In each living cell, potassium is required for maintaining the intracellular pH and for the activity of essential enzymes. Like most other bacteria, Bacillus subtilis possesses multiple low- and high-affinity potassium uptake systems. Their activity is regulated by the second messenger cyclic di-AMP. Moreover, the pools of the most abundant ions potassium and glutamate must be balanced. We report two conditions under which the low-affinity potassium channel KtrCD is able to mediate potassium uptake at low external potassium concentrations: physiologically, the presence of glutamate results in a severely increased potassium uptake. Moreover, this is achieved by a mutation affecting the selectivity filter of the KtrD channel. These results highlight the integration between potassium and glutamate homeostasis in bacteria.
KEYWORDS: potassium transport, glutamate, Bacillus subtilis, cyclic di-AMP, ion homeostasis, potassium transport
ABSTRACT
Potassium and glutamate are the major cation and anion, respectively, in every living cell. Due to the high concentrations of both ions, the cytoplasm of all cells can be regarded as a potassium glutamate solution. This implies that the concentrations of both ions need to be balanced. While the control of potassium uptake by glutamate is well established for eukaryotic cells, much less is known about the mechanisms that link potassium homeostasis to glutamate availability in bacteria. Here, we have discovered that the availability of glutamate strongly decreases the minimal external potassium concentration required for the highly abundant Bacillus subtilis potassium channel KtrCD to accumulate potassium. In contrast, the inducible KtrAB and KimA potassium uptake systems have high apparent affinities for potassium even in the absence of glutamate. Experiments with mutant strains revealed that the KtrD subunit responds to the presence of glutamate. For full activity, KtrD synergistically requires the presence of the regulatory subunit KtrC and of glutamate. The analysis of suppressor mutants of a strain that has KtrCD as the only potassium uptake system and that experiences severe potassium starvation identified a mutation in the ion selectivity filter of KtrD (Gly282 to Val) that similarly results in a strongly glutamate-independent increase of the apparent affinity for potassium. Thus, this work has identified two conditions that increase the apparent affinity of KtrCD for potassium, i.e., external glutamate and the acquisition of a single point mutation in KtrD.
IMPORTANCE In each living cell, potassium is required for maintaining the intracellular pH and for the activity of essential enzymes. Like most other bacteria, Bacillus subtilis possesses multiple low- and high-affinity potassium uptake systems. Their activity is regulated by the second messenger cyclic di-AMP. Moreover, the pools of the most abundant ions potassium and glutamate must be balanced. We report two conditions under which the low-affinity potassium channel KtrCD is able to mediate potassium uptake at low external potassium concentrations: physiologically, the presence of glutamate results in a severely increased potassium uptake. Moreover, this is achieved by a mutation affecting the selectivity filter of the KtrD channel. These results highlight the integration between potassium and glutamate homeostasis in bacteria.
INTRODUCTION
The cytoplasm of each living cell can be regarded as a potassium glutamate solution. With a concentration between 200 and 400 mM, potassium is the most abundant cation, whereas glutamate (100 mM) is the by far most abundant organic molecule (1–3). The cells need potassium for the activity of several crucial enzymes and protein complexes, including the ribosome, and for the adjustment of the intracellular pH by buffering the negative charge of nucleic acids (1, 4). Glutamate is used as building block for proteins and as the central amino group donor for most nitrogen-containing molecules in the cell. Since the affinity of transaminases for glutamate is rather low, a high intracellular concentration is required (2). In addition, both potassium and glutamate play a key role in osmoadaptation in many bacteria; upon exposure to osmotic stress, bacteria very rapidly accumulate potassium as a first response (5). Moreover, glutamate serves as an osmoprotectant in some archaea and bacteria, and it is the direct precursor for the rapid biosynthesis of proline if the bacteria experience an osmotic upshift (5). Enteric bacteria accumulate both potassium and glutamate to cope with osmotic stress (6).
In the Gram-positive model bacterium Bacillus subtilis, the levels of both molecules are subject to tight regulation. Potassium homeostasis is controlled at the levels of transporter expression and activity. Both mechanisms involve the essential second messenger cyclic di-AMP (c-di-AMP) (7). Glutamate levels are controlled by a tight regulation of the synthesis of the biosynthetic and degrading enzymes (8). Moreover, the synthesis of glutamine, which is the primary product of ammonium assimilation in B. subtilis, is subject to feedback inhibition (9). Interestingly, both potassium and glutamate have been implicated in the control of the intracellular levels of c-di-AMP, the major player in the control of potassium homeostasis (10, 11). Moreover, both ions play an important role in osmoprotection, one of the major functions of c-di-AMP (5, 12, 13). Therefore, a regulatory link between the intracellular concentrations of potassium and glutamate has been proposed (14). However, such a link has so far not been supported by experimental evidence.
B. subtilis encodes three potassium uptake systems, the high- and low-affinity potassium channels KtrAB and KtrCD (15), respectively, and the high-affinity potassium transporter KimA (11). KtrAB and KtrCD consist of a dimer of the transmembrane subunits, KtrB and KtrD, and a cytosolic octameric ring of KtrA and KtrC subunits, respectively (16). The cytosolic gating subunits consist of so-called RCK (regulator of conductance of K+) domains that regulate the ion influx (17–20). The expression of the ktrC and ktrD genes encoding the low-affinity potassium channel KtrCD is constitutive (21), whereas the expression of the kimA and ktrAB genes is barely detectable under standard experimental conditions and is strongly induced upon potassium limitation (11, 22). Both genes are controlled by a c-di-AMP-responsive riboswitch that allows transcription beyond the riboswitch only in the absence of c-di-AMP, i.e., under conditions of potassium limitation (11, 23). However, c-di-AMP not only interferes with the expression of the high-affinity potassium uptake systems, it also directly inhibits the activity of all three potassium uptake systems (7, 24). Thus, at high potassium concentrations, c-di-AMP accumulates and inhibits the expression and activity of potassium uptake systems. Interestingly, the potassium exporters also possess RCK domains, and these proteins bind c-di-AMP as well. Based on studies with the Staphylococcus aureus potassium exporter CpaA, is has been suggested that c-di-AMP stimulates the activity of the potassium exporters (7, 25).
For many Gram-positive bacteria, c-di-AMP is essential for growth on complex medium. However, strains lacking c-di-AMP have been reported for several bacteria, including Listeria monocytogenes, S. aureus, Streptococcus agalactiae, and B. subtilis. These mutants grow on specific minimal media, while the presence of osmoprotective compounds is generally toxic for such strains (11, 26–28). For B. subtilis strains lacking c-di-AMP, potassium is toxic, but the bacteria can handle this toxicity by the acquisition of mutations that either enhance potassium efflux or that reduce potassium uptake (7, 11).
To get a more comprehensive understanding of the suggested physiological link between potassium, glutamate, and c-di-AMP, we have studied growth of a strain lacking c-di-AMP in the presence of glutamate. Our results unravel a novel important link between potassium and glutamate: the apparent affinity of the major potassium uptake system KtrCD depends on the presence of glutamate. It has a low apparent affinity for potassium in the absence of glutamate but a high apparent affinity in its presence. Thus, glutamate and potassium homeostasis are directly coupled to each other in B. subtilis.
