Abstract
The aims of this study were to compare diagnostic value of anti-ribosomal P protein antibody (anti-P), anti-Smith antibody (anti-Sm), anti-double-stranded DNA antibody (anti-dsDNA), anti-nucleosome antibody (ANuA), and anti-histone antibody (AHA) for systemic lupus erythematosus (SLE) as well as explore the correlation between anti-P and SLE.
A retrospective study was performed with 487 SLE patients, 235 non-SLE rheumatic diseases, and 124 healthy subjects from January 2015 to December 2018. Clinical manifestations, laboratory results and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)-2000 scores were analyzed between anti-P/+/ and anti-P/−/ patients. SPSS19.0 statistical software was used for data analysis.
The sensitivities of anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA in SLE were 31.6%, 20.7%, 45.0%, 27.9%, and 14.6%, and the specificities were 99.2%, 99.4%, 98.9%, 98.3%, and 96.7%, respectively. Only 27.9% of SLE had a single positive anti-P while the other 4 antibodies were all negative. There were significant differences in the age of onset, skin erythema, urinary protein, creatinine and serum IgG, IgM, C3, C4 between anti-P/+/ and anti-P/−/ patients (P < .05). When anti-Sjogren syndrome A antibody, anti-P were positive and anti-dsDNA was negative, the incidence of skin erythema was the highest (35.1%). Compared with anti-P/−/ patients, anti-P/+/ patients had higher SLEDAI scores (P < .001).
Anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA have high specificity but poor sensitivity in the diagnosis of SLE; combined detection can greatly improve the detection rate. Anti-P is more valuable in the diagnosis of SLE when other specific autoantibodies are negative. SLE patients with positive anti-P have an earlier onset age and are more prone to skin erythema, lupus nephritis as well as higher disease activity.
Keywords: anti-ribosomal P protein antibody, disease activity, organ damage, skin erythema, systemic lupus erythematosus
1. Introduction
Systemic lupus erythematosus (SLE) is a connective tissue disease with dysfunction in multiple organs,[1] which is more common in women with a peak incidence of 15 to 40 years. The clinical manifestations of SLE are complex and can cause damage to skin, joints, kidneys, and central nervous system. Most patients have mild symptoms at the beginning of the disease, only 1 to 2 organs have dysfunction, and then gradually become multi-organ lesions. There are also a small number of patients with multiple organ damage or even lupus crisis in the early stage.
SLE is characterized by the presence of a variety of autoantibodies, which are closely associated with the disease progression, activity, and clinical manifestations, but few of them are directly related to the occurrence and development of SLE.[2,3] Currently, anti-Sm and anti-dsDNA have been widely used in the clinical diagnosis of SLE, anti-dsDNA is strongly associated with lupus nephritis (LN) and has been confirmed to be associated with the activity of SLE.[4,5] There are also some studies showing that anti-P, ANuA, and AHA are closely related to SLE as well, more and more attention have been paid to the diagnostic value of anti-P for SLE particularly.[6,7]
Anti-P is directed to 3 phosphoproteins (P0, P1, and P2), which are located on the 60S subunit of eukaryotic ribosome. The positive rate of anti-P in SLE is mostly between 10% to 40%, among which the Asian population is the highest.[8,9,10] Studies have found that anti-P is associated with neurological lupus,[11,12] and may be associated with LN as well.[13,14] Therefore, the current study was designed to evaluate the diagnostic performance of serum anti-P for SLE in comparison with several other autoantibodies, and further explored the correlation between anti-P on organ damage and disease activity in SLE.
2. Materials and methods
2.1. Study design
A retrospective study was performed with 487 SLE patients, 235 patients with non-SLE rheumatic diseases, and 124 normal healthy subjects from January 2015 to December 2018, who gave informed consents. The patients were all from the First Affiliated Hospital of Chongqing Medical University, SLE patients fulfilled the 1997 American College of Rheumatology (ACR) revised criteria. Patients with non-SLE rheumatic diseases included 141 cases of rheumatoid arthritis, 15 cases of Sjogren syndrome, 6 cases of autoimmune hepatitis, 20 cases of primary biliary cirrhosis, 4 cases of mixed connective tissue disease, and 49 cases of dermatomyositis. Rheumatoid arthritis (2009 ACR/Annual European Congress of Rheumatology criteria), Sjogren syndrome (2012 ACR criteria), autoimmune hepatitis (2010 (AASLD) American Association for the Study of Liver Diseases criteria), primary biliary cirrhosis (2018 AASLD criteria), mixed connective tissue disease (1991 Kahn criteria), dermatomyositis (1975 Bohan/Peter criteria). Normal healthy subjects had no history of any diseases, including rheumatic diseases.
2.2. Data collection
2.2.1. Clinical features
Including gender, age, skin erythema, butterfly erythema, discoid erythema, rash, light sensitive, hair loss, arthritis, serositis, oral ulcer, neuropsychiatric lupus.
2.2.2. Laboratory results
Including WBC, RBC, Hb, PLT, total protein, total bilirubin, ALT, AST, urea, creatinine, urine protein, serum IgA, IgG, IgM, C3, C4, CRP, ESR, and anti-nuclear antibody spectrum.
The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) 2000[15] was used to assess disease activity: inactivity, SLEDAI score ≤4; mild activity, SLEDAI score 5 to 9; moderate activity, SLEDAI score 10 to 14; severe activity, SLEDAI score ≥15.
2.3. Detection methods
Anti-dsDNA was detected by indirect immunofluorescence of the kinetoplast of Crithidia luciliae. Anti-SSA, anti-P, anti-Sm, ANuA, and AHA were detected by immunoblotting. All the reagents were produced by Germany EUROIMMUN Corporation and representative images of the results are shown in figures (Figs. 1 and 2). Biochemical tests were determined by Roche cobas e602 automatic biochemical analyzer and Beckman IMMAGE 800 immunochemistry system with original regents, blood tests were determined by Sysmex XE-2100 whole blood cell analyzer with original regents.
Figure 1.
