Introduction
The success of biomedical innovation and the development of novel therapeutics depend on the strategic integration of scientific research and clinical medicine. This process, traditionally referred to as translational medicine or “bench-to-bedside” research, allows scientists to provide clinicians with treatment strategies designed with specific biological targets in mind. Equally critical to effective research is the reverse translational pathway, whereby clinical observation of disease progression and patient outcomes informs future scientific investigation [24]. This bidirectional flow of information allows for the continued refinement of drug and product development.
Musculoskeletal medicine has its roots in reverse translation. The eighteenth-century English physician William Heberden, who characterized the osteoarthritic nodes that bear his name, championed the concept of using clinical discovery to inform treatment decisions. Considered the “father of clinical observation” of the eighteenth century, he maintained detailed records of his observations and synthesized his findings in order to improve future treatment decisions [7, 22]. Considered a true “renaissance physician-scientist” by some authors, Heberden valued collaborative investigation and encouraged other experimental scientists to study and critique his work [7]. In conjunction with a contemporary understanding of anatomy, physiology, and disease, the Heberden philosophy underlies today’s successful translational research.
Defining the Clinical Problem
In a modern context, translational research continues to be a major driver of orthopedic innovation. For example, the steady incidence of pseudarthrosis following lumbar fusion surgery has been a critical problem in the field of spine surgery over the past several decades. In 1965, Marshall Urist first described the ability of demineralized bone matrix to induce bone formation when implanted ectopically in soft tissues. He determined that this activity was driven by bone-inducing compounds, later termed bone morphogenetic proteins (BMPs) [29]. Since his discovery, extensive research has characterized this family of osteoinductive growth factors and their biochemical properties. Despite the abundance of evidence describing BMPs, some argue that this technology was not adequately tested prior to clinical translation [3].
In 2002, the US Food and Drug Administration (FDA) approved recombinant human BMP-2 (rhBMP-2) delivered via collagen sponge for use in anterior lumbar interbody fusion (ALIF), providing a substitute for iliac crest bone graft [15]. Use of the growth factor increased rapidly, reaching a peak in 2008; according to Deyo et al., rhBMP-2 was used in as many as 28.1% of all fusion cases [5]. Despite its early clinical success in a number of different biologic environments, reports of adverse effects have contributed to a decline in the use of growth-factor-based bone graft substitutes. More recent data demonstrate a dramatic decrease in use, with just 7.8% of spine surgeons reporting rhBMP-2 use in open lumbar cases in 2014 [27]. Case studies have suggested that supraphysiologic doses and off-label use of rhBMP-2 in spinal fusion can be associated with significant post-surgical complications, including radiculitis, heterotopic bone formation, bone resorption, graft subsidence, and wound infection [15]. In response to these concerns, the Yale University Open Data Access (YODA) Project organized two independent systematic reviews of industry-sponsored data pertaining to the safety and efficacy of rhBMP-2. The resulting publications indicated that the use of rhBMP-2 and iliac crest bone graft results in similarly high lumbar fusion rates, with no evidence of a clinically significant difference in long-term outcomes; the use of rhBMP-2 in the anterior cervical spine, however, leads to a significantly increased rate of complications [9, 28].
In order to mitigate the negative effects associated with adjunctive rhBMP-2 use, several investigators have sought to promote alternative bone graft substitutes with a less-harmful side effect profile, effectively returning to the bench after unsatisfactory results at the bedside.
The materials that have entered the market after the introduction of rhBMP-2, including demineralized bone matrix (DBM), ceramics, and cell-based therapies, have failed to consistently duplicate its success, however. DBM is characterized by osteoconductive and osteoinductive properties. The documented variability in concentration of growth factor between different lots of commercially available product, however, raises concerns on the reliability of its osteoinductive response [1]. Similarly, ceramic materials, though they confer substantial mechanical strength, offer no osteoinductive stimulus [13]. Autologous bone marrow aspirate has undergone significant research but like DBM is subject to variability in processing and osteoinductive potential [13]. As these materials have been unable to replicate the unique activity of rhBMP-2, our research is driven by strategies to improve, rather than replace, growth-factor-based implants.
