Abstract
Introduction
Improving health-related quality of life (hrqol) is a key goal of systemic therapy in advanced lung cancer, although routine assessment remains challenging. We analyzed the impact of a real-time electronic hrqol tool, the electronic Lung Cancer Symptom Scale (elcss-ql), on palliative care (pc) referral rates, patterns of chemotherapy treatment, and use of other supportive interventions in patients with advanced non-small-cell lung cancer (nsclc) receiving first-line chemotherapy.
Methods
Patients with advanced nsclc starting first-line chemotherapy were randomized to their oncologist receiving or not receiving their elcss-ql data before each clinic visit. Patients completed the elcss-ql at baseline, before each chemotherapy cycle, and at subsequent follow-up visits until disease progression. Prospective data about the pc referral rate, hrqol, and use of other supportive interventions were collected.
Results
For the 95 patients with advanced nsclc who participated, oncologists received real-time elcss-ql data for 44 (elcss-ql arm) and standard clinical assessment alone for 51 (standard arm). The primary endpoint, the pc referral rate, was numerically higher, but statistically similar, for patients in the elcss-ql and standard arms. The hrqol scores over time were not significantly different between the two study arms.
Conclusions
The elcss-ql is feasible as a tool for use in routine clinical practice, although no statistically significant effect of its use was demonstrated in our study. Improving access to supportive care through the collection of patient-reported outcomes and hrqol should be an important component of care for patients with advanced lung cancer.
Keywords: Patient-reported outcomes, palliative care, supportive care
INTRODUCTION
Lung cancer continues to be the most prevalent and lethal malignancy worldwide: in 2012, lung cancer alone accounted for 12.9% of all new cancer cases and for 19.4% of all cancer mortality1. Despite recent advances that improve survival for subsets of patients with advanced non-small-cell lung cancer (nsclc), the symptom burden remains high and causes significant patient distress2,3. Because patients with advanced nsclc do not have curative treatment options, maximizing health-related quality of life (hrqol) is a key management goal of systemic therapy4. Awareness of available palliative care (pc) services is fundamentally important for patients and their oncologists alike. Recent evidence shows that early pc referral might improve quality of life, mood, and survival for patients with advanced nsclc5,6. However, the rate of pc referral has remained low7, most commonly because of conflicting perceptions of the role and need for palliative care8.
Numerous strategies for improving the pc referral rate for patients with advanced nsclc undergoing chemotherapy are currently being explored. Among those strategies is the collection of patient-reported outcomes (pros) for symptoms that might trigger pc referral by treating oncologists. Patient-reported outcomes have been considered a primary method for quantitatively assessing the subjective symptomatic experiences and quality of life of patients9–11. However, the collection of pros and the routine assessment of hrqol remain challenging in clinical practice12. The Lung Cancer Symptom Scale (lcss) is a well-established and validated tool for monitoring patient-reported symptoms related to disease and treatment in lung cancer13. It has been evaluated for validity, feasibility, and reliability in nearly 1000 patients with lung cancer at 8 North American cancer centres and has been in use for more than 20 years14–21. The lcss is brief, using a simple visual analog scale to assess lung cancer symptoms, performance status, and global quality of life. It can be completed in less than 5 minutes and requires only a 2nd-grade reading level. Today’s widespread access to technology provides a unique opportunity to improve the ease and timeliness of lcss data collection22. The electronic collection of pro data has been tested, including in a large electronic Web-based survey of 660 patients with lung cancer23–25, and recently, an electronic version of the lcss (elcss-ql) on a handheld device has been validated in patients with advanced lung cancer26, permitting the capture of real-time hrqol data and pros in an accessible format.
It remains unclear whether the real-time collection of hrqol data in clinic using tools such as the elcss-ql would streamline the process of early pc referral for patients with advanced nsclc. In the present randomized trial, we aimed to analyze the effect—on the pattern of pc referral and use of other supportive interventions in patients with newly diagnosed advanced nsclc—of using the elcss-ql to collect hrqol data. We hypothesized that the elcss-ql will positively affect treatment patterns for patients with lung cancer by increasing and accelerating referral to pc and to supportive therapies, and by shortening the duration of palliative chemotherapy by more quickly identifying lack of benefit in some patients.
