Abstract
It is increasingly recognized that modulation of brain inflammation may uncover new potential therapeutic strategies for stroke. Recent studies have shifted focus from immunological implications in ischemic stroke to a more devastating form; the hemorrhagic stroke.
The aim of this study was to investigate the neuroinflammatory response in cerebrospinal fluid in patients with primary intracerebral hemorrhage (ICH) associated with intraventricular hemorrhage (IVH) in the presence of low-dose recombinant tissue plasminogen activator (rt-PA).
This retrospective study included 88 adults with primary ICH associated with IVH. Patients were divided into 2 groups: rt-PA group and non-rt-PA group, which received normal standard of care for this diagnosis. The rt-PA group was treated via catheter-based clot lysis using low-dose rt-PA injected through the external ventricular drain (EVD) system, and the non-rt-PA group was treated with saline applied to EVD system in equivalent volume. Cerebrospinal fluid samples from rt-PA were obtained from the EVD system at 4 time points: once before the drug administration, and then on day 1, 3, and 7. No attempt at randomization was made. The decision to inject rt-PA was based on the preference of the primary attending neurologist and the ability to obtain consent. Temporal interleukin-1 beta and transforming growth factor beta concentration changes were analyzed and compared between the 2 groups.
The concentration of interleukin-1 beta was significantly lower in the rt-PA group than in the non-rt-PA group on day 7. In addition, the concentration of transforming growth factor beta was significantly higher in the rt-PA group than in the non-rt-PA group on day 1. There was a significant difference in interleukin-1 beta concentration between days 0 and 1 in comparison to day 3 in the rt-PA group, and between day 0 in comparison to day 3 and 7 in the non-rt-PA group. We also observed a significant difference in transforming growth factor beta concentration between days 0 and 1 and between days 3 and 7.
The different pattern of pro- and anti-inflammatory cytokines in patients with ICH associated with IVH suggest distinct characteristics of secondary brain injury depending on the treatment modality.
Keywords: intraventricular hemorrhage, neuroinflammation, primary intracerebral hemorrhage, recombinant tissue plasminogen activator, secondary brain injury
1. Introduction
Approximately 50% of patients with primary intracerebral hemorrhage (ICH) have intraventricular hemorrhage (IVH), which is the most damaging form of stroke.[1,2] Patients with associated IVH are 3 times more likely to experience poor outcomes with high mortality rates, compared to the patients without IVH.[3] Additionally, ICH with IVH causes mechanical brain injury, and blood clot products trigger a complex sequence of events leading to secondary brain damage, including the activation of neuroinflammatory pathways,[4] which complicate the treatment. Previous studies have indicated that treatment with intraventricular administration of low-dose recombinant tissue plasminogen activator (rt-PA) is safe for most patients with ICH associated with IVH. Moreover, rt-PA treatment is associated with improvements in patient outcomes.[5,6]
As a member of the serine protease spectrum, tissue plasminogen activator plays a major role in the homeostasis of blood coagulation, fibrinolysis, and matrix regulation. Acting as a cytokine, tissue plasminogen activator, modulates the inflammatory response to tissue injury in various models and organs triggering intracellular signaling events.[7] Neuroinflammation induced by ICH and IVH involves early activation of resident microglia, the release of pro-inflammatory and anti-inflammatory mediators, and an influx of peripheral leukocytes. Although hematoma clearance is directly dependent on microglia/macrophages, these interactions also release a spectrum of potentially harmful factors; cytokines such as interleukine-1-Beta (IL-1β) and Transforming Growth Factor Beta (TGF-β), chemokines, proteases, and prostaglandins.[8,9,10]
Cytokine IL-1β isoform is strongly implicated in the sterile inflammatory response that aggravates acute cerebrovascular disease and is expressed only during or after the injury. While the precise pathophysiology of IL-1β secretion is poorly understood, recent data suggest that its secretion may depend on the force of the inflammatory stimulus.[11] Additionally, TGF-β plays a key role in microglial development and homeostasis contributing to wound regeneration by triggering responses in macrophages through the SMAD2 and SMAD3 signaling pathways. TGF-β acts as a mediator of a reparative microglial phenotype and is critical to recovery after ICH.[12]
In the present study, we aimed to investigate the dynamic changes of IL-1β and TGF-β concentrations in the cerebrospinal fluid (CSF) in patients with primary ICH with associated IVH treated via catheter-based clot lysis using low-dose rt-PA injected through the external ventricular drainage system.
