Abstract
The Canadian Cancer Society estimated that 220,400 new cases of cancer would be diagnosed in 2019. Of the affected patients, more than 60% will survive for 5 years or longer after their cancer diagnosis. Furthermore, nearly 40% will receive at least 1 course of radiotherapy (rt). Radiotherapy is used with both curative and palliative intent: to treat early-stage or locally advanced tumours (curative) and for symptom management in advanced disease (palliative). It can be delivered systemically (external-beam rt) or internally (brachytherapy).
Although technique improvements have drastically reduced the occurrence of rt-related toxicity, most patients still experience burdensome rt side effects (seffs). Radiotherapy seffs are local or locoregional, and manifest in tissues or organs that were irradiated. Side effects manifesting within weeks after rt completion are termed “early seffs,” and those occurring months or years after treatment are termed “late seffs.”
In addition to radiation oncologists, general practitioners in oncology and primary care providers are involved in survivorship care and management of rt seffs. Here, we present an overview of common seffs and their respective management: anxiety, depression, fatigue, and effects related to the head-and-neck, thoracic, and pelvic treatment sites.
Keywords: Survivorship, radiotherapy, side effects, general practitioners in oncology, primary care providers
INTRODUCTION
The Canadian Cancer Society estimated that 220,400 new cases of cancer would be diagnosed in 2019. Of the affected patients, more than 60% will survive for 5 years or longer after their cancer diagnosis1. Furthermore, nearly 40% of cancer patients receive at least 1 course of radiotherapy (rt)2. Radiotherapy is used with both curative and palliative intent: to treat early-stage or locally advanced tumours (curative) and for symptom management in advanced disease (palliative).
Although technique improvements have drastically reduced rt-related toxicity3, most patients still experience burdensome rt side effects (seffs)4. Radiotherapy seffs are local or locoregional, and manifest in tissues or organs that were irradiated. Side effects manifesting during or within weeks after rt completion are termed “early seffs,” and those occurring months or years after treatment are termed “late seffs”4.
In addition to radiation oncologists, general practitioners in oncology and primary care providers are involved in survivorship care5, including the management of rt-induced seffs. Here, we present an overview of common seffs and their respective management: anxiety, depression, fatigue, and effects related to the head-and-neck (hn), thoracic, and pelvic treatment sites.
SIDE EFFECTS AND THEIR MANAGEMENT
Distress, Anxiety, and Depression
Studies have shown an increase in distress, anxiety, and depression in patients undergoing radiation6,7. Although such problems tend to decrease upon rt completion, a significant number of patients still manifest psychological effects after treatment7. Patients with pancreatic cancer and lung cancer appear particularly vulnerable, higher rates of depression being associated with those diagnoses8. Radiotherapy-induced hypothyroidism, especially in patients with hn cancer, and secondary vitamin B12 malabsorption can contribute to psychological findings and should be ruled out8.
Regardless of stage of diagnosis or treatment intent, depression and anxiety affect approximately 20% and 10% of patients respectively9, but underrepresentation is a concern, given the lack of standardized distress screening programs across Canada10. Current guidelines therefore recommend that all patients be screened for distress at their initial post-treatment visit and at regular intervals thereafter, using validated tools such as the revised Edmonton Symptom Assessment System, the Distress Thermometer, or the Patient Health Questionnaire-210. Screening should include an assessment of psychosocial needs and fear of recurrence, with referrals to appropriate resources being promptly made as required10. In patients diagnosed with depression, a multidisciplinary approach including both nonpharmacologic and pharmacologic interventions is encouraged11.
Fatigue
Cancer-related fatigue is defined as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer and/or cancer treatment that is not proportional to recent activity and interferes with usual functioning”12.
Patients often describe fatigue as one of the most distressing adverse effects of treatment12. Regardless of treatment site, rt has been reported to cause acute fatigue in up to 80% of patients, and chronic fatigue can persist in up to 30% for months to years after treatment13. The cause for persistent fatigue is likely multifactorial, but it has been suggested potentially to be secondary to persistent immune system activation or to late effects on major organ systems14. Guidelines recommend screening for cancer-related fatigue in all patients and taking prompt action for potential contributing factors such as anemia, pain, and cardiac or endocrine dysfunction12. Nonpharmacologic and pharmacologic treatments might aid in the management of cancer-related fatigue (Table I).