METHODS
Eligibility and Accrual
Between November 2004 and May 2011, patients with incurable nsclc commencing first-line systemic therapy at the Princess Margaret Cancer Centre and Mount Sinai Hospital, Toronto, Ontario, were reviewed and recruited. Patients who were not participating in a clinical trial investigating a therapeutic intervention were eligible for the trial and were approached for trial participation in outpatient clinics. Written consent was obtained from patients before study participation.
Eligibility criteria included histologic or cytologic diagnosis of incurable stage iiib or iv nsclc, no prior chemotherapy, plans to commence first-line chemotherapy, European Cooperative Oncology Group performance status 0–3, and written fluency in English, French, Portuguese, Spanish, Italian, or Chinese (that is, the languages selected for the elcss-ql). Patients were excluded if they were unable to independently complete or understand the elcss-ql assessment process, if they were receiving concurrent radical radiotherapy, if they did not commence chemotherapy, or if they were participating in another clinical trial involving first-line therapy. Institutional review board ethics approval was obtained before conduct of the study.
Data Collection and Endpoints
Eligible patients were randomized to have their oncologist either receive their elcss-ql data in real time (the elcss-ql arm) or not receive those data (the standard arm). Patient randomization was stratified by treating oncologist, planned treatment (that is, platinum-based or non-platinum-based), and Eastern Cooperative Oncology Group performance status 0 or1 compared with 2 or greater. All patients completed the elcss-ql at baseline and before each chemotherapy cycle (every 3 weeks), and then at each follow-up visit until clinical or radiologic disease progression, the initiation of subsequent therapy, or death. The primary endpoint was the pc referral rate in each arm. Secondary endpoints included the number of first-line chemotherapy cycles administered; referral to and use of other supportive interventions such as homecare nursing, pain clinic, palliative radiation, home oxygen, bisphosphonates, transfusions, or appetite stimulants; and hrqol changes during treatment.
The elcss-ql contains 9 items—daily activities, appetite, cough, distress, dyspnea, fatigue, hemoptysis, pain, and global quality of life—that are scored by the patient using a visual analog scale. Patients completed the questionnaire using a handheld pocket personal computer with a touch screen and stylus after practice with an elcss-ql sample question. The device provides an immediate computer-generated graphic summary of an annotated elcss-ql report representing current scores and changes over time, highlighting score changes for nurse and physician review (supplemental Figure 1). The timing and administration schedule was adherent with standard lcss procedures, as detailed in the manual and published methods27. The assessment was completed immediately before the patient’s physician visit, before discussion of clinical results or the administration of chemotherapy. The hrqol was presented as a single report with a summary score and current Eastern Cooperative Oncology Group and Karnofsky performance statuses on the day of assessment. The information was provided to the treating oncologist before the patient’s assessment, with trends highlighted in a 2-page graphical report.
Statistical Analysis
The study was powered to detect an increase in the pc referral rate to 50% from 25% at any time during the study, with an alpha of 0.05 and a power of 80% (65 patients being required in each arm of the study). Primary and secondary outcomes in the two arms were compared using the Fisher exact test or the Wilcoxon–Mann–Whitney test, as appropriate; a p value less than 0.05 was considered statistically significant. Changes in hrqol during the follow-up period were compared using a mixed-effects model with random intercept. The Kaplan–Meier method and log-rank test were used for comparing time to pc referral in the study arms.