Returning to the Bench
Negative or neutral outcomes observed at any phase of the translational research pathway can often be corrected by returning to an earlier step [24]. The body of clinical evidence regarding the use of rhBMP-2 in spinal fusion surgery and adverse clinical outcomes has prompted a renewed focus on the growth factor from a basic science perspective.
The efficacy of collagen as a scaffold for rhBMP-2 is limited by its mechanical instability, which allows for compression by surrounding tissue and release of large amounts of growth factor after implantation [8]. Furthermore, many studies have demonstrated that rhBMP-2 undergoes an initial burst release when delivered on type 1 collagen sponge [18, 21]. This rapid efflux has been associated with ectopic bone formation in the spinal canal and bone resorption proximal to the implant [21]. Prior to the commercialization of rhBMP-2 combined with absorbable collagen sponge (ACS) for bone healing applications, it was well-understood that a delivery system for rhBMP-2 must retain growth factor locally for a prolonged period of time [10, 20]. The burst release profile of collagen carriers had been documented and was hypothesized to be instrumental in the recruitment of precursor cells during the initial healing response [20]. Shortly after FDA approval of rhBMP-2 for use in ALIF surgeries, Wyeth BioPharma advocated the use of a collagen carrier; however, local retention of growth factor was only compared with that from a buffer solution [10]. After the commercialization, further basic science work confirmed that there are more optimal carriers for rhBMP-2 than collagen, such as β-tricalcium phosphate, hydrogels, hydroxyapatite, and bioactive glass [16, 23, 31]. The ideal delivery vehicle would effectively bind and localize growth factor and allow for a slow, sustained release over time.
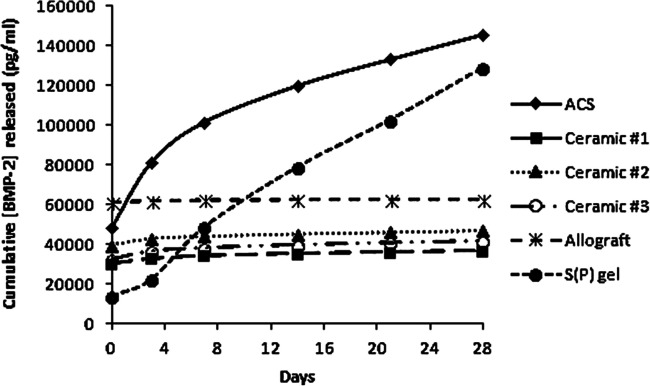
To this end, scientists at Northwestern University have investigated solutions to this clinical problem by developing improvements in carrier technology intended to mitigate the mechanism associated with adverse effects. In 2001, the laboratory of Samuel Stupp reported the development of peptide amphiphile (PA) molecules that undergo self-assembly in aqueous solution to form cylindrical micelles, or nanofibers. Reversible intramolecular disulfide bonds allow for cross-linking of nanofibers, resulting in a robust network that directs the mineralization of hydroxyapatite. The alignment of hydroxyapatite in the resulting composite material was found to be identical to the alignment observed between hydroxyapatite crystals and collagen fibrils in bone [11]. Using this foundation, we later incorporated phosphorylated serine segments within the PA molecules, which allows for the generation of a self-supporting, bioactive gel matrix that mimics bone sialoprotein, further augmenting mineralization [26]. Compared with other commercially available bone graft substitutes, including absorbable collagen sponge and ceramic-based carriers, the PA gel demonstrates superior cell adhesion capability in vitro and enabled significantly greater pre-osteoblast survival at 2 h post-inoculation. Additionally, this PA gel more efficiently binds rhBMP-2 than does other carriers and allows for sustained release of growth factor over 28 days (Fig. 1) [14].
Fig. 1.
