 provides a summary of the demographic data extracted from the included studies.
Table 1.
Demographic data extracted from the included studies. BMI body mass index, FU follow-up, LLIF lateral lumbar interbody fusion, LoE level of evidence
| Author | LoE | No. of patients | No. of LLIF | Mean age (years) | Mean BMI (kg/m2) | Mean FU (months) |
|---|---|---|---|---|---|---|
| Ahmadian et al. 2015 [1] | 4 | 59 | 96 | 60 | – | 14.6 |
| Aichmair et al. 2017 [3] | 4 | 52 | 52 | 61.9 | 29.3 | 16.1 |
| Alimi et al. 2014 [4] | 4 | 90 | 145 | 64 | 27.6 | 12.6 |
| Alimi et al. 2015 [5] | 4 | 23 | 23 | 66 | – | 11 |
| Campbell et al. 2018 [7] | 4 | 18 | 20 | 64 | 34.1 | 6.2 |
| Caputo et al. 2013 [8] | 4 | 30 | 127 | 65.9 | 28.8 | 14.3 |
| Castellvi et al. 2014 [9] | 4 | 60 | 161 | 66 | 29.2 | – |
| Dakwar et al. 2010 [11] | 3 | 25 | 76 | 62.5 | – | 11 |
| Dominguez et al. 2016 [13] | 4 | 97 | 138 | 68 | – | 12 |
| Elowitz et al. 2011 [14] | 4 | 25 | 31 | 61 | – | 6 |
| Formica et al. 2014 [15] | 4 | 39 | 41 | 58 | – | 16 |
| Gabel et al. 2015 [17] | 4 | 28 | – | 66.3 | 14 | |
| Janssen et al. 2017 [20] | 4 | 18 | 22 | 70 | – | – |
| Kepler et al. 2012 [21] | 4 | 29 | 67 | 70 | – | 19.8 |
| Khajavi et al. 2015 [22] | 4 | 160 | 197 | 61 | 28.9 | 18.5 |
| Khajavi et al. 2015 [23] | 4 | 60 | 71 | 67.7 | 29.1 | 20.3 |
| Kotwal et al. 2012 [24] | 3 | 118 | 237 | 62.1 | 27.6 | 27.5 |
| Lang et al. 2017 [25] | 4 | 21 | 28 | 70 | – | 6.6 |
| Lee et al. 2014 [27] | 4 | 90 | 116 | 65.5 | – | 8.5 |
| Malham et al. 2014 [29] | 4 | 52 | 79 | 66.4 | 25.2 | – |
| Malham et al. 2015 [30] | 4 | 122 | 169 | 62.9 | 27.3 | 22.7 |
| Malham et al. 2017 [28] | 4 | 40 | 54 | 64 | 26.8 | 12 |
| Marchi et al. 2012 [33] | 4 | 52 | 52 | 67 | 27.4 | 24 |
| Marchi et al. 2013 [32] | 3 | 74 | 98 | 56.7 | 24.7 | 12 |
| Na et al. 2012 [35] | 4 | 30 | 45 | 62 | – | 6 |
| Navarro-Ramirez et al. 2017 [37] | 4 | 37 | 74 | 68 | – | 6 |
| Navarro-Ramirez et al. 2017 [36] | 4 | 7 | 9 | 70 | – | – |
| Nemani et al. 2014 [38] | 4 | 117 | 239 | 63.6 | 27.4 | 15.6 |
| Oliveira et al. 2010 [40] | 4 | 21 | 43 | 67.6 | 25.6 | – |
| Ozgur et al. 2010 [41] | 4 | 62 | 113 | 63.8 | – | 24 |
| Park et al. 2017 [43] | 4 | 41 | 94 | 64 | – | 17.2 |
| Pawar et al. 2015 [44] | 4 | 39 | 48 | 59 | 29.6 | 16.1 |
| Pereira et al. 2016 [45] | 4 | 23 | 42 | 61 | – | 12 |
| Rodgers BW et al. 2010 [47] | 3 | 66 | 88 | 62.2 | 30.4 | 12 |
| Rodgers JA et al. 2013 [46] | 3 | 283 | 383 | 62.5 | 31.1 | 24 |
| Segawa et al. 2017 [51] | 4 | 96 | 111 | 61 | – | 18 |
| Sembrano/Isaacs et al. 2016 [19, 52] | 2 | 29 | 36 | 63 | 30.1 | 24 |
| Tessitore et al. 2016 [55] | 4 | 20 | 22 | 67.5 | 27.7 | 9.8 |
| Tohmeh et al. 2014 [56] | 3 | 140 | 223 | 60.7 | 29.1 | 15.5 |
| Wang et al. 2017 [59] | 3 | 45 | 101 | 65 | – | – |
| Yang et al. 2017 [60] | 4 | 7 | 8 | 63 | – | – |
The height of the implanted cages was available for 705 levels: 64 were 8 mm (9.1%), 229 were 10 mm (32.4%), 277 were 12 mm (39.2%), 121 were 14 mm (17.1%), and only 14 were 16 mm (2.0%) [6, 9, 12, 18–20, 31, 40]. For 1030 levels, the type of cage was described: 861 were lordotic (6 to 10°) and 169 were parallel cages [6, 9, 12, 13, 17–20, 22–24, 40]. Five authors reported the sagittal cage position in disc space, dividing the vertebral plate into anterior, middle, and posterior thirds; data were available for 379 cages: 134 were included in the anterior third (35.4%), 207 in the middle third (54.6%), and only 38 in the posterior third (10.0%) [24, 25, 27, 37, 43].
Twenty-eight authors (2686 procedures) reported that the stand-alone option was chosen in 582 cases (21.7%), 1875 procedures (69.8%) were completed with a posterior instrumentation, and in 229 cases (8.5%), a lateral instrumentation was implanted [3–5, 7–9, 11, 13, 15, 17, 19, 21–25, 28, 29, 35, 38, 40, 41, 43, 45, 46, 49, 55, 56].
Finally, information regarding the width of the implanted cage was available for a total of 1009 levels. In 677 cases, a cage of 18 mm in width was implanted (67.1%), 283 were 22 mm (28%), and 49 were 26 mm in width (4.8%) [4, 7, 9, 11, 19, 25, 27, 32, 36, 37, 40, 56]. Table 2 provides a summary of the available surgical data extracted from the included studies.
Table 2.
