Abstract
Background
Anterior cervical fusion offers surgeons a safe and reliable surgical option for single-level and multilevel pathology; however, multilevel fusions pose a higher risk of complications than single-level fusions, including possible pseudoarthrosis, adjacent segment disease, sagittal imbalance, and construct subsidence. Various techniques can be used to mitigate risk in multilevel anterior cervical fusion.
Questions/Purposes
We reviewed the literature to determine the best surgical strategies in multilevel anterior cervical fusion.
Methods
We searched the PubMed database for articles published from January 1980 through July 2019. Two authors identified relevant articles and then manually screened them for others to include in this review.
Results
We initially identified 1936 articles and included 48 in our review. We found that clinical outcomes of multilevel anterior cervical fusion can be optimized through the use of biologics and graft selection, the evaluation of pre-existing deformity, the assessment of comorbidities, and the selection of fusion levels. Meticulous surgical technique in conjunction with modern surgical tools, such as instrumentation and biologics, allow surgeons to address complex cervical problems while limiting morbidity and enhancing clinical outcomes.
Conclusions
Multilevel anterior cervical fusions offer a relatively safe and reliable treatment option for both single-level and multilevel pathology.
Electronic supplementary material
The online version of this article (10.1007/s11420-019-09738-3) contains supplementary material, which is available to authorized users.
Keywords: current concepts, fusion, multilevel anterior cervical, anterior cervical discectomy and fusion (ACDF)
Introduction
The anterior approach to the cervical spine, most notably in anterior cervical discectomy and fusion (ACDF), is a well-established method of addressing cervical degenerative diseases, fractures, and pathology that has demonstrated exceptional success rates and long-term outcomes [7]. Studies have found fusion rates upward of 90% following single-level ACDF for cervical myelopathy [14, 51]. The high fusion rate associated with this procedure has been attributed to various factors, including the addition of interbody cages and anterior plating [18, 36]. The evolution of single-level and multilevel discectomy in conjunction with single-level and multilevel corpectomy has expanded surgeons’ ability to address cervical pathology.
Simultaneously, biologics and graft extenders have played an ever-expanding role in augmenting fusion constructs. Bone grafts and substitutes have been imperative in achieving fusions [6, 21, 27, 50]. Iliac crest bone grafting is the gold standard for autogenous bone graft options, although its association with donor site morbidity has made it unattractive. Current bone graft substitutes have been designed to promote fusion by enhancing osteogenic properties. Bone graft substitutes and fusion extenders, such as demineralized bone graft (DBM), allografts, ceramics, and ingrowth/ongrowth cages, have an expanding role in anterior cervical surgery.
Established surgical techniques are well described within the literature and have a significant impact on achieving fusion. Meticulous dissection optimizing the field of view, thorough endplate preparation, appropriate graft/cage sizing and placement, and plate application serve as the fundamental steps in optimizing fusion. Pre-operative planning for multilevel fusion should account for concomitant cervical deformity, which must be assessed and corrected by an anterior and/or posterior construct, when necessary. The development of adjacent segment disease (ASD), further amplified in multilevel fusion, can significantly affect the success of these surgeries. Higher rates of pseudoarthrosis, graft dislodgement, and instrumentation failures pose real threats to achieving multilevel fusions.
We reviewed the literature and provide the following overview of evidence-based multilevel anterior cervical fusion techniques for enhancing outcomes while minimizing risks.
Methods/Search Strategy and Criteria
We sought to identify longitudinal studies including randomized controlled trials (RCTs), observational case-control studies, and prospective and retrospective cohort studies reporting on the success of multilevel ACDF, strategies for improving fusion rates, and risk factors for pseudoarthrosis. The PubMed database was queried using a combination of free and MeSH (Medical Subject Headings) search parameters related to the surgical intervention. Two authors independently performed the PubMed search to identify studies published between January 1, 1980, and July 1, 2019, using combinations of the following search terms: “anterior cervical discectomy and fusion,” “ACDF,” “anterior cervical fusion,” “multilevel anterior cervical fusion,” “multilevel ACDF,” “spine biologics,” “adjacent segment disease,” “cervical deformity,” and “cervical spine osteoporosis.” All English language studies on human subjects were included. The cited papers in the originally identified articles were also reviewed, ensuring that all eligible articles were identified and included. A list of relevant articles was identified using these search terms by two authors (M.M., J.W.) and were manually screened for inclusion in this current review.
Studies involving patients younger than 18 years old, tumors, or infection were excluded from the review (Fig. 1).
Fig. 1.
