Abstract
Background
After surgery for early-stage breast cancer (bca), adjuvant radiotherapy (rt) decreases the risk of locoregional recurrence and death from bca. It is unclear whether delays to the initiation of adjuvant rt are associated with inferior survival outcomes.
Methods
This population-based retrospective cohort study included a random sample of 25% of all women with stage i or ii bca treated with adjuvant rt in Ontario between 1 September 2001 and 31 August 2002, when, because of capacity issues, wait times for radiation were abnormally long. Pathology reports were manually abstracted and deterministically linked to population-level administrative databases to obtain information about recurrence and survival outcomes. Cox proportional hazards modelling was used to evaluate the association between waiting time and survival outcomes. A composite survival outcome was used to ensure that all possible measurable harms of delay would be captured. The composite outcome, event-free survival, included locoregional recurrence, development of metastatic disease, and bca-specific mortality.
Results
We identified 1028 women with stage i or ii bca who were treated with breast-conserving surgery and adjuvant rt. For the 599 women who were treated with adjuvant radiation without intervening chemotherapy, a waiting time of 12 weeks or more from surgery to the start of radiation appeared to be associated with worse event-free survival after a median follow-up of 7.2 years (hazard ratio for the composite outcome: 1.44; 95% confidence interval: 0.98 to 2.11; p = 0.07). For the 429 women who received intervening adjuvant chemotherapy, a waiting time of 6 weeks or more from completion of chemotherapy to start of radiation was associated with worse event-free survival after a median follow-up of 7.4 years (hazard ratio: 1.50; 95% confidence interval: 1.00 to 2.22; p = 0.047).
Conclusions
Delay to the initiation of adjuvant rt after breast-conserving surgery is associated with inferior bca survival outcomes. The good prognosis for patients with early-stage bca limits the statistical power to detect an effect of delay to rt. Given that there is no plausible advantage to delay, we agree with Mackillop that time to initiation of rt should be kept “as short as reasonably achievable.”
Keywords: Radiation oncology, time to treatment, health services research, population-based cancer outcomes, care delivery, real-world evidence
INTRODUCTION
The potential harm of waiting for radiotherapy (rt) in Ontario was first heralded back in 19941. However, because of the rising incidence of cancer and the discovery of new indications for rt, demand for rt continued to increase, and in turn, so did waiting times2,3. By 2001, median waiting times for rt in Ontario exceeded 6 weeks, with delays greater than 3 months being not uncommon3–5. Although such delays have been considered unacceptable to radiation oncologists2 and patients4 alike, their effect on clinical outcomes remains controversial, with studies producing mixed results6–17.
The effect on clinical outcomes of time to initiation of rt has never been evaluated in a randomized controlled trial. Such a trial would be unethical, because there is no plausible advantage to delayed rt. The prolonged waiting times that occurred in Ontario provide a natural cohort of patients through which the population-level effect of delays to rt initiation can be evaluated.
The derivation of the original patient cohort, based on retrospective data, and the description of waiting times was originally published in 20065. The present study prospectively followed that cohort of patients to report on long-term survival outcomes associated with delays to rt initiation.
METHODS
Study Population and Data Sources
The original study population consisted of a 25% random sample of all patients with breast cancer (bca) in the province of Ontario who received rt after lumpectomy between 1 September 2001 and 31 August 2002. To create a homogenous cohort with respect to survival outcomes, we excluded patients with stages iii and iv bca.
To create the cohort, eligible patients were identified directly from lists provided by all of the cancer centres providing rt in Ontario and by Cancer Care Ontario. Original health records were abstracted to obtain sociodemographic data, pathology, staging, date of surgery or biopsy, and dates of key events in the rt process (consultation, simulation, first treatment). The information obtained from the original health records was then deterministically linked using unique encoded patient identifiers to population-level administrative databases housed at ices. The Ontario Health Insurance Plan database provided information about dates of chemotherapy administration. The Canadian Institute for Health Information and its Discharge Abstract Database provided information about salvage surgeries, diagnoses of local recurrence, and diagnoses of new metastasis. The Registered Persons Database provided information about date of death and postal code of residence, which was then linked to Statistics Canada census data to obtain each patient’s neighbourhood income. Information about cause of death was obtained from the Ontario Cancer Registry and from vital statistics recorded by Ontario’s Office of the Registrar General.