RESULTS
Isolation of suppressor mutants that allow growth of a strain lacking c-di-AMP in the presence of glutamate.
A B. subtilis strain lacking all three diadenylate cyclases is unable to grow at potassium concentrations of 5 mM or higher (11). It was hypothesized that potassium ions accumulated to toxic concentrations in the mutant. Interestingly, we observed that the c-di-AMP-free strain was also not viable in the presence of glutamate, irrespective of the potassium concentration. We have previously isolated suppressor mutants that can grow without c-di-AMP in the presence of increased potassium concentrations (7, 11). One of these suppressor mutants was GP2223, which carries a mutation in nhaK, encoding a cation/proton antiporter (29). We postulated that the protein variant more actively exports potassium ions, thus lowering their internal concentration. Interestingly, GP2223 was also not viable in the presence of glutamate either. To identify the growth-limiting problem, we sought to isolate suppressor mutants that tolerate the presence of glutamate. For this purpose, we plated the c-di-AMP-free strain GP2222 (11) on modified sodium Spizizen minimal (MSSM) medium containing glutamate (1% wt/vol) and a low concentration of potassium (0.1 mM KCl). After 4 days at 37°C, two colonies could be isolated. One of the strains (GP2840) was subjected to whole-genome sequencing to identify the responsible mutations. The suppressor mutant carried an insertion of 5 bp at position 393 of the ktrC gene, resulting in a frameshift and the production of a truncated version of the gating subunit KtrC of the potassium channel KtrCD. Moreover, the strain carried point mutations affecting the minor NADH dehydrogenase NdhF, the motility sigma factor SigD, and the phospholipid-biosynthetic enzyme PlsC. We tested the second suppressor mutant, GP3414, for the presence of these mutations and found the same mutation in the ktrC gene.
The repeated isolation of mutations affecting the low-affinity potassium uptake system KtrCD was unexpected, as this channel is thought to not participate in potassium uptake at the low potassium concentration used for the isolation of the suppressor mutants. However, the obvious selection for the inactivation of KtrCD in the presence of glutamate supports the idea of a relevant physiological link between glutamate and potassium homeostasis. Moreover, the acquisition of mutations affecting the low-affinity potassium channel suggests that KtrCD might be stimulated under the conditions used for the selection, i.e., in the presence of glutamate.
The presence of glutamate differentially affects the activity of B. subtilis potassium uptake systems.
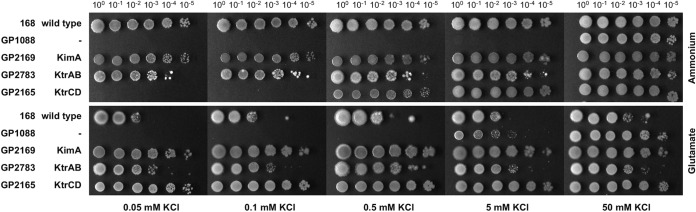
B. subtilis is able to grow at potassium concentrations as low as 0.1 mM (22). Based on the isolation of ktrC suppressor mutants in the presence of glutamate (see above), we were interested in testing the putative role of glutamate in potassium uptake. To study the contribution of the different potassium uptake systems to growth in the absence or presence of glutamate, we analyzed the growth of B. subtilis strains expressing individual potassium uptake systems (Fig. 1).
FIG 1.
Growth of B. subtilis potassium transporter mutants at different potassium concentrations in the presence of ammonium or glutamate. B. subtilis strains expressing one of the potassium uptake systems KimA, KtrAB, and KtrCD, or none at all, were cultivated in MSSM medium with 50 mM KCl and ammonium. Cells were harvested and washed, and the OD600 was adjusted to 1.0. Serial dilutions were added dropwise to MSSM minimal plates with the indicated potassium concentration and ammonium or glutamate.
In the absence of glutamate, the wild-type strain B. subtilis 168 was viable even at the lowest tested potassium concentration (0.05 mM). In contrast, strain GP1088, which lacks all known potassium uptake systems, grew only at the highest potassium concentration (50 mM). This observation supports the idea that the three characterized uptake systems are essential to accumulate potassium under physiological conditions. Moreover, B. subtilis possesses at least one additional uptake system with a very low apparent affinity for potassium.
The strains expressing only one of the high-affinity potassium uptake systems KimA and KtrAB were indistinguishable from the wild-type strain, i.e., they were able to grow even at very low potassium concentrations. Thus, both KimA and KtrAB alone are sufficient to allow growth of B. subtilis in the range of tested potassium concentrations. In contrast, strain GP2165, which expresses KtrCD as the single potassium uptake system, required at least 0.5 mM potassium for efficient growth. These observations are in excellent agreement with the previous characterization of KtrAB and KimA as high-affinity and of KtrCD as a low-affinity potassium uptake system (11).
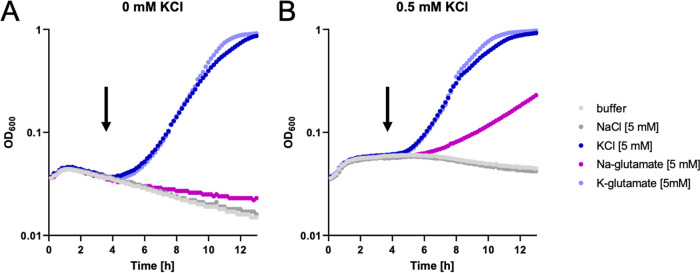
Growth of the strains on minimal media containing glutamate gave different results. Again, the wild-type strain and the mutants carrying one of the high-affinity uptake systems, KimA or KtrAB, were able to grow even at the lowest potassium concentration. However, in contrast to the results obtained for growth in the absence of glutamate, the strain expressing KtrCD as the only potassium uptake system also grew at the lowest tested potassium concentration. These observations were also confirmed by testing the growth of the strains expressing one single potassium uptake system each in liquid medium (see Fig. S1 in the supplemental material). In the experiments described above, glutamate was added as the sodium salt. To exclude the possibility that sodium was the effector of KtrCD, we cultivated B. subtilis GP2165, which has KtrCD as the only potassium uptake system under conditions of potassium limitation (no glutamate, 0.5 mM KCl or no KCl) and added potassium chloride, potassium glutamate, sodium chloride, or sodium glutamate to the culture after the cessation of growth (Fig. 2). The bacteria immediately resumed growth upon the addition of potassium chloride or potassium glutamate, indicating that the lack of potassium was the growth-limiting factor. After a longer lag phase, the addition of sodium glutamate also allowed continued growth, but only in the presence of 0.5 mM KCl in the medium. In contrast, no restoration of growth was possible for the culture without added KCl. Taken together, all these data clearly demonstrate that glutamate stimulates the ability of KtrCD to take up potassium at very low concentrations of the ion.
FIG 2.