Available surgical data extracted from the included studies. Ant anterior, AP anteroposterior, Instrum instrumentation, Lat lateral, Post posterior
| Author | Cage height (mm) | Cage angle (°) | Cage position | Technique | Cage AP width (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 10 | 12 | 14 | 16 | Parallel | Lordotic | Ant 1/3 | Middle 1/3 | Post 1/3 | Stand-alone | Post Instrum | Lat Instrum | 18 | 22 | 26 | |
| Ahmadian et al. 2015 [1] | – | – | – | – | – | – | 96 (10°) | – | – | – | – | – | – | – | – | – |
| Aichmair et al. 2017 [3] | – | – | – | – | – | – | – | – | – | – | 31 | – | – | – | – | – |
| Alimi et al. 2014 [4] | 31 | 72 | 23 | – | – | – | – | – | – | – | 15 | 111 | 19 | 82 | 63 | – |
| Alimi et al. 2015 [5] | – | – | – | – | – | – | – | – | – | – | 4 | 15 | 4 | – | – | – |
| Campbell et al. 2018 [7] | – | – | – | – | – | – | 20 (7° or 10°) | – | – | – | – | 20 | – | 3 | 15 | 2 |
| Caputo et al. 2013 [8] | – | – | – | – | – | – | – | – | – | – | – | 127 | – | – | – | – |
| Castellvi et al. 2014 [9] | – | – | – | – | – | – | – | – | – | – | – | 161 | – | 161 | – | – |
| Dakwar et al. 2010 [11] | – | – | – | – | – | – | – | – | – | – | 2 | 8 | 16 | 76 | – | – |
| Dominguez et al. 2016 [13] | – | – | – | – | – | – | – | – | – | – | 45 | 35 | 58 | – | – | – |
| Elowitz et al. 2011 [14] | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Formica et al. 2014 [15] | – | – | – | – | – | – | – | – | – | – | 4 | 37 | – | – | – | – |
| Gabel et al. 2015 [17] | – | – | – | – | – | – | – | – | – | – | – | 21 | 7 | – | – | – |
| Janssen et al. 2017 [20] | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Kepler et al. 2012 [21] | – | – | – | – | – | – | 67 (10°) | 14 | 43 | 10 | 19 | 48 | – | – | – | – |
| Khajavi et al. 2015 [22] | – | – | – | – | – | – | – | – | – | – | – | 140 | – | – | – | – |
| Khajavi et al. 2015 [23] | – | – | – | – | – | – | – | – | – | – | – | 57 | – | – | – | – |
| Kotwal et al. 2012 [24] | – | – | – | – | – | – | – | – | – | – | 32 | 205 | – | – | – | – |
| Lang et al. 2017 [25] | 7 | 19 | 2 | – | – | 24 | 4 | 20 | – | 8 | 9 | 19 | – | – | – | 28 |
| Lee et al. 2014 [27] | 6 | – | 53 | 53 | 4 | – | 116 (6°) | 32 | 83 | 1 | – | – | – | 100 | – | – |
| Malham et al. 2014 [29] | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Malham et al. 2015 [30] | – | – | – | – | – | – | 169 (10°) | – | – | – | 58 | 64 | – | – | – | – |
| Malham et al. 2017 [28] | – | – | – | – | – | – | 54 (10°) | – | – | – | 28 | 26 | – | – | – | – |
| Marchi et al. 2012 [33] | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Marchi et al. 2013 [32] | – | – | – | – | – | – | – | – | – | – | – | – | – | 60 | 38 | – |
| Na et al. 2012 [35] | – | – | – | – | – | – | 45 (6°) | – | – | – | 24 | 21 | – | – | – | – |
| Navarro-Ramirez et al. 2017 [37] | 10 | 44 | 20 | – | – | 48 | 26 | 37 | 18 | 19 | – | – | – | 14 | 50 | 10 |
| Navarro-Ramirez et al. 2017 [36] | – | 9 | – | – | – | 9 | – | – | – | – | – | – | – | – | – | 9 |
| Nemani et al. 2014 [38] | – | – | – | – | – | – | – | – | – | – | 239 | – | – | – | – | – |
| Oliveira et al. 2010 [40] | – | – | – | – | – | – | – | – | – | – | 43 | – | – | 43 | – | – |
| Ozgur et al. 2010 [41] | – | – | – | – | – | – | – | – | – | – | 23 | 83 | 7 | – | – | – |
| Park et al. 2017 [43] | – | 3 | 45 | 42 | 4 | – | 94 (6°) | 31 | 63 | – | – | 94 | – | – | – | – |
| Pawar et al. 2015 [44] | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Pereira et al. 2016 [45] | – | – | – | – | – | – | – | – | – | – | – | 42 | – | – | – | – |
| Rodgers BW et al. 2010 [47] | – | – | – | – | – | – | – | – | – | – | 1 | 61 | 4 | – | – | – |
| Rodgers JA et al. 2013 [46] | – | – | – | – | – | – | – | – | – | – | 5 | 267 | 46 | – | – | – |
| Segawa et al. 2017 [51] | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Sembrano/Isaacs et al. 2016 [19, 52] | 2 | 13 | 19 | 2 | – | 16 | 20 | – | – | – | – | 36 | – | 21 | 15 | – |
| Tessitore et al. 2016 [55] | – | – | – | – | – | – | – | – | – | – | – | 22 | – | – | – | – |
| Tohmeh et al. 2014 [56] | 8 | 69 | 115 | 24 | 6 | 72 | 150 (10°) | – | – | – | – | 155 | 68 | 117 | 102 | – |
| Wang et al. 2017 [59] | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Yang et al. 2017 [60] | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Total | 64 | 229 | 277 | 121 | 14 | 169 | 861 | 134 | 207 | 38 | 582 | 1875 | 229 | 677 | 283 | 49 |
The authors reported clinical status with different outcome scales, the most common being the ODI score and the VAS score for back and leg pain. The level of disability was evaluated with ODI in 26 studies, with a mean improvement of 21.86 ± 7.22 points (95% CI, 18.8–24.9) [4–8, 10–14, 18, 20–24, 29–32, 34, 36, 37, 39, 41, 43]. The mean ODI variation extrapolated from the included studies was 47.6 ± 10.3% (range, 33–72), which was superior to the reported minimum clinically important difference of ODI (11%) after adult spinal deformity surgery [61].
Twenty authors reported a mean improvement of back and leg pain with VAS score reduction of 4.1 ± 2.5 (95% CI, 3.6–4.6) and 3.9 ± 2.2 (95% CI, 3.4–4.4), respectively [3–5, 13–15, 20–22, 27–30, 33, 35, 37, 38, 45, 56, 60]. Table 3 provides a summary of the clinical outcomes reported by the included studies. Radiological outcomes were analyzed by 27 authors on the basis of plain radiographs, computed tomographic (CT) scans, and magnetic resonance imaging (MRI).
Table 3.
