Acinetobacter baumannii is a pathogen of worldwide importance. Due to the increasing prevalence of antibiotic resistance, these infections are becoming increasingly difficult to treat. New therapies are required to combat multidrug-resistant isolates. The role of RelA in A. baumannii is largely unknown. This study demonstrates that like in other bacteria, RelA controls a variety of functions, including virulence. Strategies to inhibit the activity of RelA and the resulting production of ppGpp could inhibit virulence and may represent a new therapeutic approach.
KEYWORDS: Acinetobacter, stringent response, quorum sensing, motility
ABSTRACT
In response to nutrient depletion, the RelA and SpoT proteins generate the signaling molecule (p)ppGpp, which then controls a number of downstream effectors to modulate cell physiology. In Acinetobacter baumannii strain AB5075, a relA ortholog (ABUW_3302) was identified by a transposon insertion that conferred an unusual colony phenotype. An in-frame deletion in relA (ΔrelA) failed to produce detectable levels of ppGpp when amino acid starvation was induced with serine hydroxamate. The ΔrelA mutant was blocked from switching from the virulent opaque colony variant (VIR-O) to the avirulent translucent colony variant (AV-T), but the rate of AV-T to VIR-O switching was unchanged. In addition, the ΔrelA mutation resulted in a pronounced hypermotile phenotype on 0.35% agar plates. This hypermotility was dependent on the activation of a LysR regulator ABUW_1132, which was required for expression of AbaR, a LuxR family quorum-sensing regulator. In the ΔrelA mutant, ABUW_1132 was also required for the increased expression of an operon composed of the ABUW_3766-ABUW_3773 genes required for production of the surfactant-like lipopeptide acinetin 505. Additional phenotypes identified in the ΔrelA mutant included (i) cell elongation at high density, (ii) reduced formation of persister cells tolerant to colistin and rifampin, and (iii) decreased virulence in a Galleria mellonella model.
IMPORTANCE Acinetobacter baumannii is a pathogen of worldwide importance. Due to the increasing prevalence of antibiotic resistance, these infections are becoming increasingly difficult to treat. New therapies are required to combat multidrug-resistant isolates. The role of RelA in A. baumannii is largely unknown. This study demonstrates that like in other bacteria, RelA controls a variety of functions, including virulence. Strategies to inhibit the activity of RelA and the resulting production of ppGpp could inhibit virulence and may represent a new therapeutic approach.
INTRODUCTION
Acinetobacter baumannii is a nosocomial pathogen responsible for a variety of human infections (1–5). The incidence of these infections is increasing worldwide, and the inability to effectively treat these infections with antibiotics is a worrisome development, as recently emphasized by both the U.S. Centers for Disease Control and Prevention and the World Health Organization (6–9). Furthermore, A. baumannii infections can be seen in the community and can cause a severe rapidly fatal disease (10, 11).
A. baumannii strain AB5075 and many clinical isolates can switch between two cell types, those that form opaque colonies on 0.5× Luria-Bertani (LB) agar plates when viewed by oblique lighting and those that form translucent colonies (12). These two cell types exhibit marked differences in virulence in both Galleria mellonella and mouse lung models of infection, where the opaque form is virulent (VIR-O) and the translucent form is avirulent (AV-T) (12, 13). Several regulatory genes can influence the rate of VIR-O to AV-T switching. For example, mutations in arpAB encoding an RND-type efflux system decrease the rate of switching, and mutations in orthologs of the EnvZ/OmpR two-component system increase the rate of VIR-O to AV-T switching (14, 15). In addition, overexpression of a TetR-type transcriptional regulator, ABUW_1645, can drive cells from the VIR-O to the AV-T state. However, a null allele in ABUW_1645 had no effect on VIR-O to AV-T switching (13).
Bacteria respond to starvation for carbon, fatty acid, phosphate, iron, and amino acids and other stress responses by inducing synthesis of the alarmone molecule (p)ppGpp via the RelA or SpoT protein, reviewed in references 16, to ,20. RelA and SpoT comprise the RSH superfamily, in which RelA synthesizes (p)ppGpp using ATP and GDP/GTP and SpoT has both weak synthetase activity and strong hydrolase (ppGpp degrading) activity. The effect of ppGpp accumulation in cells is multifactorial. Global transcription is reprogrammed utilizing ppGpp together with the DksA protein to alter the activity of RNA polymerase (RNAP), resulting in both decreased and increased transcription at various promoters (16, 19, 21, 22). A second mechanism for transcriptional reprogramming by ppGpp involves decreasing the affinity of σ70 for RNAP and allowing other sigma factors to bind (23). RelA and the resulting production of ppGpp impacts additional cellular functions, including growth rate, translation, and DNA replication (16, 20). A requirement for ppGpp in bacterial virulence has been demonstrated in a wide variety of Gram-negative bacteria, including Pseudomonas aeruginosa, Legionella pneumophila, Burkholderia pseudomallei, Francisella novicida, Vibrio cholerae, Yersinia pestis, Salmonella enterica, and Escherichia coli (24–32). The role of RelA and ppGpp in the formation of antibiotic-tolerant persister cells has also been described (33–38). Reviews on this subject highlight the multiple roles attributed to RelA, including examples in Gram-positive bacteria (16, 39, 40).
In A. baumannii, the role of RelA is largely unknown. A screen for transposon insertions that resulted in sensitivity to ascites fluid or human serum identified relA, but no additional characterization was reported (41, 42). A recent study demonstrated a role for RelA in the regulation of efflux genes, such as adeB and adeJ (43). In this study, we report a transposon insertion in relA that was initially identified by the resulting change in colony morphology. Further characterization of an in-frame relA deletion revealed that a number of phenotypes were impacted by the mutation, including (i) loss of ppGpp synthesis, (ii) loss of switching from the VIR-O to the AV-T variant, (iii) increased surface motility, and (iv) elongated cell morphology at high density. Furthermore, like in many other bacteria, the loss of relA decreased virulence when assessed in a Galleria mellonella model. This study reveals for the first time the multiple roles for RelA in the physiology of A. baumannii.
RESULTS
Identification of an A. baumannii relA ortholog.
