Abstract
Background
Pseudarthrosis after lumbar fusion can generate pain and disability and often requires revision. However, results of revision procedures have historically been relatively poor.
Questions/Purpose
The aim of this review was to examine the current evidence related to the management of lumbar pseudarthrosis, with a focus on revision after failure of posterolateral fusion or lumbar interbody fusion.
Methods
A review of orthopedic spine literature published before March 2019 was conducted using PubMed and Google Scholar. Studies addressing revision after failed posterolateral fusions and after failed interbody fusion were selected. We also present a case of successful revision after failed transforaminal lumbar interbody fusion (TLIF).
Results
The review revealed that persistent pseudarthrosis after revision posterolateral fusion occurs at rates of 35 to 51%. No significant difference has been demonstrated in rates of successful fusion after anterior lumbar interbody fusion (ALIF) and ALIF with revision posterolateral fusion for pseudarthroses after failed TLIF procedures (81% versus 88%), although ALIF alone may be appealing because it avoids further disruption of the posterior musculature. No significant differences have been observed in quality-of-life scores among patients undergoing revision after posterolateral fusion, TLIF, ALIF, or ALIF with posterior fusion. Failed TLIF cages may be extracted and replaced through an anterior or lateral approach. If the geometry of the failed cage permits insertion of a second cage, a contralateral approach may be used. Revision retroperitoneal approaches are associated with higher complication rates.
Conclusions
The management of lumbar pseudarthrosis requires careful planning, as well as intra-operative attention to detail, for revision surgery to be successful. Circumferential procedures have shown success in revision posterolateral and interbody fusion failures.
Electronic supplementary material
The online version of this article (10.1007/s11420-019-09732-9) contains supplementary material, which is available to authorized users.
Keywords: lumbar pseudarthrosis, revision, spine surgery, complex, techniques
Introduction
Lumbar arthrodesis is used in the treatment of a wide variety of spinal conditions with developmental, degenerative, infectious, neoplastic, or traumatic etiologies. The rate of lumbar fusions performed in the USA has steadily risen in recent years, growing 62.3% on a population-adjusted basis from 2004 to 2015 [9]. Despite improvements in surgical technologies and biologics, this increase in the volume of fusion procedures necessarily brings with it an uptick in the volume of revision surgery [16]. Pseudarthrosis, often defined as a failure to achieve osseous fusion by the 1-year post-operative time point [15], is a common cause of such revisions. Given the variability in patient populations, pathology, surgical techniques, and methods used to diagnose pseudarthrosis, a range of lumbar pseudarthrosis rates have been reported, but an incidence on the order of 5 to 15% is commonly observed with the use of modern operative techniques [2, 8, 12, 14].
Although not all pseudarthroses are symptomatic [21], those that generate pain and disability often require surgical intervention. The results of these revision procedures have historically been relatively poor, with rates of recurrent pseudarthrosis of 35 to 50% and clinical failure observed in as many as 40 to 70% of cases [1]. A variety of factors have been shown to influence pseudarthrosis, including smoking, metabolic disorders, surgical instrumentation and technique, and location of fusion [15]. Careful pre-operative planning and intra-operative attention to detail are therefore of paramount importance to maximize the chances of success in these challenging clinical scenarios.
Successful revision surgery for pseudarthrosis often requires a surgical approach that is different from the first, further instrumentation, and other materials such as bone grafts or biologics [15]. It is important to consider the approach used in the original fusion. The purpose of this review is to examine the current evidence related to surgical management of lumbar pseudarthrosis; our focus is on two questions: What surgical strategies might be most useful in revision surgery after posterolateral fusion failure? What strategies are currently being used in revision surgery after lumbar interbody fusion failure?
Methods
To address these questions, we reviewed the recent literature that focuses on revisions after failure of either posterolateral or interbody fusion. We also present a case of successful revision after failed transforaminal lumbar interbody fusion (TLIF).
PubMed and Google Scholar were searched for studies on orthopedic spine surgery conducted before March 2019. Only English language studies were included. We began our search broadly centered on pseudarthrosis and then focused it on revision after failed posterolateral or interbody fusion. Systematic reviews and meta-analyses were included only if the relevant studies were based on sound study design. Results are described as narrative responses to the questions proposed above.