RESULTS
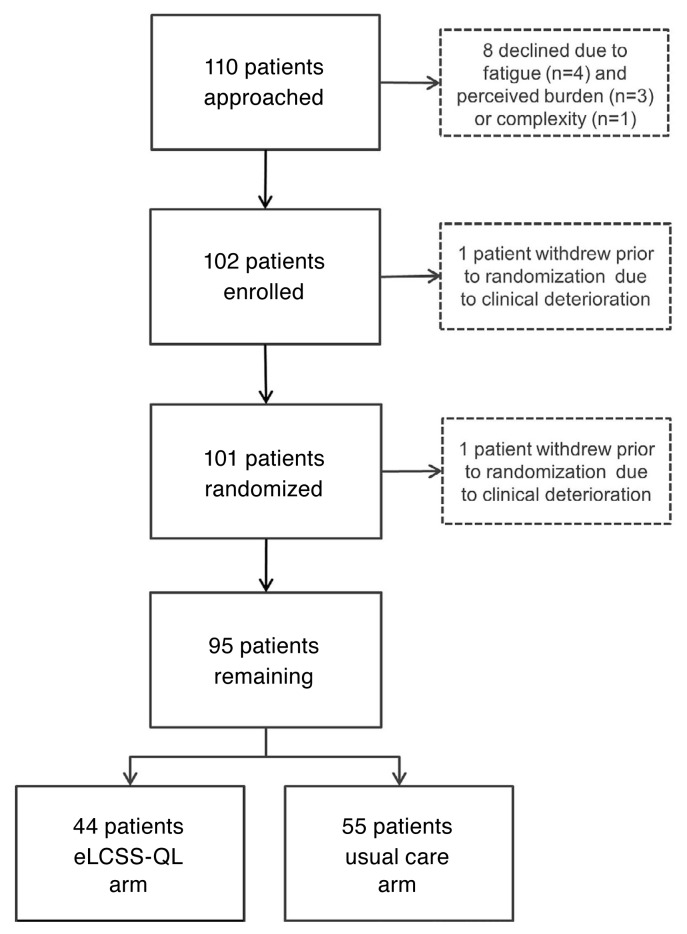
Study enrolment was halted early because of slow accrual. Of 110 patients approached, 8 declined participation because of fatigue (n = 4), perception of burden (n = 3), or complexity (n = 1). As a result, 102 patients seen by 1 of 4 attending medical oncologists at 2 centres were enrolled. Before randomization, 1 patient withdrew because of clinical deterioration, and 6 patients did not start chemotherapy as planned after randomization (5 in the elcss-ql arm, 1 in the standard arm); all 7 were thus ineligible, leaving 95 eligible patients for the analysis. Of those 95 patients, 44 were assigned to the elcss-ql arm, and 51, to the standard arm (Figure 1).
FIGURE 1.
CONSORT diagram. eLCSS-QL = electronic Lung Cancer System Scale for quality-of-life assessment.
The two arms were well-balanced with respect to patient demographic characteristics, although the patients in the standard arm were older, more likely to have stage iv disease, and more likely to have a worse performance status (Table I). Despite those differences, mean total hrqol scores at baseline were similar in the study arms.
TABLE I.
Patient characteristics at baseline
| Characteristic | Patient group | ||
|---|---|---|---|
|
| |||
| eLCSS-QL | Standard | Overall | |
| Patients (n) | 44 | 51 | 95 |
|
| |||
| Sex [n (%)] | |||
| Men | 25 (57) | 28 (55) | 53 (56) |
| Women | 19 (43) | 23 (45) | 42 (44) |
|
| |||
| Age (years) | |||
| Median | 63 | 67 | 65 |
| Range | 43–80 | 39–80 | 39–80 |
|
| |||
| Stage [n (%)] | |||
| IIIB | 21 (48) | 21 (41) | 42 (44) |
| IV | 23 (52) | 30 (59) | 53 (56) |
|
| |||
| ECOG PS [n (%)] | |||
| 0–1 | 30 (68) | 30 (59) | 60 (63) |
| 2–3 | 14 (32) | 21 (41) | 35 (37) |
|
| |||
| Chemotherapy regimen [n (%)] | |||
| Platinum doublet | 36 (82) | 45 (88) | 81 (85) |
| Non-platinum-based | 8 (18) | 6 (12) | 14 (15) |
|
| |||
| Mean HRQoL | |||
| Baseline (900 maximum) | 582±151 | 628±128 | 607±140 |
| Global (100 maximum) | 46±25 | 60±22 | 53±24 |
eLCSS-QL = electronic Lung Cancer System Scale for quality-of-life assessment; ECOG PS = Eastern Cooperative Oncology Group performance status; HRQoL = health-related quality of life.