2. Methods
A total of 88 patients in the period 2010 to 2018 with primary ICH associated with IVH (Fig. 1A, B, C, D) who had been admitted to the Clinical Hospital Center Rijeka, Rijeka, Croatia, within the first 12 hours of symptom onset and met the specific eligibility criteria (Table 1) were enrolled in the present study. The baseline characteristics of the included patients are shown in Table 2.
Figure 1.
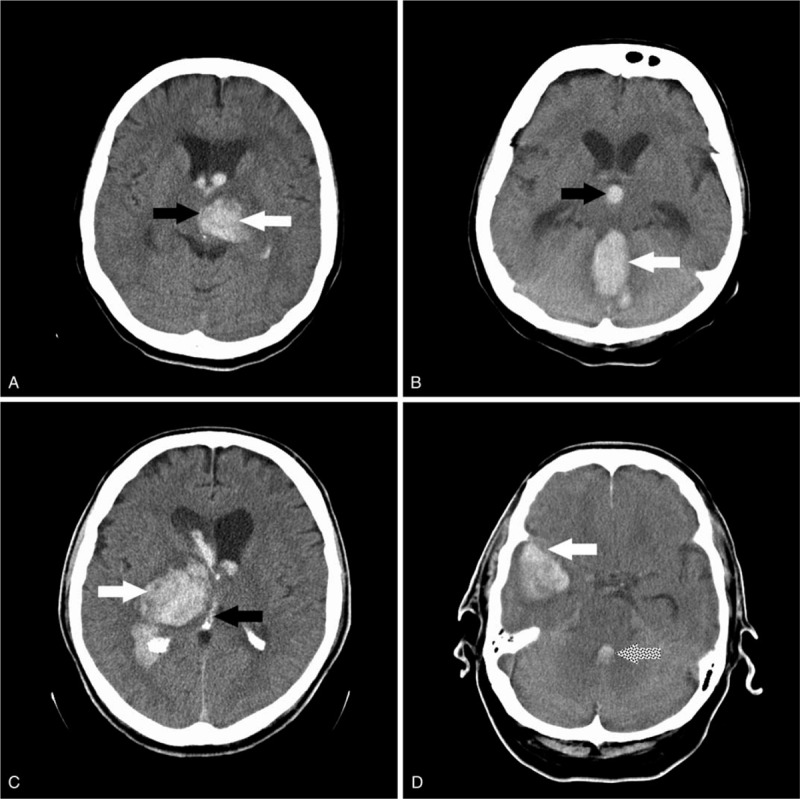
A, 1B, 1C, 1D: Computed tomography head scans of patients with intracerebral hemorrhage associated with intraventricular hemorrhage (IVH). White arrows indicate intracerebral hemorrhage, black arrows indicate IVH in 3rd ventricle, dotted arrow indicates IVH in 4th ventricle.
Table 1.
Eligibility criteria.

Table 2.
Baseline characteristics of patients.
No attempt at randomization was made. The decision to inject rt-PA was based on the preference of the primary attending neurologist and the ability to obtain consent. All patients received standard medical treatment according to an institutional protocol for ICH including early airway securing by endotracheal intubation at Glasgow Coma Scale ≤8 and proper sedation using midazolam and sufentanyl. Intracerebral pressure (ICP) was continuously monitored and treated when increased to more than 20 mm Hg for more than 5 minutes. Blood pressure was monitored using intraarterial catheters and was treated when mean arterial blood pressure exceeded 120 mm Hg (labetalol) or dropped below 90 mm Hg (noradrenalin). Patients were extubated as soon as Glasgow Coma Scale reached 8 or more, and if the brainstem reflexes were intact. In all cases of prolonged ventilation or failure weaning, tracheotomy was performed after 5 days of endotracheal intubation. A complete laboratory panel was performed daily. Cardiac enzymes were drawn when clinically indicated or when electrocardiogram changes suggested myocardial ischemia. External ventricular drain (EVD) (Vygon Neuro, Valley Forge, PA) was not placed for intraventricular thrombolysis purposes but rather as standard of care in monitoring and controlling ICP. EVDs were placed in the frontal horns of the lateral ventricles when intraventricular bleeding was present in the third and/or the fourth ventricle. Proper EVD position was confirmed by computed tomography scan of the head. All patients received the same intensive care treatment according to an institutional protocol for ICH management.[13]
Patients were divided into 2 groups depending on the treatment modality. Rt-PA group (n = 47; median age: 59 years (range: 55–76 years); male/female = 25/22) was treated with 1 mg of rt-PA (Actilyse, Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany), which was injected through the EVD system under sterile conditions every 12 hours[13] until IVH resolution in the third and/or fourth ventricle. Hemorrhage resolution was evaluated at 48 and 72 hours via head computed tomography (Siemens, Erlangen, Germany). In contrast, non-rt-PA group (n = 41; median age: 61 years (range: 56–71 years); male/female = 22/19) was not treated with rt-PA, and EVD was placed as standard institutional protocol. ICH score[14] on admission was equal in both groups (ICH score = 3 [2–3]).