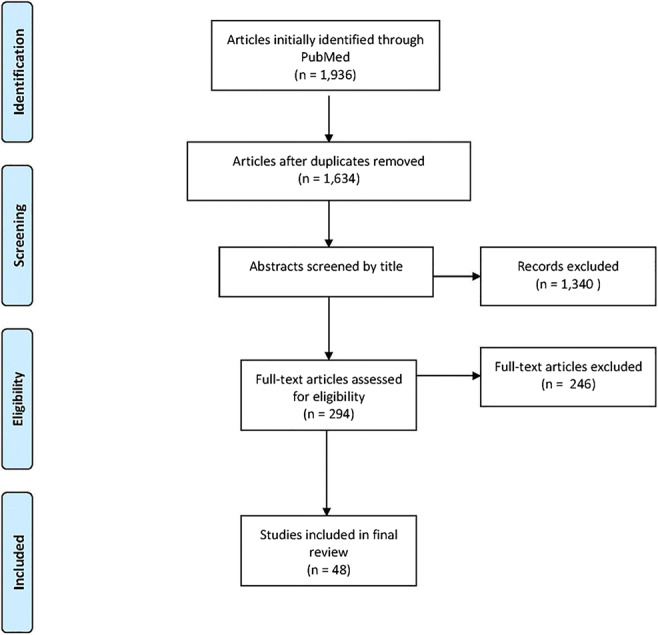
Flowchart demonstrating the literature review result.
Results
Bone grafts and novel bone graft substitutes play a key role in the successful performance of ACDF. Autogenous bone grafting serves as the gold standard for spinal fusion, with its osteoconductive, osteoinductive, and osteogenic components. The introduction of allograft as a viable alternative led to an array of studies focusing on grafting and graft extenders. The use of allograft in cervical spinal fusion has been widely reported, with many surgeons recognizing it as a mainstay treatment with dependable results. However, no one substitute encompasses the innate capabilities of autograft; thus, much of the literature has focused on bone substitute’s fusion potential against that of autogenous bone graft. It is important for spine surgeons to understand the outcomes associated with these graft options and their potential in multilevel cervical fusion.
A recent prospective randomized trial of a variety of preserved allograft implants in cervical fusion reported fusion rates greater than 95%, further confirming the favorable rates seen in other studies [20]. Another prospective study comparing autograft and allograft in ACDFs found no significant difference between fusion rates and graft collapse for both one or two level fusions, further endorsing allograft’s reliability in both single and multilevel fusions [41]. The routine application of anterior plating with allograft in single and multilevel anterior cervical fusions has further enhanced fusion rates placing allograft/plate constructs as the standard [26, 46, 47].
Demineralized bone matrix (DBM) is derived from acid extraction of allograft bone, which yields a combination of organic matrix proteins and minor amounts of growth differentiation factors [48]. DBM offers surgeons an easily accessible graft extender that demonstrates the capacity to stimulate an osteoinductive response, providing improved bone growth and fusion. DBM is osteoconductive but does not provide structural support, and its osteoinductive properties can be denuded during the sterilization process [4]. Surgeons can optimize osteogenic factors and pluripotential cells by adding bone marrow aspirate to the DBM prior to implantation into the graft or cage construct. The authors advocate mixing the graft with bone marrow aspirate, when available, or autogenous local bone graft from the surgical field.
Ceramics are one of the most widely studied groups of bone substitutes in spinal fusion. It functions as an osteoconductive material providing scaffolding for bone formation and is composed of a biodegradable composite. Ceramic implants have a porous structure resembling cancellous bone that enhances ingrowth of bone while offering scaffolding with significant mechanical strength secondary to a crystallized composite. Studies have demonstrated satisfactory outcomes and good efficacy with the use of ceramics compared to autologous bone grafts [15, 16]. Prospective and retrospective studies comparing implantable cages have demonstrated successful outcomes and high fusion rates in multilevel fusions with ceramic cages [11, 42]. One unique subset of ceramic bone substitutes is silicate substituted calcium phosphate, which is composed of both osteoconductive and osteoinductive properties. It is purported that its osteoinductive ability originates from silicate’s negative charge, which attracts osteoblasts to the ceramic implant [28]. Ceramic bone substitutes have demonstrated good clinical outcomes and fusion rates, making it a viable bone substitute in multilevel and/or revision fusion. Specifically for multilevel anterior cervical fusion, the senior author’s preferred method is a compilation of a ceramic bone graft extender in addition to autogenous bone graft (local bone or iliac crest graft) placed in and around the interbody cage. Figure 2a shows a radiograph of a patient with a prior C4–C6 ACDF, with notable C5–C6 pseudoarthrosis and C6–C7 spondylosis and radiculopathy. Figure 2b shows a post-operative radiograph of the same patient with revision C5–C6 and C6–C7 ACDF with iliac crest autograft and BioSphere® synthetic bone graft substitute (Fig. 2).
Fig. 2.