Binding/release assays for various materials loaded with BMP-2. Data represent cumulative rhBMP-2 release into surrounding media, where a flat slope represents no release. Reprinted with permission from Hsu EL et al. [14]. (Mary Ann Liebert, Inc., New Rochelle, NY, USA). rhBMP-2 recombinant human bone morphogenetic protein-2, ACS absorbable collagen sponge.
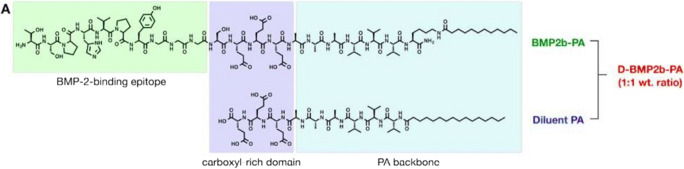
The basic structure of a PA molecule includes three domains—a hydrophobic alkyl chain, a β-sheet-forming domain, and a carboxyl-rich peptide domain—and can be further customized with the addition of bioactive epitopes (Fig. 2) [18]. Upon self-assembly, these moieties are displayed on the surface of nanofibers, where they can mediate biological activity. Heparan sulfate is a sulfated glycosaminoglycan known to interact with various proteins to direct physiologic processes [19]. In order to emulate this binding capacity using PA technology, we designed several PA molecules with differing terminal-sulfated monosaccharide domains and characterized their ability to bind BMP-2, regulate protein expression in vitro, and mediate spinal fusion in vivo [19]. Among the nanostructures tested, the PA functionalized with trisulfated 3,4,6S-N-acetyl glucosamine (3,4,6S-GlcNAc) demonstrated superior ability to induce osteoblast differentiation in BMP-2-containing medium and enhanced alkaline phosphatase activity to a significantly greater extent than naturally occurring heparan sulfate. Subsequently, we evaluated these nanostructures in a rat posterolateral lumbar fusion model, which historically requires 10 μg rhBMP-2 on collagen sponge to achieve arthrodesis [18]. Animals treated with just 100 ng rhBMP-2 when delivered with the 3,4,6S-GlcNAc PA achieved 100% fusion [19]. These data suggest that PA nanofibers can deliver growth factor more efficiently in the setting of bone regeneration and reduce the adverse effects associated with rhBMP-2 use.
Fig. 2.
Chemical structure of rhBMP-2-binding PA and diluent (non-binding) PA. Mixed in a 1:1 wt.% ratio for use as delivery system in vivo. rhBMP-2 recombinant human bone morphogenetic protein-2, PA peptide amphiphile. ACS absorbable collagen sponge
PA molecules with a BMP-2-binding motif capable of localizing both exogenous and endogenous BMP-2 have been created. The addition of this domain confers greater ability to bind BMP-2 from growth-factor-containing medium after 4 h relative to the PA nanofibers without such a domain [18]. Importantly, PAs both with and without a BMP-2-binding domain exhibited superior release kinetics than did ACS, steadily discharging growth factor over 28 days [18]. In our first in vivo translational study of this material, we compared the BMP-2-binding nanofiber scaffolds with collagen sponge as delivery vehicles for rhBMP-2 in a rat posterolateral lumbar fusion model [18]. When delivered via BMP-2-binding PA gel, rhBMP-2 elicited successful fusion at doses tenfold lower than when delivered on collagen sponge [18]. Importantly, in 42% of animals, the PA gel delivered alone led to successful bony fusion. This represents significantly enhanced functionality over the sulfated glycopeptide, which was unable to achieve fusion in this model in the absence of added rhBMP-2. This investigation again provided clear evidence that PA scaffolds significantly enhance rhBMP-2-mediated spinal fusion and if translated clinically have the potential to reduce the doses required to achieve spine arthrodesis in human patients.