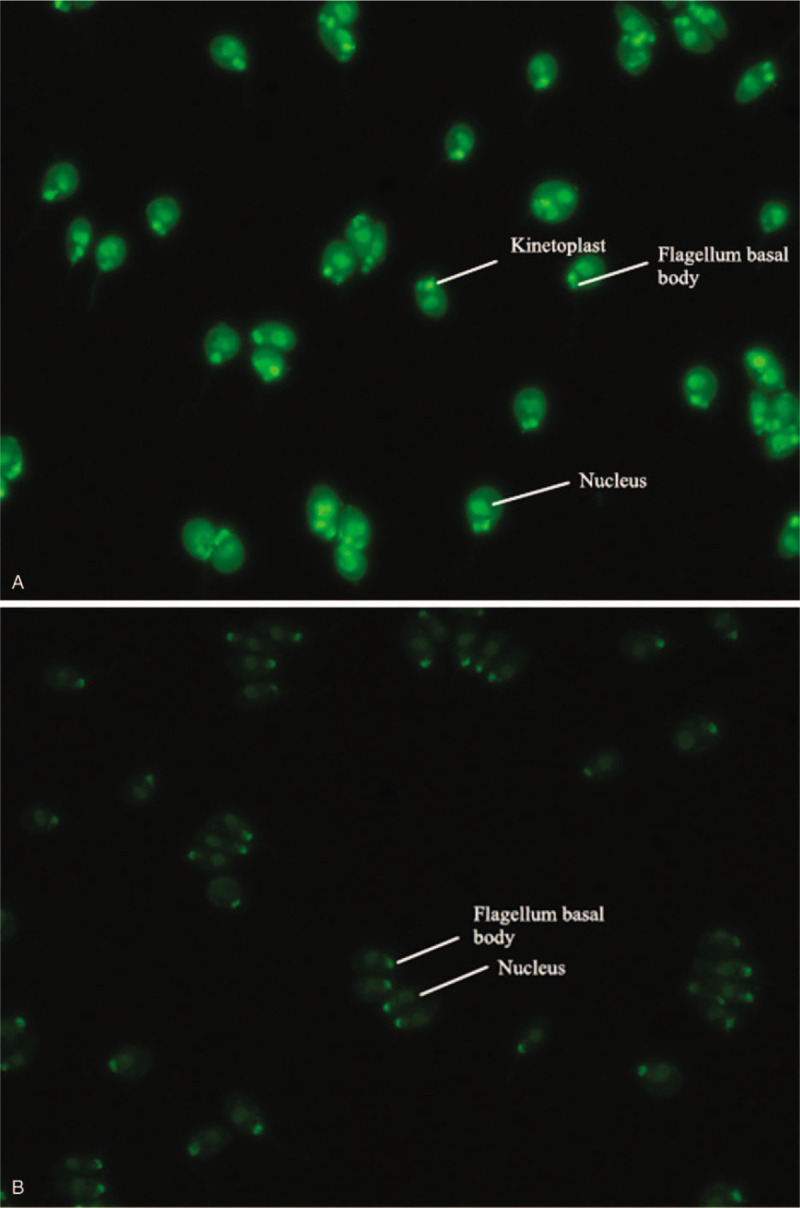
Positive and negative results of anti-double-stranded DNA antibody (anti-dsDNA) by indirect immunofluorescence (the positive expression of anti-dsDNA was homogeneous or enhanced edge fluorescence of kinetoplast of Crithidia luciliae, while only the fluorescence of nucleus or flagellum basal body was judged as negative for anti-dsDNA.).
Figure 2.
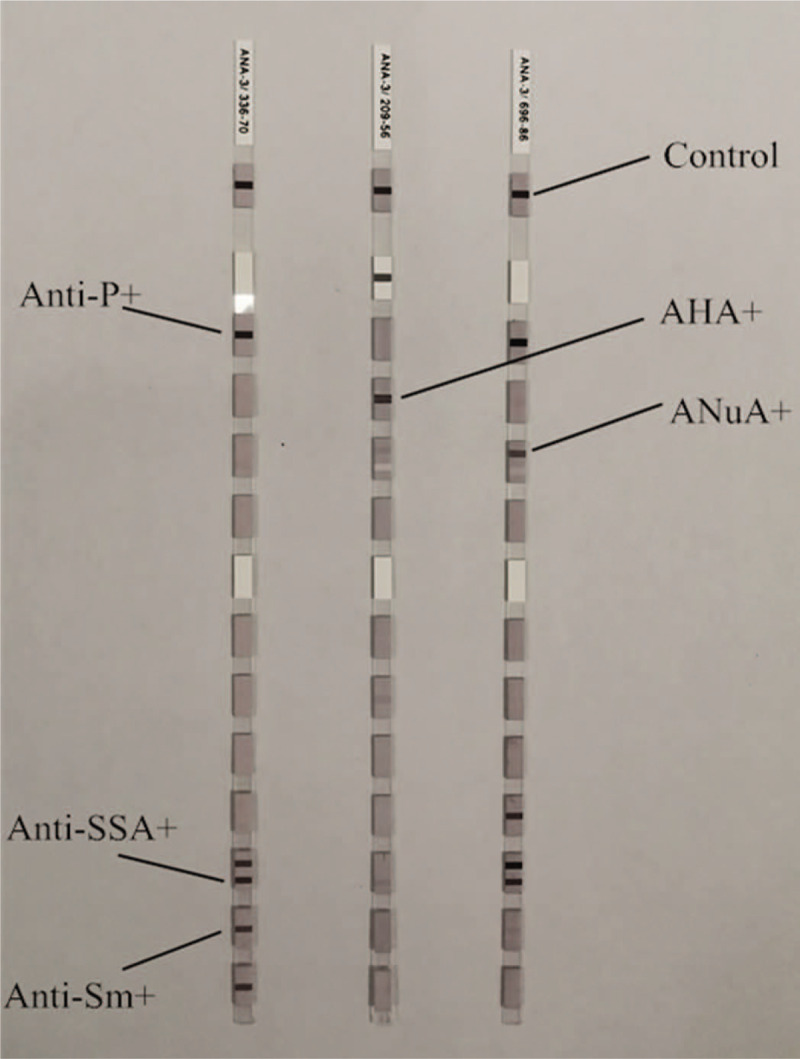
Positive results of anti-Sjogren syndrome A antibody, anti-ribosomal P protein antibody, anti-Sm, ANuA, and AHA by immunoblotting.
2.4. Statistical method
SPSS19.0 statistical software was used for data analysis. Count data were expressed as percentages and analyzed with Chi-squared test or Fisher exact probability analysis. Measurement data were expressed as median (P25, P75) and analyzed with Mann–Whitney U test, P < .05 was considered statistically significant.