Activity of KtrCD upon potassium limitation. B. subtilis GP2165 expressing only KtrCD was grown in MSSM medium with ammonium and 0.5 mM KCl (A) or 0 mM KCl (B), and growth was monitored. As soon as the cells reached stationary growth phase as a result of potassium starvation, different substances were added, and growth was monitored further. Added compounds were MSSM base buffer, 5 mM NaCl, 5 mM KCl, 5 mM sodium glutamate, and 5 mM potassium glutamate.
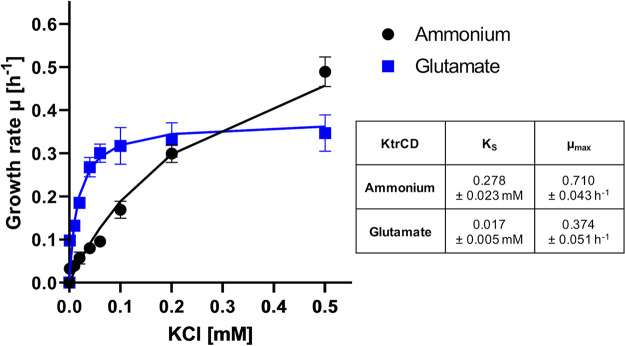
In order to study the effect of glutamate on KtrCD in more detail, we analyzed the activity of the protein in a heterologous complementation assay using Escherichia coli LB2003 (30). This strain is deficient in the major endogenous potassium uptake systems Trk, Kup, and Kdp and is therefore not able to grow at low potassium concentrations without complementation with a gene encoding a potassium uptake system. For the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible expression of ktrCD, we used plasmid pGP2989. Accordingly, E. coli LB2003 was transformed with this plasmid or the empty vector control pWH844 (31), and growth was assessed in minimal medium supplemented with increasing KCl concentrations (0.001, 0.01, 0.02, 0.04, 0.06, 0.1, 0.2, 0.5, 10, and 50 mM KCl) (Fig. 3). While 50 mM KCl were required for growth of the strain carrying the empty vector (data not shown), expression of KtrCD allowed growth at much lower KCl concentrations. The determination of the growth rates at different potassium concentrations allowed fitting according to the Monod equation, an equation describing the growth of cultures, which is based on the Michaelis-Menten equation (32). This revealed the maximum specific growth rate (μmax [per hour]) and the substrate concentration that supports the half-maximal growth rate (KS [millimolar units of KCl]) of KtrCD in the absence or presence of glutamate. The mean KS values of three independent biological replicates are as follows. In the absence of glutamate, KtrCD had a KS of 0.278 ± 0.023 mM for potassium and a μmax of 0.71 ± 0.043 h−1. In contrast, the presence of glutamate in the medium resulted in a KS for potassium of 0.017 ± 0.0045 mM and a μmax of 0.374 ± 0.051 h−1. This 16-fold increase in the apparent affinity for potassium in the presence of glutamate perfectly fits with the growth phenotype observed in B. subtilis mutants and might suggest that the presence of glutamate converts KtrCD from a low- to a high-affinity potassium uptake system.
FIG 3.
Activity of KtrCD in a heterologous complementation assay using E. coli 2003 reveals the impact of glutamate. LB2003 was transformed with pGP2989, and growth at different potassium concentrations with ammonium or glutamate was assessed over 24 h. The growth rate was determined and plotted against the potassium concentrations. The lines show the ideal progression of μ over the different potassium concentrations according to the Monod equation. Standard deviations derived from three independent experiments are indicated.
Contribution of the membrane and gating subunits to the regulation by glutamate.
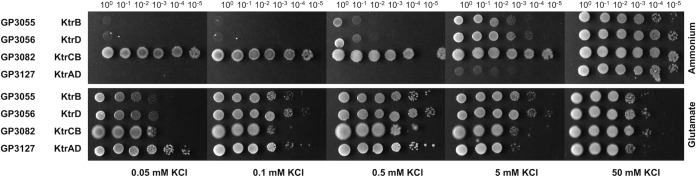
The Ktr potassium channels consist of an integral membrane and a gating subunit. In order to study the role of the subunits in the control of KtrCD by glutamate, we constructed two series of strains: a first set encoded only one of the transmembrane subunits KtrB and KtrD, whereas the second set expressed Ktr channels with swapped subunits (i.e., KtrCB and KtrAD).
The strains expressing a single transmembrane subunit (KtrB or KtrD) were unable to grow at low potassium concentrations (below 0.5 mM) in medium with ammonium as the nitrogen source (Fig. 4). However, they exhibited good growth at 5 mM potassium. If glutamate was present in the medium, both KtrB and KtrD allowed growth even at the lowest potassium concentrations. These observations demonstrate that KtrB and KtrD are functional potassium channels even in the absence of a gating subunit. Moreover, both KtrB and KtrD appear to develop more affinity for potassium in the presence of glutamate.
FIG 4.
Growth of B. subtilis potassium transporter mutants at different potassium concentrations in the presence of ammonium or glutamate. B. subtilis mutants expressing one of the transmembrane subunits KtrB and KtrD or the hybrid channels KtrCB and KtrAD as the single potassium uptake system were cultivated in MSSM medium with 50 mM KCl and ammonium. Cells were harvested and washed, and the OD600 was adjusted to 1.0. Serial dilutions were added dropwise to MSSM plates with the indicated potassium concentration and ammonium or glutamate.
It has been shown before that Ktr channels with swapped subunits are active in potassium uptake (24). Here, we tested the response of such channels to the availability of glutamate (Fig. 4). Strain GP3082, encoding KtrCB, was indistinguishable from strain GP2783 (KtrAB) in both the absence and presence of glutamate. The growth of these strains even at the lowest tested potassium concentrations confirmed that KtrB is the major determinant for the high affinity to potassium. The growth pattern of the strain expressing KtrAD (GP3127) was similar to that of the isogenic strain encoding KtrCD (GP2165). In the absence of glutamate, KtrAD allowed growth only at elevated potassium concentrations. This was even more pronounced compared to the cognate KtrCD complex: KtrAD and KtrCD sustained growth at potassium concentrations exceeding 5 and 0.5 mM KCl, respectively. In the presence of glutamate, both strains grew well at all tested potassium concentrations. These observations indicate that KtrD is the determinant for the apparently low affinity for potassium in the absence of glutamate. Moreover, the cognate complex of KtrD and KtrC is more efficient in potassium uptake in the absence of glutamate than the KtrD-KtrA complex.
Taken together, the experiments both with the isolated membrane subunits and with the cognate and swapped Ktr complexes demonstrate that both membrane subunits have a higher apparent affinity for potassium in the presence of glutamate. The presence of either of the two gating subunits strongly enhanced the apparent affinity of KtrB to potassium even in the absence of glutamate, resulting in high-affinity KtrAB and KtrCB complexes. Thus, in the case of KtrB, the gating subunits enhance the affinity of the channel to potassium irrespective of the presence of glutamate. In contrast, for KtrD, the association with either gating subunit resulted in enhanced activity, but at low potassium concentrations, KtrD remained fully dependent on the presence of glutamate even if bound to one of the gating subunits.
Identification and characterization of a high-affinity variant of KtrD.
