Abstract
The present study was carried out to investigate cyanobacteria as a potential source for biodiesel production isolated from fresh water bodies of Sri Lanka. Semi mass culturing and mass culturing were carried out to obtain biomass for extracting total lipids. Fatty acid methyl ester (FAME) or biodiesel was produced from extracted lipid by trans-esterification reaction. FAME component was identified using gas chromatography (GC). Atotal of 74 uni-algal cultures were obtained from Biofuel and Bioenergy laboratory of the National Institute of Fundamental Studies (NIFS), Kandy, Sri Lanka. The total lipid content was recorded highest in Oscillatoria sp. (31.9 ± 2.01% of dry biomass) followed by Synechococcus sp. (30.6 ± 2.87%), Croococcidiopsis sp. (22.7 ± 1.36%), Leptolyngbya sp. (21.15 ± 1.99%), Limnothrixsp. (20.73 ± 3.26%), Calothrix sp. (18.15 ± 4.11%) and Nostoc sp. (15.43 ± 3.89%), Cephalothrixsp. (13.95 ± 4.27%), Cephalothrix Komarekiana (13.8 ± 3.56%) and Westiellopsisprolifica (12.80 ± 1.97%). FAME analysis showed cyanobacteria contain Methyl palmitoleate, Linolelaidic acid methyl ester, Cis-8,11,14-eicosatrienoic acid methyl ester, Cis-10-heptadecanoic acid methyl ester, Methyl myristate, Methyl pentadecanoate, Methyl octanoate, Methyl decanoate, Methyl laurate, Methyl tridecanoate, Methyl palmitoleate, Methyl pentadeconoate, Methyl heptadeconoate, Linolaidic acid methyl ester, Methyl erucate, Methyl myristate, Myristoloeic acid, Methyl palmitate, Cis-9-oleic acid methyl ester, Methyl arachidate and Cis-8,11,14-ecosatrieconoic acid methyl ester. The present study revealed that cyanobacteria isolated from Sri Lanka are potential source for biodiesel industry because of their high fatty acid content. Further studies are required to optimize the mass culture conditions to increase thelipid content from cyanobacterial biomass along with the research in the value addition to the remaining biomass.
Keywords: Biodiesel, Cyanobacteria, Gas chromatography
1. Introduction
It is estimated that the global energy demand will grow by more than one-third over the period to 2035 (IEA, 2012). As the global petroleum reserves are shrinking at a fast pace, there is an increase in the demand for alternate fuels (Mutanda et al., 2011). But fossil fuels are non-renewable and will be depleted within next 150 years if this consumption rate stays unchanged (Ferdous et al., 2014). However, the increasing fuel demand worldwide, limited reserve and its effect on environmental pollution and contribution to global warming push scientists to look for alternative, clean and renewable source in replacement of fossil fuel (Posten & Schaub, 2009). Biodiesel has been attracted as a significant potential replacement for this.
Researchers have proposed several alternative candidates to displace fossil fuels so that the energy sectors could be made more sustainable (Korres et al., 2010). Most of the discussions for biofuel production has focused on higher plants such as corn, sugarcane, palm oil, soybean and other higher plants and these are associated with problems over utilization of arable land, loss of ecosystems along with the competition with human consumptions (Gnansounou et al., 2008, Pandey, 2009, Parmar et al., 2011). Thus far, United States of America (USA), South East Asia, Europe and South America produce biodiesel from vegetable oil extracted from food crops such as corn, canola, soybean, palm, etc. There is already an amplifying warning and rejection on food crops for biofuel production. To overcome these drawbacks for the last two decades scientists' are emphasizing on renewable sources for biodiesel which has no competition with human consumption and economically viable.
While most bio-energy options fail on both counts, several microorganism-based options have the potential to produce large amounts of renewable energy without disruptions. Since land-based biodiesel production competes with conventional food production, a water-based biomass and biodiesel production from cyanobacteria offers large potential (Steinhoff et al., 2014). The use of lipids obtained from cyanobacteria biomass has been described as a promising alternative for production of biodiesel to replace petro-diesel (dos Santos et al., 2014).