A. baumannii strain AB5075 can rapidly switch between two cell types, virulent and avirulent, that are distinguished by their opaque (VIR-O) or translucent (AV-T) colony phenotypes when viewed with a light source that illuminates the colonies from below (oblique lighting) (12, 13). In the course of screening a transposon insertion library (EZ-Tn5 <Tet-1>) in the avirulent translucent (AV-T) variant of AB5075, a colony with an unusual phenotype was observed that exhibited a metallic sheen and an irregular colony surface when viewed by oblique lighting. This colony phenotype was similar to that of mutants we had previously observed, where cells were highly filamentous (44). When this colony was restreaked, it gave rise to a second colony phenotype that was larger and flat with irregular edges. To identify this mutation, the transposon insertion was cloned out along with flanking DNA, and the insertion site was determined to be within the ABUW_3302 gene. The protein encoded by ABUW_3302 exhibited 40% identity and 62% amino acid similarity to the E. coli RelA protein. RelA functions as a ppGpp synthetase that generates the secondary messenger molecule (p)ppGpp in response to nutrient starvation and other stressors (19, 45). To confirm that the insertion in ABUW_3302 was responsible for the observed colony phenotypes, a nonpolar in-frame deletion in ABUW_3302 was constructed in both the VIR-O and AV-T variants. The AV-T ΔABUW_3302 mutant exhibited the same unusual colony phenotype as the original transposon insertion, and this phenotype is shown compared to a wild-type AV-T colony (Fig. 1A). In addition, the AV-T ΔABUW_3302 mutant was capable of switching at high frequency (∼5% to 10%) to cells that now formed large flat colonies with irregular borders that were identical in appearance to colonies observed arising in the original AV-T transposon mutant. Moreover, these colonies were identical in appearance to the VIR-O ΔABUW_3302 mutant, confirming that these represented VIR-O variants (Fig. 1B). Taken together, these data indicate that the ΔABUW_3302 mutation significantly alters colony morphology in both the VIR-O and AV-T variants.
FIG 1.

Colony morphology of wild-type and Δ3302 mutants. (A) Colonies of the wild-type AV-T and the isogenic AV-T Δ3302 mutant are shown after 24 h of growth on a 0.5× LB agar plate. (B) Colonies of the VIR-O wild type and the isogenic VIR-O Δ3302 mutant are shown after 24 h of growth on a 0.5× LB agar plate. Because of the larger size of the VIR-O Δ3302 colony, the relative picture size in panel B is approximately 50% smaller than in panel A.
A deletion of ABUW_3302 blocks the VIR-O to AV-T switch.
As noted above, the AV-T ΔABUW_3302 mutant gave rise at high frequency to colonies identical in appearance to the VIR-O ΔABUW_3302 mutant. However, the VIR-O ΔABUW_3302 mutant did not give rise to colonies of the AV-T morphology when restreaked. To determine if the rate of VIR-O to AV-T switching was reduced by the ΔABUW_3302 mutation, the frequency of switching in 24-h colonies was determined (Table 1). While the rate of VIR-O to AV-T was measured at 6.6% in wild-type cells, we could not detect any AV-T colonies arising from the VIR-O mutant in the ΔABUW_3302 mutant background. The lower limit of detection in these switching assays is estimated to be 1/105 to 1/106 cells, making the decrease in switching at least 5,000-fold. Interestingly, when the rate of AV-T to VIR-O switching was determined, the levels were similar in wild-type cells and the ΔABUW_3302 mutant, 7.5% ± 1.7% and 10.9% ± 6.8%, respectively. To determine whether providing the ΔABUW_3302 gene in trans could complement the ΔABUW_3302 mutation, an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible copy of ΔABUW_3302 was introduced into the chromosome at the glmS site on a Tn7 derivative. In the presence of 2 mM IPTG, the ΔABUW_3302/Tn7::ABUW_3302 cells now exhibited a normal colony morphology, and the rate of VIR-O to AV-T switching was restored to 20.5% ± 8.6%.
TABLE 1.
Analysis of switching frequency between VIR-O and AV-T variants
| Strain or genotype | VIR-O to AV-T (%) | AV-T to VIR-O (%) |
|---|---|---|
| Wild type | 6.6 ± 2.9 | 7.5 ± 1.7 |
| ΔABUW_3302 | None detected | 10.9 ± 6.8 |
| ΔABUW_3302/Tn7::ABUW_3302 | 20.5 ± 8.6 | Not determined |
The ABUW_3302 gene encodes a RelA ortholog.
To determine whether the ABUW_3302 gene encoded a ppGpp synthetase, the production of ppGpp was examined by thin-layer chromatography in the presence or absence of serine hydroxamate, an inducer of the RelA-dependent stringent response. In wild-type cells, the presence of serine hydroxamate induced the production of ppGpp. However, the ΔrelA mutant was unable to produce ppGpp, confirming that it functioned as a ppGpp synthetase (Fig. 2). Based on this result and the amino acid similarity of the ABUW_3302 gene product to those of the RelA proteins of other bacteria, the ABUW_3302 gene will be referred to here as relA.
FIG 2.

TLC autoradiogram of 32P-labeled nucleotides from the AB5075 VIR-O wild type (WT) and the ΔABUW_3302 (relA) mutant after exposure to 0.4 mg/ml serine hydroxamate (SHX) for 5 min. The autoradiogram shown is representative of three independent experiments.
The ΔrelA mutation results in cell elongation at high density.
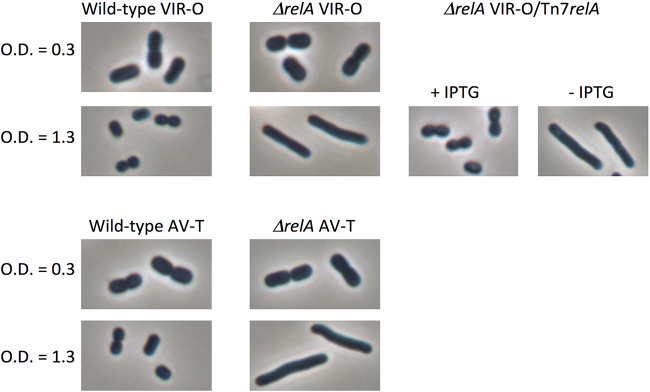
Cells of the wild-type VIR-O and AV-T variants and the corresponding isogenic ΔrelA mutants were examined by phase-contrast microscopy at mid-log phase and at early stationary phase. In mid-log-phase cultures, both wild-type and ΔrelA cells from either VIR-O or AV-T backgrounds exhibited similar morphologies (Fig. 3). However, at high cell density, the ΔrelA mutation in both the VIR-O and AV-T backgrounds resulted in elongated cells relative to the wild type, which was restored by a wild-type copy of relA (Fig. 3).
FIG 3.
Cell morphology at low and high cell densities. Cells were grown in LB and examined microscopically at early log phase (OD600 of 0.3) or at high density (OD600 of 1.3).
The ΔrelA mutation results in a hypermotile phenotype.
Based on the flat, irregular, and spreading colonies formed by the VIR-O ΔrelA mutant on 0.5× LB agar plates, we predicted that cells would be hypermotile, as a similar colony phenotype and hypermotility was previously observed in an arpB mutant (14). When motility was measured on 0.35% agar plates, the VIR-O ΔrelA mutant was indeed hypermotile relative to wild-type cells (Fig. 4A). However, this increase in motility was specific to the VIR-O form. When the AV-T ΔrelA mutant was tested for motility, it was similar to wild-type AV-T cells (data not shown). Introduction of the wild-type relA gene under the control of an IPTG-inducible tac promoter in single copy (Tn7) into the VIR-O ΔrelA mutant reduced motility back to wild-type levels when relA expression was induced with IPTG (Fig. 4A).
FIG 4.