Clinical outcomes reported by the included studies. FU follow-up, ODI Oswestry Disability Index, pre pre-operative, VAS visual analogue scale, ∆ difference
| Author | ODI pre | ODI last FU | ∆ ODI | VAS back pre | VAS back last FU | ∆ VAS back | VAS leg pre | VAS leg last FU | ∆ VAS leg |
|---|---|---|---|---|---|---|---|---|---|
| Ahmadian et al. 2015 [1] | 51.8 | 31.8 | 20 | – | – | – | – | – | – |
| Aichmair et al. 2017 [3] | – | – | – | 7.9 | 4.1 | 3.8 | 7.4 | 3.3 | 4.1 |
| Alimi et al. 2014 [4] | 50 | 29 | 21 | 7 | 3.2 | 3.8 | 6.4 | 2.8 | 3.6 |
| Alimi et al. 2015 [5] | 48 | 23 | 25 | 6.5 | 3.3 | 3.2 | 7.2 | 1.1 | 6.1 |
| Campbell et al. 2018 [7] | 49.1 | 23.1 | 26 | – | – | – | – | – | – |
| Caputo et al. 2013 [8] | – | – | – | – | – | – | – | – | – |
| Castellvi et al. 2014 [9] | 42 | 28 | 14 | 6.8 | 3.8 | 3 | – | – | – |
| Dakwar et al. 2010 [11] | 53.6 | 29.2 | 23.7 | – | – | – | – | – | – |
| Dominguez et al. 2016 [13] | – | – | – | 9 | 3 | 6 | 9 | 5.2 | 3.8 |
| Elowitz et al. 2011 [14] | 55.1 | 16.4 | 38.7 | 7.74 | 2.07 | 5.67 | 7.24 | 1.87 | 5.37 |
| Formica et al. 2014 [15] | 62.9 | 24.5 | 38.3 | 7.85 | 1.77 | 6.08 | 4.62 | 1.85 | 2.77 |
| Gabel et al. 2015 [17] | – | – | – | – | – | – | – | – | – |
| Janssen et al. 2017 [20] | – | – | – | – | – | – | – | – | – |
| Kepler et al. 2012 [21] | – | – | 10.1 | – | – | 3.3 | – | – | 2.4 |
| Khajavi et al. 2015 [22] | 32.8 | 19.8 | 13 | – | – | – | – | – | – |
| Khajavi et al. 2015 [23] | 44.1 | 23.5 | 20.6 | 6.9 | 2.8 | 4.1 | 7.1 | 3.1 | 4 |
| Kotwal et al. 2012 [24] | 43 | 21 | 22 | 8 | 2.3 | 5.7 | 7.7 | 2.7 | 5 |
| Lang et al. 2017 [25] | 30 | 17.1 | 12.9 | – | – | – | – | – | – |
| Lee et al. 2014 [27] | – | – | 13.4 | – | – | – | – | – | – |
| Malham et al. 2014 [29] | 39.9 | 11.1 | 28.8 | – | – | – | 6.3 | 2.1 | 4.2 |
| Malham et al. 2015 [30] | 50 | 29 | 21 | 6.3 | 3 | 3.3 | 5.2 | 2.1 | 3.1 |
| Malham et al. 2017 [28] | 52.7 | 30.5 | 22.2 | 6.7 | 3.2 | 3.5 | 6.4 | 2.6 | 3.8 |
| Marchi et al. 2012 [33] | 55.4 | 34.1 | 21.3 | 8.7 | 4 | 4.7 | 8.2 | 3.4 | 4.8 |
| Marchi et al. 2013 [32] | 66 | 30 | 36 | 7.8 | 3.1 | 4.7 | 5.4 | 2.3 | 3.1 |
| Na et al. 2012 [35] | – | – | – | – | – | – | – | – | – |
| Navarro-Ramirez et al. 2017 [37] | – | – | – | 4.93 | 2.01 | 2.92 | 4.87 | 1.58 | 3.29 |
| Navarro-Ramirez et al. 2017 [36] | – | – | 20 | – | – | 2.9 | – | – | 2.03 |
| Nemani et al. 2014 [38] | – | – | – | – | – | – | – | – | – |
| Oliveira et al. 2010 [40] | – | – | – | 7.2 | 2.8 | 4.4 | 6.8 | 2.2 | 4.6 |
| Ozgur et al. 2010 [41] | – | – | – | – | – | – | – | – | – |
| Park et al. 2017 [43] | – | – | – | – | – | – | – | – | – |
| Pawar et al. 2015 [44] | – | – | – | – | – | – | – | – | – |
| Pereira et al. 2016 [45] | – | – | 19.5 | – | – | – | – | – | – |
| Rodgers BW et al. 2010 [47] | 56 | 36 | 20 | 7.3 | 3.6 | 3.7 | 7.8 | 3.1 | 4.7 |
| Rodgers JA et al. 2013 [46] | – | – | – | – | – | – | – | – | – |
| Segawa et al. 2017 [51] | – | – | – | – | – | – | – | – | – |
| Sembrano/Isaacs et al. 2016 [19, 52] | 38.6 | 19.1 | 19.5 | – | – | – | – | – | – |
| Tessitore et al. 2016 [55] | 43 | 20 | 23 | – | – | – | – | – | – |
| Tohmeh et al. 2014 [56] | 41.6 | 23.5 | 18.1 | – | – | – | – | – | – |
| Wang et al. 2017 [59] | 46.1 | 25.6 | 20.5 | 7.5 | 3.8 | 3.7 | 6 | 3.1 | 2.9 |
| Yang et al. 2017 [60] | – | – | – | – | – | – | – | – | – |
The increase in disc height (DH), pre- to post-operatively, was reported by 23 authors, with a mean rise of 67.2% (3.8 ± 1.5 mm; 95% CI, 3.2–4.5) [3–5, 9, 13, 15, 19, 20, 22–25, 27, 35–37, 40, 43, 44, 46, 49, 55, 59]. Fourteen authors described a mean improvement of foraminal height (FH) of 3.5 ± 1.2 mm (95% CI, 2.4–4.6; 21.9% of mean increase) [4, 5, 8, 13, 22, 25, 30, 35, 36, 40, 44, 55, 56, 59]. In three case series, a distinction between the two foraminal sides could be performed: 3.1 ± 1.2 mm left FH versus 3.0 ± 1.4 mm right FH [30, 36, 55]. Twelve papers reported variations in both DH (X) and FH (Y) [4, 5, 8, 13, 22, 25, 35, 36, 40, 44, 55, 59]. The outcome of the corresponding regression analysis was the equation Y = 0.82X − 0.03; the estimated slope was 0.82 ± 0.46.
The FA was analyzed in ten studies. The mean variation in FA was 43.6 ± 30.9 mm2 (95% CI, 21.5–65.7; mean increase, 37.7%) [9, 20, 23, 27, 30, 37, 40, 43, 55, 59]. In seven case series, a distinction between the two foraminal sides could be performed: 37.7 ± 31.6 mm2 left FA versus 37.2 ± 36.3 mm2 right FA [9, 20, 23, 27, 30, 37, 55]. Nine papers reported variations in both DH (X) and FA (Y) [9, 20, 23, 27, 37, 40, 43, 55, 59]. The outcome of the corresponding regression analysis was the equation Y = 4.94X + 25.30; the estimated slope was 4.94 ± 7.67.
The canal area (CA) was evaluated by ten authors [9, 14, 19, 20, 37, 40, 43, 55, 59, 60]. The mean CA increase was estimated at 36.4 ± 26.3 mm2 (95% CI, 18.1–54.7; mean increase, 29.3%). Eight papers reported variations in both DH (X) and CA (Y) [9, 19, 20, 37, 40, 43, 55, 59]. The outcome of the corresponding regression analysis was the equation Y = 3.94X + 12.31; the estimated slope was 3.94 ± 3.30.
