Abstract
Postmastectomy pain syndrome (PMPS) is a frequent complication of breast surgery, and is considered a chronic neuropathic pain in the side of surgery which persists more than 3 months. We conducted a retrospective analysis of the largest reported cohort to investigate the prevalence of PMPS and to analyze its associated risk factors as well as the influence on quality of life (QoL). Two thousand thirty-three surgically-treated female patients diagnosed between 2012 and 2017 with early-stage breast cancer were asked to complete a questionnaire survey about their current chronic neuropathic pain problems and quality of life. Multivariate logistic regression analyses were applied to determine the associated risk factors of PMPS. Results have shown that 1983 (97.5%) patients responded and completed a questionnaire survey. Among them, PMPS was found in 28.2% of patients. In univariate analysis, age≤35 years, tumor staging, history of chronic pain, total mastectomy, and axillary lymph node dissection (ALND) were significantly correlated with PMPS (P < .05). Multivariate analysis showed that age≤35 years, history of chronic pain, total mastectomy, and ALND were the independent risk factors of PMPS. QoL outcomes have shown that the global QoL score, physical function score, role function score, and social function score in the PMPS group were reduced in the PMPS group (P < .05), while the difference in emotional function score and cognitive function score showed no statistical significance (P > .05). Besides, patients with PMPS have worse body image, sexual enjoyment, and more breast symptoms. In conclusion, PMPS is linked with a high incidence among breast cancer patients, and has a considerable negative influence on the quality of life. In addition, age, total mastectomy, ALND, and history of chronic pain are the independent risk factors of PMPS.
Keywords: breast cancer, chronic pain, mastectomy, postmastectomy pain syndrome
1. Introduction
Breast cancer is the most common cancer among women worldwide.[1] The treatment of this condition depends on its staging, and surgical resection constituting an important step in an attempt to cure the disease.[2] A variety of complications may occur after surgical treatment for breast cancer, one such complication is chronic pain, which may last months to years after surgery,[3] and may affect around 20% to 50% of women submitted to mastectomy.[4]
Persistent pain following mastectomy was reported as early as 1978 and this phenomenon has been named postmastectomy pain syndrome (PMPS),[5] which is defined as the chronic pain occurring in the chest, armpit, upper arm, and shoulder after mastectomy for over 3 months.[4] The International Association for the Study of Pain (IASP) defines PMPS as persistent pain soon after mastectomy/lumpectomy affecting the anterior thorax, axilla, and/or medial upper arm.[6] It usually describes as feeling of burning, stabbing, and pulling around the treatment side.[7] Despite its name, the term PMPS may also be used when discussing patients who underwent breast-conserving surgery.[8] According to epidemiologic investigation, the morbidity of PMPS may reach 20% to 68%.[9,10]
In women who survived after mastectomy, chronic pain can be extremely burdensome, reducing the quality of life and overall functional ability.[11,12] Like other neuropathic pain conditions, the treatment for PMPS is a difficult task.[13] The amount of research on the treatment of PMPS is very limited and no consensus of the treatment of PMPS has yet been made. The etiology and mechanism of PMPS remain incompletely clear yet. It is regarded as a neuropathic pain condition that might be generated due to the damage to nervous system in the axilla or the chest wall, because of surgical treatment of breast cancer.[4,13] Some risk factors are believed to be associated with PMPS, including the presence and intensity of postoperative pain,[14] the type of surgery,[15] younger women,[15,16,17,18,19] body mass index,[20] education level,[21] marital status,[3] individual susceptibility, and prior history of other types of pain.[22] Also, it has been demonstrated that pre or postoperative adjuvant therapies like chemotherapy and radiotherapy can develop acute and chronic pain after breast cancer surgery.[16,23]
In China, the incidence of breast cancer is increasing year by year; however, research on PMPS is lagging and neglected. With the increasing development of medical technology, the overall survival time of breast cancer patients after treatment in China is markedly extended, and the requirements on quality of life have been enhanced accordingly. Therefore, PMPS has been gradually brought to the forefront. Consequently, we present the largest single-institute study on PMPS. The purpose of this study was to determine the prevalence of PMPS and the factors associated with its occurrence in women submitted to surgical treatment for breast cancer, and to determine the effect of PMPS on quality of life.