CSF samples (6 mL) were obtained from the EVD system and stored in our biobank for later analysis. The CSF samples from EVD systems of rt-PA and non-rt-PA group were collected at 4 time points: once before the drug administration (day 0), and then on day 1, 3, and 7 and concentrations of IL-1β and TGF-β were measured.
Patients’ data were collected, and CSF samples were procured in accordance with published international health guidelines (2008 Declaration of Helsinki). The study protocol was approved by the local ethics committee in accordance with the World Medical Association's Declaration of Helsinki standards. Written informed consent was obtained from the patient or the patient's family members. Data were analyzed using sandwich enzyme-linked immunosorbent assays, in accordance with the manufacturer's protocol (human IL-1β and TGF-β, eBioscience, San Diego, CA,).
Quantitative data analysis was performed by examining distributions using the Shapiro–Wilk test and normality histograms. Nonparametric tests compared quantitative data, which were presented as the median and interquartile range from the 12.5th to 87.5th quartiles. Friedman and Wilcoxon rank-sum tests were used to compare time points within groups, whereas Mann–Whitney U tests, Kruskal–Wallis and Hi-quadrat tests were used to compare data between groups. Statistical processing was performed using Statistica 13 (StatSoft, Inc., Tulsa, OK,). After applying Bonferroni correction, P-values below .0125 were considered statistically significant.
3. Results
On days 1 (P = .063) and 3 (P = .064), IL-1β concentration in the CSF did not significantly differ between the groups. On day 7, IL-1β concentration was significantly lower in the rt-PA group than in the non-rt-PA group (P = .0094). In rt-PA group, significantly higher IL-1β concentration was found on days 1 (P = .0124), and day 3 (P = .0123) when compared to day 0; while no significant difference was observed between days 0 and 7 (P = .527), between day 1 and days 3 (P = .78) and 7 (P = .445), or between days 3 and 7 (P = .822). In non-rt-PA group, there was a significantly higher IL-1β concentration on days 3 (P = .0019) and 7 (P = .0017) in comparison to day 0; while no significant difference was observed between days 0 and 1 (P = .108), between day 1 and days 3 (P = .103) and 7 (P = .092), or between days 3 and 7 (P = .098) (Fig. 2).
Figure 2.

IL-1β concentrations in non- recombinant tissue plasminogen activator group (□) and recombinant tissue plasminogen activator group (▪). Data are presented as median ( ), 12.5th to 87.5th quartiles (□) and non-outlier range (I). IL-1: interleukin-1. P < .0125.
), 12.5th to 87.5th quartiles (□) and non-outlier range (I). IL-1: interleukin-1. P < .0125.
On day 1 (P = .0012), TGF-β concentration was significantly higher in rt-PA group than in non-rt-PA group. There was no significant difference in TGF-β concentration between the groups on days 3 (P = .052) and 7 (P = .082). In rt-PA group, significantly higher TGF-β concentration was found between days 0 and 1 (P = .0022), between days 0 and 3 (P = .0121), and between days 0 and 7 (P = .0122). However, no significant difference in TGF-β concentration was observed between days 1 and 3 (P = .52) or between days 3 and 7 (P = .27). In non-rt-PA group, significantly higher TGF-β concentration was observed between days 0 and 1 (P = .0021), between days 0 and 3 (P = .0123), and between days 0 and 7 (P = .0124). However, no significant difference in TGF-β concentration was found between days 1 and 3 (P = .092) or between days 3 and 7 (P = .108) (Fig. 3). We compared the mortality rate after a 90-day follow-up, and found a statistically significant decrease in mortality in the rt-PA group (10/47, 21.3%) compared to the non-rt-PA group (14/41, 34.1%), P = .011.