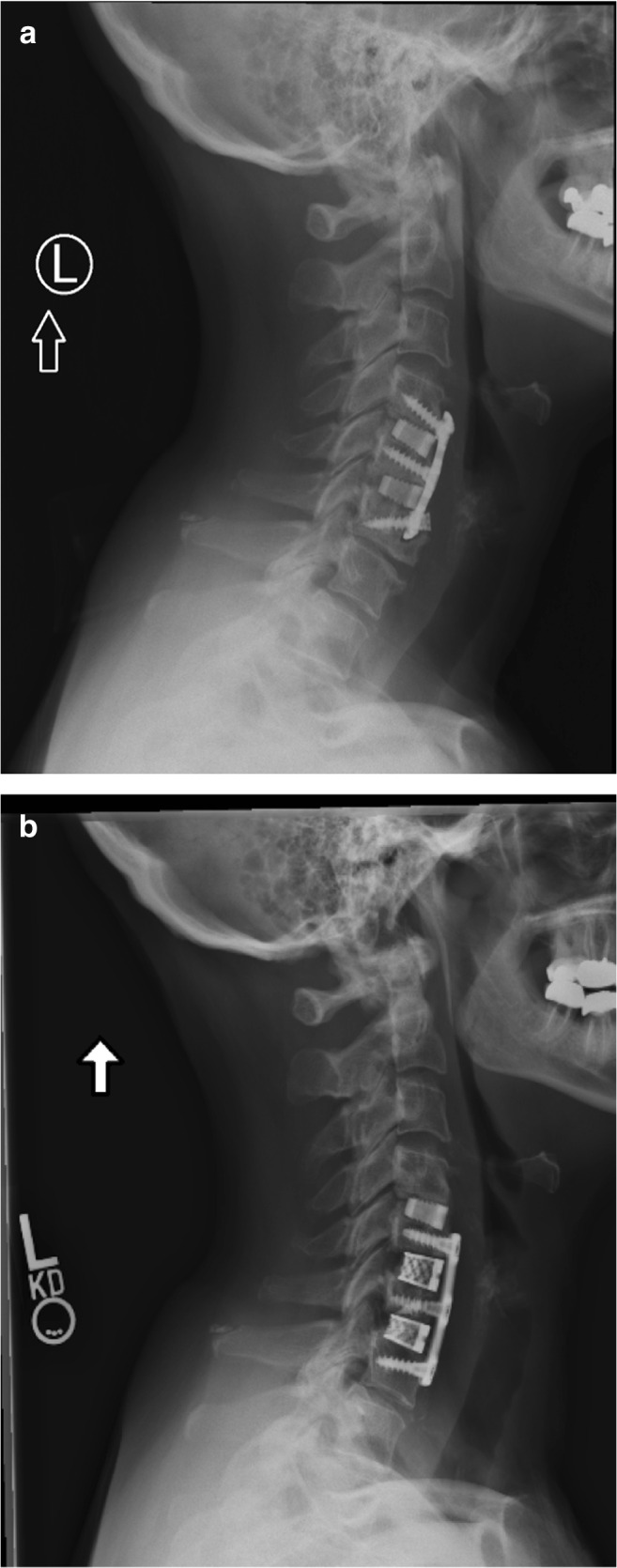
A multilevel C4–C6 revision anterior cervical discectomy and fusion (ACDF) secondary to C5–C6 pseudoarthrosis with use of a iliac crest autograft and b ceramics bone graft substitute packed in titanium interbody cages.
Bone morphogenic protein (BMP) is the most widely represented bone graft extender in the literature. BMPs are a part of the transforming growth factor beta family of proteins and have osteoinductive properties. BMP-2 is approved by the US Food and Drug Administration (FDA) to augment fusion in anterior lumbar surgery; its use in the cervical spine is an off-label application. Nonetheless, increasing trends of BMP in the cervical spine were identified in the early 2000s, with a precipitous decline of use in the anterior cervical spine that resulted from evidence of potential risks such as soft tissue swelling, dysphagia, radiculitis, seroma, heterotopic ossification, and cancer [3, 5, 8, 49]. The complication profile associated with BMP invalidates routine use of this bone graft extender in anterior cervical fusion.
Osteoporosis is a comorbidity that remains a significant risk factor for unsuccessful outcome when cervical spine fusion is contemplated. Osteoporosis affects a significant number of Americans, with the highest prevalence among the older adults and women [10]. Patients with osteoporosis have associated increases in comorbidities, and those undergoing spine surgery are at a greater risk for fusion construct failure and pseudoarthrosis [22]. Treating surgeons must identify patients at risk for osteoporosis and initiate medical optimization before surgery. A recent survey of spine surgeons found that less than 45% perform bone density tests and only 12% order metabolic bone health panels prior to spine fusion in patients with suspected osteoporosis or osteomalacia [13]. Appropriate pre-operative bone health studies in patients at high risk of osteoporosis include metabolic bone health panels (vitamin D, parathyroid hormone, thyroid-stimulating hormone, albumin, and pre-albumin levels) and dual-energy X-ray absorptiometry (DEXA) scans to evaluate bone mineral density. Quantitative computed tomography (qCT) can be used as an alternative [9]. In the setting of multilevel anterior cervical fusion, optimizing bone health can pay dividends as some of these metabolic abnormalities, such as vitamin D deficiency, can be reversed, promoting higher fusion rates [1, 33]. Pharmacotherapeutic strategies are helpful during the perioperative period in addressing both lower fusion rates and low bone density among this population. Current literature supports the use of teriparatide during the pre- and post-operative period, since it has been shown to increase fusion mass and fusion rates while decreasing the risk of bone–implant failure [17, 29]. Given the impact of vitamin D on spinal fusion and the prevalence of deficiency, we recommend pre-operative testing of serum vitamin D levels prior to both primary and revision cervical procedures. Thresholds for vitamin D levels, which are well established in the literature (Table 1) [25, 39], should be used to institute treatment. Patients deficient in vitamin D are typically prescribed 50,000 IU of oral vitamin D2 (ergocalciferol) per week for 8 weeks, followed by maintenance therapy of 1500–2000 IU/day [25]. We advocate proper screening through DEXA scans, obtaining vitamin D levels of suspected osteoporosis patients, and the use of perioperative interventions in those undergoing multilevel anterior cervical fusions.