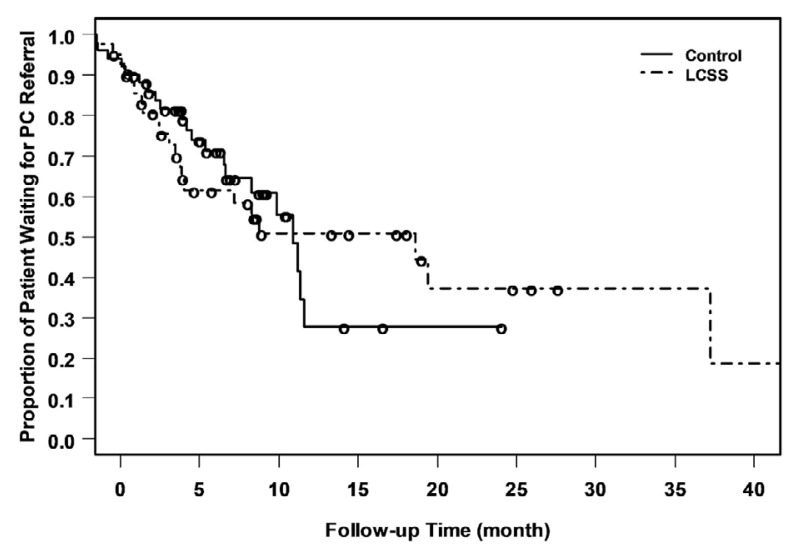
The pc referral rate during first-line chemotherapy was 48% in the elcss-ql arm and 41% in the standard arm, p = 0.54. Time to pc referral was numerically longer in the elcss-ql arm (18.6 months) than in the standard arm (11.2 months), p = 0.98. The number of chemotherapy cycles received was not significantly different between the two arms, p = 0.58. The median number of chemotherapy cycles received was 4, with similar proportions of patients in the elcss-ql and standard arms receiving 1–3 cycles (34% vs. 31% respectively) or 4–6 cycles (61% vs. 67%). No significant differences between the study arms with respect to referral for additional supportive interventions were evident (Table II).
TABLE II.
Patterns of palliative management and referral
| Variable | Patient group | p Value | ||
|---|---|---|---|---|
|
|
|
|||
| eLCSS-QL (n=44) | Standard (n=51) | Unadjusted | Adjusteda | |
| First-line chemotherapy | ||||
| Cycles [n (%)] | ||||
| Median | 4 | 4 | 0.58 | 0.45 |
| Range | 1–9 | 1–12 | ||
| 1–3 | 15 (34) | 16 (31) | ||
| 4–6 | 27 (61) | 34 (67) | ||
|
| ||||
| Palliative care referral rate [n (%)] | ||||
| Overall | 21 (48) | 21 (41) | 0.54 | 0.47 |
| At 3 months | (24) | (18) | ||
| At 6 months | (38) | (28) | ||
|
| ||||
| Time to referral (months) | ||||
| Median | 18.6 | 11.2 | ||
| 95% CI | 4.01 to NR | 8.31 to NR | ||
|
| ||||
| Referral for other support [n (%)] | ||||
| Homecare nursing | 10 (23) | 7 (14) | 0.25 | 0.28 |
| Pain clinic | 6 (14) | 3 (6) | 0.29 | 0.16 |
| Palliative radiation | 21 (47) | 23 (45) | 0.80 | 0.85 |
| Home oxygen | 5 (11) | 11 (22) | 0.19 | 0.19 |
| Bisphosphonates | 2 (5) | 3 (6) | 1.00 | 0.70 |
| Transfusions | 3 (7) | 6 (12) | 0.50 | 0.50 |
| Appetite stimulants | 10 (23) | 11 (22) | 0.89 | 0.86 |
Adjusted for age, stage, and Eastern Cooperative Oncology Group performance status.
eLCSS-QL = electronic Lung Cancer System Scale for quality-of-life assessment; CI = confidence interval; NR = not reached during study follow-up.
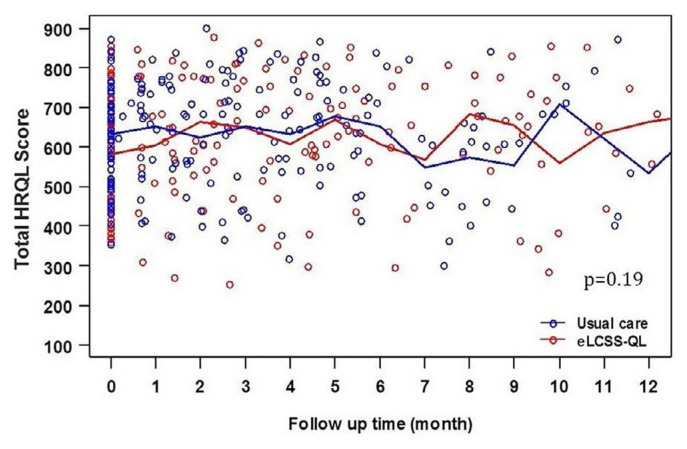
During study follow-up, total hrqol scores also did not differ between the study arms. Trends in total hrqol remained stable, including similar rates of compliance in reporting, suggesting no inherent bias in hrqol reporting or obvious imbalances between the two arms (Figures 2 and 3).