Cyanobacteria can convert up to 10% of the sun’s energy into biomass, compared to 5% achieved by eukaryotic algae and 1% recorded by conventional energy crops such as corn or sugarcane (Parmar et al., 2011). During this process they also transform carbon dioxide into biochemical compounds that can be processed into biofuels, food, feed stocks and high value bioactive compounds (Pereira et al., 2011). Due to their high biomass productivity, rapid growth potential and ability to synthesize and accumulate large amounts (approximately 20–50% of dry weight) of neutral lipid stored in cytosolic lipid bodies, cyanobacteria are viwed as promising feedstock for carbon–neutral biofuels (Halim et al., 2013, Mutanda et al., 2011). Therefore these photosynthetic microorganisms can potentially be employed for the production of biofuels in an economically effective and environmentally sustainable manner and at rates high enough to replace a substantial fraction of our society’s use of fossil fuels (Li et al., 2008).
In Sri Lanka, fossil fuel is used as the source of energy in many sectors and oil prices directly impacts the economy and development of the country. To meet sustainable national development, it is time to explore the environmental friendly, renewable and low cost alternative fuels. Fossil fuel energy consumption (% of total) in Sri Lanka was 45.87 as of 2013. Its highest value over the past 43 years was 50.55 in 2014, while its lowest value was 23.53 in 1976 (International Energy Agency, 2014).
The present study was carried out to extract total lipid and produce biodiesel from cyanobacteria isolated from freshwater bodies of Sri Lanka. The fatty acids of extracted lipids were characterized using gas chromatography. The biomass of cyanobacteria was obtained by semi-mass culturing and mass culturing of selected cyanobacteria.
2. Materials and methods
2.1. Materials
All of the chemicals employed herein were of analytical grade which were utilized as proclaimed. The mass fraction purity of NaOH, di-methyl sulphoxide (DMSO), Dichloromethane, sodium methoxide and acetic acid were 0.99, 0.98, 0.99, 0.98 and 0.99 respectively and purchased from Merk, Germany.
2.2. Semi-mass culturing and mass culturing of cyanobacteria
Semi mass culturing was carried out in 10 L aspirator bottles filled with one third of BG11 (Stanier et al., 1971) and GO (Rippka et al., 1979) media under 2000 lx light intensity, pH 7.5 and shaking at 200 rpm provided by an aerator (Risheng RS 2800). Mass culturing of cyanobacteria was carried out in 100 L size fish tank under green house environment with natural light and temperature. Media was prepared at half strength of respective BG11 (Stanier et al., 1971) and GO (Rippka et al., 1979) at pH 7.5 along with aeration (Risheng RS 2800).
2.3. Determination of higest biomass concentration
For harvesting biomass, growth concentration of cyanobacteria was measured using a spectrophotometric (UV-2450, Shimadzu) method. In brief, absorbance of the culture was taken at the range of 660 to 690 nm wavelength. The highest value of absorbance was selected to measure the growth concentration. The maximum absorbance value for each cyanobacteria was used to perform the growth curve by optical density (OD) (Rodrigues et al., 2011).
2.4. Harvesting biomass
In case of semi mass culturing, algal biomass were harvested by centrifuging the cultures in 2000 rpm for 5 min at 27 °C temperature. The cell pellet was separated in order to be dried at 60 °C for 24 h in oven to obtain dry matter (%).
For the purpose of harvesting biomass from mass culture, flocculation technique was applied. pH of the culture was increased using NaOH and the biomass was allowed to sediment. After sedimentation the water was filtered using 20 µm filter. The biomass was then oven dried and made into a fine powder for analysis.