Table 1.
Serum 25-hydroxyvitamin D [25(OH)D] concentrations and health
| nmol/L | ng/mL | Health status |
|---|---|---|
| < 30 | < 12 | Vitamin D deficiency, leading to rickets in infants and children and osteomalacia in adults |
| 30 to < 50 | 12 to < 20 | Vitamin D insufficiency |
| ≥ 50 | ≥ 20 |
Generally considered adequate for the bone and overall health in healthy individuals |
| > 125 | > 50 |
Emerging evidence links potential adverse effects to such high levels, particularly >150 nmol/L (>60 ng/mL) |
The poor bone quality posed by osteoporosis increases the risk of catastrophic failure, such as bone–implant failure and interbody cage subsidence. Decisions on fusion constructs should account for these patients’ predisposition to pseudoarthrosis, adjacent-level degeneration, progressive junctional kyphosis, and compressions fractures [12, 31]. With patients with severe osteoporosis, fixation options may be limited, compelling surgeons to choose more complex constructs, such as combined anterior-posterior fixation. In assessing the need for anterior and posterior fixation, surgeons must account for bone quality, sagittal deformity, and the integrity of fixation while calculating the risk of construct failure and future revisions.
Although a discussion of interbody cage properties is outside the scope of this review, it is important to mention technical strategies with graft sizing and selection. Graft subsidence in the setting of osteoporosis requires surgeons to consider endplate preparation, relative size of cage, and amount of Caspar distraction. Preservation of the structural integrity of endplates and graft contact with peripheral cortical bone has been shown to decrease subsidence, especially in patients with osteoporosis [37, 43, 45]. Increasing the size of the cage footprint to fit the vertebral endplate morphology has demonstrated lower rates of subsidence and is recommended [43]. Surgeons should be aware of the amount of distraction placed through Caspar pins to avoid cutout and over-distraction, which can potentiate graft subsidence. Finally, although debate continues over specific instrumentation, we feel that endplate preparation, patient selection, pre-operative medical optimization, and precise surgical technique remain the most important factors in achieving successful anterior cervical fusion.
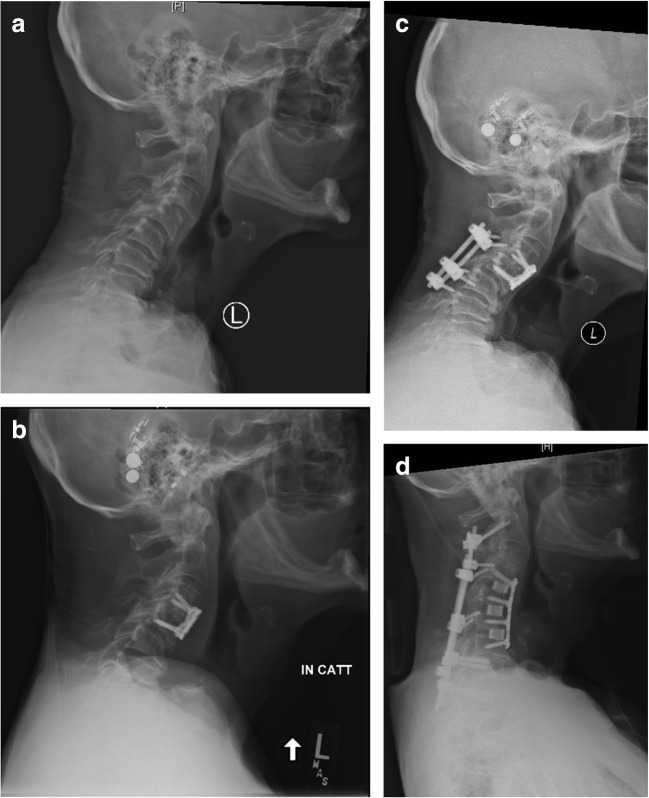
In an effort to optimize multilevel anterior cervical fusion, one would be remiss in not considering the etiology of the pathology and the overall sagittal balance of the spine. It is important to fully assess any degree of cervical deformity and determine whether the offending pathology can be treated anteriorly or if it is best addressed posteriorly. New advances in cervical interbody cages and anterior plating provide surgeons the ability to achieve and maintain some degree of cervical deformity correction. However, increasing sagittal malalignment can overwhelm the capabilities of anterior constructs and thus the role of posterior augmentation (Fig. 3). Traditional thinking regarding anterior versus posterior cervical spine procedures, first, delineates the number of compressed levels, typically three or more warrant a combined or posterior alone procedure, and second, the amount of kyphosis, which can dictate the amount of anterior column support required for correction [2, 32, 38]. Sagittal cervical Cobb angle, measured as the angle between vertical lines drawn from lines parallel to the inferior endplates of C2 and C7, and C2–C7 sagittal vertical axis (SVA), measured as the deviation of the C2 plumb line from the posterior superior endplate of C7, quantify the degree of kyphosis or lordosis and the cervical sagittal alignment, respectively. These parameters demonstrate significant impact in selection of approach, post-operative disability scores and optimal post-operative cervical sagittal alignment [2, 23, 40, 44]. Surgical decisions optimizing multilevel cervical fusion should take into account the number of levels of compression/pathology and sagittal alignment; these factors, in conjunction with patient’s comorbidities, should serve as the basis for the decision to proceed with an anterior, posterior, or combined fusion construct.