2. Methods
2.1. Patient selection
Two thousand thirty-three women with breast cancer who consulted and underwent surgical treatment in our hospital from February 2012 to November 2017 were selected. The age of the enrolled patients ranged from 23 to 82 years, with the average age of (49.3 ± 8.2) years. Inclusion criteria: all patients were confirmed with breast cancer through pathology and had undergone surgical resection, with tumor stage, nodal stage, metastasis stage Tis-T3N0-3M0, at American Society of Anesthesiologists grade I to II, and with no severe medical disease. Meanwhile, all patients had no cognitive disorder, were informed of the disease diagnosis, and agreed with the investigation. Exclusion criteria: patients combined with other malignant tumors, bilateral breast cancer, breast cancer recurrence, and distal organ metastasis; those undergoing breast implants; those combined with other diseases inducing pain (such as chronic infection); and those previously taking analgesics for a long time. The study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital, China.
2.2. Definition of PMPS
In this study, PMPS should be judged conforming to the following criteria based on the chronic pain criteria of the IASP: pain duration exceeding the normal tissue healing time after breast surgery (3 months); and pain site locating at the ipsilateral armpit, arm, shoulder, or chest wall.
2.3. Medical record collection
The general patient conditions, such as age, height, weight, and body mass index (BMI), were recorded, together with tumor site, surgical method, chemotherapy, radiotherapy, and endocrine therapy. Meanwhile, the patients were followed up through telephone and asked to complete a questionnaire survey, the site, nature, inducing or aggravating factors, interval, and attacking characteristics of postoperative chronic pain were recorded. All patients were followed up until March 2019.
2.4. Assessment of QoL
The patients were invited to finish a questionnaire, consisting of the questionnaires of the EORTC QLQ-C30 (the European Organization on the Research and Treatment of Cancer Quality of Life Questionnaire Version 3.0)[24] and QLQ-BR23 (the EORTC Breast Cancer Module).[25] All the scales were converted linearly to a scale of 0 to 100 according to the standard scoring procedures of EORTC. Higher scores stand for better health status and functioning for the scales that evaluate the global health and functioning. The outcomes of mean QoL were compared between different regimens of treatment and were compared with the reference values of EORTC.
2.5. Statistical method
Statistical analysis was carried out using SPSS20.0 software (SPSS Inc, Chicago, IL), enumeration data were compared using χ2 test, and measurement data were compared by t test. The relationship between PMPS and the related variables was analyzed through univariate analysis, and variables displaying difference in univariate analysis were performed logistic multivariate regression analysis. A difference of P < .05 was deemed as statistically significant.