FIGURE 2.
Patients awaiting palliative care (PC) referral by study arm. LCSS = electronic Lung Cancer System Scale for quality-of-life assessment.
FIGURE 3.
Health-related quality-of-life (HRQOL) scores over time. eLCSS-QL = electronic Lung Cancer System Scale for quality-of-life assessment.
DISCUSSION
The present study demonstrates that the use of automated hrqol measurement with the elcss-ql did not have a statistically significant effect on the rate of pc referral, the use of other supportive interventions, or total hrqol over time. However, a trend toward greater rates of pc referral and referral to other supportive services such as homecare nursing and pain management was observed, suggesting that routine use of the elcss-ql in clinic might have a positive effect on the use of supportive care.
The benefit of collecting pros using electronic tools has been demonstrated in a number of studies23,24,28,29. Over the course of a year, using Web-based patient self-reporting, the Ontario Health (Cancer Care Ontario) Palliative Care Integration Project demonstrated improved symptom screening, symptom control, and functional assessment for outpatients with lung cancer23,30–33. Recently, a study in which patients receiving chemotherapy were randomized to provide pro data electronically to nursing staff or to receive usual care demonstrated a 5-month median improvement in overall survival for those providing the electronic pro data34. Two other randomized trials comparing electronic methods of pro data collection with usual care showed significantly less symptom distress, a decreased need for symptom management support, and improved emotional functioning scores in patients who used electronic pro reporting24. Thus, evidence is mounting that hrqol data collected using electronic methods is effective for improving survival and symptom control in patients with advanced cancer.
The high symptom burden associated with advanced lung cancer remains a priority target in the treatment of this disease2,3. The American Society of Clinical Oncology has endorsed the integrated implementation of pc into oncology practice, including a recommendation for greater use of technology and improved education for providers, patients, and caregivers30–33,35,36. In a survey of 5422 patients with advanced lung or colon cancer, more than half had at least 1 undermanaged symptom37, highlighting the need for symptom-focused strategies to improve quality of life for patients. There is now ample evidence to suggest that earlier integration of pc improves hrqol5,38–42 and, in one study, even survival5. Other less-tangible benefits for early pc referral have also been demonstrated, including increased satisfaction with care among caregivers of patients with advanced cancer40. To our knowledge, however, the present study is the first to assess the collection of electronic pro data and its effect on pc referral.
Current research suggests that pc has not been integrated early into the trajectory of advanced cancer treatment for a number of possible reasons, including conflicting perceptions of pc and active anticancer therapy, communication barriers between patients and caregivers43, lack of standardization between symptom assessment methods, and inconsistent triggers for pc referral44. Referral for pc has been shown to be associated with a strong stigma, which can persist despite positive early pc experiences45. Variable adoption rates and disparate attitudes about pro collection on the part of health care providers have also been demonstrated46–48, further complicating the effective implementation of early pc referral. Education programs for health care providers, patients, and caregivers remain crucial to improving the perception of pc and early pc referral.