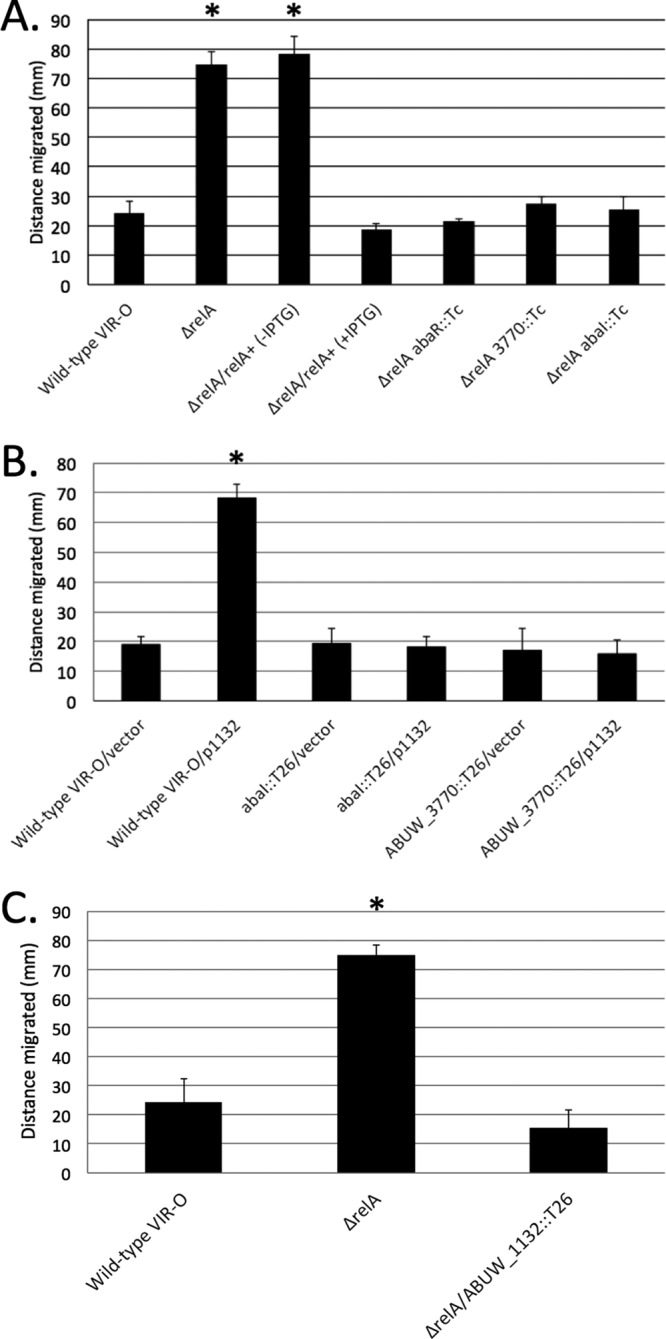
Surface motility of A. baumannii strains. Cultures of strains to be tested were grown to an optical density of 0.5, and a 1-μl aliquot was placed on the surface of a 0.35% Eiken agar plate. Plates were incubated at 37°C for 8 h. The reported values represent the averages from 6 measurements per strain, 2 from three independent experiments. *, P < 0.05 versus wild-type control determined by Student’s t test.
RelA represses quorum sensing.
Previous studies have indicated that surface motility in A. baumannii and Acinetobacter nosocomialis is dependent on a number of factors, including (i) production of the quorum-sensing signal 3-OH C12-homoserine lactone (HSL) mediated by the AbaI autoinducer synthase, which is activated by the AbaR transcriptional regulator (46), (ii) 1, 3-diaminopropane produced by the combined activity of the Dat and Ddc proteins (47), (iii) acinetin 505, a surfactant-like molecule produced by proteins encoded by a large operon, ABUW_3766-ABUW_3773 (46, 48), and (iv) loss of the histone-like protein H-NS (49). To determine if the ΔrelA mutation altered the expression of genes encoding these motility-related factors, we used quantitative real-time PCR (qRT-PCR) to measure expression of these genes in wild-type VIR-O and isogenic ΔrelA backgrounds. Expression of abaI, abaR, and ABUW_3770, a representative gene in the ABUW_3766-ABUW_3773 operon, were upregulated 3.9-fold, 41.3-fold, and 55.6-fold, respectively, in the ΔrelA mutant (Table 2). In contrast, expression of ABUW_1109 (dat) and ABUW_3609 (hns) was not significantly altered in the ΔrelA mutant (Table 2). Introduction of a wild-type copy of relA on a Tn7 derivative into the ΔrelA mutant reduced the levels of abaI, abaR, and ABUW_3770 expression back to wild-type levels (Table 2).
TABLE 2.
Real-time qRT-PCR analysis of gene expression
| Strain or genotype | Relative expression ofa
: |
|||||
|---|---|---|---|---|---|---|
| abaI | abaR | ABUW_3770 | dat (ABUW_1109) | hns (ABUW_3609) | ABUW_1132 | |
| Wild type | 1 | 1 | 1 | 1 | 1 | 1 |
| ΔrelA | 3.9 ± 1.3 | 41.3 ± 10.4b | 55.6 ± 17.1b | 1.7 ± 0.3 | 1.5 ± 0.3 | 14.2 ± 1.0b |
| ΔrelA/ABUW_1132::T26 | 1.7 ± 0.7 | 7.9 ± 3.0c | 0.9 ± 0.3c | |||
| ΔrelA Tn7relAd | 0.8 ± 0.1 | 1.2 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.1 | ||
Values represent the means from three biological replicates with standard errors. Values for each strain were calculated using the 2−ΔΔCT method with clpX as an internal control.
P < 0.05 versus wild type.
P < 0.05 versus ΔrelA.
Cells with Tn7relA were grown with 5 mM IPTG.
Next, to evaluate if hypermotility in the ΔrelA mutant was dependent on overexpression of the abaI, abaR, or ABUW_3770 gene, abaI::T26, abaR::T26, and ABUW_3770::T26 mutations were individually introduced into the ΔrelA background, and all these mutations resulted in loss of the hypermotile phenotype (Fig. 4A).
RelA controls motility and quorum sensing via ABUW_1132, a LysR-type regulator.
In the course of screening an A. baumannii plasmid library for a separate study, a transformant was identified that produced large flat colonies with irregular edges on 0.5× LB agar plates. This phenotype was very similar to that seen in the ΔrelA mutant and suggested a hypermotile phenotype. The only functional gene within this insert was ABUW_1132, encoding a predicted LysR-type transcriptional regulator. To verify that motility was increased in this transformant, surface motility was examined on 0.35% Eiken agar plates. The presence of ABUW_1132 in multicopy (p1132) resulted in a 3.8-fold increase in motility compared to that of the vector control (Fig. 4B). In a manner similar to that seen in the ΔrelA mutant, the hypermotile phenotype resulting from ABUW_1132 overexpression was lost in abaI::T26 and ABUW_3770::T26 mutants, indicating that a functional quorum-sensing pathway and acinetin 505 synthesis were required (Fig. 4B).