3. Results
3.1. The diagnostic value of anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA.
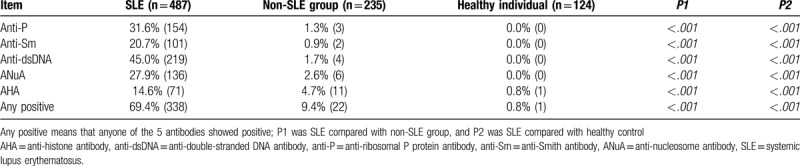
As shown in Table 1, anti-P was positive in 154 of 487 SLE patients (31.6%), in 3 of 235 patients with non-SLE rheumatic diseases and in none of 124 healthy individuals. The positive rates of anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA in SLE were significantly higher than those in non-SLE rheumatic diseases and healthy subjects.
Table 1.
Positive rates of anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA.
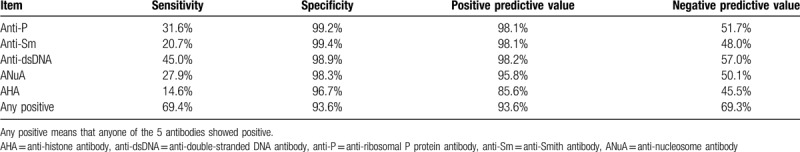
Anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA are all highly specific in the diagnosis of SLE (with specificity greater than 95%). However, their sensitivity is relatively low, and anti-dsDNA>anti-P> ANuA> anti-Sm> AHA. The sensitivity of either of the 5 antibodies positive was 69.4% and the specificity was still 93.6% (Table 2). And among them, 27.9% of SLE patients only had a single positive anti-P while the other 4 antibodies were all negative.
Table 2.
Diagnostic value of anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA.
3.2. The correlation between anti-P and SLE
SLE patients were divided into positive group and negative group according to the results of anti-P, clinical features were analyzed between the 2 groups and a comparative analysis was performed with anti-dsDNA (Table 3). SLE patients with positive anti-P or anti-dsDNA have an earlier onset age, and the incidence of skin erythema in anti-P/+/ group is significantly higher than that in anti-P/−/ group.
Table 3.
Relationship between anti-P, anti-dsDNA, and clinical features of SLE.
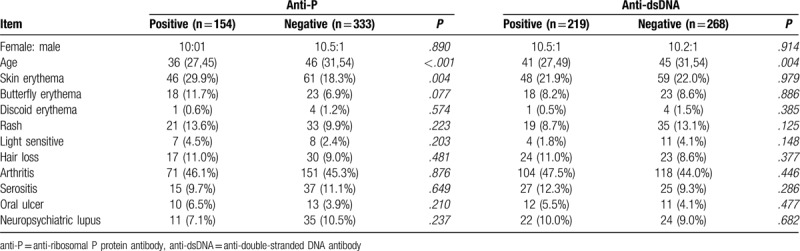
According to the results of anti-dsDNA, anti-SSA, and anti-P, their relationship with skin erythema was further analyzed. Compared with the full negative group, the incidence of skin erythema was higher in the positive anti-P or anti-SSA group, while it was lower in the positive anti-dsDNA group. When anti-SSA, anti-P were positive and anti-dsDNA was negative, the incidence of skin erythema was the highest (35.1%), and the difference was significant (Table 4).
Table 4.
The associations of anti-dsDNA, anti-SSA, anti-P, and skin erythema.
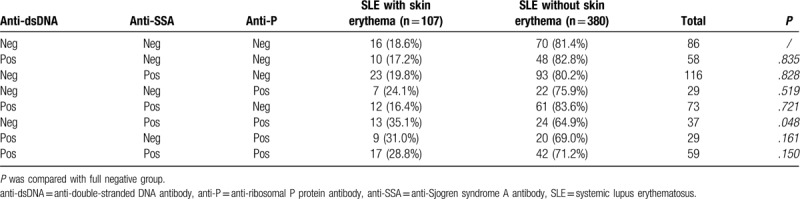
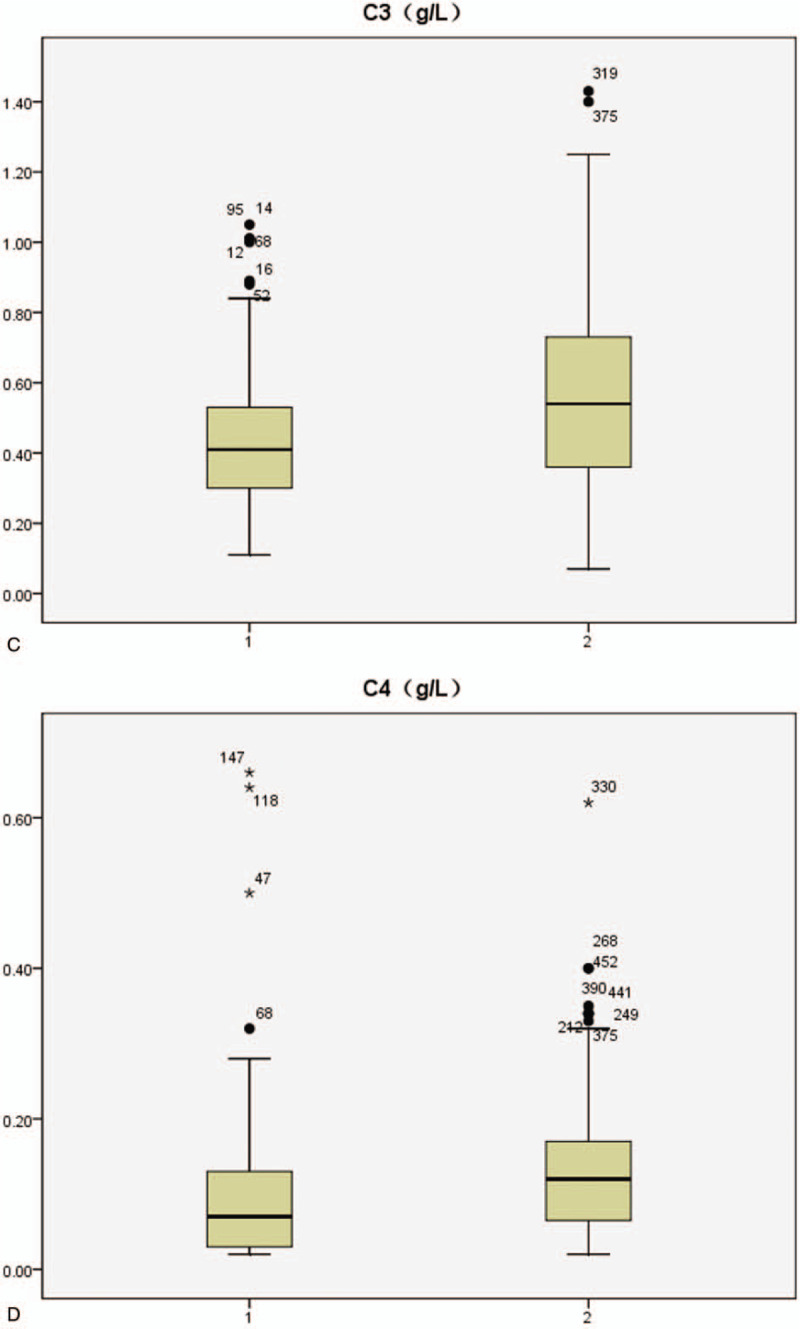
Table 5 shows the laboratory results of anti-P/+/ and anti-P/−/ SLE patients. The incidence of urine protein, the level of creatinine, the increase of immunoglobulin IgG, IgM as well as the decrease of complement C3 and C4 in anti-P/+/ group were more obvious than those in anti-P/−/ group (Fig. 3 ).