3. Results
3.1. Characteristics of the study population
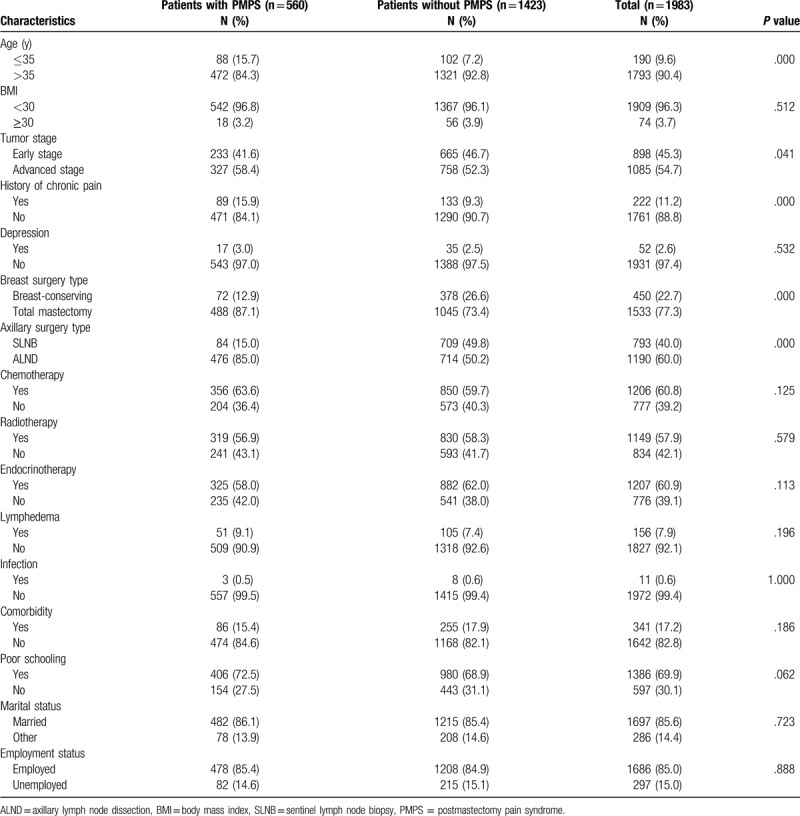
A total of 2033 women with breast cancer who underwent mastectomy procedures were enrolled in this study and sent questionnaires. Among them, 1983 cases had completed the questionnaires. So, the following analysis was based on the 1983 cases. The flowchart of study selection process was shown in Fig. 1. The age of subjects ranged from 23 to 82 years, with the average age of (49.3 ± 8.2) years, and 190 (9.6%) patients were at young age (≤35 years). The obese (BMI≥30) patients were 74 (3.7%), and the nonobese (BMI<25) patients were 1909 (96.3%). Eight hundred ninety-eight (45.3%) patients were at early stage, while 1085 (54.7%) were at advanced stage. Fifty-two (2.6%) patients had depression and 222 (11.2%) patients had a history of chronic pain. One thousand five hundred thirty-three (77.3%) patients received total mastectomy and 450 (22.7%) received breast-conserving surgery. For axillary surgery, 793 (40.0%) received sentinel lymph node biopsy, while 1190 (60.0%) received axillary lymph node dissection (ALND). One thousand two hundred six (60.8%) patients received chemotherapy, 1149 (57.9%) received radiotherapy, and 1207 (60.9%) received endocrinotherapy. One hundred fifty-six (7.9%) patients had lymphedema, 86 (15.4%) patients accompanied with other diseases. The characteristics of the study population were given in Table 1.
Figure 1.
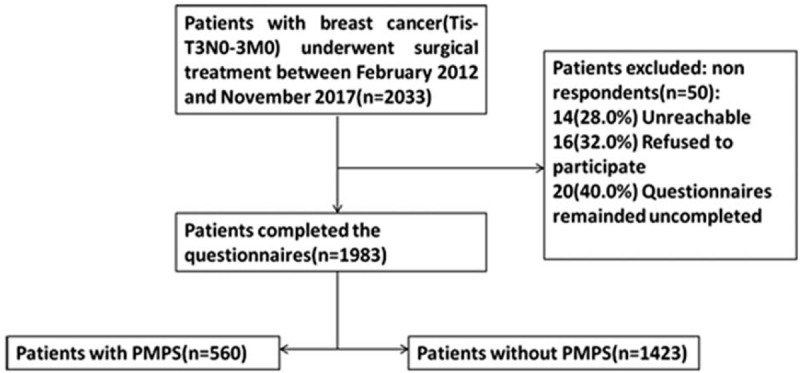
Flowchart of study selection process. T = tumor stage, N = nodal stage, M = metastasis.
Table 1.
Characteristics of the study population.
3.2. Epidemiologic features of PMPS
Of the 1983 patients followed up successfully, 560 (28.2%) cases had developed PMPS. Pain frequently occurred in the ipsilateral armpit (n = 356, 63.6%), followed by ipsilateral chest wall (n = 243, 43.4%), ipsilateral arm (n = 158, 28.2%), and other sites (n = 23, 4.1%). The pain nature was mostly numbness (n = 433, 77.3%), followed by stabbing pain (n = 285, 50.9%), swelling pain (n = 180, 32.1%), and electric overstress pain (n = 126, 22.5%). It was discovered during follows-up on patients that some patients had developed other pain symptoms, such as throbbing pain (n = 50, 8.9%), weakness (n = 32, 5.7%), and phantom pain (n = 18, 3.2%). In addition, 28 cases (5.0%) had severe and above (Numeric rating scale score of ≥ 4) pain in terms of pain intensity. Twenty-three patients had taken analgesics by themselves, and 5 had received physiotherapy. The initial occurrence of pain varied from 2 weeks to 3 months after surgery, which was usually intermittent attack.