Our study has several limitations which could have resulted in the failure to demonstrate a significant advantage with use of the elcss-ql in clinic. Slow accrual led to early closure of the study, resulting in a lack of power to detect a significant difference in effect. We believe that the accrual difficulty was a result of concurrent availability of multiple clinical trials of first-line therapy in this patient population at our centres, significantly constraining the population eligible for our study. Despite randomization, minor imbalances in prognostic factors were evident between the study arms, including a larger proportion of patients with stage iv disease and performance status of 2 or greater in the standard arm, which might have led to a higher pc referral rate in that group overall. Participation in the study might have influenced oncologists to refer for supportive services earlier in both arms of the study, independent of the use of the elcss-ql. Other studies in this area have targeted other health care staff (such as nurses) and have provided clear symptom triggers for referral or supportive interventions, demonstrating positive results. Our study did not specify qualifiers for pc referral, nor were specific qualifiers for clinical action identified. Although it is possible that the Hawthorne effect, in which outcomes appear to be better for patients in clinical trials than for those in routine practice by virtue of their participation, could have influenced outcomes, the long duration of the study makes such an influence unlikely. Although participating health care providers were aware of the study, they were unaware of whether their patients were participating until they received the data for patients randomized to the elcss-ql arm. Moreover, because only minimal endorsement of the elcss-ql was required, and no additional flags or prompts for required action were provided, the impact on treatment decisions might have been lessened, diminishing the elcss-ql effect. It was previously shown that the attitude of individual clinicians toward pros can confound pc referral rates, and some clinicians continue to rely on symptom assessment confirmed directly with the patient rather than through automated pros reports.
CONCLUSIONS
Patient-reported outcomes remain a key driver influencing treatment and decisions about pc in advanced cancer. The continued development, optimization, and standardization of pro collection methods remain important goals in the oncology community. Our novel randomized study failed to demonstrate an effect of elcss-ql data on pc referral rates or use of other supportive interventions, nor was hrqol over time altered. Despite the negative outcome and limitations of the study, electronic methods of pro collection are feasible tools that might further improve our ability to support patients with advanced cancer in routine clinical practice.
Supplementary Information
ACKNOWLEDGMENTS
We gratefully acknowledge the editorial support of Deanna McLeod and Paul Card at Kaleidoscope Strategic. This study was supported by the Princess Margaret Cancer Foundation through an Ontario Cancer Research Network infrastructure grant. NBL is supported by the Princess Margaret Cancer Foundation OSI Pharmaceuticals Foundation Chair.
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Sung MR, Patel MV, Djalalov S, et al. Evolution of symptom burden of advanced lung cancer over a decade. Clin Lung Cancer. 2017;18:274–80.e6. doi: 10.1016/j.cllc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Tishelman C, Lovgren M, Broberger E, Hamberg K, Sprangers MA. Are the most distressing concerns of patients with inoperable lung cancer adequately assessed? A mixed-methods analysis. J Clin Oncol. 2010;28:1942–9. doi: 10.1200/JCO.2009.23.3403. [DOI] [PubMed] [Google Scholar]
- 4.Masters GA, Temin S, Azzoli CG, et al. on behalf of the American Society of Clinical Oncology Clinical Practice. Systemic therapy for stage iv non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2015;33:3488–515. doi: 10.1200/JCO.2015.62.1342. [Erratum in: J Clin Oncol 2016;34:1287] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 6.Hui D, Kim SH, Roquemore J, Dev R, Chisholm G, Bruera E. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer. 2014;120:1743–9. doi: 10.1002/cncr.28628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CB, Nelson JE, Berman AR, et al. Lung cancer physicians’ referral practices for palliative care consultation. Ann Oncol. 2012;23:382–7. doi: 10.1093/annonc/mdr345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenker Y, Crowley-Matoka M, Dohan D, et al. Oncologist factors that influence referrals to subspecialty palliative care clinics. J Oncol Pract. 