Definition of Waiting Times and Outcomes
For patients who did not receive adjuvant chemotherapy after surgical resection, the waiting time was measured as the interval from the date of last surgery or biopsy to the date of first rt treatment; a prolonged waiting time was predefined as 12 weeks or more. For the subset of patients with bca who received adjuvant chemotherapy between their surgery and radiation, waiting time was measured from the date of the last chemotherapy administration to the date of first rt treatment; a prolonged waiting time was predefined as 6 weeks or more. The cut-offs for prolonged waiting time were chosen because they represented both the median waiting times identified in the original derivation of the cohort and because they are consistent with the upper limit of the recommended timeframe in which rt should be delivered5,8,18,19. Delay was also evaluated as a continuous measure per 1 week of waiting time.
The primary composite outcome was evidence of locoregional recurrence, diagnosis of metastatic disease, or bca-specific mortality. A composite outcome was used to ensure that all possible measurable harms of delay in rt initiation would be captured and to minimize the impact of competing risks. The individual components included in the composite outcome were selected based on the demonstrated survival benefits of timely rt20.
Statistical Analysis
Patient demographics and cancer stage were compared for patients with short and with prolonged waiting times, using chi-square tests for categorical variables and t-tests for continuous variables. Multivariable Cox proportional hazards modelling was used to evaluate the association between waiting time and survival outcomes. Covariates included in the model were age, income quintile, and disease stage. Hormone receptor status and hormonal treatment had not been collected in the derivation cohort, and we were therefore unable to include those known prognostic factors in the model. Composite outcome rates were estimated using the Kaplan–Meier method. The log-rank test was used to compare survival in the waiting-time groups. All analyses were stratified by receipt of adjuvant chemotherapy. All p values are two-sided, with statistical significance set at p < 0.05. Statistical analyses were performed using the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.).
Ethics Approval
For the original descriptive derivation cohort, ethics review and approval was obtained from the Sunnybrook and Women’s College Health Sciences Centre and from each rt facility in Ontario. For the outcomes analysis, ethics review and approval was obtained from the Toronto Academic Health Sciences Network Board of Record.
RESULTS
Study Population
In the original derivation cohort, 1271 women with bca received rt between 1 September 2001 and 31 August 2002 in Ontario. After the exclusion of women with stage iii or iv bca (n = 121), survival outcome follow-up data were available for 1028 women.
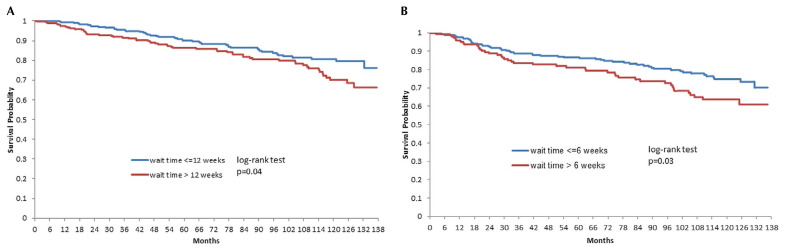
Of those 1028 women, 599 (58%) received adjuvant rt alone after surgical resection (Table I). Median waiting time to rt was 12 weeks (interquartile range: 9–15 weeks). Advancing age (p = 0.009) was the only covariate significantly associated with waiting 12 or more weeks for rt after surgical resection. After a median follow-up of 7.2 years, a waiting time of 12 or more weeks was associated with worse event-free survival in a multivariable analysis [hazard ratio (hr): 1.44; 95% confidence interval (ci): 0.98 to 2.11; p = 0.07]. Unadjusted and adjusted event rates are presented in Tables II and III respectively. Waiting time was also analyzed as a continuous variable, resulting in a hr of 1.04 (95% ci: 1.00 to 1.08; p = 0.05). Figure 1(A) presents the Kaplan–Meier curves.
TABLE I.