3.3. Risk factors for PMPS
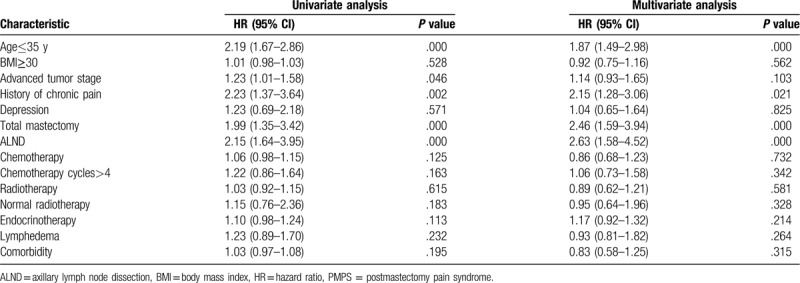
Of the various variables included in the univariate analysis, the following were associated with PMPS: age≤35 years, advanced tumor stage, history of chronic pain, total mastectomy, and ALND and normal radiotherapy method. No association was found between PMPS and BMI, advanced tumor stage, depression, chemotherapy, chemotherapy cycles, radiotherapy, radiotherapy method, endocrinotherapy, lymphedema, and comorbidity. Next the variables were performed by logistic multivariate regression analysis, and the results suggested that age≤35 years, history of chronic pain, total mastectomy, and ALND were the independent risk factors of the incidence of PMPS. The results were shown in Table 2.
Table 2.
Univariate and multivariate analysis of risk factors for PMPS.
3.4. QoL outcomes
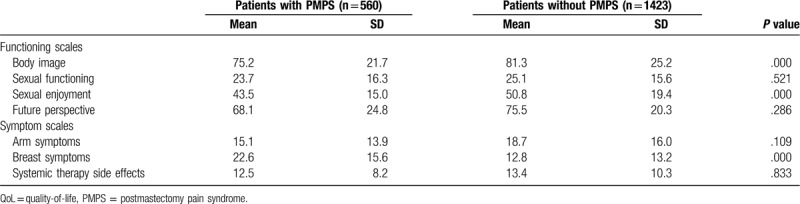
Based on the questionnaires of EORTC QLQ-C30, the global QoL score, physical function score, role function score, and social function score in the PMPS group were reduced, compared with the non-PMPS group (P < .05), while the difference in emotional function score and cognitive function score showed no statistical significance (P > .05), as shown in Table 3. Based on the questionnaires of EORTC QLQ BR23, the PMPS group reported significantly worse body image, sexual enjoyment, and more breast symptoms, which were statistically different (P < .001). There was no significant difference for other QoL domains, as shown in Table 4.
Table 3.
QoL of patients according to the QLQ-C30 questionnaire.
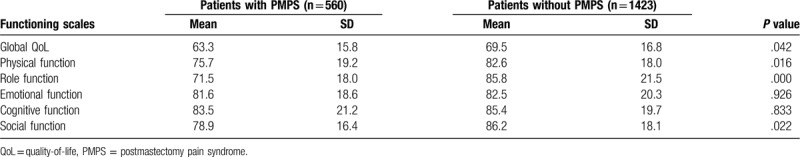
Table 4.
QoL of patients according to the QLQ-BR23 questionnaire.
4. Discussion
Postmastectomy pain syndrome is a major clinical challenge, affecting an increasing number of women worldwide.[4] The postmastectomy chronic pain belongs to the subjective feeling, which together with the lack of uniform standard of pain and different follow-up time, has resulted in great fluctuation of its incidence. In the present study, we present the largest single-institute study on PMPS, consisting of 2033 patients followed for up to 7 years. All patients were followed up for over 1 year after surgery, among them, 1983 cases had actually completed the follows-up, and 67 were lost to follow-up. According to the definition, PMPS was found in 28.2% of the patients. In all patients suffering from pain, the pain site was dominated by ipsilateral chest wall, followed by ipsilateral armpit, ipsilateral upper arm, and other sites. Additionally, the nature of pain was mainly numbness, which had accounted for 77.3%. All patients reported that the pain was intermittent, with the attack time ranging from few days to few weeks.