Results
Revising Failed Posterolateral Fusions
Pseudarthrosis of an isolated posterolateral fusion (in other words, no attempt at interbody fusion) leaves the surgeon with the largest number of revision options. One is a second attempt at posterolateral fusion, although persistent pseudarthrosis after revision posterolateral fusion has been reported at rates of 35 to 51% [6, 20]. In such an attempt, screws may be upsized, meticulous attention must be paid to decortication, and the use of iliac crest bone graft or bone morphogenetic protein 2 (BMP-2) should be considered. A screw system with a different thread pitch can help increase purchase, and cement augmentation can be used in cases of severely osteoporotic bone. It should be noted that the available studies were performed before the widespread adoption of modern pedicle screw and rod instrumentation, but the results are discouraging nonetheless.
The addition of an interbody device has been associated with higher rates of successful fusion in patients undergoing revision surgery for pseudarthrosis [1, 4, 17]. Retroperitoneal approaches, such as anterior lumbar interbody fusion (ALIF) and lateral lumbar interbody fusion (LLIF), as well as related antepsoas approaches, are attractive in that they allow for placement of interbody grafts with large surface areas, which is beneficial from a biomechanical perspective while also providing a broader region for fusion. Fusion rates of 90 to 100% have been observed with circumferential surgery (ALIF and revision posterolateral fusion) [1, 4, 17].
Given the availability of ALIF cages with built-in fixation capabilities, ALIF alone may be appealing because it avoids further disruption of the posterior musculature. A retrospective review comparing ALIF and ALIF with revision posterolateral fusion in 38 patients with pseudarthroses after failed TLIF found fusion rates of 81 and 88%, respectively, a difference that did not attain statistical significance [18]. Consistent with other studies reporting clinical outcomes after revision surgery for pseudarthrosis, functional improvement was minimal in both groups. ALIF followed by percutaneous pedicle screw instrumentation (rather than open revision posterolateral fusion) is another option that has been described in an effort to reduce surgical morbidity [7].
TLIF may also be considered but may be more technically challenging in the setting of a prior laminectomy. In a 2016 retrospective cohort study of 128 patients treated for lumbar pseudarthrosis, Owens et al. found no statistically significant difference in health-related quality-of-life scores between patients treated with revision posterolateral fusion, TLIF, ALIF, or ALIF with posterior fusion [11]. Radiographic fusion rates were not reported, but clinical outcomes overall were discouraging—depending on the treatment group, only 17 to 28% of patients reached a minimal clinically important difference on the Oswestry Disability Index. This underscores the importance of achieving robust fusion during the index procedure.
Because ALIF and LLIF do not allow for direct access to posteriorly based implants, a 540° fusion (back–front–back) may be necessary, especially if significant changes to sagittal or coronal parameters (or both) are desired despite reasonably well-fixed posterior implants. In the first stage, pedicle screws and rods are removed, any necessary releases are performed, screws are replaced, and the posterior wound is closed. This is followed by the ALIF or LLIF (the second stage). The third stage involves reopening the posterior incision and placing rods to maintain the correction. The prolonged surgical time and the logistical complexity involved in repositioning the patient multiple times intra-operatively are certainly drawbacks to this technique. If there is concern about infection or about an older patient’s ability to tolerate a three-stage procedure under one anesthetic, posteriorly based implants can be removed, cultures can be sent, and the wound can be closed to allow the patient to recover. Patients with positive intra-operative cultures can then be treated to eradicate the infection. The anterior interbody fusion and posterior reinstrumentation can be performed at a later time, in one or more operating room sessions during the same hospitalization.
In an effort to reduce the surgical burden, Kadam et al. introduced the use of hyperlordotic ALIF cages to “overpower” intact posterior fixation [5]. The authors performed two-stage surgery—ALIF followed by posterior implant removal, release, reinstrumentation, and compression—and found improvement in segmental lordosis to be approximately half that with the inserted cage (6.1°, 12.5°, and 17.7° with 12°, 20°, and 30° cages, respectively; all p values < 0.05). Use of this technique is likely ill-advised in patients with deficient bone mineral density because endplate violations or post-operative subsidence was observed in 8.3% of cases despite the fact patients with osteoporosis were excluded from the study cohort.