The results presented above suggest that specific features of KtrD cause the low affinity for potassium in the absence of glutamate even if the protein forms a complex with its cognate gating subunit, KtrC. We have observed that strains lacking the high-affinity uptake systems KimA and KtrAB experience a severe potassium limitation when cultivated at low concentrations of KCl. To cope with this limitation, such strains typically acquire mutations that result in the overexpression of the arginine biosynthetic pathway to substitute for the positive charge normally provided by potassium ions (22). However, such suppressors are unable to grow in the absence of the c-di-AMP receptor protein DarA (33; M. Weiß and J. Stülke, unpublished data). Hence, a strain lacking KimA, KtrAB, and DarA (GP2495) was used to select suppressor mutants that are able to grow at low potassium concentrations (0.5 mM KCl). We expected that such mutants would either allow the uptake of potassium at low concentrations or activate alternative pathways to provide positively charged molecules. Whole-genome sequencing of one of the mutants (GP3100) revealed a point mutation in the ktrD gene, resulting in an exchange of a glycine at position 282 by a valine. This residue is part of the selectivity filter and at the same time located close to the intramembrane loop of KtrD, which serves as a gate (34). Interestingly, the selectivity filters of related proteins (including KtrB) have a highly conserved R-T/S-A-G motif at this position, whereas KtrD has an R-S-G-G motif. The replacement of the first glycine by a larger amino acid (valine) makes the selectivity filter more similar to those in the other potassium channels at this position.
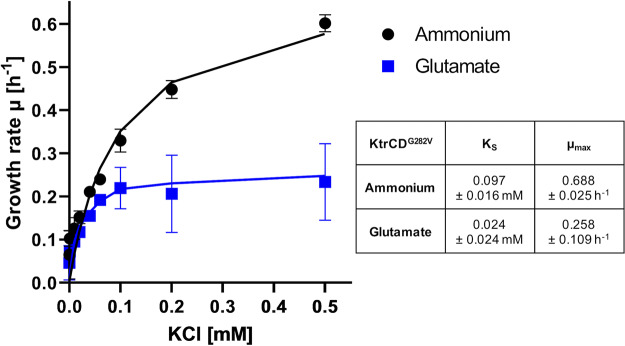
To determine the activity of the mutant KtrCDG282V channel more precisely, we performed the heterologous complementation assay using E. coli LB2003 as described above (Fig. 5). In the presence of ammonium, KtrCDG282V had a KS of 0.097 ± 0.015 mM for potassium and a μmax of 0.69 ± 0.024 h−1. The presence of glutamate in the medium resulted in an apparent KS for potassium of 0.024 ± 0.024 mM and a μmax of 0.258 ± 0.109 h−1. This result confirms the increased apparent affinity of the mutant channel for potassium compared to the wild-type protein.
FIG 5.
A single amino acid exchange, G282V in KtrD, confers high affinity for potassium independent of glutamate to KtrCD. E. coli LB2003 was transformed with pGP3303, and growth at different potassium concentrations with ammonium or glutamate was assessed over 24 h. The growth rate was determined and plotted against the potassium concentrations. The lines show the ideal progression of μ over the different potassium concentrations according to the Monod equation. Standard deviations derived from three independent experiments are indicated.
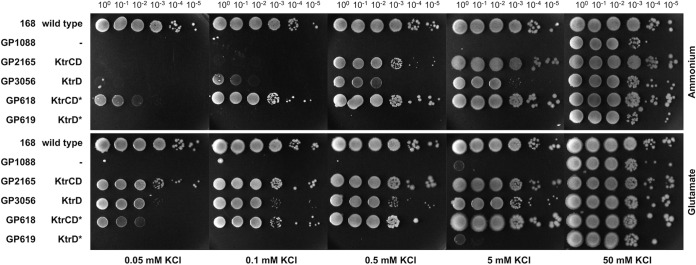
The mutant KtrDG282V protein may have a high apparent affinity for potassium in the presence of the cognate gating subunit irrespective of the presence of glutamate, as observed for KtrB. Alternatively, and unlike KtrB, the mutant protein may have an intrinsic high apparent affinity for potassium, independent of the cognate gating subunit. To distinguish between these two possibilities, we used CRISPR-Cas9-mediated genome engineering to construct the strains GP618 and GP619, which had KtrCDG282V and KtrDG282V, respectively, as the only potassium uptake systems. The activity of these channels was studied by assaying growth of the B. subtilis strains at different potassium concentrations in the presence or absence of glutamate (Fig. 6). As expected, the strain expressing KtrCDG282V grew even at the lowest tested potassium concentration in both the presence and absence of glutamate. In contrast, strain GP619 with KtrDG282V as the single potassium channel was unable to grow at low potassium concentrations. Even in the presence of glutamate, the wild-type KtrD was active at low potassium concentrations; we observed no growth at a potassium concentration lower than 50 mM KCl. Like strain GP1088, which lacks all known potassium uptake systems, GP619 was viable in the presence of 50 mM KCl whether glutamate was present or not. This finding suggests that the mutant channel protein is inactive in the absence of its gating subunit KtrC, while in its presence, KtrCDG282V shows a high glutamate-independent affinity for potassium. Thus, the mutant KtrCDG282V resembled the KtrAB complex in the requirement for the gating subunit for glutamate-independent potassium uptake at low concentrations of the ion.
FIG 6.
Growth experiments with KtrCD variants highlight the relevance of the G282V mutation for the physiology of B. subtilis. B. subtilis strains expressing KtrCD, KtrD, and the mutated variants as the only potassium uptake systems were cultivated in MSSM medium with 50 mM KCl and ammonium. Cells were harvested and washed, and the OD600 was adjusted to 1.0. Serial dilutions were added dropwise to MSSM plates with the indicated potassium concentration and ammonium or glutamate. The mutation in the ktrD gene was introduced into the chromosome via CRISPR-Cas9 gene editing.
DISCUSSION
Potassium and glutamate are essential for any living cell, and their concentrations have to be balanced (14). Indeed, glutamate-sensing potassium transporters are widespread in eukaryotic cells (35) but are present in only a few prokaryotes, such as the cyanobacteria (36). Here, we demonstrate that control of potassium uptake by glutamate can also be achieved by a classical bacterial potassium channel, KtrCD, which depends on glutamate for efficient potassium uptake at low external concentrations of the cation. This link between glutamate and KtrCD was first observed when we cultivated a B. subtilis strain lacking c-di-AMP in the presence of glutamate. To our surprise, this strain carried a mutation that resulted in inactivation of KtrC. With the results presented in this study, we can suggest that glutamate was toxic for the strain lacking c-di-AMP, since it stimulated potassium accumulation to a toxic level. This is in excellent agreement with the idea that the control and limitation of potassium uptake constitute an essential function of c-di-AMP (7, 11).