Since the motility phenotype resulting from ABUW_1132 overexpression and that of the ΔrelA mutant were similar, two additional experiments were conducted to determine if the increased motility in the ΔrelA mutant was due to ABUW_1132 overexpression. First, qRT-PCR analysis of ABUW_1132 mRNA levels in a wild-type VIR-O and the isogenic ΔrelA mutant verified that expression of ABUW_1132 was increased 14.2 ± 1.0-fold in the ΔrelA mutant (Table 2). The increased ABUW_1132 expression was brought back to wild-type levels in the ΔrelA mutant containing a wild-type copy of relA (Tn7relA). Next, to determine if the increased ABUW_1132 expression in the ΔrelA mutant was responsible for activation of the downstream abaI, abaR, and ABUW_3770 genes, the expression of these genes was examined by qRT-PCR in the ΔrelA mutant and in a ΔrelA ABUW_1132::T26 double mutant. The increased expression of abaI, abaR, and ABUW_3770 in the ΔrelA mutant was now lost or significantly reduced in the ΔrelA ABUW_1132::T26 double mutant (Table 2). In addition, the hypermotile phenotype observed in the relA mutant was now lost in the ΔrelA ABUW_1132::T26 double mutant (Fig. 4C).
Role of RelA in persister cell formation.
Strain AB5075 is highly resistant to most antibiotics and only exhibits sensitivity to a limited number of bactericidal antibiotics, such as colistin and rifampin. The MICs for colistin and rifampin were similar in both the wild-type VIR-O and the ΔrelA mutant, with the rifampin MIC at 2 μg/ml and the colistin MIC at 0.38 μg/ml. Next, to determine if RelA contributed to the development of persister cells tolerant to high levels of antibiotic, cell survival was assayed after 1 h of exposure to 50× the MIC for colistin or 80× the MIC for rifampin. For wild-type VIR-O with rifampin, the percentage of surviving VIR-O cells was 22.3% ± 10.9%, and for the ΔrelA mutant it was 6.3% ± 5.9%, representing a 3.5-fold decrease. For wild-type VIR-O, the percentage of cells surviving colistin was 7.9% ± 1.9%. For the ΔrelA mutant, the percentage of surviving cells was 1.7% ± 0.5%, a 4.6-fold decrease (P < 0.05 versus the wild type).
RelA is required for virulence in Galleria mellonella.
To investigate the role of RelA in virulence, larvae of the Galleria mellonella moth were utilized. Larvae (n = 30/strain) were infected with 9 × 105 cells of the wild-type VIR-O or isogenic ΔrelA mutant, and survival was monitored over a 5-day period. The ΔrelA mutant exhibited reduced virulence, as indicated by the increased overall survival after 5 days relative to that of larvae infected with wild-type VIR-O cells (P < 0.001) (Fig. 5). The reduced virulence of the ΔrelA mutant was restored when a copy of relA (Tn7relA) was introduced (Fig. 5).
FIG 5.

Virulence assays using Galleria mellonella. Larvae weighing between 200 and 250 mg were injected with 9 × 105 cells of the indicated strains. Larvae were monitored daily for survival for a period of 5 days. The reported values represent the averages from 30 larvae from a total of three independent experiments where 10 larvae/strain were used. *, P < 0.001 by the Mantel-Cox test.
DISCUSSION
In this study, we have identified and characterized a RelA ortholog in A. baumannii and demonstrated that it regulates multiple cellular functions. Our initial identification of the ΔrelA mutation resulted from a transposon insertion in the avirulent translucent (AV-T) colony variant that gave an unusual colony phenotype when viewed by oblique lighting under a dissecting microscope. This phenotype was likely the result of elongated cells that changed the colony appearance. This finding is consistent with previous studies that have shown that relA mutations and the resulting loss of ppGpp can also result in cell elongation (50–52). Additional changes in colony morphology due to loss of relA were evident in virulent opaque (VIR-O) cells, where colonies exhibited a spreading morphology on 0.5× agar plates, a phenotype consistent with increased surface motility.
The ΔrelA mutation in a VIR-O background resulted in a 3-fold increase in surface motility (Fig. 4A). This increased motility was dependent on the LysR-type transcriptional regulator ABUW_1132, AbaI (autoinducer synthase), AbaR (LuxR-type regulator), and ABUW_3770. Previous studies in A. nosocomialis M2 have shown that abaI, encoding an autoinducer synthase responsible for the production of 3-OH C12-HSL, was required for surface motility (46). In addition, the AbaI-dependent quorum-sensing pathway in A. nosocomialis was responsible for activating a large operon highly similar to A1S_0112-A1S_0119 in A. baumannii strain ATCC 17978 and to ABUW_3766-ABUW_3773 in A. baumannii AB5075 (46). Recent work has shown the A1S_0112-A1S_0119 gene products direct the production of acinetin 505, a surfactant-like lipopeptide (48). In the ΔrelA mutant, expression of abaR and ABUW_3770 (a representative gene for the ABUW_3766-ABUW_3773 operon) were upregulated 41-fold and 56-fold, respectively. This suggests the following mechanism to explain the hypermotile phenotype in the ΔrelA mutant (Fig. 6). First, loss of relA increases ABUW_1132 expression, which directly or indirectly activates AbaR. In turn, together with 3-OH C12-HSL, AbaR then activates the ABUW_3766-ABUW_3773 operon, which increases acinetin 505 production and aids cell motility by its surfactant-like qualities. However, the loss of relA may also increase abaR expression in a manner independent of ABUW_1132, as the levels of expression in the relA ABUW_1132::T26 double mutant are still elevated above wild-type levels (Table 2). Interestingly, the effect of RelA on the quorum-sensing response in A. baumannii differs from that seen in Pseudomonas aeruginosa, where quorum sensing is activated in a RelA-dependent manner (53).
FIG 6.

A model for the RelA-dependent control of downstream pathways. RelA is predicted to act as a negative regulator of both the LysR-type regulator ABUW_1132 and the LuxR-type regulator AbaR. ABUW_1132 then activates the abaR gene and, together with 3-OH C12-HSL, AbaR then activates the abaI gene and the ABUW_3766-ABUW_3773 operon.
The ΔrelA mutation completely blocked the ability of A. baumannii AB5075 to switch from the virulent opaque form (VIR-O) to the avirulent translucent form (AV-T). This phenotype was fully complemented by expressing a single copy of the relA gene from an IPTG-inducible promoter. Interestingly, the reciprocal AV-T to VIR-O switch was unaffected by the ΔrelA mutation. A similar effect on switching was also observed in arpAB mutants, where switching was decreased in the VIR-O to AV-T direction only (14). This adds support to the hypothesis that separate pathways can control the directionality of this virulence switch. Since arpAB mutants exhibited a defect in switching, we determined if the ΔrelA mutation decreased expression of these genes, but similar levels were observed in the wild type and the ΔrelA mutant (M. Perez-Varela and P. Rather, unpublished data). Despite being locked in the virulent opaque form, the ΔrelA mutant exhibited a significant decrease in virulence when tested in a Galleria mellonella model. Reduced virulence is a common phenotype resulting from loss of RelA in other bacteria (24–32). The contribution of decreased switching from the VIR-O to the AV-T form to the overall levels of virulence in vivo are likely to be minimal, as the majority of cells will be in the VIR-O form either way. Therefore, in the absence of other genetic changes, simply locking a strain in the VIR-O state may not increase virulence. The reduced virulence of the ΔrelA mutant is likely due to global pleiotropic changes that negatively impact virulence.