Baseline characteristics of breast cancer patients with short and long waiting times for radiotherapy, by use of intervening adjuvantchemotherapy
| Variable | Intervening adjuvant chemotherapy | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No | Yes | |||||||
|
|
|
|||||||
| Waiting time | p Value | Waiting time | p Value | |||||
|
|
|
|||||||
| ≤12 Weeks (n=314) | >12 Weeks (n=285) | Overall (n=599) | ≤12 Weeks (n=275) | >12 Weeks (n=154) | Overall (n=429) | |||
| Mean age (years) | 61.93±11.73 | 64.48±11.94 | 63.14±11.89 | 0.009 | 52.17±10.54 | 53.46±10.01 | 52.64±10.36 | 0.218 |
|
| ||||||||
| Socioeconomic status [n (%)] | 0.399 | 0.153 | ||||||
| 1 | 42 (55) | 35 (45) | 77 | 29 (52) | 27 (48) | 56 | ||
| 2 | 58 (48) | 63 (52) | 121 | 40 (63) | 23 (37) | 63 | ||
| 3 | 49 (48) | 54 (53) | 103 | 49 (58) | 35 (42) | 84 | ||
| 4 | 63 (58) | 45 (42) | 108 | 56 (70) | 24 (30) | 80 | ||
| 5 | 67 (57) | 50 (43) | 117 | 59 (71) | 24 (29) | 83 | ||
|
| ||||||||
| Stage [n (%)] | 0.388 | 0.02 | ||||||
| I | 242 (53) | 211 (47) | 453 | 88 (73) | 33 (27) | 121 | ||
| II | 72 (49) | 74 (51) | 146 | 187 (61) | 121 (39) | 308 | ||
TABLE II.
Unadjusted event ratesa for patients with short and long waiting times for radiotherapy and no intervening adjuvant chemotherapy
| Event | Waiting time [n (%)] | p Value | |
|---|---|---|---|
| ≤12 Weeks (n=314) | >12 Weeks (n=285) | ||
| None | 266 (85) | 227 (80) | 0.264 |
| Death | ≤6 | 6 (2) | |
| Metastasis | 31 (10) | 31 (11) | |
| Local recurrence | ≤15 | 21 (7) | |
Per policy at ICES, all small cells must be suppressed to prevent patient re-identification. Any results with counts between 1 and 5 are therefore reported as ≤5.
TABLE III.
Adjusted hazard ratios (HRs) for the primary composite outcome in patients with short and long waiting times for radiotherapya and no intervening adjuvant chemotherapy
| Parameter | Comparator | HR | 95% CI | p Value |
|---|---|---|---|---|
| Long waiting time | Short waiting time | 1.44 | 0.98 to 2.11 | 0.07 |
| Age | Per 10 years | 1.138 | 0.96 to 1.35 | 0.14 |
| Stage II | Stage I | 1.469 | 0.97 to 2.23 | 0.07 |
≤12 Weeks or >12 weeks.
CI = confidence interval.
FIGURE 1.
Survival probability after radiotherapy for breast cancer patients who (A) did not receive adjuvant chemotherapy and (B) did receive adjuvant chemotherapy.
The remaining 429 women (42%) received adjuvant chemotherapy before rt (Table I). Their median waiting time to rt was 5 weeks (25%–75% interquartile range: 4–7 weeks). Disease stage (p = 0.02) was the only covariate significantly associated with a waiting time of 6 or more weeks for rt after completion of chemotherapy. After a median follow-up of 7.4 years, a waiting time of 6 or more weeks was associated with worse event-free survival in a multivariable analysis (hr: 1.50; 95% ci: 1.00 to 2.22; p = 0.047). Unadjusted and adjusted event rates are presented in Tables IV and V respectively. Waiting time was also analyzed as a continuous variable (hr: 1.01; 95% ci: 0.97 to 1.05; p = 0.58). Figure 1(B) presents the Kaplan–Meier curves.
TABLE IV.
Unadjusted event ratesa for patients with short and long waiting times for radiotherapy and with intervening adjuvant chemotherapy
| Event | Waiting time [n (%)] | p Value | |
|---|---|---|---|
| ≤6 Weeks (n=275) | >6 Weeks (n=154) | ||
| None | 219 (80) | 110 (71) | 0.15 |
| Death | ≤6 | ≤6 | |
| Metastasis | 36 (13) | 26 (17) | |
| Local recurrence | ≤15 | 16 (10) | |
Per policy at ICES, all small cells must be suppressed to prevent patient re-identification. Any results with counts between 1 and 5 are therefore reported as ≤5.
TABLE V.
Adjusted hazard ratios (HRs) for the primary composite outcome in patients with short and long waiting times for radiotherapya and with intervening adjuvant chemotherapy
| Parameter | Comparator | HR | 95% CI | p Value |
|---|---|---|---|---|
| Long waiting time | Short waiting time | 1.50 | 1.00 to 2.22 | 0.047 |
| Age | Per 10 years | 1.01 | 0.83 to 1.23 | 0.94 |
| Stage II | Stage I | 1.68 | 1.01 to 2.78 | 0.04 |
≤6 weeks > 6 weeks
CI= confidence interval.