Fig. 3.
a, b, c A 76-year-old man who initially presented with myelopathy and was treated with a C3–C4 anterior cervical discectomy and fusion (ACDF) complicated by pseudoarthrosis that was addressed with a posterior spinal fusion. c Iatrogenic kyphotic deformity was addressed with a C4–C7 ACDF and d C2–T2 posterior spinal fusion.
The development of adjacent segment disease (ASD) is a matter of major concern, given the widespread use of anterior cervical spine fusion. Hilibrand et al., in a classic study assessing long-term outcomes of ACDF, demonstrated the rate of developing symptomatic ASD to be 2.9%, with a prevalence of roughly 25% within 10 years of the index fusion [24]. Also, the same study found multilevel fusion to have lower rates of ASD, which are likely to include at-risk segments, primarily C5–C7. Regardless of the fusion length, ASD is a significant clinical problem that should be addressed prior to anterior fusion and monitored post-operatively. The etiology of ASD remains somewhat controversial, with the literature supporting both notions of increased biomechanical stress placed on adjacent disk space and the continued natural history of cervical spondylosis. The etiology and prevalence of ASD is outside the scope of this review.
When considering strategies for preventing ASD, surgeons should recognize that the majority of ASD is not a result of poor surgical execution or planning. Although ASD is considered largely unavoidable, surgeons should consider pre- and intra-operative tactics to minimize risk of future ASD. First, as previously mentioned, it is important to keep in mind sagittal alignment parameters associated with cervical deformity and the intended preserved motion segments. Reducing lordosis and increasing kyphosis in the cervical spine alters biomechanics leading to abnormal stress concentrations and degeneration at adjacent segments [35]. Intra-operatively, surgeons should pay close attention to using an appropriate-size interbody and contoured plate so as to not under- or over-distract the intervertebral segment.
Second, thoughtful selection of fusion level is an important ASD-prevention strategy; studies have demonstrated that pre-existing degenerative changes and surgery performed adjacent to C5–C6 and C6–C7 are associated with ASD [24, 30]. Fusions adjacent to C5–C6 and C6–C7 should give pause for critical assessment and surgeons should have a low threshold to include adjacent degenerative levels in the planned fusion. Additionally, flexion and extension radiographs can be useful in identifying hypermobile adjacent segments that are also at greater risk for ASD.
Last, meticulous dissection and precise surgical technique are vital in preserving key structures, such as the adjacent annulus, cranial and caudal anterior longitudinal ligament (ALL), and longus colli. Seemingly trivial aberrant placement of the localizing needle or excessive dissection of the longus colli have been associated with increased risk of developing ASD, further stressing the importance of detailed surgical techniques [19, 34]. Of the strategies to minimize surgical dissection and optimize instrumentation, we recommend the use of a hemostat clamp as a localizing tool in order to avoid violating the ALL and/or annulus. Regrading plate selection and placement, the shortest plate is recommended so as to maximize distance between the cranial and caudal endplates and the end of the plate, which optimally is 5 mm or more. Furthermore, drilling should proceed in a trajectory away from the fused disc space both cranially and caudally so as to improve the working length of the construct.
Discussion
Anterior cervical fusion offers surgeons a dependable and reproducible treatment option for both single-level and multilevel cervical spine disease. Although anterior cervical fusion is one of the most common spine surgeries, it has inherent risk but appropriately addressing those risks makes it a safe and powerful surgery. Advances in fusion biologics and graft extenders have provided a host of new options that provide similar fusion rates to autogenous bone graft without the associated morbidity. Combining graft extenders to potentiate osteoconductive and osteoinductive properties in concert exploit current technologies to maximize fusion rates and improve clinical outcomes. Understanding patient comorbidities, such as osteoporosis, allow surgeons to proactively address pre-operative bone quality while decreasing the risk of intra- or post-operative instrumentation failure and/or graft subsidence. Surgeons who critically analyze pre-operative imaging offer their patients solutions to focal cervical pathologies while simultaneously employing prevention strategies to improve or maintain overall sagittal alignment. Clinically relevant adjacent segment pathology can significantly impact long term outcomes of multilevel anterior cervical fusions and surgeons should consider techniques to decrease potential ASD. In this review article, we offer a variety of strategies for surgeons looking to optimize outcomes for patients undergoing multilevel anterior cervical fusions.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
Compliance with Ethical Standards
Conflict of Interest
Michael H. McCarthy, MD, MPH, and Joseph A. Weiner, MD, declare that they have no conflicts of interest. Alpesh A. Patel, MD, FACS, reports royalties from Amedica, Nuvasive, and Zimmer Biomet; stock ownership from Amedica, Nocimed, Vital 5, and Cytonics; personal fees as a consultant from Amedica, Relievant, Pacira, Nuvasive, and Zimmer Biomet; and board of director membership from Cervical Spine Research Society, outside the submitted work.