The reoperation rate was extrapolated in seven studies [5, 17, 23, 29, 35, 38, 40]; 38 patients out of 385 (9.9%) needed a direct decompression because of persistent radicular or axial pain. The main reasons reported were severe pre-operative canal stenosis, cage mobilization, spondylolisthesis with facet instability, and bony lateral recess stenosis. Table 4 describes the available radiological results extrapolated by the included studies.
Table 4.
Radiological results extrapolated by the included studies. CA canal area, DH disc height, FA foraminal area, FH foraminal height, Lf left, post post-operative, pre pre-operative, Rx right, ∆ difference
| Author | DH pre mm | DH post mm | Δ DH | Rx FH pre mm | Rx FH post mm | Δ Rx FH | Lf FH pre mm | Lf FH post mm | Δ Lf FH | FH pre mm | FH post mm | ∆ FH | Rx FA pre mm² | Rx FA post mm² | ∆ Rx FA | Lf FA pre mm² | Lf FA post mm² | ∆ Lf FA | FA pre mm² | FA post mm² | ∆ FA | CA pre mm | CA post mm² | ∆ CA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmadian et al. 2015 [1] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Aichmair et al. 2016 [3] | - | - | 3.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Alimi et al. 2014 [4] | 4.1 | 6.8 | 2.7 | - | - | - | - | - | - | 15.4 | 17.5 | 2.1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Alimi et al. 2015 [5] | 5.1 | 8.2 | 3.1 | - | - | - | - | - | - | 13.95 | 18.2 | 4.25 | - | - | - | - | - | - | - | - | - | - | - | - |
| Campbell et al. 2018 [7] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Caputo et al. 2013 [8] | 4.8 | 10.2 | 5.4 | - | - | - | - | - | - | 11.1 | 19.7 | 8.6 | - | - | - | - | - | - | - | - | - | - | - | - |
| Castellvi et al. 2014 [9] | 3 | 5 | 2 | - | - | - | - | - | - | - | - | - | 91 | 113 | 22 | 87 | 114 | 27 | 89 | 113.5 | 24.5 | 136 | 159 | 23 |
| Dakwar et al. 2010 [11] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Dominguez et al. 2016 [13] | 8.4 | 13.8 | 5.4 | - | - | - | - | - | - | 10.5 | 13.1 | 2.6 | - | - | - | - | - | - | - | - | - | - | - | - |
| Elowitz et al. 2011 [14] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 107.9 | 190.9 | 83 |
| Formica et al. 2014 [15] | 3.6 | 4.8 | 1.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Gabel et al. 2015 17] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Isaacs et al. 2016 [19] | 7.6 | 9.1 | 1.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 135 | 153.9 | 18.9 |
| Janssen et al. 2017 [20] | 3.9 | 8.69 | 4.79 | - | - | - | - | - | - | - | - | - | 81.26 | 193.5 | 112.24 | 84.03 | 179.5 | 95.47 | 82.65 | 189.08 | 106.43 | 167.49 | 222.6 | 55.11 |
| Kepler et al. 2012 [21] | 4.95 | 8.05 | 3.1 | - | - | - | - | - | - | - | - | - | 103 | 137 | 34 | 104 | 143 | 39 | 103.5 | 140 | 36.5 | - | - | - |
| Khajavi et al. 2015 [22] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Khajavi et al. 2015 [23] | 6.6 | 11.3 | 4.7 | - | - | - | - | - | - | 19.4 | 23.2 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| Kotwal et al. 2012 [24] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Lang et al. 2017 [25] | 4.06 | 9.61 | 5.55 | - | - | - | - | - | - | 19.02 | 22.01 | 2.99 | - | - | - | - | - | - | - | - | - | - | - | - |
| Lee et al. 2014 [27] | 8.8 | 15.4 | 6.6 | - | - | - | - | - | - | - | - | - | 102.9 | 151.2 | 48.3 | 99.5 | 159.2 | 59.7 | 101.2 | 155.2 | 54 | - | - | - |
| Malham et al. 2014 [29] | - | - | - | 14 | 17 | 3 | 15 | 17 | 2 | 14.5 | 17 | 2.5 | 110 | 140 | 30 | 120 | 150 | 30 | 115 | 145 | 30 | - | - | - |
| Malham et al. 2015 30] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Malham et al. 2017 [28] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Marchi et al. 2012 [33] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Marchi et al. 2013 [32] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Na et al. 2012 [35] | 7.93 | 11.49 | 3.56 | - | - | - | - | - | - | 19.17 | 20.49 | 1.32 | - | - | - | - | - | - | - | - | - | - | - | - |
| Navarro-Ramirez et al. 2017 [37] | 3.56 | 8.33 | 4.77 | 15.2 | 19.8 | 4.41 | 15.1 | 19.6 | 4.43 | 15.2 | 19.7 | 4.5 | - | - | - | - | - | - | - | - | - | - | - | - |
| Navarro-Ramirez et al. 2017 [36] | 4.5 | 7.5 | 3 | - | - | - | - | - | - | - | - | - | 46 | 56 | 10 | 47 | 56.2 | 9.2 | 45.2 | 55.1 | 9.9 | 102.3 | 119.5 | 17.2 |
| Nemani et al. 2014 [38] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Oliveira et al. 2010 [40] | 7.1 | 10.1 | 3 | - | - | - | - | - | - | 20.9 | 23.7 | 2.8 | - | - | - | - | - | - | 243 | 303 | 60 | 147.4 | 159.8 | 12.4 |
| Ozgur et al. 2010 [41] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Park et al. 2017 [43] | 7.55 | 13.65 | 6.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 61.5 | 94.1 | 32.6 | 91.4 | 116.5 | 25.1 |
| Pawar et al. 2015 [44] | 7.7 | 13.3 | 5.6 | - | - | - | - | - | - | 15 | 18.9 | 3.9 | - | - | - | - | - | - | - | - | - | - | - | - |
| Pereira et al. 2016 [45] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Rodgers BW et al. 2010 [47] | 6.2 | 9.3 | 3.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Rodgers JA et al. 2013 [46] | 6.2 | 9.0 | 2.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Segawa et al. 2017 [51] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sembrano et al. 2016 [52] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Tessitore et al. 2016 [55] | 7 | 10.7 | 3.7 | 14.9 | 16.5 | 1.6 | 15.1 | 17.7 | 2.6 | 15 | 17.1 | 2.1 | 78.7 | 82.3 | 3.6 | 88.5 | 91.8 | 3.3 | 83.6 | 87.05 | 3.45 | 115.7 | 136.5 | 20.8 |
| Tohmeh et al. 2014 [56] | - | - | - | - | - | - | - | - | - | 15.7 | 21.2 | 5.5 | - | - | - | - | - | - | - | - | - | - | - | - |
| Wang et al. 2017 [59] | 7 | 10 | 3 | - | - | - | - | - | - | 18.8 | 20.8 | 2 | - | - | - | - | - | - | 232.5 | 307.29 | 74.79 | 175.47 | 208.03 | 32.56 |
| Yang et al. 2017 [60] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 65 | 141 | 76 |
Discussion
LLIF has gained in popularity in the treatment of degenerative lumbar spinal conditions in the past decade. The literature reports the following advantages of LLIF: (1) improved indirect neural decompression and (2) improved restoration of sagittal and coronal patient profile, compared with posterior techniques [6, 19, 52]. Moreover, some authors also suggest that lateral access in revisions offers a new surgical avenue to the spine, avoiding the scar tissue created by the previous posterior and anterior approaches and thus reducing surgical time, blood loss, and surgical complications [16, 48]. Several authors have focused their attention on the specific complications related to this surgical approach [2, 11, 12, 18, 46, 47, 50, 58, 62, 63]. However, evidence on the effectiveness, potential benefits, and limitations of LLIF in neural decompression in different lumbar degenerative conditions is still lacking. The supposed mechanisms of indirect decompression are the recovery of disc height with consequent ligamentotaxis of the ligamentum flavum and posterior longitudinal ligament, the reduction of slippage in the case of spondylolisthesis, and the correction of coronally asymmetrical disc spaces in adult scoliosis [6, 33, 44].