DISCUSSION
Radiotherapy is an integral component of multidisciplinary care for patients with bca. Randomized trials have consistently shown that adjuvant rt is associated with reductions in local recurrence and bca-specific mortality after surgery for early-stage bca20. Because all the major randomized trials that established the efficacy of adjuvant rt mandated that treatment be initiated within 6–12 weeks after surgery21–23, there is little evidence to understand its efficacy, or lack thereof, when initiated beyond those timelines24,25. Our large prospective population-based study of women with early-stage bca in Ontario demonstrates that prolonged waiting times for adjuvant rt appear to be associated with inferior bca outcomes. The fact that statistical significance was not achieved in the present study likely reflects the good overall prognosis for women with early-stage bca, resulting in lack of statistical power rather than lack of a true association. Despite a median 7.3 years of follow-up in the present study, 80% of patients did not experience an adverse survival event, thereby limiting the statistical power to detect a differential treatment effect. Consideration of the effect estimates and cis, together with the totality of prior evidence, supports the conclusion that delays to rt are associated with inferior bca outcomes.
The present study adds to a growing body of literature examining the outcomes associated with delays to rt initiation. The available literature consists of studies evaluating heterogeneous patient populations, using disparate definitions of delay, and evaluating multiple different endpoints, making it difficult to clearly establish the association between delay to rt and survival outcomes and, accordingly, to establish safe evidence-based waiting-time benchmarks. Attempts to overcome those limitations have come in the form of meta-analyses.
In 2008, Chen et al.6 performed a systematic review and meta-analysis to evaluate the association between waiting time to rt and survival outcomes. The meta-analysis concluded that delays to rt for early-stage bca were associated with decrements in local control for patients with bca (hr per 4 weeks of delay: 1.11; 95% ci: 1.04 to 1.19). However, that result was driven mainly by an increased risk of local recurrence in patients who received intervening adjuvant chemotherapy (hr per 4 weeks of delay, no adjuvant chemotherapy: 1.11; 95% ci: 0.94 to 1.33; hr per 4 weeks of delay, received adjuvant chemotherapy: 1.11; 95% ci: 1.03 to 1.19).
There are several potential problems with performing a combined analysis of patients who did and did not receive intervening adjuvant chemotherapy in this type of meta-analysis:
■ The patients treated with adjuvant chemotherapy would almost certainly have had higher-risk and more aggressive disease and might therefore have been more likely to experience recurrence regardless of waiting time.
■ Chen et al. defined “waiting time” as the interval from surgery to rt, without consideration of time spent receiving intervening chemotherapy—a problematic approach because the analysis would therefore include delays to rt of up to 9 months, which would act as strong outliers in the subsequent analysis.
Because the meta-analytic technique used by Chen et al. relied on the assumption of a log-linear relationship between waiting time and recurrence, it is possible that the relationship does not hold when extending to such long time intervals.
In 2016, the meta-analysis was updated to include eighteen more studies, and it similarly found that every 4 weeks of waiting time for rt was associated with an 8% relative increase in the risk of locoregional recurrence25.
The impact of intervening adjuvant chemotherapy on the relationship between time to rt and outcomes is worth considering further. The optimal sequencing and timing of adjuvant treatments for early-stage bca has been a topic of considerable historical controversy26. Based mainly on the results of a two small randomized trials, current practice is commonly to sequence adjuvant chemotherapy before rt in patients deemed to be at sufficient risk of distant metastasis based on their clinical or genomic profile (or both)22,27,28. Long-term follow-up from the two major adjuvant sequencing studies demonstrated no differences in the rates of distant and local recurrence27,28. Most earlier studies evaluating the potential harm of delay to rt have used, as a definition of waiting time, the interval from surgery to rt. However, when the decision has been made to proceed with chemotherapy, the interval between surgery and rt will be dictated largely by the duration of the chosen chemotherapy regimen5. We feel that, in this circumstance, the interval between last chemotherapy administration and start of radiation is more relevant to the clinician and patient. The amount of time spent waiting for rt during that time interval, while the patient is not receiving any form of adjuvant treatment, is the interval prone to delays from system-level issues such as delayed referrals and is therefore potentially modifiable14.