Human/Animal Rights
N/A
Informed Consent
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Andreassen Troels T, Fledelius Christian, Ejersted Charlotte, Oxlund Hans. Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthopaedica Scandinavica. 2001;72(3):304–307. doi: 10.1080/00016470152846673. [DOI] [PubMed] [Google Scholar]
- 2.Bakhsheshian Joshua, Mehta Vivek A., Liu John C. Current Diagnosis and Management of Cervical Spondylotic Myelopathy. Global Spine Journal. 2017;7(6):572–586. doi: 10.1177/2192568217699208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benglis David, Wang Michael Y., Levi Allan D. A Comprehensive Review of the Safety Profile of Bone Morphogenetic Protein in Spine Surgery. Operative Neurosurgery. 2008;62(suppl_5):ONS423–ONS431. doi: 10.1227/01.neu.0000326030.24220.d8. [DOI] [PubMed] [Google Scholar]
- 4.Bostrom Mathias P. G., Seigerman Daniel A. The Clinical Use of Allografts, Demineralized Bone Matrices, Synthetic Bone Graft Substitutes and Osteoinductive Growth Factors: A Survey Study. HSS Journal. 2005;1(1):9–18. doi: 10.1007/s11420-005-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BURKUS J. KENNETH, SANDHU HARVINDER S., GORNET MATTHEW F., LONGLEY MICHAEL C. USE OF RHBMP-2 IN COMBINATION WITH STRUCTURAL CORTICAL ALLOGRAFTS. The Journal of Bone and Joint Surgery-American Volume. 2005;87(6):1205–1212. doi: 10.2106/JBJS.D.02532. [DOI] [PubMed] [Google Scholar]
- 6.Buser Zorica, Brodke Darrel S., Youssef Jim A., Meisel Hans-Joerg, Myhre Sue Lynn, Hashimoto Robin, Park Jong-Beom, Tim Yoon S., Wang Jeffrey C. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. Journal of Neurosurgery: Spine. 2016;25(4):509–516. doi: 10.3171/2016.1.SPINE151005. [DOI] [PubMed] [Google Scholar]
- 7.Buttermann Glenn R. Anterior Cervical Discectomy and Fusion Outcomes over 10 Years. SPINE. 2018;43(3):207–214. doi: 10.1097/BRS.0000000000002273. [DOI] [PubMed] [Google Scholar]
- 8.Cahill Kevin S. Prevalence, Complications, and Hospital Charges Associated With Use of Bone-Morphogenetic Proteins in Spinal Fusion Procedures. JAMA. 2009;302(1):58. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 9.Carlson BC, Robinson WA, Wanderman NR, et al. A review and clinical perspective of the impact of osteoporosis on the spine. Geriatr Orthop Surg Rehabil. 2019;10:2151459319861591. doi: 10.1177/2151459319861591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng H., Gary L. C., Curtis J. R., Saag K. G., Kilgore M. L., Morrisey M. A., Matthews R., Smith W., Yun H., Delzell E. Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporosis International. 2009;20(9):1507–1515. doi: 10.1007/s00198-009-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai Li-Yang, Jiang Lei-Sheng. Anterior cervical fusion with interbody cage containing β-tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2-year follow-up. European Spine Journal. 2008;17(5):698–705. doi: 10.1007/s00586-008-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeWald Christopher J., Stanley Thomas. Instrumentation-Related Complications of Multilevel Fusions for Adult Spinal Deformity Patients Over Age 65. Spine. 2006;31(Suppl):S144–S151. doi: 10.1097/01.brs.0000236893.65878.39. [DOI] [PubMed] [Google Scholar]
- 13.Dipaola Christian P., Bible Jesse E., Biswas Debdut, Dipaola Matthew, Grauer Jonathan N., Rechtine Glenn R. Survey of spine surgeons on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. The Spine Journal. 2009;9(7):537–544. doi: 10.1016/j.spinee.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Emery Sanford E., Bolesta Michael J., Banks Michael A., Jones Paul K. Robinson Anterior Cervical Fusion. Spine. 1994;19(Supplement):660–663. [PubMed] [Google Scholar]
- 15.Epstein NE. Efficacy of posterior cervical fusions utilizing an artificial bone graft expander, beta tricalcium phosphate. Surg Neurol Int. 2011;2:15. doi: 10.4103/2152-7806.76458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein NancyE. Preliminary documentation of the comparable efficacy of vitoss versus NanOss bioactive as bone graft expanders for posterior cervical fusion. Surgical Neurology International. 2015;6(5):164. doi: 10.4103/2152-7806.156559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer CR, et al. A systematic review of treatment strategies for degenerative lumbar spine fusion surgery in patients with osteoporosis. Geriatr Orthop Surg Rehabil. 2016;7(4):188–196. doi: 10.1177/2151458516669204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser Justin F., Härtl Roger. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. Journal of Neurosurgery: Spine. 2007;6(4):298–303. doi: 10.3171/spi.2007.6.4.2. [DOI] [PubMed] [Google Scholar]