Empiric evidence of the effectiveness of LLIF on clinical symptoms (disability expressed with ODI scores and back and leg pain with VAS scores) is demonstrated by all authors. Nevertheless, evidence based on direct linear correlation between improvement of clinical symptoms (expressed as numerical scores) and variations of radiological parameters (FH, FA, and CA) is lacking and characterized by huge variability.
Conversely, evidence of the effectiveness of LLIF in indirect decompression of neural elements based exclusively on radiological parameters is suggested.
In pooling the data, we calculated a mean DH recovery of 3.8 ± 1.5 mm. Foraminal height and area are the most commonly evaluated parameters for assessing foraminal decompression. A mean increase of 21.9% of FH was reported. The mean rise of FA was 37.7%. No significant variations were recorded when analyzing the difference of FH in relation to foramen side (3.1 ± 1.2 mm left vs. 3.0 ± 1.4 mm right). Similar results were observed also for FA (37.7 ± 31.6 mm2 left vs. 37.2 ± 36.3 mm2 right). A careful bilateral annulus release and large footprint interbody cages were reported as key points of the surgical technique to guarantee symmetrical foraminal decompression [6]. Nevertheless, a global analysis of influence of access side and of pre-operative diagnosis, mainly in adult scoliosis (convex vs. concave side), cannot be performed. Only one author referred to the access side reporting a 60% increase of FA at the surgical approach side versus 48% at the other side [27].
The correlation between DH increase (X) and foraminal decompression, expressed as FH rise (Y), is easily assessable: 3.8 ± 1.5 mm versus 3.5 ± 1.2 mm. The outcome of the corresponding regression analysis was the equation Y = 0.82X − 0.03; the estimated slope was 0.82 ± 0.46. Conversely, the correlation between DH increase (X) and foraminal decompression, expressed as FA rise (Y), appears more arduous to interpret (3.8 ± 1.5 mm vs. 43.6 ± 30.9 mm2), with a high variability of data in the included studies. The outcome of the corresponding regression analysis was the equation Y = 4.94X + 25.30; the estimated slope was 4.94 ± 7.67. The observed discrepancy between DH–FH and DH–FA correlation is probably due to the fewer FA data available in the included studies.
LLIF seems to be an efficient technique for indirect foramen decompression according to radiological parameters (FH, FA). Some authors have hypothesized the role of this technique in treating adult spinal degenerative pathologies [19, 52, 57]. Unfortunately, it is not possible to establish a careful and global analysis of the relation of pre-operative diagnosis, approach side (especially in lumbar scoliosis: convex vs. concave side), additional instrumentation, cage geometry (parallel/lordotic), dimension (height and width), and position (anterior/medium/posterior third of disc space), with DH restoration and consecutively with effectiveness of foraminal indirect decompression (FH and FA); this thus represents an intrinsic study limitation.
Kepler et al. [23] used 10° lordotic cages positioned for 65% of the levels in the middle third, 20% in the anterior, and 15% in the posterior portion; they observed no differences in post-operative FA, regardless of cage position. However, Lee et al. [27] reported that cage position affected the entity of segmental lordosis restoration: anterior third position guarantees an increase of lordosis of 3.8 ± 5.5° versus 0.3 ± 5.5° for the middle third position. Similarly, Park et al. [43] demonstrated that positioning the cage in the anterior part of the vertebral end plate led to a better segmental lordosis restoration without compromising LLIF effectiveness in increasing CA and FA. Cage subsidence is one of the critical points reported in the literature regarding the potential benefits and limitations of indirect foraminal decompression in LLIF [4, 26, 56]. Cage subsidence can occur during surgical procedure in disc space preparation or in the post-operative period. Risk factors referred by several authors are age, sex, bone quality, aggressive end-plate preparation, and cage insertion [32]. Nevertheless, Tohmeh et al. [56] reported a constant foraminal height regardless 67% of cage subsidence at 1-year follow-up. Large cage footprint is referred to as a protective factor for subsidence [48]. Lang et al. [25] observed a linear reduction in cage subsidence with the increase of cage width and consequently maintaining indirect decompression.
The efficacy of LLIF in restoring the CA was described by ten authors reporting data about 303 patients with a mean increase, pre- to post-operatively, of 36.4 ± 26.4 mm2 (95% CI, 18.08–54.73; mean increase, 29.3%). The correlation between DH increase (X) and central canal decompression, expressed as CA rise (Y), is challenging to analyze. The outcome of the corresponding regression analysis was the equation Y = 3.94X + 12.31; the estimated slope was 3.94 ± 3.30. High variability between the results of the studies included can be detected. Elowitz et al. [14] reported an increase in dural sac area of 77%. Other groups have not observed this. Castellvi et al. [9] detected a 17% improvement in CA at 1-year follow-up. The evaluation of central canal area was performed with CT scan in three studies (pre-operative mean CA, 122.8 ± 52.5 mm2; post-operative mean CA, 174.2 ± 42.9 mm2) [9, 20, 60] and MRI in four studies (pre-operative mean CA, 124.9 ± 44.6 mm2; post-operative mean CA, 171.8 ± 48.7 mm2) [40, 43, 59], and three used both imaging techniques (pre-operative mean CA, 117.7 ± 16.4 mm2; post-operative mean CA 136.6 ± 17.2 mm2) [36, 52, 55]. The authors who performed measurements with both modalities did not specify the method to extrapolate the declared CA.
Analysis of the available demographic data resulted in homogeneous findings for gender (Pearson’s χ2 test, p = 0.227) and age (ANOVA test, p = 0.685). However, other demographic data such as race, height, and body mass index were not available.
Data on indirect decompression of the central canal are inconclusive and contradictory. Moreover, it is not possible to analyze the relationship of pre-operative diagnosis, additional instrumentation, cage geometry (parallel/lordotic), dimension (height and width), and position (anterior/medium/posterior third of disc space) with DH restoration and consecutively with the effectiveness of central canal indirect decompression. This represents another intrinsic limitation to the study.