Considerable theoretical evidence supports the concept that timely rt could be important, summarized previously by Mackillop24. Apart from potential decrements in survival, delay can adversely affect patients by necessitating larger rt fields and therefore potentially increasing the toxicity of treatment. Finally, the potential effect on the mental well-being and health-related quality of life for patients and their families of waiting for medically necessary treatment can never be discounted.
Although the effect of delays to rt on the foregoing important patient-centred outcomes has not been adequately studied before, Canadians have consistently identified waiting too long for care as the largest barrier to accessing health care in Canada29,30. Furthermore, there is evidence that the existence of a longer waiting list can actually deter referrals for radiation that is otherwise indicated30. Therefore, although our study did not identify a statistically significant association between waiting time to rt and survival outcomes, given that prolonged delays to treatment offer no plausible advantage, we agree with Mackillop that time to rt should be kept “as short as reasonably achievable”24.
The major limitation of the present study, as for all previous analyses evaluating the effect of delays on survival outcomes, is that the results are based on nonrandomized observational data. Baseline differences in the balance of prognostic factors, comorbidities, and postoperative complications might therefore confound the relationship between waiting time for rt and survival outcomes. Furthermore, in the inception cohort, baseline information about hormone receptor status or adjuvant hormonal treatments was not collected—factors that are recognized to be associated with improved rates of local recurrence and overall survival independent of the effect of rt31. Likewise, we lacked information about rt doses and completion rates, and whether boost radiation was applied to the tumour bed. Finally, despite a large sample size and a median follow-up of 7.3 years, the low event rates limited the power to identify a statistically significant difference between waiting-time groups. That consideration is supported by the high hrs for the composite survival outcome (seen whether the patients were treated with or without chemotherapy), together with the wide cis and borderline p values
CONCLUSIONS
Our study demonstrates that prolonged waiting times to rt appear to be associated with inferior survival outcomes for patients with early-stage bca. Given that there is no plausible advantage to delay, we agree with Mackillop that that time to rt initiation should be kept “as short as reasonably achievable.”
ACKNOWLEDGMENTS
This study was conducted with the support of the Ontario Institute for Cancer Research and Ontario Health (Cancer Care Ontario) [oh(cco)] through funding provided by the Government of Ontario. The study was supported by ices, which is funded by an annual grant from the Ontario Ministry of Health (omh). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ices or the omh is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (cihi). However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not necessarily those of cihi. Parts of this material are based on data and information provided by oh(cco). The opinions, results, views, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of oh(cco). No endorsement by oh(cco) is intended or should be inferred.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: SS has received honoraria from Novartis and Ipsen; institutional research funding from Novartis and emd Serono; and grants for travel, accommodations, and expenses from Novartis and Pfizer.The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Mackillop WJ, Fu H, Quirt CF, Dixon P, Brundage M, Zhou Y. Waiting for radiotherapy in Ontario. Int J Radiat Oncol Biol Phys. 1994;30:221–8. doi: 10.1016/0360-3016(94)90538-X. [DOI] [PubMed] [Google Scholar]
- 2.Mackillop WJ, Zhou Y, Quirt CF. A comparison of delays in the treatment of cancer with radiation in Canada and the United States. Int J Radiat Oncol Biol Phys. 1995;32:531–9. doi: 10.1016/0360-3016(94)00662-5. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Quality Council of Ontario. Synopsis. In: Sullivan T, Evans W, Angus H, Hudson A, editors. Strengthening the Quality of Cancer Services in Ontario. Ottawa, ON: Canadian Healthcare Association Press; 2003. [Google Scholar]
- 4.McGowan T. Private management of a public service: what can be learned from the cros experience? Hosp Q. 2003;6:33–8. doi: 10.12927/hcq..16479. [DOI] [PubMed] [Google Scholar]