The etiology of PMPS is complex and poorly understood, but it is mainly suggested that intraoperative nerve injury is the main cause of the symptom,[8] associated risk factors include the presence and intensity of postoperative pain,[14] the type of surgery,[15] young age,[15,16,17,18,19] body mass index,[20] education level,[21] marital status,[3] adjuvant chemotherapy and radiotherapy, and psychological factors such as preoperative anxiety.[16] Similar to published studies in the Western world, we observed that age, NSR score, ALND, and total mastectomy were the risk factors of PMPS. With respect to age, most scientific evidence suggests that the younger the woman, the more likely she is to develop PMPS. Cairns et al reported that the incidence of PMPS was 65% among the age group of 30 to 49 years, 40% among the age group of 50 to 59 years, and 26% among the age group of 70 and above.[26] In addition, Couceiro et al[9] suggested in a cross-sectional cohort study involving 250 women that, the morbidity of PMPS among the female samples aged 18 to 75 years was about 44.4%, and the age of < 50 years was related to the incidence of PMPS. Our results were consistent with above reports, a younger age was associated with higher incidence of PMPS, the average age of PMPS patients was (47.0 ± 11.5) years, while that of non-PMPS patients was (53.4 ± 12.0) years, and the difference was statistically significant (P < .05). Age is closely correlated with the incidence of PMPS, which may be attributed to the following causes[27]: young patients are more sensitive to nerve injury and have a wider range of ALND, the morbidity of breast cancer in premenstrual women is higher, and young patients are more susceptible to anxiety. Therefore, they have reduced thresholds to a variety of adverse feelings. However, a case-control study suggested younger age might not be associated with increased risk of PMPS development.[28] The different result which was observed in that study might be due to different assessment of pain and its consequences, classification of the type of pain, different types of surgery and treatment modalities, and the type of study.
The pathogenesis of PMPS is largely related to intraoperative nerve injury. Not surprisingly, the type of surgery was also a risk factor for PMPS base on our result. Recent findings have identified that the morbidity of PMPS after breast-conserving surgery is lower than that after modified radical mastectomy.[21] In addition, the morbidity after sentinel lymph node dissection (SLND) is much higher than that of ALND.[29] In the present study, in agreement with data reported by above investigators, the incidence of PMPS in patients undergoing breast-conserving surgery was obviously lower than that in those receiving total mastectomy, and that in patients undergoing ALND was dramatically higher than that in those without ALND. Analysis showed that total mastectomy and ALND were the independent risk factors of the incidence of breast cancer PMPS, this may be related to the operation region and injury to chest wall muscle and skin of total mastectomy are greater than those of breast-conserving surgery. ALND may induce numerous complications, including nerve injury, and nerve injury will result in paresthesia in arm and armpit, thus leading to long-term paresthesia and pain. It is proposed that intercostal nerve injury is the most common during breast surgery, particularly those involving ALND. However, nerves governing breast and the deep nerves may also be damaged, including the medial pectoral nerve, lateral pectoral nerve, thoracodorsal nerve, and long thoracic nerve.[30] Besides, injury derived from traction or scar during the surgery may also lead to PMPS. In this study, the long thoracic nerve and thoracodorsal nerve in all patients undergoing ALND were protected. Nonetheless, the intercostal brachial nerve may also be injured during ALND, at the same time, other tiny sensory nerves in the armpit may also be damaged. In this way, SLND can markedly reduce the injury to intercostal branchial nerve, as well as the sensory nerves in chest wall and armpit. The prosthesis in patients receiving breast prosthesis implantation may be an unknown influence factor that cannot be acutely judged. Therefore, patients receiving breast prosthesis implantation were excluded in this study. Currently, most studies suggested that, completely preserving the intercostal bronchial nerve during ALND can guarantee the thoroughness of surgery without increasing the risk of local tumor recurrence. As a result, the intercostal bronchial nerve should be completely preserved as far as possible during surgery, which may contribute to lessening the incidence of breast cancer PMPS.