2.5. Quantitative analysis of total pigments
The phycoerytherin (PE), phycocyanin (PC) & allophycocyanin (APC) pigments were determined using the method below:
0.5 g of biomass was added to 10 mL of DMSO and sonicated for 30 min. Homogenized sample was then centrifuged for 15 min at 10,000 rpm at 4 °C. The supernatant (0.5 mL) was mixed with 4.5 mL of DMSO and this mixure was used for the experiment. Absorbance was recorded at 480 nm, 562 nm, 615 nm, 649.1 nm, 652 nm and 665.1 nm (UV-2450, Shimadzu). The quantities of PE, PC, APC, Chlorophyll-a (Ch-a), Chlorophyll-b (Ch-b) and Carotene (Cx+c) in the different extracts were calculated using following equations (Bennett and Bogorad, 1973, Bryant et al., 1979, Sumanta et al., 2014):
APC = {A652 – 0.208(A615)}/5.09 mg mL−1
PC = {A615 −0.474(A652)}/5.34 mg mL−1
PE = {A562-2.41(PC) – 0.849(APC)} /9.62 mg mL−1
Ch-a = 12.47A665.1 – 3.62A649.1 µg mL−1
Ch-b = 25.06A649.1 – 6.5A665.1 µg mL−1
Cx+c = (1000A480 – 129Ca – 53.78Cb)/220 µg mL−1
2.6. Gravimetric quantification of total lipids
The intial weight of biomass was recorded and total lipid was extracted using sohxlet extraction apparatus where hexane was used as extraction solvent. Extracted solvent was evaporated using rotor evaporator (Heidolph, Hei-VAP). The remaining oil was transfered in a pre-weighed screw capped glass vial and the amount of lipid content was measured gravimetrically on w/w % of dry biomass by taking the difference in the pre- and final weights of the vial.
2.7. Preparation of FAME (Biodiesel)
0.2 mL of extracted oil was taken for analysis. Dichloromethane (0.3 mL) and 2.0 mL of 0.5 M sodium methoxide were added into screw capped tubes containing the sample. The tubes were vortexed and then heated for 30 min at 50 °C in a heat block. The reaction was stopped by careful drop wise addition of 5.0 mL of distilled water containing 0.1 mL of glacial acetic acid. Esterified fatty acids were extracted into 0.5 mL of hexane and hexane layer was separated by centrifugation (1500 rpm for 10 min) at 5 °C and hexane layer was removed by using a Pasteur pipette. Esterified samples were stored at −20 °C until they were analyzed by using GC (Agilent 7860B).
2.8. GC analysis of FAME
A fused silica gas chromatography capillary column (Supelco 2560) was used in a gas chromatograph equipped with flame ionization detector (Agilent 7890B) by the method of Yaniv et al., (1996). FAME standard was purchased from Sigma Aldrich to identify the fatty acid in cyanobacteria sample.
2.9. Statistical analysis
Statistical analysis were done using ANOVA in SAS 9.1 (SAS, 1999), MINITAB-14 and SPSS-16 statistical software packages. Data were presented as mean ± standard deviation (SD) of three replicates. The P-values less than 0.05 were considered significant. Experiments were carried out in triplicate.
3. Results and discussion
3.1. Biomass and total pigments production in cyanobacteria
Biomass, is defined as the mass of living biological organisms in a given area or ecosystem at a given time. In a natural water body the higher cyanobacterial biomass may sometimes cause the formation of algal blooms leading to releasing of cyanotoxins. According to previous report cyanobacteria had been considered as “blooms” at a biomass concentration of about 200 µg/L in the mixed upper 10 cm of the water (Olenina et al., 2009, Wasmund et al., 2011).