TABLE I.
Management strategies for cancer-related fatigue
| Strategy | Application |
|---|---|
| Nonpharmacologic | |
| Pharmacologic |
Effects of HN RT
Approximately 80% of patients with hn cancer will receive at least 1 course of rt as part of their treatment20. A frequent early seff of hn rt is oral mucositis: acute inflammation or ulceration, or both, of the oral or oropharyngeal mucosal membranes. Oral mucositis can cause pain and negatively affect capacity to swallow, eat, and speak, which can be very distressing to patients21. Oral mucositis is graded on a scale of 1–4 based on severity; Table II summarizes its management22.
TABLE II.
Clinical practice guidelines for oral mucositisa
| Recommendationsb | Suggestionsc |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
I = high-power studies; II = low-power studies; III = nonrandomized or case–control studies; IV = descriptive and case studies; V = case-report evidence or clinical examples.
From the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology. Reprinted with permission (https://creativecommons.org/licenses/by-nc-nd/3.0/legalcode) from Lalla et al.21.
Based on level I or II evidence.
Based on level III, IV, or V evidence, with panel consensus about the interpretation of such evidence.
Other common seffs of hn rt include alterations of taste, dysphagia, xerostomia, and hypothyroidism. The latter condition should be recognized because thyroid hormone can readily be replaced. Screening for thyroid dysfunction based on thyroid stimulating hormone levels should be performed every 6–12 months after rt23. Alterations of taste occur in more than 70% of patients24. Taste dysfunction can be partial or complete, and typically occurs 4–5 weeks after rt start25. Taste recovery can occur as early as 1 month after rt, and most survivors experience a complete return of taste 6–12 months after rt26.
The risk of dysphagia in patients with hn cancer who receive rt is high, and its occurrence can negatively affect quality of life27. Radiotherapy-induced fibrosis can impair the swallowing musculature28 and could lead to nutritional intake through enteral feeding. Radiotherapy-induced fibrosis is dose- and site-dependent28, and concomitant chemotherapy can further affect swallowing29. The mainstay of management is behavioural swallowing interventions with exercise aids provided by speech–language pathologists30. Thus, early referral to a speech–language pathologist is warranted; interventions can be performed to prevent dysphagia onset (before or during treatment) or to minimize existing dysphagia (after treatment)31. For persistent and debilitating dysphagia, referral to an experienced gastroenterologist for endoscopic dilatation might be beneficial31.
Lastly, xerostomia results from salivary gland dysfunction causing hyposalivation and is associated with swallowing, speech, and oral health problems20. Despite technique advancements such as intensity-modulated rt, approximately 40% of patients still experience burdensome xerostomia20. Increasing existing salivary flow (or replacing lost salivary secretions) and maintaining oral health (including treating dental caries and possible infections) are the mainstays of management32. After rt, dental visits are recommended at least once every 6 months23. Treatment options depend on the presence or absence of residual gland function. If gland function remains, mechanical gland stimulation with sugar-containing gums or xylitol- or sorbitol-containing candy can be attempted32,33. Salivary flow can also be stimulated by cholinergic medications such as pilocarpine at a recommended dose of 5 mg 3 times daily32,33. In the absence of gland function or upon saliva stimulation failure, mouthwashes and saliva substitutes can be used33.
Notably, hn rt is also associated with other late seffs, including lymphedema and carotid artery stenosis (cas). Lymphedema presents as local swelling because of damage to the lymphatic system, which can affect swallowing, speaking, and body image. Lymphedema management includes lymph drainage and use of compression garments: referral to a certified lymphedema therapist is recommended22. If cas occurs after hn rt, the risk for cerebrovascular disease increases. The risk appears greater in patients with other cas risk factors, including smoking, dyslipidemia, diabetes, and coronary and peripheral artery disease34. In addition to carotid artery surveillance, screening and optimal management of cas comorbid conditions are therefore recommended34.