Revising Failed Interbody Fusions
The presence of a previously placed interbody device has implications for the choice of revision strategy. The above considerations and techniques apply, but additional thought must be given to removal of the previously placed cage if another attempt at interbody fusion is desired. Previously placed TLIF cages are most easily extracted using an anterior or lateral approach and can be replaced with larger ALIF or LLIF cages [7, 10]. Revision of a TLIF through an oblique lateral approach has also been described [13]. Loose cages may come out easily after being freed up with an osteotome, but partial corpectomies may be necessary to explant cages that are more stably fixed or have subsided into the subchondral bone. A high-speed burr can be used to obliterate interbody spacers made of bone or polyether ether ketone (PEEK) but not metallic cages. Eom et al. described a technique for the removal of difficult-to-remove PEEK interbody cages: a tap hole is created into which a rod with a threaded tip is screwed to function as a joystick to control the cage for removal [3]. If the geometry and orientation of the original TLIF cage allow for insertion of a second, revision TLIF can be performed using a contralateral approach.
Revision retroperitoneal approaches, although possible, are associated with higher surgical complication rates if there is difficulty mobilizing scarred-down vessels and viscera [19]. Given the larger footprints of ALIF and LLIF cages, associated pseudarthroses that are relatively stable may simply require a revision posterolateral fusion. However, if removal of a previously placed LLIF cage is necessary, the level can be approached from the other side, as long as the initial cage does not have integral fixation. Failed ALIFs can be addressed using a lateral approach, but a revision anterior approach is necessary to extract ALIF cages with associated anterior fixation. If the level of interest is L5–S1, the second anterior approach might be easier when a course through the contralateral retroperitoneum is taken, but this is less feasible at more cranial interspaces [19]. The level of experience of the surgeon performing the approach, as well as patient factors such as obesity and vascular anatomy, also comes into the equation when weighing the risks and benefits of revision ALIF.
Clinical Case
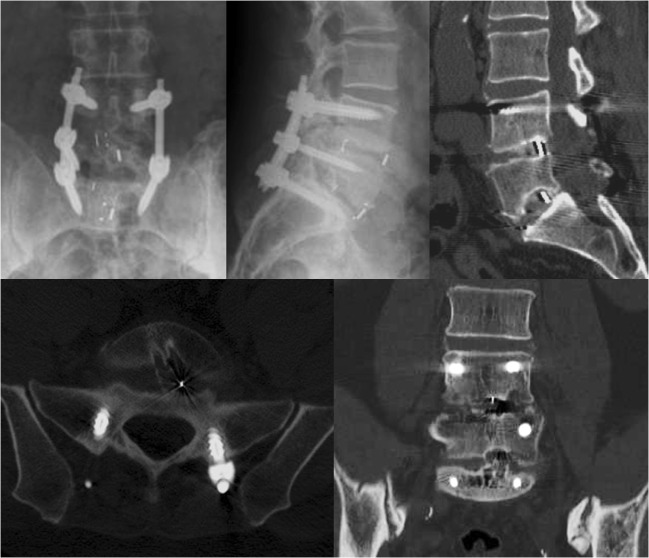
A 58-year-old man experienced axial back pain and intermittent left lower extremity paresthesias despite nonoperative treatments, which included physical therapy and anti-inflammatory medications. He had undergone an L4–S1 decompression and TLIF at another hospital 2 years earlier. His symptoms initially improved after the index procedure but began to worsen again within a year. A computed tomographic scan demonstrated pseudarthrosis at L4–L5 and L5–S1 with haloing about the right S1 pedicle screw (Fig. 1). Cage subsidence and endplate violation were also present.
Fig. 1.
Pre-operative anteroposterior and lateral X-rays (first two images) and selected computed tomographic cuts demonstrating cage subsidence and pseudarthrosis.
For this patient, revision fusion through L4–S1 ALIF with retention of the posterior instrumentation was indicated, given that the instrumentation remained relatively well fixed. After standard retroperitoneal exposure of the L5–S1 disc space, an annulotomy was performed, and residual disc material was removed using a series of pituitaries and curettes. The subsided PEEK TLIF cage was identified. There was gross motion at this level with no evidence of bridging bone. In order to facilitate extraction, a high-speed burr was used to perform partial corpectomies, removing 5 mm from the caudal aspect of the L5 vertebral body and 5 mm from the cranial aspect of S1. The TLIF cage was then removed using a heavy pituitary. Once the L5–S1 intervertebral space was cleared, an appropriately sized PEEK ALIF cage was selected, packed with BMP-2, and secured using an upgoing screw placed through the cage. Next, the identical steps were performed at L4–L5, where pseudarthrosis was also clearly present. The procedure and post-operative course were uncomplicated. After the revision, the patient reported significant symptom improvement. Post-operative imaging demonstrated well-positioned grafts with evidence of osseous fusion (Fig. 2).
Fig. 2.