2014;10:e37–44. doi: 10.1200/JOP.2013.001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson TM, Andreotti CF, Roberts KE, Saracino RM, Hernandez M, Basch E. The level of association between functional performance status measures and patient-reported outcomes in cancer patients: a systematic review. Support Care Cancer. 2015;23:3645–52. doi: 10.1007/s00520-015-2923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–55. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 11.Sun V, Raz DJ, Ruel N, et al. A multimedia self-management intervention to prepare cancer patients and family caregivers for lung surgery and postoperative recovery. Clin Lung Cancer. 2017;18:e151–9. doi: 10.1016/j.cllc.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung CH, Hays RD. Prospects and challenges in using patient-reported outcomes in clinical practice. Qual Life Res. 2008;17:1297–302. doi: 10.1007/s11136-008-9379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanvetyanon T, Soares HP, Djulbegovic B, Jacobsen PB, Bepler G. A systematic review of quality of life associated with standard chemotherapy regimens for advanced non–small cell lung cancer. J Thorac Oncol. 2007;2:1091–7. doi: 10.1097/JTO.0b013e31815cff64. [DOI] [PubMed] [Google Scholar]
- 14.Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies. Psychometric assessment of the Lung Cancer Symptom Scale. Cancer. 1994;73:2087–98. doi: 10.1002/1097-0142(19940415)73:8<2087::AID-CNCR2820730813>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Hollen PJ, Gralla RJ, Kris MG, Potanovich LM. Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (lcss) Eur J Cancer. 1993;29A(suppl 1):S51–8. doi: 10.1016/S0959-8049(05)80262-X. [DOI] [PubMed] [Google Scholar]
- 16.Hollen PJ, Gralla RJ, Kris MG. An overview of the Lung Cancer Symptom Scale. In: Gralla RJ, Moinpour CM, editors. Assessing Quality of Life in Patients with Lung Cancer: A Guide for Clinicians [monograph] New York, NY: NCM Publishers; 1995. pp. 57–63. [Google Scholar]
- 17.Burke M, Gralla R, Kris M, Howard J, Monras P. Subjective evaluation in non–small cell lung cancer: comparison of Karnofsky performance status with a patient generated visual analogue scale measuring activity [abstract] Lung Cancer. 1986;2:181. doi: 10.1016/S0169-5002(86)80655-9. [DOI] [Google Scholar]
- 18.Monras P, Gralla R, Burke M, et al. Development of specific instruments for subjective evaluation of patients with lung cancer: comparison of observer assessment with patient generated visual analogue scales (vas) [abstract] J Clin Oncol. 1985;4:251. [Google Scholar]
- 19.Hollen PJ, Gralla RJ, Kris MG, Cox C. Quality of life during clinical trials: conceptual model for the Lung Cancer Symptom Scale (lcss) Support Care Cancer. 1994;2:213–22. doi: 10.1007/BF00365725. [DOI] [PubMed] [Google Scholar]
- 20.Hollen PJ, Gralla RJ, Cox C, Eberly SW, Kris MG. A dilemma in analysis: issues in the serial measurement of quality of life in patients with advanced lung cancer. Lung Cancer. 1997;18:119–36. doi: 10.1016/S0169-5002(97)00059-7. [DOI] [PubMed] [Google Scholar]
- 21.Hollen PJ, Gralla RJ, Kris MG, Eberly SW, Cox C. Normative data and trends in quality of life from the Lung Cancer Symptom Scale (lcss) Support Care Cancer. 1999;7:140–8. doi: 10.1007/s005200050244. [DOI] [PubMed] [Google Scholar]
- 22.King J, Patel V, Furukawa MF. Physician Adoption of Electronic Health Record Technology to Meet Meaningful Use Objectives: 2009–2012. Washington, DC: Office of the National Coordinator for Health Information Technology; 2012. [Google Scholar]
- 23.Gilbert JE, Howell D, King S, et al. Quality improvement in cancer symptom assessment and control: the Provincial Palliative Care Integration Project (ppcip) J Pain Symptom Manage. 2012;43:663–78. doi: 10.1016/j.jpainsymman.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe J, Orellana L, Cook EF, et al. Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the pediquest randomized controlled trial. J Clin Oncol. 2014;32:1119–26. doi: 10.1200/JCO.2013.51.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gralla RJ, Hollen PJ, Msaouel P, Davis BV, Petersen J. An evidence-based determination of issues affecting quality of life and patient-reported outcomes in lung cancer: results of a survey of 660 patients. J Thorac Oncol. 2014;9:1243–8. doi: 10.1097/JTO.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 26.Hollen PJ, Gralla RJ, Stewart JA, Meharchand JM, Wierzbicki R, Leighl N. Can a computerized format replace a paper form in pro and hrql evaluation? Psychometric testing of the computer-assisted lcss instrument (elcss-ql) Support Care Cancer. 2013;21:165–72. doi: 10.1007/s00520-012-1507-7. [DOI] [PubMed] [Google Scholar]