Effects of Thoracic RT
Common effects of thoracic rt include radiation-induced lung injury (rili) and radiation-induced heart disease. Radiation-induced lung injury is a known complication in patients with lung, breast, esophageal, thymic, and hematologic malignancies who have undergone thoracic rt35. It affects 5%–20% of patients and can lead to dyspnea and chronic lung fibrosis, which can negatively affect quality of life36.
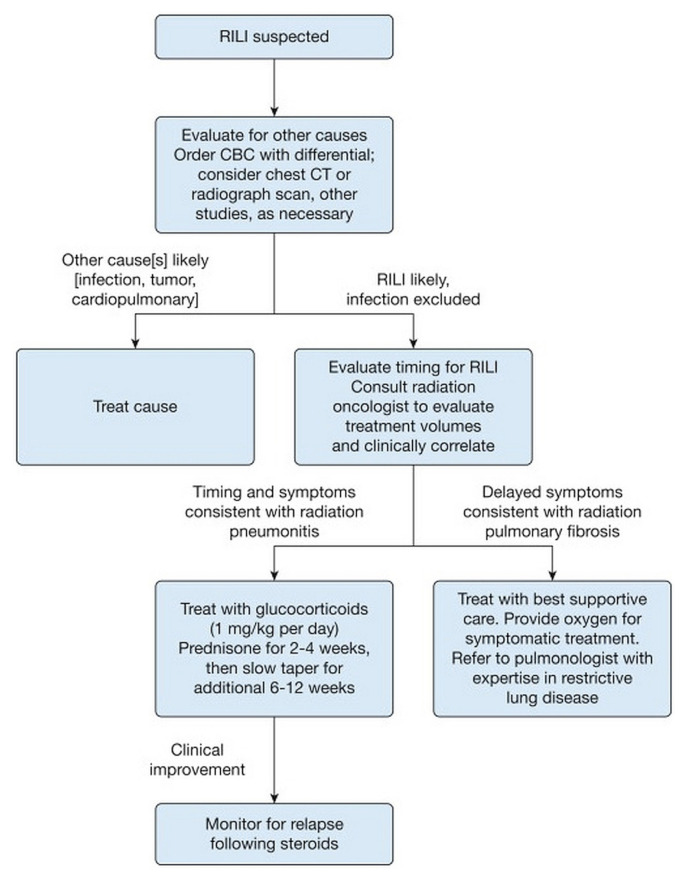
Radiation-induced lung injury consists of an acute inflammatory phase, defined as radiation pneumonitis (1–3 months after rt), and a chronic fibrotic phase, also known as radiation fibrosis (6–24 months after rt)37. Although most patients receiving thoracic rt are at risk of developing rili, certain factors such as smoking history, chronic obstructive pulmonary disease, and interstitial lung disease might increase the risk35,36. Older age and selected chemotherapies, immunotherapies, and targeted therapies also predispose patients to a higher risk of radiation recall pneumonitis. “Radiation recall” is a phenomenon in which patients develop pneumonitis after active rt treatments have been completed35. Radiation pneumonitis often presents with dyspnea, dry cough, and sometimes fever. A physical exam could be normal, but rare signs include pleural friction rub and rales37. Given those nonspecific findings, rili must always be included in the differential diagnosis for these patients. Although investigations can guide its identification, radiation pneumonitis is a clinical diagnosis: treatment includes steroids in symptomatic patients37. Figure 1 summarizes rili assessment and management.
FIGURE 1.
Clinical algorithm for the assessment and management of radiation-induced lung injury (RILI). Suspicion of RILI should be raised when a patient’s physical examination findings correlate temporally (typically within 3 months) with completion of thoracic radiation. CBC = complete blood count; CT = computed tomography. Reprinted with permission (Elsevier) from Hanania et al.35.
Radiation-induced heart disease can present years after rt completion and can manifest as valvular disease, pericardial disease, coronary artery disease, cardiomyopathy, or conduction abnormalities38. Although rt dose is the most significant risk factor, other traditional cardiovascular disease risk factors such as diabetes, hypertension, obesity, and smoking increase the risk39. Survivors should have an annual physician visit and scheduled screening for radiation-induced heart disease, together with targeted symptom investigation. Promotion of healthy lifestyle habits— including diet, regular exercise, weight control, and abstinence from smoking—are of utmost importance40. Moreover, a baseline echocardiogram 6–12 months after rt should be considered for high-risk survivors40. Lastly, adult survivors of childhood cancers should also receive periodic evaluation for cardiac toxicity and cardiology referral, typically 5–10 years after rt, especially for survivors exposed to a 35 Gy dose to the chest (or at least 15 Gy if they also received an anthracycline)41.