Figure 3.
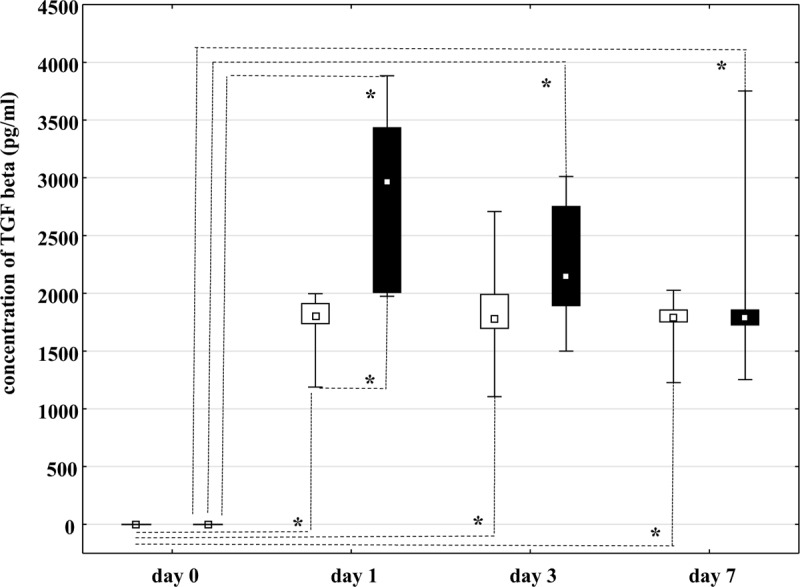
= transforming growth factor beta concentration in recombinant tissue plasminogen activator group (▪) and non- recombinant tissue plasminogen activator group (□). Data are presented as median ( ), 12.5th to 87.5th quartiles (□) and non-outlier range (I). TGF-β: transforming growth factor beta. P < .0125.
), 12.5th to 87.5th quartiles (□) and non-outlier range (I). TGF-β: transforming growth factor beta. P < .0125.
4. Discussion
Intensive treatment aims in patients with primary ICH associated with IVH include neuroprotection and the prevention of secondary brain damage following brain hemorrhage. Previous reports suggest better results for patients treated via intraventricular fibrinolysis, including lower ICP, fewer EVD obstructions necessitating replacement, and an absence of significant ventriculitis when compared with controls treated using EVDs only.[5,6] These results may be attributed to the improvement of blood clearance and clot lysis; together with favorable neuroinflammation modulation and potential reduction of secondary brain damage due to the administration of fibrinolytic agents per se.[6,7,8,9,10] Even though intraventricular fibrinolysis shows promising results, and new methods to enhance local fibrinolysis have been studied,[15] they are still debatable because of different origins and various forms and volumes of ICH included in the research. This fact remains the main impediment of gathering more precise data regarding which ICH forms would gain most benefit from fibrinolysis.[5,16,17,18,19] It is being increasingly recognized that modulation of brain inflammation may uncover new potential therapeutic strategies for stroke. Recent studies have shifted focus from immunological implications in ischemic stroke to hemorrhagic stroke, and it seems that the future holds new approaches to stroke management based on immunological targets and inflammation modulation.[20] A review article investigated the role of neuroinflammation and the immune system in the formation of perihematomal edema and secondary brain injury, and the increasing attention on adaptive immunity in ICH.[8] A phase-II randomized controlled trial found fingolimod, a sphingosine 1-phosphate receptor modulator approved for multiple sclerosis, to reduce perihematomal edema and neurologic deficit, promoting recovery.[21] IL-1 is a pro-inflammatory cytokine which could be a target for neuroprotection by reducing neuronal injury.[11,22] The interleukin-1 Receptor antagonist (IL-1Ra) has been demonstrated to be neuroprotective in ischemic stroke models and to reduce peripheral inflammation in patients suffering from aneurysmal subarachnoid hemorrhage.[22] The SCIL-STROKE subcutaneous interleukin-1 receptor antagonist in ischemic stroke is a recent phase 2 randomized clinical trial on IL-1Ra investigating its effect in ischemic stroke.[23] IL-1Ra was not associated with favorable outcome despite the reduction of plasma inflammatory markers. Also, 2 meta-analysis revealed that increased neutrophil-to-lymphocyte serum ratio is associated with poor outcomes in primary ICH[24] and that baseline leukocytosis may have a role in predicting outcomes in ICH patients.[25] However, inflammation may not necessarily be a negative phenomenon in acute ICH[26], and there is interesting data on stroke-induced immunodepression and secondary complications after ICH.[27] A study with smaller sample size of patients with subarachnoid hemorrhage, suggested transient rise in inflammatory cytokines in CSF,[28] which has not been noted in our study.