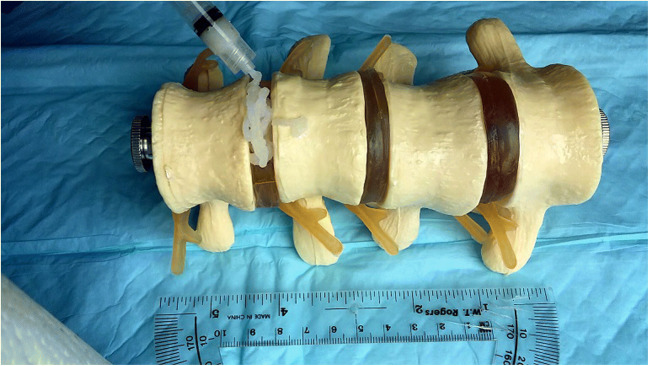
We have continued to adjust this technology and explore its potential for translation in a larger animal model. Boden et al. first described the utility of a rabbit model of lumbar fusion, noting that it more closely approximates surgical technique, graft environment, and rate of nonunion observed in humans than smaller animal models [2]. Pre-clinical feasibility studies continue to use this standard because rabbits reach skeletal maturity quickly, and their larger body size relative to rodents allows for assessing larger-scale implants [25, 30]. Our BMP-2-binding PA solution may be delivered on collagen sponge or combined with lyophilized collagen particles to create an injectable implant. This material is easily deployed using a syringe and retains its shape after delivery (Fig. 3). Our recent data suggest that, at doses of just 5 μg per implant, treatment with the injectable formulation resulted in significantly greater fusion rates and bridging bone volumes than treatment with BMP-2-binding PA on ACS [4]. After extensive investigation and continued modification, we believe this combination of PA design and delivery formulation represent the ideal carrier for rhBMP-2. We are optimistic that this material will reduce the amount of rhBMP-2 required to achieve fusion, allowing surgeons to utilize this highly osteoinductive growth factor with minimal risk of associated complications.
Fig. 3.
Injectable BMP-2-binding PA delivered via syringe. Image courtesy of Mark T. McClendon, PhD, Simpson Querrey Institute and Department of Materials Science & Engineering, Northwestern University, Chicago, IL, USA. BMP-2 recombinant human bone morphogenetic protein-2, PA peptide amphiphile.
The Importance of a Team-Oriented Approach
Stressing the importance of multidisciplinary collaboration and interactive partnerships in developing translational research strategies, Duda et al. argue that translational investigators must identify unmet clinical needs and create a bridge between stakeholders, including researchers, funding agencies, and regulatory authorities [6]. Translational research requires a shift away from traditional academic investigations, in which individual departments tend to function as discrete, non-overlapping units [17].
Our laboratory team has evolved organically around the goal of enhancing orthobiologic implants to reflect this set of recommendations. Bringing together surgeons, materials engineers, and basic scientists with expertise in toxicology, biochemistry, and regenerative medicine allows for creativity and efficiency in study design and implementation. A surgical perspective is particularly important in the research and development of new materials. If surgical handling properties of new materials are not assessed prior to implementation in an animal model, for example, any demonstrated efficacy may be inconsequential if it is difficult to deploy. Conversely, without a depth of understanding of physical and mechanical properties, degradation profiles, and other parameters, construction of effective materials is not possible.
The appropriate trajectory of studies, including safety investigations, feasibility testing, and pre-clinical efficacy, is necessary to make well-supported claims about novel osteobiologics. A pre-clinical investigation of the efficacy of a given material in a rodent model, for example, may require up to 100 animal subjects in order to guarantee sufficient statistical power. After spinal fusion procedures, which may take several weeks to complete, animals must be housed between 8 and 12 weeks to allow time for a sufficient healing response to occur. After animal sacrifice, specimens are harvested and subject to testing specific to the outcomes of interest. We routinely test specimens for fusion by blinded manual palpation, quantify new bone growth via microCT imaging and analysis, and conduct histological staining for visualization of mineralization and bone growth. Repeating this process for consecutive study aims quickly multiplies the number of animal subjects and personnel hours required to complete an investigation. The volume of animal work driving our research necessitates a coordinated effort between all members of the research team.