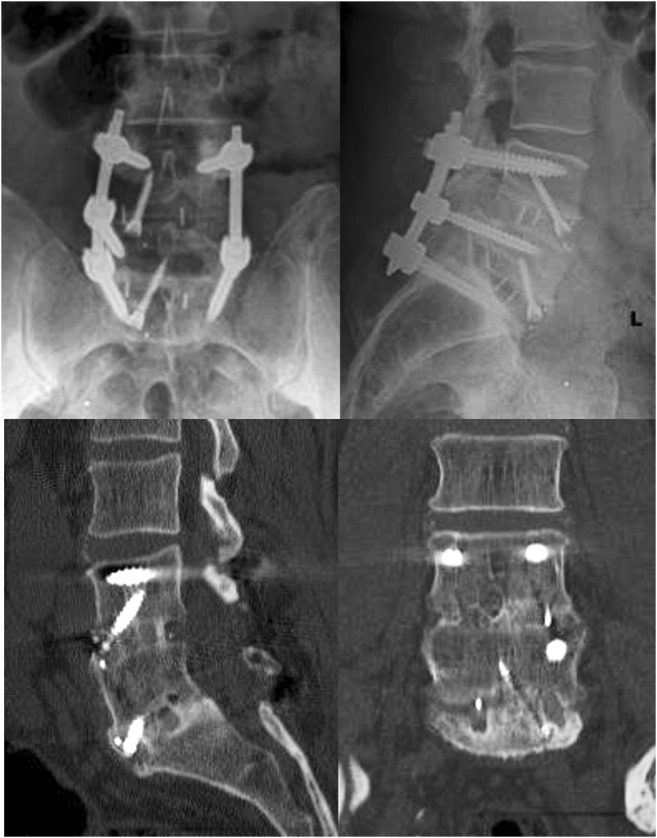
Anteroposterior and lateral X-rays (first two images) and selected computed tomographic cuts at follow-up after revision surgery demonstrating appropriately placed implants with evidence of robust osseous fusion.
Discussion
Successfully managing lumbar spine pseudarthrosis involves complex decision-making and technical expertise. Each case is unique and must be approached on an individual basis. Surgeons must be proficient with a variety of surgical approaches so that they can tailor operative plans to the nuances of each case. Although a surgeon’s comfort in managing these challenging cases grows with experience, a thorough understanding of the information presented above will help maximize rates of successful fusion and optimize patient outcomes.
Electronic supplementary material
(PDF 1225 kb)
Compliance with Ethical Standards
Conflict of Interest
Peter B. Derman, MD, MBA, declares that he has no conflicts of interest. Kern Singh, MD, reports receiving consulting fees or honoraria from Zimmer Biomet, K2M, Stryker, Pioneer, Lippincott Williams and Wilkins, Thieme, Jaypee Publishing, Slack Publishing, Avaz Surgical, Vital 5, and DuPuy Synthes; royalties or licensing fees from Zimmer Biomet, RTI Surgical, and Stryker; is on the board of directors at the Cervical Spine Research Society, the International Society for the Advancement of Spine Surgery (ISASS), the American Academy of Orthopaedic Surgeons, the Scoliosis Research Society, and Vertebral Columns (ISASS); and has received a Cervical Spine Research Society resident grant, outside the submitted work.