Effects of Pelvic RT
Compared with other cancer sites, pelvic cancers more frequently involve treatment with rt. Pelvic rt can lead to gastrointestinal toxicity, sexual dysfunction, and fertility concerns.
Pelvic radiation disease (prd) is defined as mild-to-severe transient or long-term gastrointestinal symptoms secondary to rt of a pelvic tumour. Patients have reported prd to have the greatest adverse effect on their quality of life42. Patients can present with up to 22 gastrointestinal symptoms, and given that each symptom can have more than one cause, symptoms should be investigated systematically43. Frequent seffs of pelvic rt are diarrhea, rectal bleeding, urgency, and fecal incontinence, all reported in up to 50% of patients42,44. In addition to pelvic rt, patient-related risk factors for prd include diabetes, inflammatory bowel disease, collagen vascular disease, low body mass index, and smoking45. Table III summarizes the proposed work-up and management for gastrointestinal symptoms linked to prd. Other pharmacologic (aminosalicylates, sucralfate, amifostine, corticosteroid enemas, bile acid sequestrants, famotidine, and selenium) and nonpharmacologic interventions (dietary modifications, green tea tablets, glutamine) currently have lower-certainty evidence of potential benefit46.
TABLE III.
Common gastrointestinal symptoms and managementa
| Symptom | Investigations | Potential results | Management | Alternative diagnoses |
|---|---|---|---|---|
| Rectal bleeding | Complete blood count, coagulation profile, referral for flexible sigmoidoscopy | Radiation proctopathy with bleeding from telangiectasia |
|
Hemorrhoids, primary inflammatory bowel disease, diverticular bleeding, new neoplasm |
| Bloating or abdominal cramps | Dietary history with or without test for carbohydrate malabsorption with or without biliary tree ultrasonography | Carbohydrate intolerance, irritable bowel disease, gallstones |
|
Tumour recurrence |
| Diarrhea | Dietary and lifestyle assessment, medication review, referral for flexible sigmoidoscopy | Radiation proctopathy or colopathy and pelvic floor dysfunction |
|
Infectious causes, celiac disease, dietary causes, drug-induced causes |
| Fecal incontinence | Rectal exam, referral for flexible sigmoidoscopy | Pelvic floor dysfunction with radiation proctopathy and fecal incontinence or leakage |
|
Constipation with overflow diarrhea, previous sphincter surgery, childbirth |
| Tenesmus | Referral for flexible sigmoidoscopy | Radiation proctopathy |
|
New neoplasm, irritable bowel disease, anterior resection syndrome |
Adapted with permission from: Andreyev et al.43 (https://creativecommons.org/licenses/by-nc/3.0/legalcode).
Sexual dysfunction after pelvic rt is typically multifactorial and negatively affects patients47. In men, erectile dysfunction is a common late seff, being reported in up to 50% of patients at 5 years after rt48. Bladder and bowel dysfunction can also occur and lead to decreased intimacy and self-esteem49. Phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, have been described as effective to treat rt-associated erectile dysfunction and should be considered for first-line treatment47,49,50. In women, seffs related to pelvic rt include vaginal dryness and stenosis, decreased sexual interest, and dyspareunia49. Vaginal dilators can help to improve vaginal elasticity and reduce fibrosis: their use has been associated with lesser rates of self-reported vaginal stenosis51. Experts recommend starting dilation 4 weeks after rt, at a frequency of 2–3 times weekly (1–3 minutes) for 9–12 months52. Referral to a trained physiotherapist for pelvic physiotherapy and education might facilitate dilator use and progress monitoring. Vaginal morbidity should be assessed before treatment, once every 3 months for the first 2 years after treatment, and then every 6 months thereafter53. Water-based non-hormonal lubricants might help vaginal dryness during intercourse54. Sexual counselling before treatment start might be beneficial, and referral to a psychologist or sexual health specialist could be warranted if sexual concerns arise49,55.