- 19.Goffin Jan, van Loon Johan, Calenbergh Frank Van, Plets Chris. Long-Term Results After Anterior Cervical Fusion and Osteosynthetic Stabilization for Fractures and/or Dislocations of the Cervical Spine. Journal of Spinal Disorders & Techniques. 1995;8(6):500–508. [PubMed] [Google Scholar]
- 20.Graham R. Scott, Samsell Brian J., Proffer Allison, Moore Mark A., Vega Rafael A., Stary Joel M., Mathern Bruce. Evaluation of glycerol-preserved bone allografts in cervical spine fusion: a prospective, randomized controlled trial. Journal of Neurosurgery: Spine. 2015;22(1):1–10. doi: 10.3171/2014.9.SPINE131005. [DOI] [PubMed] [Google Scholar]
- 21.Greene Allison C., Hsu Wellington K. Orthobiologics in minimally invasive lumbar fusion. Journal of Spine Surgery. 2019;5(S1):S11–S18. doi: 10.21037/jss.2019.04.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzman Javier Z., Feldman Zachary M., McAnany Steven, Hecht Andrew C., Qureshi Sheeraz A., Cho Samuel K. Osteoporosis in Cervical Spine Surgery. SPINE. 2016;41(8):662–668. doi: 10.1097/BRS.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 23.Hardacker JW, et al. Radiographic standing cervical segmental alignment in adult volunteers without neck symptoms. Spine (Phila Pa 1976). 1997;22(13):1472–1480. [DOI] [PubMed]
- 24.HILIBRAND ALAN S., CARLSON GREGORY D., PALUMBO MARK A., JONES PAUL K., BOHLMAN HENRY H. Radiculopathy and Myelopathy at Segments Adjacent to the Site of a Previous Anterior Cervical Arthrodesis*. The Journal of Bone & Joint Surgery. 1999;81(4):519–28. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Holick Michael F. Vitamin D Deficiency. New England Journal of Medicine. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser Michael G., Haid Regis W., Subach Brian R., Barnes Bryan, Rodts Gerald E. Anterior Cervical Plating Enhances Arthrodesis after Discectomy and Fusion with Cortical Allograft. Neurosurgery. 2002;50(2):229–238. doi: 10.1097/00006123-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser Michael G., Groff Michael W., Watters William C., Ghogawala Zoher, Mummaneni Praveen V., Dailey Andrew T., Choudhri Tanvir F., Eck Jason C., Sharan Alok, Wang Jeffrey C., Dhall Sanjay S., Resnick Daniel K. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: Bone graft extenders and substitutes as an adjunct for lumbar fusion. Journal of Neurosurgery: Spine. 2014;21(1):106–132. doi: 10.3171/2014.4.SPINE14325. [DOI] [PubMed] [Google Scholar]
- 28.Khan AF, et al. Bioactive behavior of silicon substituted calcium phosphate based bioceramics for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2014;35:245–252. doi: 10.1016/j.msec.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Kleerekoper Michael, Greenspan Susan L, Lewiecki E. Michael, Miller Paul D, Kendler David L, Maricic Michael, Keaveny Tony M, Kopperdahl David L, Ruff Valerie A, Wan Xiaohai, Janos Boris, Krohn Kelly. Assessing the Effects of Teriparatide Treatment on Bone Mineral Density, Bone Microarchitecture, and Bone Strength. The Journal of Bone and Joint Surgery-American Volume. 2014;96(11):e90-1-9. doi: 10.2106/JBJS.L.01757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komura Shingo, Miyamoto Kei, Hosoe Hideo, Iinuma Nobuki, Shimizu Katsuji. Lower Incidence of Adjacent Segment Degeneration After Anterior Cervical Fusion Found With Those Fusing C5-6 and C6-7 Than Those Leaving C5-6 or C6-7 as an Adjacent Level. Journal of Spinal Disorders & Techniques. 2012;25(1):23–29. doi: 10.1097/BSD.0b013e31820bb1f8. [DOI] [PubMed] [Google Scholar]
- 31.Lehman Ronald A., Kang Daniel Gene, Wagner Scott Cameron. Management of Osteoporosis in Spine Surgery. Journal of the American Academy of Orthopaedic Surgeons. 2015;23(4):253–263. doi: 10.5435/JAAOS-D-14-00042. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, et al. Anterior corpectomy versus posterior laminoplasty for multilevel cervical myelopathy: a systematic review and meta-analysis. Eur Spine J. 2014;23(2):362–372. doi: 10.1007/s00586-013-3043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima A, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34) J Bone Miner Res. 2002;7(11):2038–2047. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 34.Nassr Ahmad, Lee Joon Y., Bashir Rubin S., Rihn Jeffrey A., Eck Jason C., Kang James D., Lim Moe R. Does Incorrect Level Needle Localization During Anterior Cervical Discectomy and Fusion Lead to Accelerated Disc Degeneration? Spine. 2009;34(2):189–192. doi: 10.1097/BRS.0b013e3181913872. [DOI] [PubMed] [Google Scholar]