Recent structural analyses of the gating subunit KtrC in the presence of ATP and ADP uncovered a ligand-dependent assembly-disassembly of the gating ring. These observations had already suggested a distinct physiological role of the KtrCD system compared to KtrAB and KimA (19). This role may lie in the link between the glutamate and the potassium homeostasis. In contrast to KtrAB and KimA, KtrCD is strongly constitutively expressed in B. subtilis (21) and can readily be activated. In the absence of glutamate, KtrCD serves the general potassium homeostasis. Additionally, we established here that in the presence of a high external glutamate concentration, the apparent affinity of KtrCD for potassium increases 16-fold, allowing the accumulation of potassium even if it is limited on the outside. Prior to this stimulation, glutamate likely is taken up into the cells, as deduced from the delayed growth initiation after potassium starvation (Fig. 2A). The resulting imbalance between negative and positive charges may consequently require the rapid uptake of potassium ions to counterbalance the negative charges. This immediate uptake could always be achieved by the constitutively present KtrCD. Binding of glutamate or a downstream metabolite of it may then result in the more effective uptake of potassium ions. Alternatively, an increased membrane potential resulting from the increased utilization of glutamate could promote potassium fluxes through KtrCD. Due to the more efficient potassium uptake in the presence of glutamate, it also becomes obvious why KtrCD is inactivated by the direct binding of c-di-AMP: too high internal potassium concentrations accumulated via KtrCD would again be toxic for the cells (7, 24).
Interesting future questions are of course how glutamate stimulates the potassium uptake through KtrCD and what distinguishes the KtrCD system from the paralogous KtrAB potassium channel. KtrD and KtrB share 37% identity and are predicted to be structurally very similar. KtrC and KtrA are 55% identical, and both form octameric rings that associate to the dimeric channel subunits. Despite the apparently high similarity of these systems, KtrCD was assumed to have 10-fold-less affinity for K+ (15). This lower apparent affinity could result either from a lower binding affinity at the slightly different selectivity filter or from a second selective step, the gating. In KtrAB, KtrA facilitates the gating of the channel, while KtrB initially binds K+ at its selectivity filter (16, 19, 37, 38). It has recently been shown that it is in fact the gating that determines the high apparent affinity of KtrAB: while potassium ions also bind to KtrB alone with micromolar affinity (low KD value), a low Km value in the micromolar range was observed only in the presence of the gating subunit KtrA and sodium ions (39). The respective Km values for KtrB and KtrAB in the presence of Na+ are in good agreement with the apparent affinities deduced from growth curves (KS values) shown here (Fig. 1). In analogy to the stimulatory effect of Na+ on KtrAB, it is tempting to speculate that internal glutamate might play a similar role for KtrCD. By means of the binding to either the regulatory subunit KtrC or the gating region of KtrD, the gating could be facilitated, allowing efficient potassium uptake even at very low external concentrations. Our data favor the binding to the gating region, since an increased apparent affinity was observed in the presence of glutamate already for KtrD alone. However, the isolation of KtrC suppressor mutants of a c-di-AMP-free strain in the presence of glutamate suggests a significant contribution of KtrC to the glutamate-dependent gating, and its role is further reinforced by the improved growth in the presence of KtrC and glutamate at low external potassium concentrations. Gating may thus underlie a synergistic effect between the glutamate binding to KtrD and the regulatory effect of KtrC. In agreement with this hypothesis are the results obtained with the selectivity filter mutant in KtrD: the selectivity filter and the gate are in close proximity, and the identified mutation (G282V) is located on the inner side of the filter. The Gly-to-Val exchange may thus facilitate the KtrC-mediated gating, rendering KtrCD independent of glutamate, while in the absence of KtrC, the gate opens less efficiently than in wild-type KtrD.
The idea of a link between glutamate and potassium homeostasis is not entirely new for bacteria (36, 40). Glutamate receptors sense an increased external glutamate concentration, leading to transporter activation. However, the physiological role of bacterial glutamate receptors has not been established. A potassium channel suggested to sense the internal glutamate concentration is the B. subtilis potassium exporter YugO (41). YugO is proposed to function in electrical signaling within a bacterial biofilm. Upon glutamate starvation in the interior cells of a biofilm, the channel would be activated, resulting in the efflux of potassium ions. This efflux consequently would depolarize the neighboring outer cells, which thus would consume less glutamate. Without any loss of signal intensity, the electrical signal would propagate through a biofilm, making glutamate more accessible for the interior cell. For the benefit of the exterior cells, the interior cells in return would provide sufficient concentrations of ammonium, allowing the survival of the whole biofilm (41, 42). KtrCD appears to function in the opposite direction by accumulating potassium ions, if high internal concentrations of glutamate are present. KtrCD could consequently be involved in the signaling within bacterial biofilms. It may serve the second step, the uptake of glutamate by the hyperpolarized interior cells, by allowing the rapid accumulation of both potassium and glutamate. This way, the interior cells could accumulate the required glutamate and restore their potassium pool.
It is striking that the B. subtilis potassium import and export systems, KtrCD and YugO, are stimulated and inhibited, respectively, by glutamate. Moreover, the integration of potassium and glutamate homeostasis by the essential second messenger c-di-AMP suggests that we are only starting to discover the tip of an iceberg. A complete picture of the interdependency between glutamate and potassium concentrations and the underlying regulatory mechanisms will be essential for a full understanding of living cells.
MATERIALS AND METHODS
Strains, media, and growth conditions.
E. coli DH5α (43) was used for cloning and for the expression of recombinant proteins. E. coli LB2003 (30) was used to assay potassium uptake activity. All B. subtilis strains used in this study are derivatives of the laboratory strain 168 and are listed in Table 1. B. subtilis was grown in Luria-Bertani (LB) medium or in sporulation (SP) medium (43, 44). For the assay of potassium channel activity, B. subtilis and E. coli were cultivated in MSSM medium (11). In this medium, KH2PO4 was replaced with NaH2PO4, and KCl was added as indicated. The media were supplemented with ampicillin (100 μg/ml), kanamycin (10 and 50 μg/ml for B. subtilis and E. coli, respectively), chloramphenicol (5 μg/ml), tetracycline (12.5 μg/ml), spectinomycin (150 μg/ml), or erythromycin and lincomycin (2 and 25 μg/ml, respectively) if required.
TABLE 1.