Table 5.
Laboratory results of anti-P/+/ and anti-P/−/ SLE patients.
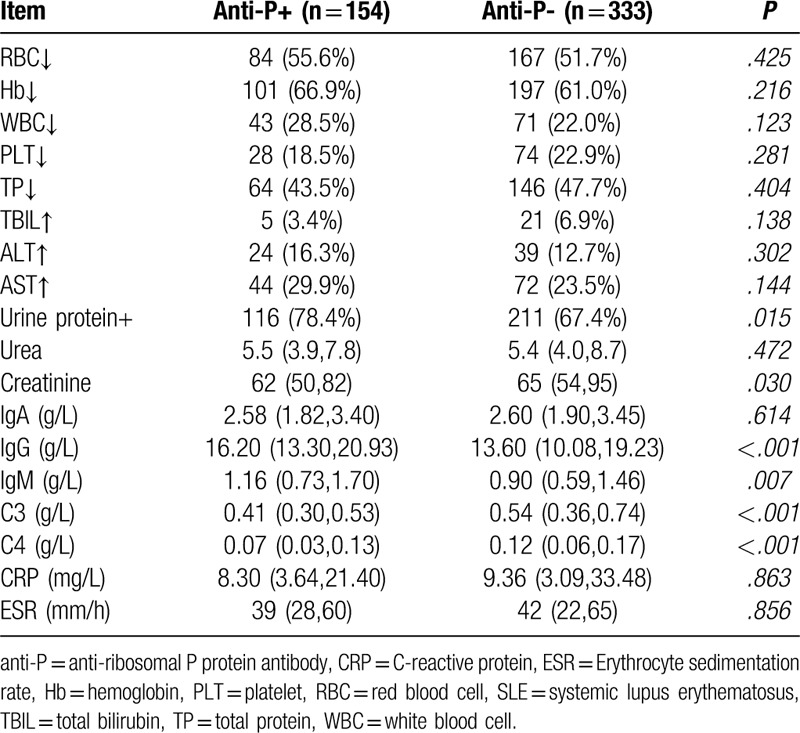
Figure 3.
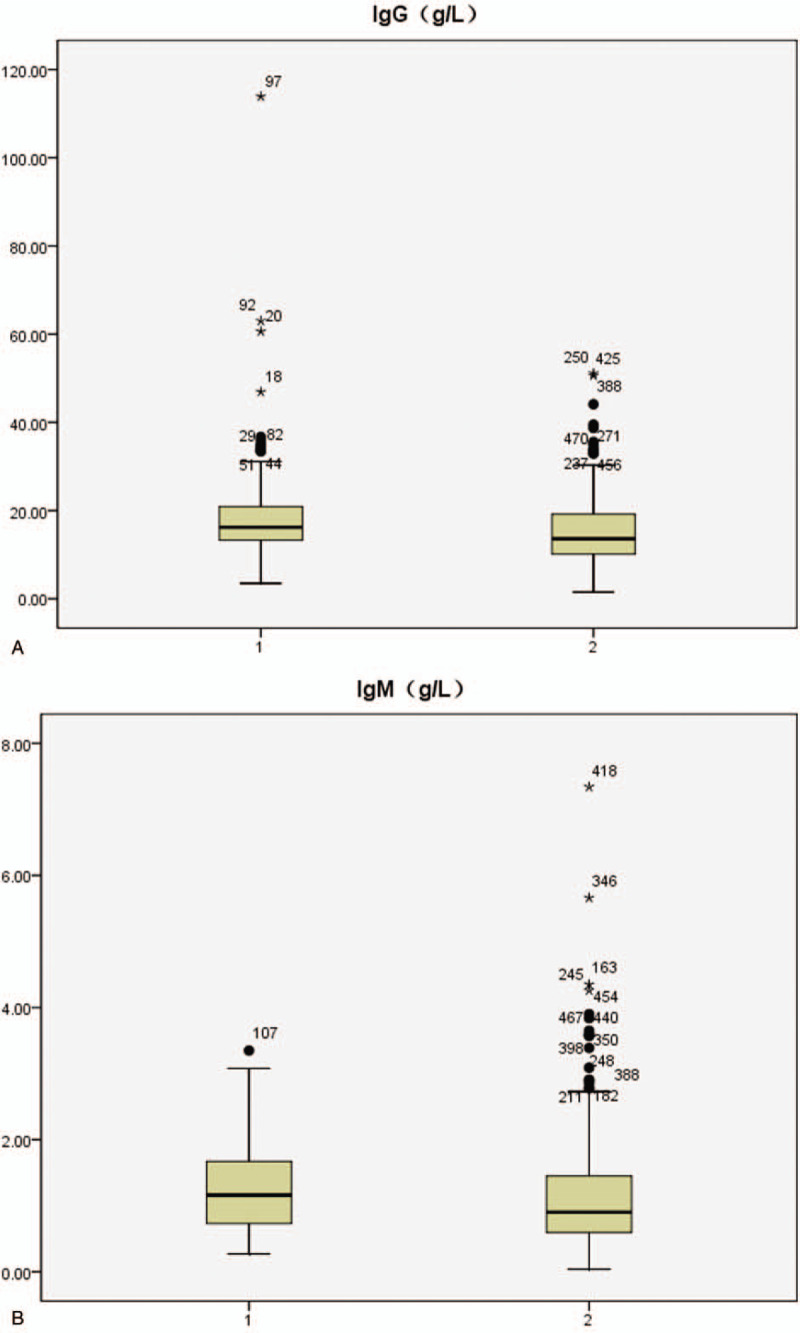
Serum IgG, IgM, C3,C4 profiles in systemic lupus erythematosus patients with positive/negative anti-ribosomal P protein antibody (anti-P) (1: anti-P/+/ group, 2:anti-P/−/ group).