Hobin et al. emphasized the importance of early career exposure to translational medicine for medical and graduate students, arguing for a greater effort to teach strategies for successful collaboration between basic investigators, physician-scientists, and clinical practitioners [12]. Given our location at a research-oriented institution, student and resident physician involvement in ongoing animal projects has become critical to the success of our work.
Future Research
Reintroducing rhBMP-2 to surgical practice with an enhanced carrier material will require researchers to identify the factors contributing to adverse effects and devise reliable methods to address them. The return back to “bedside” has not proven a linear, single-step endeavor. Each investigation, however, has allowed us to narrow the gap between the laboratory and the operating room. Our approach to translational research has allowed for the development of promising new materials such as the BMP-2-binding PA nanofibers highlighted here. Ongoing investigations in the rabbit model represent the next step in the evolution of a product suitable for use in humans. As we continue to progress toward a product designed to improve patient outcomes, this work remains a rewarding challenge.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1803 kb)
Compliance with Ethical Standards
Conflict of Interest
Allison C. Greene, MPH, reports no conflicts of interest. Wellington K. Hsu, MD, reports personal fees as a consultant from Medtronic, Stryker, Allosource, and Asahi and as a member of the advisory board for Bioventus, Mirus, Wright Medical, Lumbar Spine Research Society, Cervical Spine Research Society, and the National Football League, and has received institutional/research support from Medtronic, Baxter, and Pioneer Surgical. He receives royalties from Stryker.
Human and Animal Rights and Informed Consent
N/A
Informed Consent
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine. 2006;31(12):1299–1306. doi: 10.1097/01.brs.0000218581.92992.b7. [DOI] [PubMed] [Google Scholar]
- 2.Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 1995;20(4):412–420. doi: 10.1097/00007632-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Chang KY, McClendon M, Driscoll JA, et al. Friday, September 28, 2018 4:05–5:05 PM abstracts: basic science of spinal fusion: 235. Peptide amphiphile nanoscaffolds potentiates the delivery of rh-BMP2 in a rabbit spine fusion model. Spine J. 2018;18(8):S116–S117. doi: 10.1016/j.spinee.2018.06.500. [DOI] [Google Scholar]
- 5.Deyo RA, Ching A, Matsen L, et al. Use of bone morphogenetic proteins in spinal fusion surgery for older adults with lumbar stenosis: trends, complications, repeat surgery, and charges. Spine. 2012;37(3):222–230. doi: 10.1097/BRS.0b013e31821bfa3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duda GN, Grainger DW, Frisk ML, et al. Changing the mindset in life sciences toward translation: a consensus. Sci Transl Med. 2014;6(264):264cm212. doi: 10.1126/scitranslmed.aaa0599. [DOI] [PubMed] [Google Scholar]
- 7.Edelman ER, LaMarco K. William Heberden and reverse translation. Sci Transl Med. 2015;7(287):287fs220. doi: 10.1126/scitranslmed.aaa2250. [DOI] [PubMed] [Google Scholar]
- 8.El Bialy I, Jiskoot W, Reza NM. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm Res. 2017;34(6):1152–1170. doi: 10.1007/s11095-017-2147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu R, Selph S, McDonagh M, et al. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013;158(12):890–902. doi: 10.7326/0003-4819-158-12-201306180-00006. [DOI] [PubMed] [Google Scholar]