Bearing in mind the promising results of intraventricular fibrinolytic therapy in patients with ICH associated with IVH and recent studies that focus on potential biomarkers and new therapeutic targets in stroke, we hypothesized that significant change in local neuroinflammatory response in CSF may be present after the application of rt-PA and that the investigated cytokines may pose as contributing factors in the unveiling search for potential specific targets in ICH treatment. We measured the concentrations of proinflammatory (IL-1β) and anti-inflammatory (TGF-β) cytokines in CSF obtained using the EVD system, and compared the neuroinflammatory response in patients with primary ICH and IVH treated via catheter-based clot lysis using low-dose rt-PA injected through the EVD system and those treated with saline. Our results indicate that intraventricular fibrinolysis is associated with an initial increase in anti-inflammatory response and delayed decrease in proinflammatory response in rt-PA group. This anti-inflammatory effect might have a positive impact on secondary brain injury, which may explain the improvements in outcomes in previously reported studies. The present trial possesses some limitations of note. A comparison between local intraventricular and systemic inflammatory response in the serum would contribute to a more complete interpretation of our findings. Additionally, the patients’ different volumes and nonidentical (but similar) localizations of the ICH with IVH may have had an effect on the results which must be interpreted with caution. Bearing these limitations in mind, our findings suggest that intraventricular clot lysis using low-dose rt-PA can amplify the initial anti-inflammatory response and diminish the proinflammatory response during the later stages in patients with primary ICH associated with IVH. Better understanding of the role and regulation of neuroinflammation in secondary brain injury, and the detection of capital inflammatory modulators, may unfold the path to more effective therapeutic targets.
Acknowledgment
We thank Dunatov S, Antoncic I, Bralic M for their contribution to our present work.
The authors thank www.editage.com for language editing.
Author contributions
Conceptualization: Igor Antončić, Alan Šustić, Vlatka Sotošek
Data curation: Janja Tarčuković, Siniša Dunatov
Formal analysis: Matija Sošić, Janja Tarčuković, Vlatka Sotošek
Investigation: Matija Sošić, Janja Tarčuković, Vlatka Sotošek, Božena Ćurko-Cofek
Methodology: Matija Sošić, Vlatka Sotošek, Božena Ćurko-Cofek
Supervision: Alan Šustić, Igor Antončić
Writing – original draft: Matija Sošić, JanjaTarčuković, Vlatka Sotošek, Božena Ćurko-Cofek
Footnotes
Abbreviations: CSF = cerebrospinal fluid, CT = computed tomography, EVD = external ventricular drain, GCS = Glasgow Coma Scale, ICH = intracerebral hemorrhage, ICP = intracranial pressure, IL-1β = interleukin-1 beta, IL-1Ra = interleukin-1 receptor antagonist, IVH = intraventricular hemorrhage, rt-PA = recombinant tissue plasminogen activator, TGF-β = transforming growth factor beta.
How to cite this article: Sošić M, Antončić I, Tarčuković J, Dunatov S, Šustić A, Ćurko-Cofek B, Sotošek V. Effect of intraventricularly administered low-dose recombinant tissue plasminogen activator on interleukin 1-beta and transforming growth factor beta concentrations in cerebrospinal fluid of patients with primary intracerebral hemorrhage associated with intraventricular hemorrhage: a retrospective study. Medicine. 2020;99:20(e19966).
This work was funded by the University of Rijeka, Rijeka, Croatia (project no. 13.06.1.1.12).