- 35.Oda Itaru, Cunningham Bryan W., Buckley Rudolph A., Goebel Michael J., Haggerty Charles J., Orbegoso Carlos M., McAfee Paul C. Does Spinal Kyphotic Deformity Influence the Biomechanical Characteristics of the Adjacent Motion Segments? Spine. 1999;24(20):2139. doi: 10.1097/00007632-199910150-00014. [DOI] [PubMed] [Google Scholar]
- 36.Park Yung, Maeda Takeshi, Cho Woojin, Riew K. Daniel. Comparison of anterior cervical fusion after two-level discectomy or single-level corpectomy: sagittal alignment, cervical lordosis, graft collapse, and adjacent-level ossification. The Spine Journal. 2010;10(3):193–199. doi: 10.1016/j.spinee.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Polikeit Anne, Ferguson Stephen J., Nolte Lutz P., Orr Tracy E. The importance of the endplate for interbody cages in the lumbar spine. European Spine Journal. 2003;12(6):556–561. doi: 10.1007/s00586-003-0556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao Raj D., Gourab Krishnaj, David Kenny S. Operative Treatment of Cervical Spondylotic Myelopathy. The Journal of Bone & Joint Surgery. 2006;88(7):1619–1640. doi: 10.2106/JBJS.F.00014. [DOI] [PubMed] [Google Scholar]
- 39.Rosen Clifford J. Vitamin D Insufficiency. New England Journal of Medicine. 2011;364(3):248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 40.Shamji MF, et al. The association of cervical spine alignment with neurologic recovery in a prospective cohort of patients with surgical myelopathy: analysis of a series of 124 cases. World Neurosurg. 2016;86:112–119. doi: 10.1016/j.wneu.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 41.Suchomel Petr, Barsa Pavel, Buchvald Pavel, Svobodnik Adam, Vanickova Eva. Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: a prospective study with respect to bone union pattern. European Spine Journal. 2004;13(6):510–515. doi: 10.1007/s00586-003-0667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugawara Taku, Itoh Yasunobu, Hirano Yoshitaka, Higashiyama Naoki, Mizoi Kazuo. β-Tricalcium Phosphate Promotes Bony Fusion After Anterior Cervical Discectomy and Fusion Using Titanium Cages. Spine. 2011;36(23):E1509–E1514. doi: 10.1097/BRS.0b013e31820e60d9. [DOI] [PubMed] [Google Scholar]
- 43.Suh Paul B., Puttlitz Christian, Lewis Chad, Bal B. Sonny, McGilvray Kirk. The Effect of Cervical Interbody Cage Morphology, Material Composition, and Substrate Density on Cage Subsidence. Journal of the American Academy of Orthopaedic Surgeons. 2017;25(2):160–168. doi: 10.5435/JAAOS-D-16-00390. [DOI] [PubMed] [Google Scholar]
- 44.Tang JA, et al. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery. 2012;71(3):662–669. [DOI] [PubMed]
- 45.Truumees Eeric, Demetropoulos Constantine K., Yang King H., Herkowitz Harry N. Failure of Human Cervical Endplates: A Cadaveric Experimental Model. Spine. 2003;28(19):2204–2208. doi: 10.1097/01.BRS.0000084881.11695.50. [DOI] [PubMed] [Google Scholar]
- 46.Wang Jeffrey C., McDonough Paul W., Endow Kevin K., Delamarter Rick B. Increased Fusion Rates With Cervical Plating for Two-Level Anterior Cervical Discectomy and Fusion. Spine. 2000;25(1):41. doi: 10.1097/00007632-200001010-00009. [DOI] [PubMed] [Google Scholar]
- 47.Wang Jeffrey C., McDonough Paul W., Kanim Linda E. A., Endow Kevin K., Delamarter Rick B. Increased Fusion Rates With Cervical Plating for Three-Level Anterior Cervical Discectomy and Fusion. Spine. 2001;26(6):643–646. doi: 10.1097/00007632-200103150-00015. [DOI] [PubMed] [Google Scholar]
- 48.Wang Jeffrey C., Alanay A., Mark Davies, Kanim Linda E. A., Campbell Pat A., Dawson Edgar G., Lieberman Jay R. A comparison of commercially available demineralized bone matrix for spinal fusion. European Spine Journal. 2007;16(8):1233–1240. doi: 10.1007/s00586-006-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Marjorie C., Chan Leighton, Maiman Dennis J., Kreuter William, Deyo Richard A. Complications and Mortality Associated With Cervical Spine Surgery for Degenerative Disease in the United States. Spine. 2007;32(3):342–347. doi: 10.1097/01.brs.0000254120.25411.ae. [DOI] [PubMed] [Google Scholar]
- 50.Zadegan Shayan Abdollah, Abedi Aidin, Jazayeri Seyed Behnam, Vaccaro Alexander R., Rahimi-Movaghar Vafa. Demineralized bone matrix in anterior cervical discectomy and fusion: a systematic review. European Spine Journal. 2016;26(4):958–974. doi: 10.1007/s00586-016-4858-9. [DOI] [PubMed] [Google Scholar]
- 51.ZHANG ZHI-HU, YIN HUAFU, YANG KEQIN, ZHANG TANCHEN, DONG FANGCHUN, DANG GENGDING, LOU SI-QUAN, CAI QINLIN. Anterior Intervertebral Disc Excision and Bone Grafting in Cervical Spondylotic Myelopathy. Spine. 1983;8(1):16–19. doi: 10.1097/00007632-198301000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)