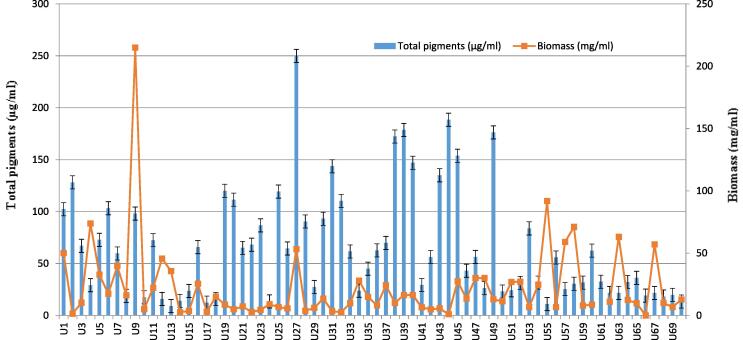
In the present study, the rate of biomass production of cyanobacteria in laboratory condition was measured and expressed as milligram of biomass per milliliter of water (mg/mL). As the aim of the present study was to extract fatty acid from cyanobacterial biomass, the fatty acid yield was calculated based on the percentage of dry biomass. Furthermore, higher biomass had a greater effect on harvesting higher fatty acid, as more biomass results with a dense growth of cyanobacteria in the media (Tsygankov et al., 2002). In this context, the use of biomass for energy production is regarded as a suitable alternative owing to its renewable and carbon neutral features (Cho et al., 2011). The highest biomass was recorded from the isolate U9 (215 mg/mL) followed by U55 (91.8 mg/mL), U4 (73.8 mg/mL), U58 (71 mg/mL), U57 (59 mg/mL), U63 (63 mg/mL), U67 (57 mg/mL), U27 (53.3 mg/mL), U1 (50 mg/mL), U13 (35.6 mg/mL) respectively (Fig. 1).
Fig. 1.
Total pigments and biomass production in different cyanobacteria strain. The identities of the isolates denoted by the codes (U1, U2…) are shown in Annex- 1.1.
At the same time the highest total pigments were recorded for the isolate U27 (249.90 µg/mL) followed by U44 (188.4 µg/mL), U39 (178.61 µg/mL), U49 (176.26 µg/mL), U38 (172.3 µg/mL), U45 (153.75 µg/mL), U40 (147.05 µg/mL), U43 (134.98 µg/mL), U31 (143.61 µg/mL), U2 (128.14 µg/mL), U19 (120.03 µg/mL), U20 (111.46 µg/mL), U1 (102.32 µg/mL), U22 (68.12 µg/mL) and U55 (10.99 µg/mL) respectively (Fig. 1).
A study in Brazil reports, Geitlerinema amphibium produced up to 2.74 mg g−1 of astaxanthin and 5.49 mg g−1 of lutein, which is seven times more lutein than Marigold, currently the main raw material used commercially (D’Alessandro et al., 2019). Cyanobacteria possess a wide range of pigmentation due to their photosynthetic pigments consisting of chlorophyll-a (which all of them possess) together with different concentrations of accessory phyco-biliprotein pigments, phycocyanin (blue) and phycoerythrin (red). The final external colour of a specimen is largely dependent upon the concentrations of these pigments. Characteristically all cyanobacteria have thin or thick gelatinous sheaths outside their cell walls, their thickness and sometimes their colour contribute to the final appearance of the organism. Recently a few species of cyanobacteria have been investigated for biofuel production because their ability to convert solar energy to chemical energy has been found to be the most efficient among all living organisms. The efficiency rate for solar energy conversion in corn and sugarcane as 1–2%, for algae it is 5% and for cyanobacteria it is estimated as 10%. This is possible because of the photosynthetic pigments variations of cyanobacteria. In addition to chlorophyll-a cyanobacteria have phycobilisomes with the thylakoid membranes which act as antennae to harvest light for photosystem II. Among the pigments contained in the phycobilisomes, phycoerythrin absorbs energy between the wave lengths of 500 – 600 nm, phycocyanin absorbs between 550 and 650 nm and allophycocyanin absorbs between 600 and 675 nm. Together with chlorophyll-a, their light absorption capacity will therefore extend right across the entire spectrum of visible light. Researchers showed that certain part of the oil synthesis machinery in the chloroplasts of the plants and cyanobacteria are from same origin. The result showed cyanobacteria are capable of producing lipid which could be converted into fatty acid methyl ester to produce oil (Mohammed et al., 2020).