Fertility should be explored before treatment in patients who are considering pregnancy after treatment completion. A multidisciplinary approach involving reproductive endocrinologists, gynecologists, and maternal–fetal medicine specialists is recommended56. Women who have had pelvic rt can be at increased risk for spontaneous miscarriages, preterm labor, low birth weight, and placental abnormalities56. These survivors should be closely followed by a multidisciplinary team throughout pregnancy56.
SUMMARY
Radiotherapy treatments are associated with significant side effects that can negatively affect quality of life for cancer survivors. Although newer techniques in the field of radiation oncology have helped to reduce some of the adverse effects, further extensive research is needed to minimize rt-induced deleterious outcomes. All providers caring for cancer survivors, including general practitioners in oncology, should carefully assess and provide management for rt-related effects.
Key Points.
■ Radiation-induced side effects adversely affect quality of life for cancer survivors.
■ Screening and management of rt-induced early and late effects are crucial parts of the survivorship care agenda.
■ Family physicians and general practitioners in oncology are key providers in the management of comorbid conditions, promotion of healthy lifestyles, and treatment of rt-induced side effects.
Footnotes
This series is brought to you in partnership with the Canadian Association of General Practitioners in Oncology.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society. Cancer Statistics at a Glance [Web page, Quebec focused] Toronto, ON: Canadian Cancer Society; 2019. [Available at: https://www.cancer.ca/en/cancer-information/cancer-101/cancer-statistics-at-aglance/?region=qc; cited 1 October 2019] [Google Scholar]
- 2.Lalani N, Cummings B, Halperin R, et al. The practice of radiation oncology in Canada. Int J Radiat Oncol Biol Phys. 2017;97:876–80. doi: 10.1016/j.ijrobp.2016.11.055. [DOI] [PubMed] [Google Scholar]
- 3.Citrin DE. Recent developments in radiotherapy. N Engl J Med. 2017;377:2200–1. doi: 10.1056/NEJMra1608986. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 5.Chaput G, Med CP, Sussman J. Integrating primary care providers through the seasons of survivorship. Curr Oncol. 2019;26:48–54. doi: 10.3747/co.26.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi T, Hondo M, Nishimura K, et al. Evaluation of quality of life and psychological response in cancer patients treated with radiotherapy. Radiat Med. 2008;26:396–401. doi: 10.1007/s11604-008-0248-5. [DOI] [PubMed] [Google Scholar]
- 7.Stiegelis HE, Ranchor AV, Sanderman R. Psychological functioning in cancer patients treated with radiotherapy. Patient Educ Couns. 2004;52:131–41. doi: 10.1016/S0738-3991(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 8.Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi: 10.1136/bmj.k1415. [DOI] [PubMed] [Google Scholar]
- 9.Kawase E, Karasawa K, Shimotsu S, et al. Estimation of anxiety and depression in patients with early stage breast cancer before and after radiation therapy. Breast Cancer. 2012;19:147–52. doi: 10.1007/s12282-010-0220-y. [DOI] [PubMed] [Google Scholar]
- 10.Howell D, Keshavarz H, Esplen MJ, et al. on behalf of the Cancer Journey Advisory Group of the Canadian Partnership Against Cancer (cpac) A Pan Canadian Practice Guideline: Screening, Assessment and Care of Psychosocial Distress, Depression, and Anxiety in Adults with Cancer. Toronto, ON: CPAC and the Canadian Association of Psychosocial Oncology; 2015. [Available online at: https://capo.ca/resources/documents/guidelines/3apan-~1.pdf; cited 1 November 2019[ [Google Scholar]
- 11.Li M, Kennedy EB, Byrne N, et al. Management of depression in patients with cancer: a clinical practice guideline. J Oncol Pract. 2016;12:747–56. doi: 10.1200/JOP.2016.011072. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue. Ver. 1.2020. Fort Washington, PA: NCCN; 2019. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf (free registration required); cited 26 October 2019] [Google Scholar]
- 13.Turriziani A, Mattiucci GC, Montoro C, et al. Radiotherapy-related fatigue: incidence and predictive factors. Rays. 2005;30:197–203. [PubMed] [Google Scholar]