Compared with anti-P/−/ patients, anti-P/+/ SLE patients had higher SLEDAI scores and the difference was statistically significant (Table 6). SLE with inactivity or mild activity in anti P/+/ group were significantly lower than that in anti P /-/ group, while the proportion of severe activity was significantly higher than the anti P /-/group (Fig. 4).
Table 6.
Disease activity of anti-P/+/ and anti-P/−/ SLE patients.
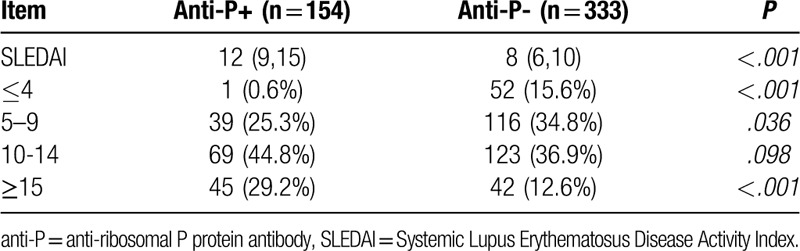
Figure 3 (Continued).
Serum IgG, IgM, C3,C4 profiles in systemic lupus erythematosus patients with positive/negative anti-ribosomal P protein antibody (anti-P) (1: anti-P/+/ group, 2:anti-P/−/ group).
Figure 4.
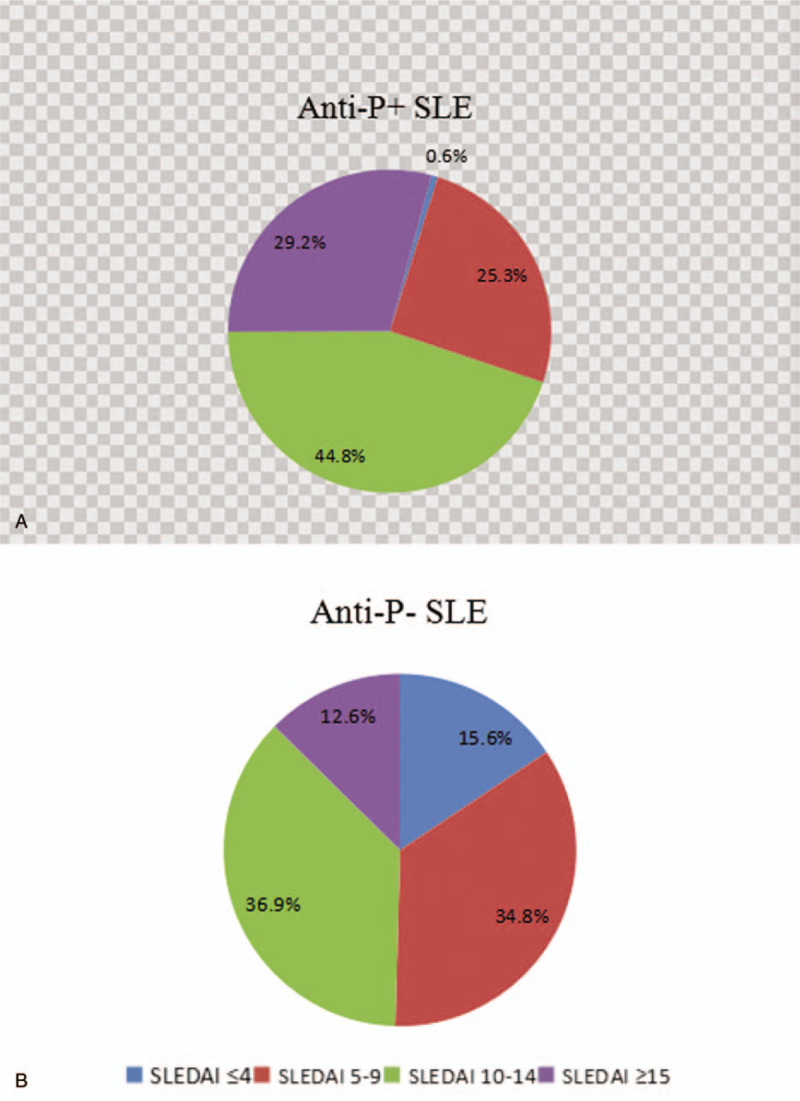
Systemic Lupus Erythematosus Disease Activity Index scores in anti-ribosomal P protein antibody/+/ and anti-ribosomal P protein antibody/−/ patients.
4. Discussion
In present report, we found that anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA were all highly specific in the diagnosis of SLE. Anti-P, anti-Sm, anti-dsDNA, and ANuA have comparable specificity and sensitivity for the diagnosis of SLE, which is consistent with other studies.[8,16] Of note, the sensitivity of either of the 5 antibodies positive increased to 69.4% and the specificity remained 93.6%, which suggest that combined detection of the 5 antibodies might significantly enhance the sensitivity and negative predictive value to diagnose SLE without reduction of specificity and positive predictive value.
Many studies have found that anti-P can be detected early in the pathogenesis of SLE, earlier than anti-Sm, anti-dsDNA, and other antibodies.[17] Our data also showed that 27.9% of SLE patients have only a single positive anti-P and the other 4 antibodies were all negative, thus further confirming the diagnostic value of anti-P for SLE in the absence of other autoantibodies. The appearance of anti-P can predict the occurrence of SLE, contribute to the early diagnosis of SLE as well as avoiding missed detection.
The data in Table 3 show that SLE patients with positive anti-P or anti-dsDNA are younger compared with negative groups and the age of onset in anti-P/+/ group is the youngest with a median of 36 years, which is consistent with Olesińska's research.[18] Studies of Reichlin and Pisoni also showed that the positive rate of anti-P in adolescent SLE patients was much higher than that of adult SLE patients.[19,20] SLE patients with early onset age have a higher positive rate of anti-P and more serious clinical manifestations, but the specific reasons are still unclear and need further exploration.