- 10.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55(12):1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 12.Hobin JA, Deschamps AM, Bockman R, et al. Engaging basic scientists in translational research: identifying opportunities, overcoming obstacles. J Transl Med. 2012;10:72. doi: 10.1186/1479-5876-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu WK, Nickoli MS, Wang JC, et al. Improving the clinical evidence of bone graft substitute technology in lumbar spine surgery. Global Spine J. 2012;2(4):239–248. doi: 10.1055/s-0032-1315454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu EL, Ghodasra JH, Ashtekar A, et al. A comparative evaluation of factors influencing osteoinductivity among scaffolds designed for bone regeneration. Tissue Eng Part A. 2013;19(15–16):1764–1772. doi: 10.1089/ten.tea.2012.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James AW, LaChaud G, Shen J, et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev. 2016;22(4):284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kissling S, Seidenstuecker M, Pilz IH, Suedkamp NP, Mayr HO, Bernstein A. Sustained release of rhBMP-2 from microporous tricalciumphosphate using hydrogels as a carrier. BMC Biotechnol. 2016;16(1):44. doi: 10.1186/s12896-016-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurpinski K, Johnson T, Kumar S, Desai T, Li S. Mastering translational medicine: interdisciplinary education for a new generation. Sci Transl Med. 2014;6(218):218fs212. doi: 10.1126/scitranslmed.3006858. [DOI] [PubMed] [Google Scholar]
- 18.Lee SS, Hsu EL, Mendoza M, et al. Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv Healthc Mater. 2015;4(1):131–141. doi: 10.1002/adhm.201400129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SS, Fyrner T, Chen F, et al. Sulfated glycopeptide nanostructures for multipotent protein activation. Nat Nanotechnol. 2017;12(8):821–829. doi: 10.1038/nnano.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li RH, Wozney JM. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001;19(7):255–265. doi: 10.1016/S0167-7799(01)01665-1. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Johnson NR, Usas A, et al. Sustained release of bone morphogenetic protein 2 via coacervate improves the osteogenic potential of muscle-derived stem cells. Stem Cells Transl Med. 2013;2(9):667–677. doi: 10.5966/sctm.2013-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lian TY, Lim KK. The legacy of William Heberden the Elder (1710–1801) Rheumatology. 2004;43(5):664–665. doi: 10.1093/rheumatology/keg007. [DOI] [PubMed] [Google Scholar]
- 23.Liu W-C, Robu IS, Patel R, Leu MC, Velez M, Gabriel Chu T-M. The effects of 3D bioactive glass scaffolds and BMP-2 on bone formation in rat femoral critical size defects and adjacent bones. Biomed Mater. 2014;9(4):045013. doi: 10.1088/1748-6041/9/4/045013. [DOI] [PubMed] [Google Scholar]
- 24.Lobo M, Ibanez B. Take a deep (nitric oxide) breath and follow the reverse translational research pathway. Eur Heart J. 2018;39(29):2726–2729. doi: 10.1093/eurheartj/ehy355. [DOI] [PubMed] [Google Scholar]
- 25.Mapara M, Thomas BS, Bhat KM. Rabbit as an animal model for experimental research. Dent Res J (Isfahan) 2012;9(1):111–118. doi: 10.4103/1735-3327.92960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mata A, Geng Y, Henrikson KJ, et al. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials. 2010;31(23):6004–6012. doi: 10.1016/j.biomaterials.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder GD, Hsu WK, Kepler CK, et al. Use of recombinant human bone morphogenetic protein-2 in the treatment of degenerative spondylolisthesis. Spine. 2016;41(5):445–449. doi: 10.1097/BRS.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 28.Simmonds MC, Brown JVE, Heirs MK, et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158(12):877–889. doi: 10.7326/0003-4819-158-12-201306180-00005. [DOI] [PubMed] [Google Scholar]
- 29.Urist MR. Bone: formation by autoinduction. Clin Orthop Relat Res. 1965;2002(395):4–10. doi: 10.1097/00003086-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Wancket LM. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. Vet Pathol. 2015;52(5):842–850. doi: 10.1177/0300985815593124. [DOI] [PubMed] [Google Scholar]
- 31.Xie G, Sun J, Zhong G, Liu C, Wei J. Hydroxyapatite nanoparticles as a controlled-release carrier of BMP-2: absorption and release kinetics in vitro. J Mater Sci Mater Med. 2010;21(6):1875–1880. doi: 10.1007/s10856-010-4038-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1803 kb)