The mechanism by which the ΔrelA mutation results in loss of switching from the VIR-O to AV-T state is under investigation. However, a ΔrelA ABUW_1132 double mutant did not exhibit restored switching, indicating that the RelA-mediated upregulation of ABUW_1132 was not involved (Perez-Varela and Rather, unpublished). It is possible that ppGpp directly influences the activity of a transcriptional regulatory protein in a manner similar to that seen with SlyA in S. enterica serovar Typhimurium and PigR in Francisella tularensis (54, 55). Alternatively, the loss of switching may result from a more general mechanism due to the global changes in gene expression brought about by reprogramming RNA polymerase. We are currently investigating these possibilities by conducting suppressor screens to identify mutations or high-copy-number suppressors that restore VIR-O to AV-T switching in the ΔrelA mutant. Additionally, a SpoT ortholog (ABUW_0309) is present in A. baumannii, and its role in A. baumannii is under investigation.
MATERIALS AND METHODS
Strains and growth conditions.
A. baumannii AB5075 was grown in modified Luria-Bertani (LB) broth (10 g tryptone, 5 g yeast extract, 5 g NaCl per liter) at 37°C with shaking at 270 rpm. LB agar plates were prepared either full strength (1× LB), using 10 g tryptone, 5 g yeast extract, and 5 g NaCl per liter and 1.5% agar, or half strength (0.5× LB), using 5 g tryptone, 2.5 g yeast extract, and 2.5 g NaCl per liter and 0.8% agar. To prepare competent AB5075 AV-T, cells were grown to an optical density at 600 nm (OD600) of 0.8, pelleted by centrifugation, and washed twice with 1 ml of ice-cold 10% glycerol. Competent cells were resuspended in 70 μl of ice-cold 10% glycerol per electroporation. Following electroporation, cells were resuspended in 1 ml of LB broth and recovered by stationary incubation at 37°C for 30 min followed by 30 min of incubation at 270 rpm. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a final concentration of 2 μM to media.
Measurement of (p)ppGpp pools.
Nucleotides were measured as described previously (56, 57). A. baumannii cells were grown in morpholinepropanesulfonic acid (MOPS) minimal medium supplemented with 0.4% d-glucose, 40 μg/ml of each amino acid except serine, and 0.4 mM phosphate. MOPS minimal medium consisted of 40 mM MOPS buffer, 4 mM tricine, 0.4% d-glucose, 40 μg/ml of each amino acid except serine, 2 mM K2HPO4, 10 μM FeSO4·7H2O, 9.5 mM NH4Cl, 276 μM K2SO4, 500 nM CaCl2, 50 mM NaCl, 525 μM MgCl2, 2.9 nM (NH4)6Mo7O24·4H2O, 400 nM H3BO3, 30 nM CoCl2, 9.6 nM CuSO4, 80.8 nM MnCl2, and 9.74 nM ZnSO4 (pH 7.2) (58). Logarithmically grown bacterial cultures (OD600 of 0.25) were labeled with 10 μCi/ml of [32P]phosphorous (PerkinElmer, Waltham, MA) for approximately 1.5 doubling times (60 min). Cells were treated with 0.4 mg/ml serine hydroxamate for 5 min before 0.4 ml of ice-cold 50% formic acid was added to the cultures. Samples incubated on ice for at least 20 min were centrifuged at 13,000 rpm for 5 min. Five microliters of formic acid extracts was spotted along the bottom of polyethyleneimine-cellulose thin-layer chromatography (TLC) plates (20 cm by 20 cm; Millipore, Billerica, MA), and air-dried plates were separated with a 1.25 M KH2PO4 (pH 3.4) solvent system in a TLC chamber for 1 h. TLC autoradiograms were visualized using a phosphorimager (Bio-Rad, Hercules, CA).
Transposon mutagenesis.
The AB5075 AV-T variant was mutagenized with EZ-Tn5 <Tet-1> (Lucigen) per the manufacturer’s instructions. The transposon solution (0.5 μl transposon, 0.5 μl transposase, 1.5 μl 10% glycerol) was incubated at 37°C for 1 h prior to electroporation into competent AB5075 AV-T cells. Electroporations were performed in Bio-Rad Gene Pulser 0.2-cm-gap cuvettes with a MicroPulser electroporator (Bio-Rad, Hercules, CA). Cells were plated on LB agar plates with tetracycline and incubated at 37°C for 24 h. Plates were inspected using a dissecting microscope with oblique lighting.
Identification of the EZ-Tn5 <Tet-1> insertion site.
To identify the insertion site of the EZ-Tn5 <Tet-1> insertion, total genomic DNA from the mutant was partially digested with Sau3A, and fragments in the 2- to 4-kb range were gel purified, ligated into the BamHI site of pBC SK(−), and electroporated into EC100D competent cells (Lucigen, Middleton, WI). Cells were plated on LB agar plates containing 10 μg/ml tetracycline and incubated overnight at 37°C. To confirm that the insertions were present, plasmid DNA was purified from Tetr colonies using the QIAprep Spin Miniprep kit (Qiagen, Hilden, Germany) prior to digestion with XbaI. Plasmids containing insertions were sequenced with primers FP1 and RP1 provided by the EZ-Tn5 <Tet-1> insertion kit (Lucigen, Middleton, WI).
Microscopy.
Cells were grown in LB to either mid-log phase (OD600 of 0.3) or to early stationary phase (OD600 of 1.3). Cells at mid-log phase were concentrated 10-fold prior to phase-contrast microscopy at ×1,000 magnification under an oil immersion lens.
Construction of an in-frame deletion in relA.
To create an in-frame deletion mutant, a fragment upstream of the relA gene (RelA Up) which contains the first few codons of relA and flanking DNA was PCR amplified using primers Up-1 (5′-AAAAAGGATCCGCTTGAGCCTTTTTCTCACC-3′) and Up-2 (5′-GCACGACGCATGATTCAAG-3′). A second fragment downstream of relA (RelA Down) containing the last few relA codons and flanking DNA was PCR amplified using primers Down-1 (5′-CTGTTCACGTACTGTGACCAT-3′) and Down-2 (5′-AAAAAGGATCCTAAGCGCCATCCAACTTTAGT-3′). Primers Up-2 and Down-1 were previously phosphorylated using T4 polynucleotide kinase (New England BioLabs, Beverly, MA). RelA Up and RelA Down were ligated for 2 h, run on a 0.8% Tris-acetate-EDTA (TAE) gel, and the gel section corresponding to the size of the combined fragments was gel purified. The fragment was PCR amplified using primers Up-1 and Down-2 to create the ΔrelA fragment, which was digested and ligated into the BamHI site of pEX18Tc. The ligation was transformed into EC100D cells and plated on LB plates containing tetracycline (10 μg/ml). Plasmid DNA was isolated from Tetr colonies and digested to confirm the presence of pEX18Tc-ΔrelA construct. pEX18Tc-ΔrelA was electroporated and maintained in E. coli SM10 λpir. E. coli SM10 λpir pEX18Tc-ΔrelA was conjugated to the VIR-O variant of AB5075, and an Ampr Tetr Sucs AB5075 exconjugant was grown in LB broth at 37°C for 1.5 h at 250 rpm. Cells were plated on LB (without NaCl) containing 10% sucrose and incubated at room temperature. Sucrose-resistant colonies were screened via PCR using primers Up-1 and Down-2 to confirm the deletion of relA.