- 14.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–11. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Juvet LK, Thune I, Elvsaas IKO, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166–77. doi: 10.1016/j.breast.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Josef AM, Chen J, Wileyto P, et al. Effect of Eischens yoga during radiation therapy on prostate cancer patient symptoms and quality of life: a randomized phase ii trial. Int J Radiat Oncol Biol Phys. 2017;98:1036–44. doi: 10.1016/j.ijrobp.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarty J, Vidyasagar M, Fernandes D, Joisa G, Varghese P, Mayya S. Effectiveness of pranayama on cancer-related fatigue in breast cancer patients undergoing radiation therapy: a randomized controlled trial. Int J Yoga. 2015;8:47–53. doi: 10.4103/0973-6131.146062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengacher CA, Reich RR, Paterson CL, et al. Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2016;34:2827–34. doi: 10.1200/JCO.2015.65.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balk J, Day R, Rosenzweig M, Beriwal S. Pilot, randomized, modified, double-blind, placebo-controlled trial of acupuncture for cancer-related fatigue. J Soc Integr Oncol. 2009;7:4–11. [PubMed] [Google Scholar]
- 20.Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79–92. doi: 10.1016/j.ctrv.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalla RV, Bowen J, Barasch A, et al. on behalf of the Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (mascc/isoo) mascc/isoo clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2015;120:1453–61. doi: 10.1002/cncr.28592. [DOI] [Google Scholar]
- 22.Tyker A, Franco J, Massa ST, Desai SC, Walen SG. Treatment for lymphedema following head and neck cancer therapy: a systematic review. Am J Otolaryngol. 2019;40:761–9. doi: 10.1016/j.amjoto.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Colevas AD, Yom SS, Pfister DG, et al. nccn guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw. 2018;16:479–90. doi: 10.6004/jnccn.2018.0026. [DOI] [PubMed] [Google Scholar]
- 24.Baharvand M, ShoalehSaadi N, Barakian R, Moghaddam EJ. Taste alteration and impact on quality of life after head and neck radiotherapy. J Oral Pathol Med. 2013;42:106–12. doi: 10.1111/j.1600-0714.2012.01200.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita H, Nakagawa K, Tago M, et al. Taste dysfunction in patients receiving radiotherapy. Head Neck. 2006;28:508–16. doi: 10.1002/hed.20347. [DOI] [PubMed] [Google Scholar]
- 26.Sandow PL, Hejrat-Yazdi M, Heft MW. Taste loss and recovery following radiation therapy. J Dent Res. 2006;85:608–11. doi: 10.1177/154405910608500705. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:772–8. doi: 10.1016/j.ijrobp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by imrt? Int J Radiat Oncol Biol Phys. 2004;60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 29.O’Sullivan B, Levin W. Late radiation-related fibrosis: pathogenesis, manifestations, and current management. Semin Radiat Oncol. 2003;13:274–89. doi: 10.1016/S1053-4296(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 30.Greco E, Simic T, Ringash J, Tomlinson G, Inamoto Y, Martino R. Dysphagia treatment for patients with head and neck cancer undergoing radiation therapy: a meta-analysis review. Int J Radiat Oncol Biol Phys. 2018;101:421–44. doi: 10.1016/j.ijrobp.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 31.Chapuy CI, Annino DJ, Tishler RB, Haddad RI, Snavely A, Goguen LA. Success of endoscopic pharyngoesophageal dilation after head and neck cancer treatment. Laryngoscope. 2013;123:3066–73. doi: 10.1002/lary.24268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinna R, Campus G, Cumbo E, Mura I, Milia E. Xerostomia induced by radiotherapy: an overview of the physiopathology, clinical evidence, and management of the oral damage. Ther Clin Risk Manag. 2015;11:171–88. doi: 10.2147/TCRM.S70652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salum FG, Medella-Junior FAC, Figueiredo MAZ, Cherubini K. Salivary hypofunction: an update on therapeutic strategies. Gerodontology. 2018;35:305–16. doi: 10.1111/ger.12353. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter DJ, Mowery YM, Broadwater G, et al. The risk of carotid stenosis in head and neck cancer patients after radiation therapy. Oral Oncol. 