Furthermore, we also found that the patients with a prior history of chronic pain had a higher risk of developing PMPS. It is believed that this could be explained by central sensitization. Frequent chronic pain can produce a state of central hypersensitivity that initially is merely functional but which may consolidate by perpetuating the pain process due to neuronal plasticity. Consistent with our results, Cui et al[10] reported that history of chronic pain was an independent risk factor related to the PMPS occurrence, and Couceiro et al[9] reported that prior history of headache was a risk factor for PMPS.
PMPS may affect the patient's quality of life. This study uses the simple healthy life quality scale to assess the quality of life, higher scores stand for better health status and functioning. Our results showed that the global QoL score, physical function score, role function score, and social function score were reduced in PMPS group. Patients with PMPS also had worse body image, sexual enjoyment, and breast symptoms. The results suggested that PMPS has a considerable negative influence on the quality of life of the affected women, in agreement with data reported by other investigators.[31] Therefore, PMPS needs our attention to carry out effective intervention and prevention, which is of positive significance to improve the postoperative quality of life of breast cancer patients. However, the amount of research on the treatment of PMPS is very limited and no consensus of the treatment of PMPS has yet been made. Therefore, we should pay attention to the prevention of PMPS. According to the results of the analysis on risk factor for PMPS in the present study, preservation of the intercostal nerve during surgery could decrease chronic pain, the use of sentinel lymph node biopsy could reduce the need for axillary dissection and thereby reduce intercostal nerve damage. Besides, it is of high clinical relevance to find a safe, reliable, and tolerated treatment with a substantial effect on PMPS.
Our study has a few limitations. It was a retrospective study, although the sample size was not small, but they were from a single center. It is also important to point out that other important variables including socioeconomic conditions and religiousness were not investigated. Further high-quality, prospective, multicenter studies are needed to investigate the prevalence of PMPS and its related risk factors as well as the influence on quality of life.
In conclusions, PMPS is linked with a high incidence among postmastectomy patients, and has a considerable negative influence on the quality of life. In addition, age, total mastectomy, ALND, and history of chronic pain are the independent risk factors of PMPS.
Author contributions
Data curation: Youwei Gong.
Funding acquisition: Changyuan Wei.
Investigation: Youwei Gong, Qixing Tan.
Methodology: Qinghong Qin, Changyuan Wei.
Project administration: Changyuan Wei.
Software: Qinghong Qin.
Writing – original draft: Qixing Tan.
Writing – review & editing: Qinghong Qin.
Footnotes
Abbreviations: ALND= axillary lymph node dissection, BMI = body mass index, CI = confidence interval, HR= hazard ratio, PMPS = postmastectomy pain syndrome, QoL= quality of life.
How to cite this article: Gong Y, Tan Q, Qin Q, Wei C. Prevalence of post-mastectomy pain syndrome and associated risk factors: A large single-institution cohort study. Medicine. 2020;99:20(e19834).
YG and QT have contributed equally to this work.
This work was supported by the foundation of Guangxi Science and Technology Infrastructure Project (Grant 15-235-05), the Natural Science Foundation of China (Grant 81360396), and the Youth Science Foundation of Guangxi Medical University (GXMUYSF201628) and the Natural Science Foundation of Guangxi (2019GXNSFAA245067).