Construction of a relA-complemented strain.
A wild-type copy of the relA gene with its native ribosome binding site was generated by PCR using the primers AGGAGAATGGTATGGTCACAGTACG and ACTCTCAACAATAAGTCCCCAG. This PCR-generated fragment was then cloned into the SmaI site of pUC18Tn7 LAC Apr (kindly provided by Ayush Kumar at the University of Manitoba) (59). A recombinant containing the relA gene in the correct orientation such that expression could be driven by the IPTG-inducible tac promoter was electroporated along with a plasmid containing the Tn7 transposase (pTNS2) into the ΔrelA mutant. A strain with the correct insertion of the Tn7/relA into the glmS region of the chromosome was verified by PCR. This strain was then grown in the presence of IPTG at the indicated concentrations for complementation experiments.
Moving T26 transposon insertions into new backgrounds.
A recent study reported that chromosomal markers could be moved between A. baumannii strains by phage-mediated generalized transduction (60). Based on this study, we determined that insertions from the comprehensive insertion library constructed by Colin Manoil’s group at the University of Washington could be moved between strains by the same procedure. Overnight saturated cultures containing a given transposon insertion were spun down, and 1 ml of supernatant was filter sterilized (0.22 μm) to generate a phage lysate. To transduce the recipient strain, 20 μl of a mid-log-phase culture was mixed with 20 μl of lysate and spotted on the surface of a well-dried LB plate to allow the spot to quickly soak into the agar. After incubation at 37°C for 4 h, cells were scraped from the plate, resuspended in 2 ml of LB broth, and plated on LB plus tetracycline (5 μg/ml). Colonies were screened by PCR for the correct insertion.
Plasmid p1132 construction.
Plasmid pQF1266 is a derivative of pQF50 (61) and contains a hygromycin resistance cassette cloned into the ScaI site of the β-lactamase gene and an origin of replication from pWH1266. A partial Sau3A digest of AB5075 chromosomal DNA was performed, and fragments in the size range of 3 to 6 kb were cloned into the BamHI site of pQF1266 (62). This genomic library was used in a variety of genetic screens, and a recombinant plasmid that generated a hypermotile colony was designated p1132. The only complete gene within this plasmid was ABUW_1132.
Analysis of VIR-O and AV-T switching frequencies.
Aliquots of AB5075 VIR-O and AV-T variants in the wild-type and ΔrelA mutant backgrounds were serially diluted, plated on 0.5× LB plates, and incubated at 37°C for 24 h. Plates containing fewer than 50 colonies were used, and 6 well-isolated colonies from each strain were removed from the plate as agar plugs and resuspended in 2 ml LB broth. Serial dilutions of 10−4, 10−5, and 10−7 were plated on 0.5× LB plates and incubated at 37°C for 24 h. Plates were inspected under a dissecting microscope via indirect light, and VIR-O and AV-T variants were quantified.
Motility assays.
Motility assays were carried out on Eiken agar motility plates consisted of 10 g tryptone, 5 g yeast extract, 5 g NaCl, and 3.5 g Eiken agar (Eiken Chemical Ltd., Tokyo, Japan) per liter. The plates were prepared the same day of the assay and they were allowed to dry for 30 min before use. Strains were inoculated in LB broth medium and grown at 37°C with shaking until an OD600 of 0.5 was reached. A 1-μl aliquot of the corresponding strain culture was spotted onto the 0.35% Eiken agar motility plates, and the plates were incubated at 37°C for approximately 8 h, which was previously determined to be the time required for the ΔrelA mutant strain to reach the plate border under experimental conditions. Wild-type and ΔrelA mutant strains were compared on the same plates. Motility diameters were measured after the designated time, and statistical analysis was performed using Student's t test. The relative migration was calculated as the ratio of the motility diameter of the studied strain to that of the corresponding control strain in each case. All assays were conducted a minimum of two independent times.
Galleria mellonella infections.
G. mellonella larvae were purchased from Speedy Worm, Alexandria, MN. For larval infections, the wild-type VIR-O variant of AB5075 and isogenic ΔrelA mutant cultures were grown in 2 ml of LB broth at 37°C with shaking to an optical density (OD600) of approximately 0.5. Serial dilutions were prepared in LB with 20% glycerol and stored in aliquots at −80°C. For infections, 9 × 105 cells were injected in an 8-μl volume with IPTG (10 mM) into the last proleg of G. mellonella larvae weighing between 200 and 250 mg. Larvae were incubated in a petri plate at 37°C in a humidified incubator for up to 5 days. The number of dead larvae was evaluated at 24-h intervals; larvae were considered dead when they were dark brown to black and showed no movement in response to touch with a pipette tip. Injections of LB alone did not result in killing. For all experiments, the serial dilutions used were plated onto regular LB plates to determine CFU per milliliter for each bacterial strain. The reported data correspond to three independent experiments carried out with 10 larvae for each strain.
RNA extraction and quantitative real-time PCR.
Cultures of wild-type VIR-O strain AB5075 and the ΔrelA mutant were grown in 2 ml of LB broth at 37°C with shaking to an OD600 of approximately 1.500. The cultures were centrifuged, and the RNA was isolated using the MasterPure RNA isolation kit (Lucigen, Middleton, WI) according to the manufacturer’s instructions. The samples were treated with Turbo DNA-free DNase (Ambion) to remove the contaminating DNA. The RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer, and cDNA was obtained by using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) with random primers. The reverse transcription reaction was performed as follows: 25°C for 5 min, 42°C for 45 min, and 85°C for 5 min. Diluted cDNA (1:10) was used as the template for experimental reactions. Specific oligonucleotides pairs for quantitative real-time PCR (qRT-PCR) to amplify approximately 150-bp fragments from each gene of study were generated by using the Primer-BLAST program (www.ncbi.nlm.nih.gov/tools/primer-blast/). Gene expression was determined using iQ SYBR green Supermix (Bio-Rad, Hercules, CA) on a CFX Connect cycler (Bio-Rad). Cycle conditions to amplify and quantify fragments were the following: 95°C for 3 min and then 95°C for 10 s, 55°C for 10 s, and 72°C for 20 s, repeated 40 times. Melt curve data were then collected to confirm the specificity of the oligonucleotide primer set. Data were generated from two independent RNA isolation and cDNA preparations and three replicates for each primer pair. The expression level of the target genes was standardized relative to the transcription level of the housekeeping gene clpX using the threshold cycle (2−ΔΔCT) method (63). The statistical significance of the observed differences was confirmed by the analysis of variance (ANOVA) test.