2018;80:9–15. doi: 10.1016/j.oraloncology.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156:150–62. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giuranno L, Ient J, De Ruysscher D, Vooijs MA. Radiation-induced lung injury (rili) Front Oncol. 2019;9:877. doi: 10.3389/fonc.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng G, Liang N, Xie J, et al. Pulmonary toxicity generated from radiotherapeutic treatment of thoracic malignancies. Oncol Lett. 2017;14:501–11. doi: 10.3892/ol.2017.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Wei J, Zheng Q, et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15:2128–38. doi: 10.7150/ijbs.35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Chuy K, Nahhas O, Dominic P, et al. Cardiovascular complications associated with mediastinal radiation. Curr Treat Options Cardiovasc Med. 2019;21:31. doi: 10.1007/s11936-019-0737-0. [DOI] [PubMed] [Google Scholar]
- 40.Armenian SH, Lacchetti C, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract. 2017;13:270–5. doi: 10.1200/JOP.2016.018770. [DOI] [PubMed] [Google Scholar]
- 41.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Monrovia, CA: Children’s Oncology Group; 2018. [Available online at: http://www.survivorshipguidelines.org; cited 20 October 2019] [Google Scholar]
- 42.Adams E, Boulton MG, Horne A, et al. The effects of pelvic radiotherapy on cancer survivors: symptom profile, psychological morbidity and quality of life. Clin Oncol (R Coll Radiol) 2014;26:10–17. doi: 10.1016/j.clon.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Andreyev HJ, Muls AC, Norton C, et al. Guidance: the practical management of the gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol. 2015;6:53–72. doi: 10.1136/flgastro-2014-100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuccio L, Frazzoni L, Guido A. Prevention of pelvic radiation disease. World J Gastrointest Pharmacol Ther. 2015;6:1–9. doi: 10.4292/wjgpt.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuccio L, Guido A, Andreyev HJ. Management of intestinal complications in patients with pelvic radiation disease. Clin Gastroenterol Hepatol. 2012;10:1326–34.e4. doi: 10.1016/j.cgh.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Lawrie TA, Green JT, Beresford M, et al. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers. Cochrane Database Syst Rev. 2018;(1):CD012529. doi: 10.1002/14651858.CD012529.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Incrocci L, Jensen PT. Pelvic radiotherapy and sexual function in men and women. J Sex Med. 2013;10(suppl 1):53–64. doi: 10.1111/jsm.12010. [DOI] [PubMed] [Google Scholar]
- 48.Gaither TW, Awad MA, Osterberg EC, et al. The natural history of erectile dysfunction after prostatic radiotherapy: a systematic review and meta-analysis. J Sex Med. 2017;14:1071–8. doi: 10.1016/j.jsxm.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician. 2010;82:381–8. [PubMed] [Google Scholar]
- 50.Mahmood J, Shamah AA, Creed TM, et al. Radiation-induced erectile dysfunction: recent advances and future directions. Adv Radiat Oncol. 2016;1:161–9. doi: 10.1016/j.adro.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:CD007291. doi: 10.1002/14651858.CD007291.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakker RM, ter Kuile MM, Vermeer WM, et al. Sexual rehabilitation after pelvic radiotherapy and vaginal dilator use: consensus using the Delphi method. Int J Gynecol Cancer. 2014;24:1499–506. doi: 10.1097/IGC.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 53.Morris L, Do V, Chard J, Brand AH. Radiation-induced vaginal stenosis: current perspectives. Int J Womens Health. 2017;9:273–9. doi: 10.2147/IJWH.S106796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canadian Cancer Society. Sex, Intimacy and Cancer. Toronto, ON: Canadian Cancer Society; 2018. [Google Scholar]
- 55.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Survivorship. Ver. 1.2020. Fort Washington, PA: NCCN; 2020. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf (free registration required); cited 26 October 2019] [Google Scholar]
- 56.Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73:1304–12. doi: 10.1016/j.ijrobp.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]