Human/Animal Rights
N/A
Informed Consent
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Albert TJ, Pinto M, Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion. A functional and radiographic outcome study. Spine (Phila Pa 1976) 2000;25(1):123–129. doi: 10.1097/00007632-200001010-00021. [DOI] [PubMed] [Google Scholar]
- 2.Berjano P, Langella F, Damilano M, et al. Fusion rate following extreme lateral lumbar interbody fusion. Eur Spine J. 2015;24(Suppl 3):369–371. doi: 10.1007/s00586-015-3929-7. [DOI] [PubMed] [Google Scholar]
- 3.Eom JS, Jeon I, Kim SW. Application of lateral approach for the removal of migrated interbody cage: taphole and fixing technique. Korean J Spine. 2017;14(1):23–26. doi: 10.14245/kjs.2017.14.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gertzbein SD, Hollopeter MR, Hall S. Pseudarthrosis of the lumbar spine. Outcome after circumferential fusion. Spine (Phila Pa 1976) 1998;23(21):2352–2356. doi: 10.1097/00007632-199811010-00021. [DOI] [PubMed] [Google Scholar]
- 5.Kadam A, Wigner N, Saville P, Arlet V. Overpowering posterior lumbar instrumentation and fusion with hyperlordotic anterior lumbar interbody cages followed by posterior revision: a preliminary feasibility study. J Neurosurg Spine. 2017;27(6):650–660. doi: 10.3171/2017.5.SPINE16926. [DOI] [PubMed] [Google Scholar]
- 6.Lauerman WC, Bradford DS, Ogilvie JW, Transfeldt EE. Results of lumbar pseudarthrosis repair. J Spinal Disord. 1992;5(2):149–157. doi: 10.1097/00002517-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lee S-H, Kang B-U, Jeon SH, et al. Revision surgery of the lumbar spine: anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation. J Neurosurg Spine. 2006;5(3):228–233. doi: 10.3171/spi.2006.5.3.228. [DOI] [PubMed] [Google Scholar]
- 8.Levin JM, Tanenbaum JE, Steinmetz MP, Mroz TE, Overley SC. Posterolateral fusion (PLF) versus transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis: a systematic review and meta-analysis. Spine J. 2018;18(6):1088–1098. doi: 10.1016/j.spinee.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976) 2019;44(5):369–376. doi: 10.1097/BRS.0000000000002822. [DOI] [PubMed] [Google Scholar]
- 10.Moisi M, Page J, Paulson D, Oskouian RJ. Technical note—lateral approach to the lumbar spine for the removal of interbody cages. Cureus. 2015;7(5):e268. doi: 10.7759/cureus.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owens RK, Djurasovic M, Crawford CH, Glassman SD, Dimar JR, Carreon LY. Impact of surgical approach on clinical outcomes in the treatment of lumbar pseudarthrosis. Global Spine J. 2016;6(8):786–791. doi: 10.1055/s-0036-1582390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parajón A, Alimi M, Navarro-Ramirez R, et al. Minimally invasive transforaminal lumbar interbody fusion: meta-analysis of the fusion rates. what is the optimal graft material? Neurosurgery. 2017;81(6):958–971. doi: 10.1093/neuros/nyx141. [DOI] [PubMed] [Google Scholar]
- 13.Phan K, Mobbs RJ. Oblique lumbar interbody fusion for revision of non-union following prior posterior surgery: a case report. Orthop Surg. 2015;7(4):364–367. doi: 10.1111/os.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion—systematic review and meta-analysis. Br J Neurosurg. 2015;29(5):705–711. doi: 10.3109/02688697.2015.1036838. [DOI] [PubMed] [Google Scholar]
- 15.Raizman NM, O’Brien JR, Poehling-Monaghan KL, Yu WD. Pseudarthrosis of the spine. J Am Acad Orthop Surg. 2009;17(8):494–503. doi: 10.5435/00124635-200908000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Rajaee SS, Kanim LEA, Bae HW. National trends in revision spinal fusion in the USA: patient characteristics and complications. Bone Joint J. 2014;96-B(6):807–816. doi: 10.1302/0301-620X.96B6.31149. [DOI] [PubMed] [Google Scholar]
- 17.Slosar PJ, Reynolds JB, Schofferman J, Goldthwaite N, White AH, Keaney D. Patient satisfaction after circumferential lumbar fusion. Spine (Phila Pa 1976) 2000;25(6):722–726. doi: 10.1097/00007632-200003150-00012. [DOI] [PubMed] [Google Scholar]
- 18.Vargas-Soto HA, Mehbod A, Mullaney KJ, et al. Salvage procedures for pseudarthrosis after transforaminal lumbar interbody fusion (TLIF)-anterior-only versus anterior–posterior surgery: a clinical and radiological outcome study. J Surg Orthop Adv. 2009;18(4):200–204. [PubMed] [Google Scholar]
- 19.Wagner WH, Regan JJ, Leary SP, et al. Access strategies for revision or explantation of the Charité lumbar artificial disc replacement. J Vasc Surg. 2006;44(6):1266–1272. doi: 10.1016/j.jvs.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 20.West JL, Bradford DS, Ogilvie JW. Results of spinal arthrodesis with pedicle screw-plate fixation. J Bone Joint Surg Am. 1991;73(8):1179–1184. doi: 10.2106/00004623-199173080-00006. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Steinmetz MP, Lieberman IH, Modic MT, Mroz TE. The utility of repeated postoperative radiographs after lumbar instrumented fusion for degenerative lumbar spine. Spine (Phila Pa 1976) 2011;36(23):1955–1960. doi: 10.1097/BRS.0b013e31820125b5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)