Seven authors have reported incidence of second-stage direct decompression surgery. The main causes reported were severe pre-operative central canal stenosis, spondylolisthesis with facet instability, and bony lateral recess stenosis; those diagnoses might be assumed as contraindications to this surgical procedure [5, 17, 23, 29, 35, 38, 40].
Congenital short pedicles, calcified discs, severe facet hypertrophy, synovial cysts, osteophytes arising from the posterior endplates, severe central canal stenosis, and uncontained disc herniations were reported as risk factors for canal decompression failure [40, 48, 59]. Two authors excluded a correlation with facet degeneration [30, 37]; nevertheless, a systematic evaluation of the degree of arthropathy was not performed in a majority of the included studies.
Severe central canal stenosis is seen as a relative contraindication for indirect decompression [26, 40]. Unfortunately, no well-defined and unambiguous radiological criteria to define and characterize lumbar spinal stenosis are established in literature; consequently, it is not possible to extrapolate clear guidelines in terms of potential benefits and limitations of indirect canal decompression [10, 31, 54]. The adoption of a consensus regarding morphological and quantitative parameters could permit to the standardization of central spinal stenosis grading in the future, thus allowing for homogenization of results and reliable decision-making for indirect decompression.
With reference to lateral recess stenosis, the evidence is poorly investigated. Severe facet hypertrophy, synovial cysts, and osteophytes arising from the posterior endplates were reported as risk factors for lateral recess decompression failure [26, 40, 59]. Only one study analyzed this aspect and reported that bony stenosis is an independent predictor of failure for indirect neural decompression of the lateral recess [59].
A lumbarized sacrum (L5–L6) is another contraindication for indirect decompression via the transpsoas approach, as described by Smith et al. in 2012 [53].
This study has limitations. The lack of standardized imaging modalities and radiological parameters for the assessment of indirect decompression in the literature resulted in heterogeneous data that were difficult to analyze. Our analysis on radiological parameters was based on data originally declared by the authors in the included studies. However, different imaging modalities were used, and the specific method of radiographic measurements was not accurately described by the different authors. This represents an evident limitation of the study. The lack of standardized morphological and/or quantitative classification systems of central, foraminal, and lateral recess stenosis is the main barrier to extrapolating evidence about the potentials and limitations of LLIF in indirectly decompressing neural elements. Moreover, the population analyzed appeared homogeneous for age and gender, but some potentially relevant demographic data were lacking, such as race, height, and body mass index. Most of the studies included were based on retrospective data with low levels of evidence, including highly heterogeneous study populations in terms of indications and surgical strategy (cage dimension/geometry/position and additional instrumentation).
In conclusion, this review supports the clinical effectiveness of LLIF in indirect neural decompression in degenerative lumbar diseases. Radiological results’ analysis recommends LLIF as an efficient technique in symmetrical decompression of foraminal stenosis, particularly in restoring foramen height and area. Data on indirect decompression of the central canal are inconclusive and contradictory. The evidence on decompression of lateral recess stenosis via LLIF is low. The analysis of the influence of pre-operative diagnosis, additional instrumentation, and cage geometry/dimension/position on effectiveness of indirect decompression cannot be established. Large cage footprint is seen as a key factor to avoid subsidence and consequently potential decompression failure. Severe central canal stenosis and facet joint degeneration appear to be contraindications to indirect decompression. This systematic review provides a reference for surgeons to define the potential benefits and limitations of LLIF in indirect neural element decompression. Further high-quality studies will better clarify the correct indications of this promising technique.
Electronic Supplementary Material
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
Compliance with Ethical Standards
Conflict of Interest
Matteo Formica, MD; Emanuele Quarto, MD; Andrea Zanirato, MD; Lorenzo Mosconi, MD; Davide Vallerga, MD; Irene Zotta, MD; Maddalena Lontaro Baracchini, MD; Carlo Formica, MD; and Lamberto Felli, MD, declare that they have no conflicts of interest.
Human/Animal Rights
N/A
Informed Consent
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level IV: Systematic Review of Level II-Level IV Studies
References
- 1.Ahmadian A, Bach K, Bolinger B, Malham GM, Okonkwo DO, Kanter AS, Uribe JS. Stand-alone minimally invasive lateral lumbar interbody fusion: multicenter clinical outcomes. J Clin Neurosci. 2015;22:740–746. doi: 10.1016/j.jocn.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadian A, Deukmedjan AR, Abel N, Dakwar E, Uribe JS. Analysis of lumbar plexopathies and nerve injury after lateral retroperitoneal transpsoas approach: diagnostic standardization. A review. J Neurosurg Spine. 2013;18:289–297. doi: 10.3171/2012.11.SPINE12755. [DOI] [PubMed] [Google Scholar]
- 3.Aichmar A, Alimi M, Hughes AP, et al. Single-level lateral lumbar interbody fusion for the treatment of adjacent segment disease. Spine (Phila Pa 1976). 2017;42(9):E515–E522. doi: 10.1097/BRS.0000000000001871. [DOI] [PubMed] [Google Scholar]
- 4.Alimi M, Hofstetter CP, Cong GTC, Tsouris AJ, James AR, Paulo D, Elowitz E, Hartl R. Radiological and clinical outcomes following extreme lateral interbody fusion. J Neurosurg Spine. 2014;20:623–635. doi: 10.3171/2014.1.SPINE13569. [DOI] [PubMed] [Google Scholar]
- 5.Alimi M, Hofstetter CP, Tsiouris AJ, Elowitz E, Hartl R. Extreme lateral interbody fusion for unilateral symptomatic vertical foraminal stenosis. Eur Spine J. 2015;24(Suppl 3):346–352. doi: 10.1007/s00586-015-3940-z. [DOI] [PubMed] [Google Scholar]
- 6.Berjano P, Lamartina C. Far lateral approaches (XLIF) in adult scoliosis. Eur Spine J. 2013;22(Suppl 2):S242–S253. doi: 10.1007/s00586-012-2426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell PG, Nunley PD, Cavanaugh D, Kerr E, Utter PA, Frank K, Stone M. Short-term outcome of lateral interbody fusion without decompression for the treatment of symptomatic degenerative spondylolithesis at L4-L5. Neurosurg Focus. 2018;44(1):E6. doi: 10.3171/2017.10.FOCUS17566. [DOI] [PubMed] [Google Scholar]
- 8.Caputo AM, Michael KW, Chapmann TM, et al. Extreme lateral interbody fusion for the treatment of adult degenerative scoliosis. J Clin Neurosci. 2013;20:1558–1563. doi: 10.1016/j.jocn.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Castellvi AE, Nienke TW, Marulanda GA, Murtagh RD, Santoni BG. Indirect decompression of lumbar stenosis with transpsoas interbody cages and percutaneous posterior instrumentation. Clin Orthop Relat Res. 2014;472:1784–1791. doi: 10.1007/s11999-014-3464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowley P. Neuroimaging of spinal canal stenosis. Magn Reson Imaging Clin N Am. 2016;24(3):523–539. doi: 10.1016/j.mric.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcome and safety of the minimally invasive, lateral retroperitoneal trans psoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28(3):E8. doi: 10.3171/2010.1.FOCUS09282. [DOI] [PubMed] [Google Scholar]