In this study, the incidence of skin erythema was 29.9% and 18.3% in anti-P/+/ group and in anti-P/−/ group, respectively. The difference was statistically significant (P < .001), which indicated that anti-P is associated with skin damage in SLE patients. Many studies have also found that SLE patients with positive anti-P have higher incidence of facial erythema and light sensitivity.[13,18] SLE patients with skin lesions have a higher positive rate of autoantibody compared with patients with no skin lesions, which may be associated with 2 specific antigens (acidicribosomal protein P0 and galectin 3).[21] However, the molecular mechanisms of skin damage mediated by these 2 antigens still need to be further explored. A hypothesis about SLE skin damage caused by autoantibodies has been put forward. Patients with lupus genetic background may induce skin inflammation due to external factors (e.g., ultraviolet light), and skin keratinocytes exposed to ultraviolet light produce pro-inflammatory cytokines. The expression of SSA and high mobility group box 1 (HMGB1) are up-regulated and released to the outside of cells when apoptosis of keratinocytes occurs. These proteins form complexes with immunoglobulins and complements, mainly in the form of SSA-anti-SSA and DNA-HMGB1-IgG, which stimulate B lymphocytes to produce anti-HMGB1 and anti-dsDNA antibodies. At the same time, these immune complexes also stimulate CD8+ T lymphocytes to directly localize to keratinocytes as well as CD4+ T lymphocytes to cause keratinocyte damage by producing more inflammatory factors and chemotactic inflammatory cells. In addition, they also stimulate plasmacytoid dendritic cells to up-regulate the secretion of IFN, which is one of the key cytokines in the pathogenesis of SLE.[22,23,24] So we speculate that anti-P is probably to cause skin lesions by synergy with anti-dsDNA and anti-SSA. In order to further study the relationship between anti-P and skin erythema, we divided SLE patients into 8 groups based on the results of anti-dsDNA, anti-SSA, and anti-P. We found that positive anti-SSA or anti-P was positively correlated with the occurrence of skin erythema, while positive anti-dsDNA was negatively correlated, and when anti-SSA, anti-P were positive, and anti-dsDNA was negative, the incidence rate of skin erythema was the highest, which further confirmed our conclusion. Therefore, we believe that both anti-P and anti-SSA can be used as important indicators of SLE skin damage.
Neuropsychiatric systemic lupus erythematosus (NPSLE) is one of the complications of SLE patients, although the incidence is not high, the clinical manifestations are complex and prognosis is poor.[25,26] Mild patients only have dizziness, headache, and memory loss, while severe cases may have cerebrovascular accident, coma, and epilepsy. Anti-P has long been considered to be associated with NPSLE, especially in patients with psychiatric manifestations (psychosis, depression, and mood disorder).[27,28,29] There are also some new clinical and experimental evidence revealing a pathogenic role of anti-P antibodies in cognitive dysfunction recently.[30] Karassa believed that the sensitivity and specificity of anti-P to diagnose NPSLE are 26% and 80%. And for psychosis, mood disorder, or both, the sensitivity, and specificity were 27% and 80% respectively.[31] A study of Valões found that 13% of childhood-onset SLE patients in the anti-P-positive group developed acute confusion and 18% had symptoms of mood disorder, while the positive rate of the above symptoms in the negative group was 5% and 8%.[32] The pathogenesis is that the presence of P antigen on the surface of nerve cell membrane can specifically bind to anti-P and cause cell damage, which leads to corresponding clinical manifestations in patients.[13] Moreover, in vitro assay, anti-P induce the production of proinflammatory cytokine from peripheral activated T cells or monocytes by directly binding to the surface of them.[33,34] Therefore, anti-P influence not only diffuse NPSLE pathogenesis, but also induce the systemic inflammation. The results of this study exhibited that 11 of 154 anti-P/+/ SLE patients developed NPSLE symptoms, while 35 of 333 anti-P/−/ patients had above symptoms, the difference was not statistically significant. The results are inconsistent with previous research and the reasons may be as follows. Firstly, racial factors may affect the expression of ribosomal P protein. Secondly, the incidence of NPSLE in this study is very low, only 46 of 487 SLE patients had central nervous system lesions, the limited sample representation may lead to this discrepancy as well.
LN is the most common complication of SLE patients, and renal failure is also an important cause of death. Clinical renal involvement occurs in 50% to 70% of patients with SLE, and renal biopsy shows renal pathology changes in almost all SLE. In present study, the positive rate of urinary protein between anti-P/+/ and anti-P/−/ group was significantly different (78.4% vs 67.4%, P = .015), while patients with negative anti-P had higher levels of creatinine (P = .030). do Nascimento's study introduced anti-P antibody as a novel serologic marker for membranous lupus nephritis, he found that anti-P-positive SLE patients have higher levels of proteinuria and the great majority of them who had both isolated anti-P and class V LN have normal renal function,[35] which is in agreement with our results. The positive rate of anti-P in membranous nephropathy was also higher than that in control group, which could be used as a marker of membranous nephropathy.[16] Some studies have found anti-dsDNA antibodies cross-react with ribosomal P proteins,[36] anti-P coexisting with anti-dsDNA was associated with LN, suggesting that the coexistence of 2 antibodies is nephritogenic to a greater extent.[37,38] But there were also some reports confirming anti-P is cross reactive with Sm but not with dsDNA and accelerate the development of glomerulonephritis in lupus prone mice.[39] In recent years, there is increasing evidence that DNA is not the only antigen causing lupus nephritis, binding kidney tissue via other intracellular proteins such as α-actinin or by direct binding of anti-laminin antibodies to laminin within the glomerular mesangium may also result in kidney injury.[40,41] These findings further indicate that anti-P is closely related to the occurrence of LN, and may also have a certain correlation with the pathological type of LN.