Persister cell assays.
Wild-type and ΔrelA cells were grown to an OD600 of 1.1 and incubated for 1 h at 37°C with either rifampin at a final concentration of 160 μg/ml or colistin at a final concentration of 20 μg/ml. Dilutions were then plated for CFU on LB agar plates.
ACKNOWLEDGMENTS
This work was supported by funding from the Department of Veterans Affairs (I01BX001725 and IK6BX004470) and NIH (R21AI142489 to P.N.R., and R01AI36520, R01AI05449 and VAI01BX002073 to A.V.T.).
We thank Ayush Kumar at the University of Manitoba for providing pUC18-miniTn7-Lac-Apra. We are grateful to Sarah Dayman, Marjan Farokhyfar, and Daniel Knight for help with strain and plasmid constructions.
REFERENCES
- 1.Gootz TD, Marra A. 2008. Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev Anti Infect Ther 6:309–325. doi: 10.1586/14787210.6.3.309. [DOI] [PubMed] [Google Scholar]
- 2.Joly-Guillou ML. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect 11:868–873. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 3.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visca P, Seifert H, Towner KJ. 2011. Acinetobacter infection–an emerging threat to human health. IUBMB Life 63:1048–1054. doi: 10.1002/iub.534. [DOI] [PubMed] [Google Scholar]
- 5.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, Aboderin AO, Al-Abri SS, Awang Jalil N, Benzonana N, Bhattacharya S, Brink AJ, Burkert FR, Cars O, Cornaglia G, Dyar OJ, Friedrich AW, Gales AC, Gandra S, Giske CG, Goff DA, Goossens H, Gottlieb T, Guzman Blanco M, Hryniewicz W, Kattula D, Jinks T, Kanj SS, Kerr L, Kieny M-P, Kim YS, Kozlov RS, Labarca J, Laxminarayan R, Leder K, et al. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 7.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 8.Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 9.Rice LB. 2006. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 43:S100–S105. doi: 10.1086/504487. [DOI] [PubMed] [Google Scholar]
- 10.Telang NV, Satpute MG, Dhakephalkar PK, Niphadkar KB, Joshi SG. 2011. Fulminating septicemia due to persistent pan-resistant community-acquired metallo-beta-lactamase (IMP-1)-positive Acinetobacter baumannii. Indian J Pathol Microbiol 54:180–182. doi: 10.4103/0377-4929.77397. [DOI] [PubMed] [Google Scholar]
- 11.Lowman W, Kalk T, Menezes CN, John MA, Grobusch MP. 2008. A case of community-acquired Acinetobacter baumannii meningitis - has the threat moved beyond the hospital? J Med Microbiol 57:676–678. doi: 10.1099/jmm.0.47781-0. [DOI] [PubMed] [Google Scholar]
- 12.Tipton KA, Dimitrova D, Rather PN. 2015. Phase-variable control of multiple phenotypes in Acinetobacter baumannii strain AB5075. J Bacteriol 197:2593–2599. doi: 10.1128/JB.00188-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin CY, Tipton KA, Farokhyfar M, Burd EM, Weiss DS, Rather PN. 2018. A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii. Nat Microbiol 3:563–569. doi: 10.1038/s41564-018-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tipton KA, Farokhyfar M, Rather PN. 2017. Multiple roles for a novel RND-type efflux system in Acinetobacter baumannii AB5075. Microbiologyopen 6:e00418. doi: 10.1002/mbo3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tipton KA, Rather PN. 2017. An ompR/envZ two-component system ortholog regulates phase variation, osmotic tolerance, motility, and virulence in Acinetobacter baumannii strain AB5075. J Bacteriol 199:e00705-16. doi: 10.1128/jb.00705-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 17.Irving SE, Corrigan RM. 2018. Triggering the stringent response: signals responsible for activating (p)ppGpp synthesis in bacteria. Microbiology 164:268–276. doi: 10.1099/mic.0.000621. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson GC, Tenson T, Hauryliuk V. 2011. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One 6:e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. 2015. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol 13:298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivatsan A, Wang JD. 2008. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol 11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL. 2004. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Paul BJ, Berkmen MB, Gourse RL. 2005. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci U S A 102:7823–7828. doi: 10.1073/pnas.0501170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jishage M, Kvint K, Shingler V, Nystrom T. 2002. Regulation of sigma factor competition by the alarmone ppGpp. Genes Dev 16:1260–1270. doi: 10.1101/gad.227902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erickson DL, Lines JL, Pesci EC, Venturi V, Storey DG. 2004. Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect Immun 72:5638–5645. doi: 10.1128/IAI.72.10.5638-5645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer BK, Swanson MS. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol Microbiol 33:721–731. doi: 10.1046/j.1365-2958.1999.01519.x. [DOI] [PubMed] [Google Scholar]
- 26.Muller CM, Conejero L, Spink N, Wand ME, Bancroft GJ, Titball RW. 2012. Role of RelA and SpoT in Burkholderia pseudomallei virulence and immunity. Infect Immun 80:3247–3255. doi: 10.1128/IAI.00178-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean RE, Ireland PM, Jordan JE, Titball RW, Oyston PC. 2009. RelA regulates virulence and intracellular survival of Francisella novicida. Microbiology 155:4104–4113. doi: 10.1099/mic.0.031021-0. [DOI] [PubMed] [Google Scholar]
- 28.Haralalka S, Nandi S, Bhadra RK. 2003. Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors. J Bacteriol 185:4672–4682. doi: 10.1128/JB.185.16.4672-4682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun W, Roland KL, Branger CG, Kuang X, Curtiss R III. 2009. The role of relA and spoT in Yersinia pestis KIM5 pathogenicity. PLoS One 4:e6720. doi: 10.1371/journal.pone.0006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pizarro-Cerdá J, Tedin K. 2004. The bacterial signal molecule, ppGpp, regulates Salmonella virulence gene expression. Mol Microbiol 52:1827–1844. doi: 10.1111/j.1365-2958.2004.04122.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi N, Abe H, Ogura Y, Hayashi T, Tashiro K, Kuhara S, Sugimoto N, Tobe T. 2006. ppGpp with DksA controls gene expression in the locus of enterocyte effacement (LEE) pathogenicity island of enterohaemorrhagic Escherichia coli through activation of two virulence regulatory genes. Mol Microbiol 61:194–205. doi: 10.1111/j.1365-2958.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- 32.Tapscott T, Kim J-S, Crawford MA, Fitzsimmons L, Liu L, Jones-Carson J, Vázquez-Torres A. 2018. Guanosine tetraphosphate relieves the negative regulation of Salmonella pathogenicity island-2 gene transcription exerted by the AT-rich ssrA discriminator region. Sci Rep 8:9465. doi: 10.1038/s41598-018-27780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korch SB, Henderson TA, Hill TM. 2003. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol 50:1199–1213. doi: 10.1046/j.1365-2958.2003.03779.x. [DOI] [PubMed] [Google Scholar]