- 12.Dakwar E, Le VT, Baaj AA, Le AX, Smith WD, Akbarnia BA, Uribe JS. Abdominal wall paresis as a complication of minimally invasive lateral transpsoas interbody fusion. Neurosurg Focus. 2011;31(4):E18. doi: 10.3171/2011.7.FOCUS11164. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez I, Luque R, Noriega M, Rey J, Alia J, Marco-Martinez F. Extreme lateral lumbar interbody fusion. Surgical technique, outcomes and complications after a minimum of 1-year follow-up. Rev Esp Cir Ortop Traumatol. 2018;61(1):8–18. doi: 10.1016/j.recot.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Elowitz EH, Yanni DS, Chwajol M, Starke RM, Perin NI. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and clinical analysis. Minim Invas Neurosurg. 2011;54:201–206. doi: 10.1055/s-0031-1286334. [DOI] [PubMed] [Google Scholar]
- 15.Formica M, Berjano P, Cavagnaro L, Zanirato A, Piazzolla A, Formica C. Extreme lateral approach to the spine in degenerative and post traumatic lumbar disease: selection process, results and complications. Eur Spine J. 2014;23(Suppl 6):648–692. doi: 10.1007/s00586-014-3545-y. [DOI] [PubMed] [Google Scholar]
- 16.Formica M, Zanirato A, Cavagnaro L, Basso M, Divano S, Felli L, Formica C. Extreme lateral interbody fusion in spinal revision surgery: clinical results and complications. Eur Spine J. 2017;26(Suppl 4):464–470. doi: 10.1007/s00586-017-5115-6. [DOI] [PubMed] [Google Scholar]
- 17.Gabel BC, Hoshide R, Taylor W. An algorithm to predict success of indirect decompression using the extreme lateral lumbar interbody fusion procedure. Cereus. 2015;7(9):e317. doi: 10.7759/cureus.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hijji FY, Narain AS, Bohl DD, Ahn J, Long WW, DiBattista JV, Kudaravalli KT, Singh K. Lateral lumbar interbody fusion: a systematic review of complication rates. Spine J. 2017;17:1412–1419. doi: 10.1016/j.spinee.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs RE, Sembrano JN, Tohmeh AGSOLAS Degenerative Study Group Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part II: radiographic findings. Spine (Phila Pa 1976) 2016;41(Suppl 8):133–144. doi: 10.1097/BRS.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Lang G, Navarro-Ramirez R, et al. Can fan beam computed tomography accurately predict indirect decompression in minimally invasive spine surgery fusion procedures? World Neurosurg. 2017;107:322–333. doi: 10.1016/j.wneu.2017.07.167. [DOI] [PubMed] [Google Scholar]
- 21.Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012;16:329–333. doi: 10.3171/2012.1.SPINE11528. [DOI] [PubMed] [Google Scholar]
- 22.Khajavi K, Shen A, Hutchinson A. Substantial clinical benefit of minimally invasive lateral interbody fusion for degenerative spondylolisthesis. Eur Spine J. 2015;24(Suppl 3):314–321. doi: 10.1007/s00586-015-3841-1. [DOI] [PubMed] [Google Scholar]
- 23.Khajavi K, Shen A, Lagina M, Hutchinson A. Comparison of clinical outcomes following minimally invasive lateral interbody fusion stratified by preoperative diagnosis. Eur Spine J. 2015;24(Suppl 3):322–330. doi: 10.1007/s00586-015-3840-2. [DOI] [PubMed] [Google Scholar]
- 24.Kotwal S, Kawaguchi S, Lebl D, et al. Minimally invasive lateral lumbar interbody fusion. clinical and radiological outcome at a minimum 2-year follow-up. J Spinal Disord Tech. 2012;28:119–125. doi: 10.1097/BSD.0b013e3182706ce7. [DOI] [PubMed] [Google Scholar]
- 25.Lang G, Navarro-Ramirez R, Gandevia L, et al. Elimination of subsidence with 26-mm-wide cages in extreme lateral interbody fusion. World Neurosurg. 2017;104:644–652. doi: 10.1016/j.wneu.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Lang G, Perrech M, Navarro-Ramirez R, et al. Potential and limitations of neural decompression in extreme lateral interbody fusion—a systematic review. World Neurosurg. 2017;101:99–113. doi: 10.1016/j.wneu.2017.01.080. [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Park SW, Kim YB. Direct lateral lumbar interbody fusion: clinical and radiological outcomes. J Korean Neurosurg Soc. 2014;55(5):248–254. doi: 10.3340/jkns.2014.55.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malham GM, Ellis NJ, Parker RM, Blecher CM, Goss B, Seex KA. Maintenance of segmental lordosis and disc height in standalone and instrumented extreme lateral interbody fusion (XLIF) Clin Spine Surg. 2017;30(2):E90–E98. doi: 10.1097/BSD.0b013e3182aa4c94. [DOI] [PubMed] [Google Scholar]
- 29.Malham GM, Parker RM, Goss B, Blecher CM, Ballok ZE. Indirect foraminal decompression is independent of metabolically active facet arthropathy in extreme lateral interbody fusion. Spine (Phila Pa 1976). 2014;39(22):E1303–E1310. doi: 10.1097/BRS.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 30.Malham GM, Parker RM, Goss B, Blecher CM. Clinical results and limitations of indirect decompression in spinal stenosis with laterally implanted interbody cages: results from a prospective cohort study. Eur Spine J. 2015;24(Suppl 3):339–345. doi: 10.1007/s00586-015-3807-3. [DOI] [PubMed] [Google Scholar]
- 31.Mamisch N, Brumann M, Hodler J, Lumbar Spinal Stenosis Outcome Study Working Group et al. Radiologic criteria for the diagnosis of spinal stenosis: results of a Delphi survey. Radiology. 2012;264(1):174–179. doi: 10.1148/radiol.12111930. [DOI] [PubMed] [Google Scholar]
- 32.Marchi L, Abdala N, Oliveira L, Amaral R, Couthino E, Pimenta L. Radiographic and clinical evaluation of cage subsidence after stand-alone interbody fusion. J Neurosurg Spine. 2013;19:110–118. doi: 10.3171/2013.4.SPINE12319. [DOI] [PubMed] [Google Scholar]
- 33.Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal. 2012;2012:456346. doi: 10.1100/2012/456346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin B, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004-2015. Spine (Phila Pa 1976). 2019;44(5):369–376. doi: 10.1097/BRS.0000000000002822. [DOI] [PubMed] [Google Scholar]
- 35.Na YC, Lee HS, Shin DA, Ha Y, Kim KN, Yoon DH. Initial clinical outcomes of minimally invasive lateral lumbar interbody fusion in degenerative lumbar disease: a preliminary report on the experience of a single institution with 30 cases. Korean J Spine. 2012;9(3):187–192. doi: 10.14245/kjs.2012.9.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro-Ramirez R, Berlin C, Lang G, et al. A new volumetric radiologic method to asses indirect decompression after extreme lateral interbody fusion using high-resolution intraoperative computed tomography. World Neurosurg. 2018;109:59–67. doi: 10.1016/j.wneu.2017.07.155. [DOI] [PubMed] [Google Scholar]
- 37.Navarro-Ramirez R, Lang G, Moriguchi Y, et al. Are locked facets a contraindication for extreme lateral interbody fusion? World Neurosurg. 2017;100:607–618. doi: 10.1016/j.wneu.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 38.Nemani VM, Aichmar A, Taher F, Lebl DR, Hughes AP, Sama AA. Rate of revision surgery after stand-alone lateral lumbar interbody fusion: clinical article. Spine (Phila Pa 1976) 2014;39:E326–E331. doi: 10.1097/BRS.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 39.OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Center for Evidence-Based Medicine. 2016. Available from http://www.cebm.net/index .aspx?o=5653. Accessed August 2017.