B. subtilis strains used in this studya
| Strain | Genotype | Reference or description |
|---|---|---|
| 168 | trpC2 | Laboratory collection |
| GHB6 | trpC2 pheA1 ΔktrC::spc | 15 |
| GHB12 | trpC2 pheA1 ΔktrD::tet | 15 |
| GP92 | trpC2 ΔktrAB::aphA3 | 11 |
| GP93 | trpC2 ΔkimA::cat | 11 |
| GP616 | trpC2 ktrDG282V | This study |
| GP617 | trpC2 ktrDG282V ΔktrAB::spec | GP2716 → GP616 |
| GP618 | trpC2 ktrDG282V ΔktrAB::spec ΔkimA::ermC | GP2721 → GP617 |
| GP619 | trpC2 ktrDG282V ΔktrAB::spec ΔkimA::ermC ΔktrC::cat | GP2048 → GP618 |
| GP1054 | trpC2 ΔktrC::spec | GHB6 → 168 |
| GP1055 | trpC2 ΔktrAB::aphA3 ΔkimA::cat ΔktrD::tet | GP2030 → GP2165 |
| GP1088 | trpC2 ΔktrAB::aphA3 ΔkimA::cat ΔktrD::tet ΔktrC::spec | GP1054 → GP1055 |
| GP2030 | trpC2 ΔktrD::tet | 11 |
| GP2048 | trpC2 ΔktrC::cat | 11 |
| GP2079 | trpC2 ΔktrC::tet | 22 |
| GP2136 | trpC2 ΔktrD::tet ΔktrAB::aphA3 | 11 |
| GP2165 | trpC2 ΔktrAB::aphA3 ΔkimA::cat | 11 |
| GP2166 | trpC2 ΔktrC::tet ΔkimA::cat | 22 |
| GP2167 | trpC2 ΔktrD::tet ΔkimA::cat | 11 |
| GP2168 | trpC2 ΔktrC::cat ΔktrD::tet | GP2048 → GP2030 |
| GP2169 | trpC2 ΔktrAB::aphA3 ΔktrC::cat ΔktrD::tet | 11 |
| GP2185 | trpC2 ΔahrC::ermC | 22 |
| GP2222 | trpC2 ΔcdaA::cat ΔcdaS::ermC ΔdisA::tet | 11 |
| GP2415 | trpC2 ΔdarA::spc | This study |
| GP2461 | trpC2 ΔktrAB::aphA3 ΔkimA::cat ΔdarA::spc | GP2415 → GP2165 |
| GP2495 | trpC2 ΔktrAB::aphA3 ΔkimA::cat ΔdarA::spc ΔahrC::ermC | GP2185 → GP2461 |
| GP2716 | trpC2 ΔktrAB::spc | This study |
| GP2721 | trpC2 ΔkimA::ermC | This study |
| GP2783 | trpC2 ΔktrC::cat ΔktrD::tet ΔkimA::ermC | GP2721 → GP2168 |
| GP2840 | trpC2 ΔcdaA::cat ΔcdaS::ermC ΔdisA::tet plsCA61V ndhFFS ktrCFS sigDG88D | This study; suppressor mutant of GP2222 |
| GP3055 | trpC2 ΔktrC::cat ΔktrD::tet ΔkimA::ermC ΔktrA::aphA3 | GP3065 → GP2783 |
| GP3056 | trpC2 ΔktrAB::aphA3 ΔkimA::cat ΔktrC::tet | GP2079 → GP2165 |
| GP3064 | trpC2 ΔktrB::aphA3 | This study |
| GP3065 | trpC2 ΔktrA::aphA3 | This study |
| GP3082 | trpC2 ΔktrD::tet ΔkimA::cat ΔktrA::aphA3 | GP3065 → GP2167 |
| GP3100 | trpC2 ΔktrAB::aphA3 ΔkimA::cat ΔdarA::spc ΔahrC::ermC ktrDG282V | This study, suppressor mutant of GP2495 |
| GP3127 | trpC2 ΔktrC::tet ΔkimA::cat ΔktrB::aphA3 | GP3064 → GP2166 |
| GP3414 | trpC2 ΔcdaA::cat ΔcdaS::ermC ΔdisA::tet ktrCFS | This study; suppressor mutant of GP2222 |
FS, frameshift.
DNA manipulation and genome sequencing.
Transformation of E. coli and plasmid DNA extraction were performed using standard procedures (43). All commercially available plasmids, restriction enzymes, T4 DNA ligase, and DNA polymerases were used as recommended by the manufacturers. Chromosomal DNA of B. subtilis was isolated as previously described (44). B. subtilis was transformed with plasmid and genomic DNA according to the two-step protocol (44).
To identify the mutations in the suppressor mutant strains GP2840 and GP3100, their genomic DNA was subjected to whole-genome sequencing (3). Briefly, the reads were mapped on the reference genome of B. subtilis 168 (GenBank accession number NC_000964) (45). Mapping of the reads was performed using the Geneious software package (Biomatters Ltd., Auckland, New Zealand) (46). Single nucleotide polymorphisms were considered significant when the total coverage depth exceeded 25 reads with a variant frequency of ≥90%. All identified mutations were verified by PCR amplification and Sanger sequencing.
Construction of mutant strains by allelic replacement.
Deletion of the darA, kimA, ktrA, and ktrB genes as well as of the ktrAB operon was achieved by transformation of B. subtilis 168 with a PCR product constructed using oligonucleotides to amplify DNA fragments flanking the target genes and an appropriate intervening resistance cassette as described previously (47). The integrity of the regions flanking the integrated resistance cassette was verified by sequencing PCR products of about 1,000 bp amplified from chromosomal DNA of the resulting mutant strains.
Genome editing.
A single amino acid substitution in KtrD (G282V) was generated at the native ktrD locus using CRISPR editing as described previously (48). Briefly, oligonucleotides encoding a 20-nucleotide guide RNA (gRNA) with flanking BsaI sites and a repair fragment carrying mutations of interest with flanking SfiI restriction sites were cloned sequentially into the vector pJOE8999 (48). The resulting plasmid, pGP2833, was used to transform recipient B. subtilis strain 168, and cells were plated on 15-μg/ml kanamycin plates with 0.2% mannose. Transformation was carried out at 30°C, since replication of pJOE8999 derivatives is temperature sensitive. The transformants were plated on LB agar plates and incubated at the nonpermissive temperature of 50°C. The loss of the vector was verified by the inability of the bacteria to grow on kanamycin plates. The presence of the desired mutations in the resulting strain, GP616, was confirmed by Sanger sequencing.
Plasmid constructions.
The ktrD alleles were amplified using chromosomal DNA of B. subtilis 168 or GP3100 as the template and appropriate oligonucleotides that attached PstI and HindIII sites and a ribosomal binding site to the ktrD fragment. The ktrD alleles were cloned between the PstI and HindIII sites of pGP2907 (7), resulting in pGP2989 and pGP3303. These plasmids allow the expression of wild-type and mutant (KtrDG282V) ktrCD operons, respectively, in E. coli.
Determination of specific growth parameters.
The growth characteristics of E. coli LB2003 complemented with plasmid-based KtrCD, KtrCDG282V, or empty vector were determined as follows. Potassium was used as the growth-limiting factor. The bacteria were inoculated in LB medium containing 50 mM KCl and precultured in MSSM medium, supplemented with thiamine (1 mg/ml) and 50 mM KCl. The cultures were grown to exponential phase and harvested, and the cells were incubated for 1 h in potassium-free medium and washed three times in MSSM buffer. Afterwards, the cells were adjusted to an optical density at 600 nm (OD600) of 1.0 and used to inoculate a 96-well plate (Microtest plate; Sarstedt) containing MSSM medium with either ammonium or glutamate and the required potassium concentrations. Growth was tracked in an Epoch 2 microplate spectrophotometer (BioTek Instruments) at 37°C with linear shaking at 237 range of cycles/min, (range of plate movement, 4 mm) for 20 h, and optical density at 600 nm was measured at 10-min intervals. The exponential growth phase was used to determine the growth rate (μ [per hour]). The growth rates were then plotted against the potassium concentrations. This allowed fitting to the Monod equation and calculation of μmax [per hour] and the apparent KS [millimolar units of KCl] using the solver tool of Excel 2012 (Microsoft). Experiments were repeated with three biological replicates.