- 34.Fung DK, Chan EW, Chin ML, Chan RC. 2010. Delineation of a bacterial starvation stress response network which can mediate antibiotic tolerance development. Antimicrob Agents Chemother 54:1082–1093. doi: 10.1128/AAC.01218-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amato SM, Brynildsen MP. 2015. Persister heterogeneity arising from a single metabolic stress. Curr Biol 25:2090–2098. doi: 10.1016/j.cub.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 36.Cohen NR, Lobritz MA, Collins JJ. 2013. Microbial persistence and the road to drug resistance. Cell Host Microbe 13:632–642. doi: 10.1016/j.chom.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 39.Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. 2010. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev 74:171–199. doi: 10.1128/MMBR.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalebroux ZD, Swanson MS. 2012. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol 10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- 41.Umland TC, Schultz LW, MacDonald U, Beanan JM, Olson R, Russo TA. 2012. In vivo-validated essential genes identified in Acinetobacter baumannii by using human ascites overlap poorly with essential genes detected on laboratory media. mBio 3:e00113-12. doi: 10.1128/mBio.00113-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez-Larrayoz AF, Elhosseiny NM, Chevrette MG, Fu Y, Giunta P, Spallanzani RG, Ravi K, Pier GB, Lory S, Maira-Litrán T. 2017. Complexity of complement resistance factors expressed by Acinetobacter baumannii needed for survival in human serum. J Immunol 199:2803–2814. doi: 10.4049/jimmunol.1700877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung H-W, Kim K, Islam MM, Lee JC, Shin M. 12 February 2020. Role of ppGpp-regulated efflux genes in Acinetobacter baumannii. J Antimicrob Chemother doi: 10.1093/jac/dkaa014. [DOI] [PubMed] [Google Scholar]
- 44.Knight D, Dimitrova DD, Rudin SD, Bonomo RA, Rather PN. 2016. Mutations decreasing intrinsic beta-lactam resistance are linked to cell division in the nosocomial pathogen Acinetobacter baumannii. Antimicrob Agents Chemother 60:3751–3758. doi: 10.1128/AAC.00361-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown DR, Barton G, Pan Z, Buck M, Wigneshweraraj S. 2014. Nitrogen stress response and stringent response are coupled in Escherichia coli. Nat Commun 5:4115. doi: 10.1038/ncomms5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clemmer KM, Bonomo RA, Rather PN. 2011. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology 157:2534–2544. doi: 10.1099/mic.0.049791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skiebe E, de Berardinis V, Morczinek P, Kerrinnes T, Faber F, Lepka D, Hammer B, Zimmermann O, Ziesing S, Wichelhaus TA, Hunfeld KP, Borgmann S, Grobner S, Higgins PG, Seifert H, Busse HJ, Witte W, Pfeifer Y, Wilharm G. 2012. Surface-associated motility, a common trait of clinical isolates of Acinetobacter baumannii, depends on 1,3-diaminopropane. Int J Med Microbiol 302:117–128. doi: 10.1016/j.ijmm.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Rumbo-Feal S, Perez A, Ramelot TA, Alvarez-Fraga L, Vallejo JA, Beceiro A, Ohneck EJ, Arivett BA, Merino M, Fiester SE, Kennedy MA, Actis LA, Bou G, Poza M. 2017. Contribution of the A. baumannii A1S_0114 gene to the interaction with eukaryotic cells and virulence. Front Cell Infect Microbiol 7:108. doi: 10.3389/fcimb.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eijkelkamp BA, Stroeher UH, Hassan KA, Elbourne LD, Paulsen IT, Brown MH. 2013. H-NS plays a role in expression of Acinetobacter baumannii virulence features. Infect Immun 81:2574–2583. doi: 10.1128/IAI.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M. 1991. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem 266:5980–5990. [PubMed] [Google Scholar]
- 51.Dahl JL, Arora K, Boshoff HI, Whiteford DC, Pacheco SA, Walsh OJ, Lau-Bonilla D, Davis WB, Garza AG. 2005. The relA homolog of Mycobacterium smegmatis affects cell appearance, viability, and gene expression. J Bacteriol 187:2439–2447. doi: 10.1128/JB.187.7.2439-2447.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatnaparat T, Li Z, Korban SS, Zhao Y. 2015. The bacterial alarmone (p)ppGpp is required for virulence and controls cell size and survival of Pseudomonas syringae on plants. Environ Microbiol 17:4253–4270. doi: 10.1111/1462-2920.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Delden C, Comte R, Bally AM. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J Bacteriol 183:5376–5384. doi: 10.1128/JB.183.18.5376-5384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao G, Weatherspoon N, Kong W, Curtiss R III, Shi Y. 2008. A dual-signal regulatory circuit activates transcription of a set of divergent operons in Salmonella typhimurium. Proc Natl Acad Sci U S A 105:20924–20929. doi: 10.1073/pnas.0807071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charity JC, Blalock LT, Costante-Hamm MM, Kasper DL, Dove SL. 2009. Small molecule control of virulence gene expression in Francisella tularensis. PLoS Pathog 5:e1000641. doi: 10.1371/journal.ppat.1000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitzsimmons LF, Liu L, Kim J-S, Jones-Carson J, Vázquez-Torres A. 2018. Salmonella reprograms nucleotide metabolism in its adaptation to nitrosative stress. mBio 9:e00211-18. doi: 10.1128/mBio.00211-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cashel M. 1974. Preparation of guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) from Escherichia coli ribosomes. Anal Biochem 57:100–107. doi: 10.1016/0003-2697(74)90056-6. [DOI] [PubMed] [Google Scholar]
- 58.Neidhardt FC, Bloch PL, Smith DF. 1974. Culture medium for enterobacteria. J Bacteriol 119:736–747. doi: 10.1128/JB.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ducas-Mowchun K, De Silva PM, Crisostomo L, Fernando DM, Chao T-C, Pelka P, Schweizer HP, Kumar A. 2019. Next generation of Tn7-based single-copy insertion elements for use in multi- and pan-drug-resistant strains of Acinetobacter baumannii. Appl Environ Microbiol 85:e00066-19. doi: 10.1128/AEM.00066-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wachino J-I, Jin W, Kimura K, Arakawa Y. 2019. Intercellular transfer of chromosomal antimicrobial resistance genes between Acinetobacter baumannii strains mediated by prophages. Antimicrob Agents Chemother 63:e00334-19. doi: 10.1128/AAC.00334-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farinha MA, Kropinski AM. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol 172:3496–3499. doi: 10.1128/JB.172.6.3496-3499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunger M, Schmucker R, Kishan V, Hillen W. 1990. Analysis and nucleotide sequence of an origin of DNA replication in Acinetobacter calcoaceticus and its use for Escherichia coli shuttle plasmids. Gene 87:45–51. doi: 10.1016/0378-1119(90)90494-C. [DOI] [PubMed] [Google Scholar]
- 63.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]