- 40.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976). 2010;35(26S):331–337. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 41.Ozgur BM, Agarwal V, Nail E, Pimenta L. Two-year clinical and radiographic success of minimally invasive lateral transpsoas approach for the treatment of degenerative lumbar conditions. SAS Journal. 2010;4:41–46. doi: 10.1016/j.esas.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Park SJ, Lee CS, Chung SS, Kang SS, Park HJ, Kim SH. The ideal cage position for achieving both indirect neural decompression and segmental angle restoration in lateral lumbar interbody fusion (LLIF) Clin Spine Surg. 2017;30(6):E784–E790. doi: 10.1097/BSD.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 44.Pawar AY, Hughes AP, Sama AA, Girardi FP, Lebl DR, Cammisa FP. A comparative study of lateral lumbar interbody fusion and posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Asian Spine J. 2015;9:668–674. doi: 10.4184/asj.2015.9.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira EAC, Farwana M, Lam KS. Extreme lateral interbody fusion relieves symptoms of spinal stenosis and low-grade spondylolisthesis by indirect decompression in complex patients. J Clin Neurosci. 2017;35:56–61. doi: 10.1016/j.jocn.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Rodgers JA, Gerber EJ, Lehmen JA, Rodgers BW. Clinical and radiographic outcome in less invasive lumbar fusion: XLIF at two year follow-up. J Spine Neurosurg. 2013;2:3. [Google Scholar]
- 47.Rodgers W, Cox C, Gerber E. Minimally invasive treatment (XLIF) of adjacent segment disease after prior lumbar fusion. Internet J Minim Invasive Spinal Technol. 2009;3(4).
- 48.Rodgers WB, Cox CS, Gerber EJ. Early complications of extreme lateral interbody fusion in the obese. J Spinal Disord Tech. 2010;23:393–397. doi: 10.1097/BSD.0b013e3181b31729. [DOI] [PubMed] [Google Scholar]
- 49.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion. an analysis of 600 cases. Spine (Phila Pa 1976) 2010;36(1):26–33. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally invasive lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4:63–66. doi: 10.1016/j.esas.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segawa T, Inanami H, Koga H. Clinical evaluation of microendoscopy-assisted extreme lateral interbody fusion. J Spine Surg. 2017;3(3):398–402. doi: 10.21037/jss.2017.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sembrano JN, Tohmeh AG, Isaacs RE, SOLAS Degenerative Study Group Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part I: clinical findings. Spine (Phila Pa 1976). 2016;41(Suppl8):123–132. doi: 10.1097/BRS.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 53.Smith WD, Youssef JA, Christian G, Serrano S, Hyde J. Lumbarized sacrum as a relative contraindication for lateral transpsoas interbody fusion at L5-6. J Spinal Disord Tech. 2012;25:285–291. doi: 10.1097/BSD.0b013e31821e262f. [DOI] [PubMed] [Google Scholar]
- 54.Steurer J, Roner S, Gnannt R, Hodler J, LumbSten Research Collaboration Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011;12:175. doi: 10.1186/1471-2474-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tessitore E, Molliqaj G, Schaller K, Gautschi OP. Extreme lateral interbody fusion (XLIF): a single-center clinical and radiological follow-up study of 20 patients. J Clin Neurosci. 2017;36:76–79. doi: 10.1016/j.jocn.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Tohmeh AG, Khorsand D, Watson B, Zielinski X. Radiographical and clinical evaluation of extreme lateral interbody fusion. Spine (Phila Pa 1976) 2014;39:E1582–E1591. doi: 10.1097/BRS.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 57.Uribe JS, Beckman J, Mummaneni PV, Okonkwo D, Nunley P, Wang MY, Mundis GM, Park P, Eastlack R, Anand N, Kanter A, Lamarca F, Fessler R, Shaffrey CI, Lafage V, Chou D, Deviren V, The MIS-ISSG Group Does MIS surgery allow for shorter construct in the surgical treatment of adult spinal deformity? Neurosurgery. 2017;80:489–497. doi: 10.1093/neuros/nyw072. [DOI] [PubMed] [Google Scholar]
- 58.Walker CT, Farber SH, Cole TS, Xu DS, Godzik J, Whiting AC, Hartman C, Porter RW, Turner JD, Uribe J. Complications for minimally invasive lateral interbody arthrodesis: a systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J Neurosurg Spine. 2019;January 25. [DOI] [PubMed]
- 59.Wang TY, Nayar G, Brown CR, Pimenta L, Karikari IO, Isaacs RE. Bony lateral recess stenosis and other radiographic predictors of failed indirect decompression vie extreme lateral interbody fusion: multi-institutional analysis of 101 consecutive spinal levels. World Neurosurg. 2017;106:819–826. doi: 10.1016/j.wneu.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 60.Yang Yang, Zhang Liangming, Dong Jianwen, Chen Zihao, Xie Peigen, Chen Ruiqiang, He Lei, Feng Feng, Rong Limin, Liu Bin. Intraoperative Myelography in Transpsoas Lateral Lumbar Interbody Fusion for Degenerative Lumbar Spinal Stenosis: A Preliminary Prospective Study. BioMed Research International. 2017;2017:1–8. doi: 10.1155/2017/3742182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida G, Hasegawa T, Yamato Y, et al. Minimum clinically important differences in Oswestry Disability Index domains and their impact on adult spinal deformity surgery. Asian Spine J. 2019;13(1):35–44. doi: 10.31616/asj.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion. result and review. Spine (Phila Pa 1976) 2010;35(26S):S302–311. doi: 10.1097/BRS.0b013e3182023438. [DOI] [PubMed] [Google Scholar]
- 63.Zanirato A, Damilano M, Formica M, et al. Complications in adult spine deformity surgery: a systematic review of the recent literature with reporting of aggregated incidences. Eur Spine J. 2018;27(9):2272–2284. doi: 10.1007/s00586-018-5535-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)
(PDF 1.19 mb)