To assay growth of B. subtilis mutants at different potassium concentrations, a drop dilution assay was performed. Briefly, precultures in MSSM medium with ammonium and 50 mM KCl were washed three times, and the cells were adjusted to an OD600 of 1.0 in MSSM buffer. Dilution series were then spotted onto MSSM plates with ammonium or glutamate and different KCl concentrations (0.001, 0.01, 0.02, 0.04, 0.06, 0.1, 0.2, 0.5, 10, and 50 mM KCl).
For the determination of growth characteristics in liquid medium, cells were treated as described above, and the cells were adjusted to an OD600 of 1.0 and used to inoculate a 96-well plate (Microtest plate; Sarstedt) containing MSSM medium with either ammonium or glutamate and the required potassium concentration.
Supplementary Material
ACKNOWLEDGMENTS
We thank Iljana Gerlitzki, Julius Fülleborn, Jan Gundlach, and Alexander Lockhorn for help with strain construction.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft via Priority Program SPP1879 (to I.H. and J.S.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Epstein W. 2003. The roles and regulation of potassium in bacteria. Prog Nucleic Acid Res Mol Biol 75:293–320. doi: 10.1016/S0079-6603(03)75008-9. [DOI] [PubMed] [Google Scholar]
- 2.Bennet BD, Kimball EH, Gao M, Osterhout R, van Dien SJ, Rabinowitz JD. 2009. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol 5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuß DR, Altenbuchner J, Mäder U, Rath H, Ischebeck T, Sappa PK, Thürmer A, Guérin C, Nicolas P, Steil L, Zhu B, Feussner I, Klumpp S, Daniel R, Commichau FM, Völker U, Stülke J. 2017. Large-scale reduction of the Bacillus subtilis genome: consequences for the transcriptional network, resource allocation, and metabolism. Genome Res 27:289–299. doi: 10.1101/gr.215293.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozov A, Khusainov I, El Omari K, Duman R, Mykhaylyk V, Yusupov M, Westhof E, Wagner A, Yusupova G. 2019. Importance of potassium ions for ribosome structure and function revealed by long-wavelength X-ray diffraction. Nat Commun 10:2519. doi: 10.1038/s41467-019-10409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremer E, Krämer R. 2019. Responses of microorganisms to osmotic stress. Annu Rev Microbiol 73:313–334. doi: 10.1146/annurev-micro-020518-115504. [DOI] [PubMed] [Google Scholar]
- 6.Booth IR, Higgins CF. 1990. Enteric bacteria and osmotic stress: intracellular potassium glutamate as a secondary signal of osmotic stress? FEMS Microbiol Rev 6:239–246. doi: 10.1111/j.1574-6968.1990.tb04097.x. [DOI] [PubMed] [Google Scholar]
- 7.Gundlach J, Krüger L, Herzberg C, Turdiev A, Poehlein A, Tascón I, Weiss M, Hertel D, Daniel R, Hänelt I, Lee VT, Stülke J. 2019. Sustained sensing in potassium homeostasis: cyclic di-AMP controls potassium uptake by KimA at the levels of expression and activity. J Biol Chem 294:9605–9614. doi: 10.1074/jbc.RA119.008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Commichau FM, Gunka K, Landmann JJ, Stülke J. 2008. Glutamate metabolism in Bacillus subtilis: gene expression and enzyme activities evolved to avoid futile cycles and to allow rapid responses to perturbations in the system. J Bacteriol 190:3557–3564. doi: 10.1128/JB.00099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher MA, Chinnam NB, Cuthbert B, Tonthat NK, Whitfill T. 2015. Structures of regulatory machine reveal novel molecular mechanisms controlling B. subtilis nitrogen homeostasis. Genes Dev 29:451–464. doi: 10.1101/gad.254714.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundlach J, Mehne FMP, Herzberg C, Kampf J, Valerius O, Kaever V, Stülke J. 2015. An essential poison: synthesis and degradation of cyclic di-AMP in Bacillus subtilis. J Bacteriol 197:3265–3274. doi: 10.1128/JB.00564-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gundlach J, Herzberg C, Kaever V, Gunka K, Hoffmann T, Weiß M, Gibhardt J, Thürmer A, Hertel D, Daniel R, Bremer E, Commichau FM, Stülke J. 2017. Control of potassium homeostasis is an essential function of the second messenger cyclic di-AMP in Bacillus subtilis. Sci Signal 10:eaal3011. doi: 10.1126/scisignal.aal3011. [DOI] [PubMed] [Google Scholar]
- 12.Commichau FM, Gibhardt J, Halbedel S, Gundlach J, Stülke J. 2018. A delicate connection: c-di-AMP affects cell integrity by controlling osmolyte transport. Trends Microbiol 26:175–185. doi: 10.1016/j.tim.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Commichau FM, Heidemann JL, Ficner R, Stülke J. 2019. Making and breaking of an essential poison: the cyclases and phosphodiesterases that produce and degrade the essential second messenger cyclic di-AMP in bacteria. J Bacteriol 201:e00462-18. doi: 10.1128/JB.00462-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gundlach J, Commichau FM, Stülke J. 2018. Perspective of ions and messengers: an intricate link between potassium, glutamate and cyclic di-AMP. Curr Genet 64:191–195. doi: 10.1007/s00294-017-0734-3. [DOI] [PubMed] [Google Scholar]
- 15.Holtmann G, Bakker EP, Uozumi N, Bremer E. 2003. KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J Bacteriol 185:1289–1298. doi: 10.1128/jb.185.4.1289-1298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vieira-Pires RS, Szollosi A, Morais-Cabral JH. 2013. The structure of the KtrAB potassium transporter. Nature 496:323–328. doi: 10.1038/nature12055. [DOI] [PubMed] [Google Scholar]
- 17.Albright RA, Ibar JL, Kim CU, Gruner SM, Morais-Cabral JH. 2006. The RCK domain of the KtrAB K+ transporter: multiple conformations of an octameric ring. Cell 126:1147–1159. doi: 10.1016/j.cell.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Szollosi A, Vieira-Pires RS, Teixeira-Duarte CM, Rocha R, Morais-Cabral JH. 2016. Dissecting the molecular mechanism of nucleotide-dependent activation of the KtrAB K+ transporter. PLoS Biol 14:e1002356. doi: 10.1371/journal.pbio.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diskowski M, Mehdipour AR, Wunnicke D, Mills DJ, Mikusevic V, Bärland N, Hoffmann J, Morgner N, Steinhoff HJ, Hummer G, Vonck J, Hänelt I. 2017. Helical jackknives control the gates of the double-pore K+ uptake system KtrAB. Elife 6:e24303. doi: 10.7554/eLife.24303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrecker M, Wunnicke D, Hänelt I. 2019. How RCK domains regulate gating of K+ channels. Biol Chem 400:1303–1322. doi: 10.1515/hsz-2019-0153. [DOI] [PubMed] [Google Scholar]