This paper is an extension of a previous study (Dunatov S, Antoncic I, Bralic M. Intraventricular thrombolysis with rt-PA in patients with intraventricular hemorrhage. Acta Neurol Scand. 2011;124:343–348).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Brott T, Thalinge K, Hertzberg V. Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke 1986;17:1078–83. [DOI] [PubMed] [Google Scholar]
- [2].Daverat P, Castel JP, Dartigues JF. Death and functional outcome after spontaneous intracerebral hemorrhage. A prospective study of 166 cases using multivariate analysis. Stroke 1991;22:1–6. [DOI] [PubMed] [Google Scholar]
- [3].Hallevi H, Albright KC, Aronowski J. Intraventricular hemorrhage: anatomic relationships and clinical implications. Neurology 2008;70:848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 2011;42:1781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dunatov S, Antoncic I, Bralic Intraventricular thrombolysis with rt-PA in patients with intraventricular hemorrhage. Acta Neurol Scand 2011;124:343–8. [DOI] [PubMed] [Google Scholar]
- [6].Hanley DF, Lan K, McBee N. CLEAR III Investigators. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet 2017;389:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ling L, Kebin H. Tissue plasminogen activator and inflammation: from phenotype to signaling mechanisms. Am J Clin Exp Immunol 2014;3:30–6. [PMC free article] [PubMed] [Google Scholar]
- [8].Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci 2014;388:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 2010;92:463–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Ceres Blood Flow Metab 2007;27:894–908. [DOI] [PubMed] [Google Scholar]
- [11].Galea J, Brough D. The role of inflammation and interleukin-1 in acute cerebrovascular disease. J Inflamm Res 2013;6:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taylor RA, Chang CF, Goods BA. TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest 2017;127:280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]
- [14].Hemphill JC, Bonovich DC, Besmertis L. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–7. [DOI] [PubMed] [Google Scholar]
- [15].Masomi-Bornwasser J, Winter P, Neulen A, et al. Doppler sonography enhances rtPA-induced fibrinolysis in an in vitro clot model of spontaneous intracerebral hemorrhages. PLoS One 2019;14:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Castaño AS, Corral Lozano E, Vallejo De La Cueva A. Intraventricular hemorrhage treated with intraventricular fibrinolysis: a 10-year experience. Med Intensiva 2013;37:61–6. [DOI] [PubMed] [Google Scholar]
- [17].Gaberel T, Magheru C, Parienti JJ. Intraventricular fibrinolysis versus external ventricular drainage alone in intraventricular hemorrhage: a meta-analysis. Stroke 2011;42:2776–81. [DOI] [PubMed] [Google Scholar]
- [18].Naff NJ, Hanley DF, Key PM. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery 2004;54:577–83. [DOI] [PubMed] [Google Scholar]
- [19].Starnoni D, Oddo M, Maduri R. Thrombolysis for non-traumatic intra-ventricular hemorrhage in adults: a critical reappraisal. Minerva Anestesiol 2017;83:982–93. [DOI] [PubMed] [Google Scholar]
- [20].Shi K, Tian DC, Li ZG, et al. Global brain inflammation in stroke. Lancet Neurol 2019;18:1058–66. [DOI] [PubMed] [Google Scholar]
- [21].Fu Y, Hao J, Zhang N, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol 2014;71:1092–101. [DOI] [PubMed] [Google Scholar]
- [22].Galea J, Ogungbenro K, Hulme S, et al. Reduction of inflammation after administration of interleukin-1 receptor antagonist following aneurysmal subarachnoid hemorrhage: results of the subcutaneous interleukin-1Ra in SAH (SCIL-SAH) study. J Neurosurg 2018;128:515–23. [DOI] [PubMed] [Google Scholar]
- [23].Smith CJ, Hulme S, Vail A, et al. SCIL-STROKE (subcutaneous interleukin-1 receptor antagonist in ischemic stroke): a randomized controlled phase 2 trial. Stroke 2018;49:1210–6. [DOI] [PubMed] [Google Scholar]
- [24].Liu S, Liu X, Chen S, et al. Neutrophil-lymphocyte ratio predicts the outcome of intracerebral hemorrhage: a meta-analysis. Medicine (Baltimore) 2019;98:e16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu Z, Zheng J, Guo R, et al. Prognostic impact of leukocytosis in intracerebral hemorrhage: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2019;98:631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morotti A, Phuah CL, Anderson CD, et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke 2016;47:1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morotti A, Marini S, Jessel MJ, et al. Lymphopenia, infectious complications, and outcome in spontaneous intracerebral hemorrhage. Neurocrit Care 2017;26:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kramer AH, Jenne CN, Zygun DA. Intraventricular fibrinolysis with tissue plasminogen activator is associated with transient cerebrospinal fluid inflammation: a randomized controlled trial. J Cereb Blood Flow Metab 2015;35:1241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