- 5.Benk V, Przybysz R, McGowan T, Paszat L. Waiting times for radiation therapy in Ontario. Can J Surg. 2006;49:16–21. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3–16. doi: 10.1016/j.radonc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Desai S, Hurley J, Takita C, et al. Impact of surgery–radiation interval on locoregional outcome in patients receiving neoadjuvant therapy and mastectomy. Breast J. 2013;19:427–30. doi: 10.1111/tbj.12140. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21:555–63. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 9.Livi L, Borghesi S, Saieva C, et al. Radiotherapy timing in 4,820 patients with breast cancer: University of Florence experience. Int J Radiat Oncol Biol Phys. 2009;73:365–9. doi: 10.1016/j.ijrobp.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 10.Mackillop WJ, Bates JH, O’Sullivan B, Withers HR. The effect of delay in treatment on local control by radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:243–50. doi: 10.1016/0360-3016(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 11.Olivotto IA, Lesperance ML, Truong PT, et al. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. J Clin Oncol. 2009;27:16–23. doi: 10.1200/JCO.2008.18.1891. [DOI] [PubMed] [Google Scholar]
- 12.Punglia RS, Saito AM, Neville BA, Earle CC, Weeks JC. Impact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysis. BMJ. 2010;340:c845. doi: 10.1136/bmj.c845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys. 2003;56:399–412. doi: 10.1016/S0360-3016(02)04469-3. [DOI] [PubMed] [Google Scholar]
- 14.Vujovic O, Yu E, Cherian A, Dar AR, Stitt L, Perera F. Time interval from breast-conserving surgery to breast irradiation in early stage node-negative breast cancer: 17-year follow-up results and patterns of recurrence. Int J Radiat Oncol Biol Phys. 2015;91:319–24. doi: 10.1016/j.ijrobp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Fortin A, Bairati I, Albert M, Moore L, Allard J, Couture C. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:929–36. doi: 10.1016/S0360-3016(01)02606-2. [DOI] [PubMed] [Google Scholar]
- 16.Lopez S, Calugaru V, Lamproglou I, et al. The effect of waiting list for radiotherapy for glioblastoma [French] Cancer Radiother. 2008;12:497–9. doi: 10.1016/j.canrad.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 17.EC, Dahrouge S, Samant R, Mirzaei A, Price J. Radical radiotherapy for cervix cancer: the effect of waiting time on outcome. Int J Radiat Oncol Biol Phys. 2005;61:1071–7. doi: 10.1016/j.ijrobp.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Whelan TJ, Lada BM, Laukkanen E, Perera FE, Shelley WE, Levine MN. Breast irradiation in women with early stage invasive breast cancer following breast conserving surgery. Breast Cancer Disease Site Group. Cancer Prev Control. 1997;1:228–40. [PubMed] [Google Scholar]
- 19.Ontario Health (Cancer Care Ontario) [oh(cco)]; Radiation Treatment Wait Times [Web page] Toronto, ON: OH(CCO); 2016. [Available at: http://ocp.cancercare.on.ca/cms/One.aspx?portalId=327895&pageId=8851; cited 23 August 2016] [Google Scholar]
- 20.Darby S, McGale P, Correa C, et al. on behalf of the Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–73. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 22.Recht A, Come SE, Henderson IC, et al. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996;334:1356–61. doi: 10.1056/NEJM199605233342102. [DOI] [PubMed] [Google Scholar]
- 23.Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. J Natl Cancer Inst. 1996;88:1659–64. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 24.Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1–4. doi: 10.1016/j.radonc.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, King WD, Korzeniowski M, Wallace DL, Mackillop WJ. The effect of waiting times for post-operative radiotherapy on outcomes for women receiving partial mastectomy for breast cancer: a systematic review and meta-analysis. Clin Oncol (R Coll Radiol) 2016;28:739–49. doi: 10.1016/j.clon.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Bellon JR, Harris JR. Chemotherapy and radiation therapy for breast cancer: what is the optimal sequence? J Clin Oncol. 2005;23:5–7. doi: 10.1200/JCO.2005.09.962. [DOI] [PubMed] [Google Scholar]
- 27.Pinnarò P, Rambone R, Giordano C, Giannarelli D, Strigar L, Arcangeli G. Long-term results of a randomized trial on the sequencing of radiotherapy and chemotherapy in breast cancer. Am J Clin Oncol. 2011;34:238–44. doi: 10.1097/COC.0b013e3181dea9b8. [DOI] [PubMed] [Google Scholar]
- 28.Bellon JR, Come SE, Gelman RS, et al. Sequencing of chemotherapy and radiation therapy in early-stage breast cancer: updated results of a prospective randomized trial. J Clin Oncol. 2005;23:1934–40. doi: 10.1200/JCO.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Statistics Canada. Access to Health Care Services in Canada: January to December 2005. Cat. no. 82-525-XIE. Ottawa, ON: Statistics Canada; 2006. [Google Scholar]
- 30.Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. doi: 10.1186/1748-717X-7-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]