- 21.Zhu B, Stülke J. 2018. SubtiWiki in 2018: from genes and proteins to functional network annotation of the model organism Bacillus subtilis. Nucleic Acids Res 46:D743–D748. doi: 10.1093/nar/gkx908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundlach J, Herzberg C, Hertel D, Thürmer A, Daniel R, Link H, Stülke J. 2017. Adaptation of Bacillus subtilis to life at extreme potassium limitation. mBio 8:e00861-17. doi: 10.1128/mBio.00861-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson JW, Sudarsan N, Furukawa K, Weinberg Z, Wang JX, Breaker RR. 2013. Riboswitches in eubacteria sense the second messenger cyclic di-AMP. Nat Chem Biol 9:834–839. doi: 10.1038/nchembio.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha R, Teixeira-Duarte CM, Jorge JMP, Morais-Cabral JH. 2019. Characterization of the molecular properties of KtrC, a second RCK domain that regulates a Ktr channel in Bacillus subtilis. J Struct Biol 205:34–43. doi: 10.1016/j.jsb.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Chin KH, Liang JM, Yang JG, Shih MS, Tu ZL, Wang YC, Sun XH, Hu NJ, Liang ZX, Dow JM, Ryan RP, Chou SH. 2015. Structural insights into the distinct binding mode of cyclic di-AMP with SaCpaA_RCK. Biochemistry 54:4936–4951. doi: 10.1021/acs.biochem.5b00633. [DOI] [PubMed] [Google Scholar]
- 26.Whiteley AT, Pollock AJ, Portnoy DA. 2015. The PAMP c-di-AMP is essential for Listeria growth in macrophages and rich but not minimal medium due to a toxic increase in (p)ppGpp. Cell Host Microbe 17:788–798. doi: 10.1016/j.chom.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeden MS, Schuster CF, Bowman L, Zhong Q, Williams HD, Gründling A. 2018. Cyclic di-adenosine monophosphate (c-di-AMP) is required for osmotic regulation in Staphylococcus aureus but dispensable for viability in anaerobic conditions. J Biol Chem 293:3180–3200. doi: 10.1074/jbc.M117.818716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devaux L, Sleiman D, Mazzuoli MV, Gominet M, Lanotte P, Trieu-Cuot P, Kaminski PA, Firon A. 2018. Cyclic di-AMP regulation of osmotic homeostasis is essential in group B Streptococcus. PLoS Genet 14:e1007342. doi: 10.1371/journal.pgen.1007342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujisawa M, Kusumoto A, Wada Y, Tsuchiya T, Ito M. 2005. NhaK, a novel monovalent cation/H+ antiporter of Bacillus subtilis. Arch Microbiol 183:411–420. doi: 10.1007/s00203-005-0011-6. [DOI] [PubMed] [Google Scholar]
- 30.Stumpe S, Bakker EP. 1997. Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch Microbiol 167:126–136. doi: 10.1007/s002030050425. [DOI] [PubMed] [Google Scholar]
- 31.Schirmer F, Ehrt S, Hillen W. 1997. Expression, inducer spectrum, domain structure, and function of MopR, the regulator of phenol degradation in Acinetobacter calcoaceticus NCIB8250. J Bacteriol 179:1329–1336. doi: 10.1128/jb.179.4.1329-1336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monod J. 1949. The growth of bacterial cultures. Annu Rev Microbiol 3:371–394. doi: 10.1146/annurev.mi.03.100149.002103. [DOI] [Google Scholar]
- 33.Gundlach J, Dickmanns A, Schröder-Tittmann K, Neumann P, Kaesler J, Kampf J, Herzberg C, Hammer E, Schwede F, Kaever V, Tittmann K, Stülke J, Ficner R. 2015. Identification, characterization and structure analysis of the c-di-AMP binding PII-like signal transduction protein DarA. J Biol Chem 290:3069–3080. doi: 10.1074/jbc.M114.619619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diskowski M, Mikusevic V, Stock C, Hänelt I. 2015. Functional diversity of the superfamily of K+ transporters to meet various requirements. Biol Chem 396:1003–1014. doi: 10.1515/hsz-2015-0123. [DOI] [PubMed] [Google Scholar]
- 35.Sobolevsky AI. 2015. Structure and gating of tetrameric glutamate receptors. J Physiol 593:29–38. doi: 10.1113/jphysiol.2013.264911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ger MF, Rendon G, Tilson JL, Jakobsson E. 2010. Domain-based identification and analysis of glutamate receptor ion channels and their relatives in prokaryotes. PLoS One 5:e12827. doi: 10.1371/journal.pone.0012827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kröning N, Willenborg M, Tholema N, Hänelt I, Schmid R, Bakker EP. 2007. ATP binding to the KTN/RCK subunit KtrA from the K+-uptake system KtrAB of Vibrio alginolyticus: its role in the formation of the KtrAB complex and its requirement in vivo. J Biol Chem 282:14018–14027. doi: 10.1074/jbc.M609084200. [DOI] [PubMed] [Google Scholar]
- 38.Tholema N, Vor der Brüggen M, Mäser P, Nakamura T, Schroeder JI, Kobayashi H, Uozumi N, Bakker EP. 2005. All four putative selectivity filter glycine residues in KtrB are essential for high affinity and selective K+ uptake by the KtrAB system from Vibrio alginolyticus. J Biol Chem 280:41146–41154. doi: 10.1074/jbc.M507647200. [DOI] [PubMed] [Google Scholar]
- 39.Mikušević V, Schrecker M, Kolesova N, Patiño-Ruiz M, Fendler K, Hänelt I. 2019. A channel profile report of the unusual K+ channel KtrB. J Gen Physiol 151:1357–1368. doi: 10.1085/jgp.201912384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen GQ, Cui C, Mayer ML, Gouaux E. 1999. Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature 402:817–821. doi: 10.1038/45568. [DOI] [PubMed] [Google Scholar]
- 41.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Süel GM. 2015. Ion channels enable electrical communication in bacterial communities. Nature 527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang KC. 2017. Staying in touch while on the go. Cell 168:15–17. doi: 10.1016/j.cell.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 44.Kunst F, Rapoport G. 1995. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J Bacteriol 177:2403–2407. doi: 10.1128/jb.177.9.2403-2407.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbe V, Cruveiller S, Kunst F, Lenoble P, Meurice G, Sekowska A, Vallenet D, Wang T, Moszer I, Médigue C, Danchin A. 2009. From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology 155:1758–1775. doi: 10.1099/mic.0.027839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diethmaier C, Pietack N, Gunka K, Wrede C, Lehnik-Habrink M, Herzberg C, Hübner S, Stülke J. 2011. A novel factor controlling bistability in Bacillus subtilis: the YmdB protein affects flagellin expression and biofilm formation. J Bacteriol 193:5997–6007. doi: 10.1128/JB.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altenbuchner J. 2016. Editing of the Bacillus subtilis genome by the CRISPR-Cas9 system. Appl Environ Microbiol 82:5421–5427. doi: 10.1128/AEM.01453-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.