At present, clinicians evaluate disease activity of SLE patients mainly through the SLEDAI score. In this study, anti-P/+/ patients had significantly higher SLEDAI scores than anti-P/−/ ones (P < .001), and 29.2% of SLEDAI scores in anti-P/+/ patients were greater than 15, compared with 12.6% in the negative group, suggesting that positive anti-P is associated with high disease activity of SLE. In SLE patients, autoantibodies bind to the corresponding antigens and deposit in tissues and organs, which activates complement to produce inflammatory reaction and reduces the content of serum free complements. Since the increase of serum immunoglobulin is mainly IgG during the active period of SLE,[42] the increase of IgG and the decrease of complement C3,C4 can indirectly reflect the activity of SLE as well. Table 5 shows that the levels of serum IgG and IgM in anti-P/+/ group were significantly higher than those in anti-P/−/ group (P < .05), while the levels of C3 and C4 were significantly lower than those in anti-P/−/ group (P < .05), further suggesting that anti-P was correlated with the activity of SLE.
The present study has several limitations that should be recognized. First, this is a retrospective study, there were no treatment and follow-up results. Second, only 1 method was used to detect anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA, which may affect the accuracy of results to a certain extent. Third, in order to collect complete case information, only inpatients were included in SLE group without outpatients, which may introduce selection bias.
5. Conclusion
Anti-P, anti-Sm, anti-dsDNA, ANuA, and AHA have high specificity but poor sensitivity in the diagnosis of SLE, combined detection can greatly improve the detection rate of SLE, which will be more conducive to the diagnosis and differential diagnosis of SLE. Anti-P is more valuable in the diagnosis of SLE when other specific autoantibodies are negative, which contributes to the early diagnosis and avoids missed detection. SLE patients with positive anti-P have an earlier onset age and are more prone to skin erythema and proteinuria, it can be used as an important indicator of skin and kidney damage in SLE. Anti-P is also associated with the disease activity of SLE, which can reflect the severity of SLE to some extent.
Author contributions
Conceptualization: Yanping Wang, Peng Luo.
Data curation: Ting Guo, Lin Zou.
Formal analysis: Yanping Wang, Peng Luo.
Funding acquisition: Peng Luo.
Investigation: Jing Shi,Pu Chen.
Methodology: Yanping Wang, Peng Luo.
Writing – original draft: Yanping Wang, Peng Luo.
Writing – review & editing: Yanping Wang.
Footnotes
Abbreviations: ACR = American College of Rheumatology, AHA = anti-histone antibody, anti-dsDNA = anti-double-stranded DNA antibody, anti-P = anti-ribosomal P protein antibody, anti-Sm = anti-Smith antibody, anti-SSA = anti-Sjogren syndrome A antibody, ANuA = anti-nucleosome antibody, LN = lupus nephritis, NPSLE = neuropsychiatric systemic lupus erythematosus, SLE = systemic lupus erythematosus, SLEDAI = Systemic Lupus Erythematosus Disease Activity Index.
How to cite this article: Wang Y, Luo P, Guo T, Zou L, Shi J, Chen P. Study on the correlation between anti-ribosomal P protein antibody and systemic lupus erythematosus. Medicine. 2020;99:20(e20192).
YW and PL contributed equally to this work and are the co-first authors.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Oku K, Atsumi T. Systemic lupus erythematosus: nothing stale her infinite variety. Mod Rheumatol 2018;28:758–65. [DOI] [PubMed] [Google Scholar]
- [2].Yaniv G, Twig G, Shor DB, et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev 2015;14:75–9. [DOI] [PubMed] [Google Scholar]
- [3].Sherer Y, Gorstein A, Fritzler MJ, et al. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum 2004;34:501–37. [DOI] [PubMed] [Google Scholar]
- [4].Rastin M, Mahmoudi M, Sahebari M, et al. Immunological characteristics in systemic lupus erythematosus patients. Indian J Med Res 2017;146:224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jia Y, Zhao L, Wang C, et al. Anti-double-stranded DNA isotypes and anti-C1q antibody improve the diagnostic specificity of systemic lupus erythematosus. Dis Markers 2018;27:4528547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kiss E, Shoenfeld Y. Are anti-ribosomal P protein antibodies relevant in systemic lupus erythematosus? Clin Rev Allergy Immunol 2007;32:37–46. [DOI] [PubMed] [Google Scholar]
- [7].Toubi E, Shoenfeld Y. Clinical and biological aspects of anti-P-ribosomal protein autoantibodies. Autoimmun Rev 2007;6:119–25. [DOI] [PubMed] [Google Scholar]
- [8].Carmona-Fernandes D, Santos MJ, Canhão H, et al. Anti-ribosomal P protein IgG autoantibodies in patients with systemic lupus erythematosus:diagnostic performance and clinical profile. BMC Med 2013;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ahmed N, Shigidi M, Agib AI, et al. Clinical features and antinuclear antibodies profile among adults with systemic lupus erythematosus and lupus nephritis: a cross-sectional study. Pan Afr Med J 2017;27:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mahler M, Kessenbrock K, Szmyrka M, et al. International multicenter evaluation of autoantibodies to ribosomal P proteins. Clin Vaccine Immunol 2006;13:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zandman-Goddard G, Chapman J, Shoenfeld Y. Autoantibodies involved in neuropsychiatric SLE and antiphospholipid syndrome. Semin Arthritis Rheum 2007;36:297–315. [DOI] [PubMed] [Google Scholar]
- [12].Briani C, Lucchetta M, Ghirardello A, et al. Neurolupus is associated with anti-ribosomal P protein antibodies: an inception cohort study. J Autoimmun 2009;32:79–84. [DOI] [PubMed] [Google Scholar]
- [13].Abraham M, Derk CT. Anti-ribosomal-P antibodies in lupus nephritis, neuropsychiatric lupus, lupus hepatitis, and Chagas’ disease: promising yet limited in clinical utility. Rheumatol Int 2015;35:27–33. [DOI] [PubMed] [Google Scholar]