- 27.Hollen PJ, Gralla RJ, Rittenberg CN. Quality of life as a clinical trial endpoint: determining the appropriate interval for repeated assessments in patients with advanced lung cancer. Support Care Cancer. 2004;12:767–73. doi: 10.1007/s00520-004-0639-9. [DOI] [PubMed] [Google Scholar]
- 28.Ruland CM, Holte HH, Roislien J, et al. Effects of a computer-supported interactive tailored patient assessment tool on patient care, symptom distress, and patients’ need for symptom management support: a randomized clinical trial. J Am Med Inform Assoc. 2010;17:403–10. doi: 10.1136/jamia.2010.005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steel JL, Geller DA, Kim KH, et al. Web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer. 2016;122:1270–82. doi: 10.1002/cncr.29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ontario Health (Cancer Care Ontario) [oh(cco)] Interactive Symptom Assessment and Collection (ISAAC) tool [Web page] Toronto, ON: OH(CCO); n.d.. [Available at: http://ocp.cancercare.on.ca/cms/One.aspx?portalId=77515&pageId=57699; cited 29 April 2020] [Google Scholar]
- 31.Ontario Health (Cancer Care Ontario) [oh(cco)] Symptom Assessment Tools: Your Symptoms Matter [Web page] Toronto, ON: OH(CCO); n.d.. [Available at: https://www.cancercareontario.ca/en/guidelines-advice/symptom-side-effect-management/symptom-assessment-tool; cited 29 April 2020] [Google Scholar]
- 32.Ontario Health (Cancer Care Ontario) [oh(cco)] Program in Evidence-Based Care [Web page] Toronto, ON: OH(CCO); n.d.. [ https://www.cancercareontario.ca/en/cancer-careontario/programs/data-research/evidence-based-care; cited 29 April 2020] [Google Scholar]
- 33.Ontario Health (Cancer Care Ontario) [oh(cco)] Ontario’s Palliative Care Integration Project [slide presentation] Toronto, ON: OH(CCO); n.d.. [ http://ocp.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13750; cited 29 April 2020] [Google Scholar]
- 34.Basch EM, Deal AM, Dueck AC, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–8. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Partridge AH, Seah DS, King T, et al. Developing a service model that integrates palliative care throughout cancer care: the time is now. J Clin Oncol. 2014;32:3330–6. doi: 10.1200/JCO.2013.54.8149. [DOI] [PubMed] [Google Scholar]
- 36.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:96–112. doi: 10.1200/JCO.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 37.Walling AM, Weeks JC, Kahn KL, et al. Symptom prevalence in lung and colorectal cancer patients. J Pain Symptom Manage. 2015;49:192–202. doi: 10.1016/j.jpainsymman.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35:834–41. doi: 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–30. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 40.McDonald J, Swami N, Hannon B, et al. Impact of early palliative care on caregivers of patients with advanced cancer: cluster randomised trial. Ann Oncol. 2017;28:163–8. doi: 10.1093/annonc/mdw438. [DOI] [PubMed] [Google Scholar]
- 41.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316:2104–14. doi: 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannon B, Swami N, Pope A, et al. Early palliative care and its role in oncology: a qualitative study. Oncologist. 2016;21:1387–95. doi: 10.1634/theoncologist.2016-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssens A, Derijcke S, Lefebure A, et al. Addressing the palliative setting in advanced lung cancer should not remain a barrier: a multicenter study. Clin Lung Cancer. 2017;18:e283–7. doi: 10.1016/j.cllc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Hui D, Mori M, Watanabe SM, et al. Referral criteria for outpatient specialty palliative cancer care: an international consensus. Lancet Oncol. 2016;17:e552–9. doi: 10.1016/S1470-2045(16)30577-0. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann C, Swami N, Krzyzanowska M, et al. Perceptions of palliative care among patients with advanced cancer and their caregivers. CMAJ. 2016;188:E217–27. doi: 10.1503/cmaj.151171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hui D, Meng YC, Bruera S, et al. Referral criteria for outpatient palliative cancer care: a systematic review. Oncologist. 2016;21:895–901. doi: 10.1634/theoncologist.2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bainbridge D, Seow H, Sussman J, et al. Multidisciplinary health care professionals’ perceptions of the use and utility of a symptom assessment system for oncology patients. J Oncol Pract. 2011;7:19–23. doi: 10.1200/JOP.2010.000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chasen M, Bhargava R, Dalzell C, Pereira JL. Attitudes of oncologists towards palliative care and the Edmonton Symptom Assessment System (esas) at an Ontario cancer center in Canada. Support Care Cancer. 2015;23:769–78. doi: 10.1007/s00520-014-2411-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.