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Chatterjee A, Pyfer B, Czerniecki B, et al. Early postoperative outcomes in lumpectomy versus simple mastectomy. J Surg Res 2015;198:143–8. [DOI] [PubMed] [Google Scholar]
- [3].Gartner R, Jensen MB, Nielsen J, et al. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009;302:1985–92. [DOI] [PubMed] [Google Scholar]
- [4].Macdonald L, Bruce J, Scott NW, et al. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer 2005;92:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wood KM. Intercostobrachial nerve entrapment syndrome. South Med J 1978;71:662–3. [DOI] [PubMed] [Google Scholar]
- [6].Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1–226. [PubMed] [Google Scholar]
- [7].Smith WC, Bourne D, Squair J, et al. A retrospective cohort study of post mastectomy pain syndrome. Pain 1999;83:91–5. [DOI] [PubMed] [Google Scholar]
- [8].Couceiro TC, Menezes TC, Valenca MM. Post-mastectomy pain syndrome: the magnitude of the problem. Rev Bras Anestesiol 2009;59:358–65. [DOI] [PubMed] [Google Scholar]
- [9].Couceiro TC, Valenca MM, Raposo MC, et al. Prevalence of post-mastectomy pain syndrome and associated risk factors: a cross-sectional cohort study. Pain Manag Nurs 2014;15:731–7. [DOI] [PubMed] [Google Scholar]
- [10].Cui L, Fan P, Qiu C, et al. Single institution analysis of incidence and risk factors for post-mastectomy pain syndrome. Sci Rep 2018;8:11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caffo O, Amichetti M, Ferro A, et al. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat 2003;80:39–48. [DOI] [PubMed] [Google Scholar]
- [12].Amichetti M, Caffo O. Pain after quadrantectomy and radiotherapy for early-stage breast cancer: incidence, characteristics and influence on quality of life. Results from a retrospective study. Oncology 2003;65:23–8. [DOI] [PubMed] [Google Scholar]
- [13].Vilholm OJ, Cold S, Rasmussen L, et al. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer 2008;99:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [15].Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain 2006;7:626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bokhari F, Sawatzky JA. Chronic neuropathic pain in women after breast cancer treatment. Pain Manag Nurs 2009;10:197–205. [DOI] [PubMed] [Google Scholar]
- [17].Gulluoglu BM, Cingi A, Cakir T, et al. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med 2006;51:75–82. [PubMed] [Google Scholar]
- [18].Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain 2005;119:16–25. [DOI] [PubMed] [Google Scholar]
- [19].Lundstedt D, Gustafsson M, Steineck G, et al. Risk factors of developing long-lasting breast pain after breast cancer radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:71–8. [DOI] [PubMed] [Google Scholar]
- [20].Helyer LK, Varnic M, Le LW, et al. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J 2010;16:48–54. [DOI] [PubMed] [Google Scholar]
- [21].Alves Nogueira Fabro E, Bergmann A, do Amaral ESB, et al. Post-mastectomy pain syndrome: incidence and risks. Breast 2012;21:321–5. [DOI] [PubMed] [Google Scholar]
- [22].Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [23].Castel LD, Abernethy AP, Li Y, et al. Hazards for pain severity and pain interference with daily living, with exploration of brief pain inventory cutpoints, among women with metastatic breast cancer. J Pain Symptom Manage 2007;34:380–92. [DOI] [PubMed] [Google Scholar]
- [24].Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- [25].Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 1996;14:2756–68. [DOI] [PubMed] [Google Scholar]
- [26].Hovind IL, Bredal IS, Dihle A. Women's experience of acute and chronic pain following breast cancer surgery. J Clin Nurs 2013;22:1044–52. [DOI] [PubMed] [Google Scholar]
- [27].Fenlon D, Powers C, Simmonds P, et al. The JACS prospective cohort study of newly diagnosed women with breast cancer investigating joint and muscle pain, aches, and stiffness: pain and quality of life after primary surgery and before adjuvant treatment. BMC Cancer 2014;14:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shahbazi R, Akbari ME, Hashemian M, et al. High body mass index and young age are not associated with post-mastectomy pain syndrome in breast cancer survivors: a case-control study. Iran J Cancer Prev 2015;8:29–35. [PMC free article] [PubMed] [Google Scholar]
- [29].Canavese G, Bruzzi P, Catturich A, et al. Sentinel lymph node biopsy versus axillary dissection in node-negative early-stage breast cancer: 15-year follow-up update of a randomized clinical trial. Ann Surg Oncol 2016;23:2494–500. [DOI] [PubMed] [Google Scholar]
- [30].Rai AS, Khan JS, Dhaliwal J, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: a systematic review and meta-analysis of randomized controlled trials. J Plast Reconstr Aesthet Surg 2017;70:1317–28. [DOI] [PubMed] [Google Scholar]
- [31].Peuckmann V, Ekholm O, Rasmussen NK, et al. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain 2009;13:478–85. [DOI] [PubMed] [Google Scholar]