- [14].Monova D, Argirova T, Monov S. Antiribosomal P antibodies in patients with lupus glomerulonephritis. Clin Nephrol 2001;55:425–6. [PubMed] [Google Scholar]
- [15].Giadman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosusdisease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- [16].Hirohata S, Kasama T, Kawahito Y, et al. Efficacy of anti-ribosomal P protein antibody testing for diagnosis of systemic lupus erythematosus. Mod Rheumatol 2014;24:939–44. [DOI] [PubMed] [Google Scholar]
- [17].Zhigang Hu, Guoqian Chen, Yaohong Zou, et al. Clinical application of anti-ribosomal P0 protein antibody in the diagnosis of systemic lupus erythematosus. Chin J Nosocomiology 2010;22:3630–2. [Google Scholar]
- [18].Olesińska M, Chwalińska-Sadowska H, Wiesik-Szewczyk E, et al. Clinical manifestation of systemic lupus erythematosus in patients with antiribosomal P protein antibodies. Pol Arch Med Wewn 2010;120:76–81. [PubMed] [Google Scholar]
- [19].Reichlin M, Broyles TF, Hubscher O, et al. Prevalence of autoantibodies to ribosomal P proteins in juvenile-onset systemic lupus erythematosus compared with the adult disease. Arthritis Rheum 1999;42:69–75. [DOI] [PubMed] [Google Scholar]
- [20].Pisoni CN, Muñoz SA, Carrizo C, et al. Multicentric prevalence study of anti P ribosomal autoantibodies in juvenile onset systemic lupus erythematosus compared with adult onset systemic lupus erythematosus. Reumatol Clin 2015;11:73–7. [DOI] [PubMed] [Google Scholar]
- [21].Shi ZR, Tan GZ, Meng Z, et al. Association of anti-acidic ribosomal protein P0 and anti-galectin 3 antibodies with the development of skin lesions in systemic lupus erythematosus. Arthritis Rheumatol 2015;67:193–203. [DOI] [PubMed] [Google Scholar]
- [22].Oke V, Wahren-Herlenius M. Cutaneous lupus erythematosus: clinical aspects and molecular pathogenesis. J Intern Med 2013;273:544–54. [DOI] [PubMed] [Google Scholar]
- [23].Yu C, Chang C, Zhang J. Immunologic and genetic considerations of cutaneous lupus erythematosus: a comprehensive review. J Autoimmun 2013;41:34–45. [DOI] [PubMed] [Google Scholar]
- [24].Abdulahad DA, Westra J, Limburg PC, et al. HMGB1 in systemic lupus erythematosus: its role in cutaneous lesions development. Autoimmun Rev 2010;9:661–5. [DOI] [PubMed] [Google Scholar]
- [25].Arinuma Y, Kikuchi H, Hirohata S. Anti-ribosomal P protein antibodies influence mortality of patients with diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematous involving a severe form of the disease. Mod Rheumatol 2019;29:612–8. [DOI] [PubMed] [Google Scholar]
- [26].Hanly JG, McCurdy G, Fougere L, et al. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol 2004;31:2156–62. [PubMed] [Google Scholar]
- [27].Katzav A, Solodeev I, Brodsky O, et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum 2007;56:938–48. [DOI] [PubMed] [Google Scholar]
- [28].Sciascia S, Bertolaccini ML, Roccatello D, et al. Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus: a systematic review. J Neurol 2014;261:1706–14. [DOI] [PubMed] [Google Scholar]
- [29].Abdel-Nasser AM, Ghaleb RM, Mahmoud JA, et al. Association of anti-ribosomal P protein antibodies with neuropsychiatric and other manifestations of systemic lupus erythematosus. Clin Rheumatol 2008;27:1377–85. [DOI] [PubMed] [Google Scholar]
- [30].González A, Massardo L. Antibodies and the brain: antiribosomal P protein anti-body and the clinical effects in patients with systemic lupus erythematosus. Curr Opin Neurol 2018;31:300–5. [DOI] [PubMed] [Google Scholar]
- [31].Karassa FB, Afeltra A, Ambrozic A, et al. Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis. Arthritis Rheum 2006;54:312–24. [DOI] [PubMed] [Google Scholar]
- [32].Valões CC, Molinari BC, Pitta AC, et al. Anti-ribosomal P antibody: a multicenter study in childhood-onset systemic lupus erythematosus patients. Lupus 2017;26:484–9. [DOI] [PubMed] [Google Scholar]
- [33].Hirohata S, Nakanishi K. Antiribosomal P protein antibody in human systemic lupus erythematosus reacts specifically with activated T cells. Lupus 2001;10:612–21. [DOI] [PubMed] [Google Scholar]
- [34].Nagai T, Arinuma Y, Yanagida T, et al. Anti-ribosomal P protein antibody in human systemic lupus erythematosus up-regulates the expression of proinflammatory cytokines by human peripheral blood monocytes. Arthritis Rheum 2005;52:847–55. [DOI] [PubMed] [Google Scholar]
- [35].do Nascimento AP, Viana Vdos S, Testagrossa Lde A, et al. Antibodies to ribosomal P proteins: a potential serologic marker for lupus membranous glomerulonephritis. Arthritis Rheum 2006;54:1568–72. [DOI] [PubMed] [Google Scholar]
- [36].Sun KH, Liu WT, Tsai CY, et al. Anti-dsDNA antibodies cross-react with ribosomal P proteins expressed on the surface of glomerular mesangial cells to exert a cytostatic effect. Immunology 1995;85:262–9. [PMC free article] [PubMed] [Google Scholar]
- [37].Quintana G, Coral-Alvarado P, Aroca G, et al. Single anti-P ribosomal antibodies are not associated with lupus nephritis in patients suffering from active systemic lupus erythematosus. Autoimmun Rev 2010;9:750–5. [DOI] [PubMed] [Google Scholar]
- [38].Reichlin M, Wolfson-Reichlin M. Correlations of anti-dsDNA and anti-ribosomal P autoantibodies with lupus nephritis. Clin Immunol 2003;108:69–72. [DOI] [PubMed] [Google Scholar]
- [39].Ben-Ami Shor D, Blank M, Reuter S, et al. Anti-ribosomal-P antibodies accelerate lupus glomerulonephritis and induce lupus nephritis in naïve mice. J Autoimmun 2014;54:118–26. [DOI] [PubMed] [Google Scholar]
- [40].Amital H, Heilweil-Harel M, Ulmansky R, et al. Antibodies against the VRT101 laminin epitope correlate with human SLE disease activity and can be removed by extracorporeal immunoadsorption. Rheumatology (Oxford) 2007;46:1433–7. [DOI] [PubMed] [Google Scholar]
- [41].Renaudineau Y, Deocharan B, Jousse S, et al. Anti-alpha-actinin antibodies: a new marker of lupus nephritis. Autoimmun Rev 2007;6:464–8. [DOI] [PubMed] [Google Scholar]
- [42].Qing Zhong, Hua Gan, Pu Chen, et al. Study on the correlation between anti-nucleosome antibodies and systemic lupus erythematosus. Chin J Rheumatol 2003;2:111–3. [Google Scholar]