3.2. Mass culturing
3.2.1. Biomass harvesting time
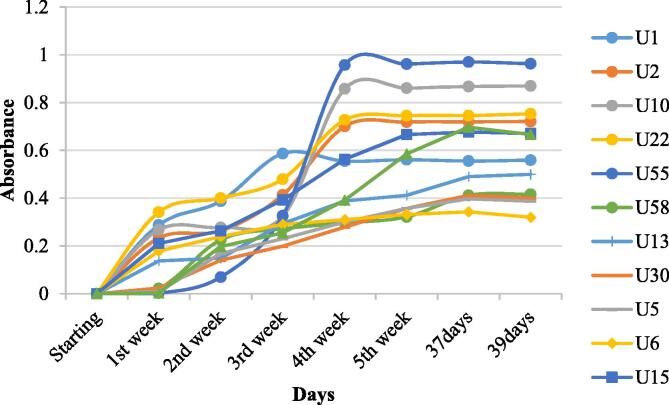
The light absorbances of cyanobacteria selected (based on total lipid, biomass and pigments production) for mass culturing in the present study at different growth stages are shown in Fig. 2. An increased concentration of biomass with the increase in the number of days was observed. A slight steady growth was observed from 5th week onwards (Fig. 2). Wavelength was scanned and maximum absorbance was recorded between 687 and 690 nm for the present study. The maximum absorbance was measured up to 39th day of growing, which was found to have same characteristics for all culture. The present study was supported by another study carried out by Santos and others (Santos-Ballardo et al., 2015). The maximum absorbance difference in cyanobacteria could be due to the different contents of pigments, such as chlorophyll-a, chlorophyll-b and carotenoids present in the cells (Bricaud et al., 1998). These results are similar to previous reports, where, for cell growth of some microalgae species, wavelengths were from 664 to 678 nm (Padovan, 1992), 680 nm (Geis et al., 2000), 684 nm (Rodrigues et al., 2011), and 687 nm (Valer & Glock, 1998).
Fig. 2.
Absorbance of cyanobacterial concentration in mass culture.
However, the standard tests of microalgal growth measurement using spectrophotometry, the wavelength 664–690 nm range is recommended, as these values are correlated with the absorbance of chlorophyll (Bricaud et al., 1998). In the present study, the cyanobacterial mass culture was found to grow continuously up to 35th day of its culture and showed steady growth curve thereafter. Therefore the optimum time of harvesting biomass from mass culture was 5th weeks onwards (Fig. 2).
U1: Leptolyngbya sp., U2: Phormidium sp., U5: Lyngbya sp., U6: Anabaena sp., U10: Synechococcus sp., U13: Croococcidiopsis sp., U15: Calothrix sp., U22: Cephalothrix sp., U30: Limnothrix sp., U55: Oscillatoria sp., U58: Westiellopsis sp., U67: Limnothrix sp.
3.2.2. Harvesting technique
Harvesting biomass from mass culture is one of the major hurdles in cyanobacterial research. In the presesent study flocculation technique was applied to harvest biomass. High molecular weight extracellular metabolites produced by cyanobacteria accumulate rapidly during the late log phase. These polymeric molecules comprise of long chain polysaccharides, proteins and nucleic acids and are of sufficient length to form bridges between algal particles, hence enhancing flocculation. Due to pH increase in the culture the extracellular polymers on the surface of the cyanobacterial cell colonies binds together causing them to become too dense to remain afloat. In the present study flocculating pH for different cyanobacteria strains were found to be different (Table 1).
Table 1.
Flocculating pH of samples.
| Samples tested | pH value at 39th Day of culture | Flocculating pH |
|---|---|---|
| U 01 | 9.01 | 11.51 |
| U 02 | 9.80 | >12.00 |
| U 05 | 9.19 | 11.52 |
| U 10 | 10.17 | >12.00 |
| U 13 | 10.36 | 11.52 |
| U 22 | 9.40 | >12.00 |
| U 30 | 10.32 | 11.85 |
| U 15 | 9.61 | 12.00 |
| U 6 | 9.81 | 11.81 |
| U 55 | 9.59 | 11.55 |
| U 58 | 9.36 | 11.51 |
| U 67 | 9.46 | 11.60 |
U1: Leptolyngbya sp., U2: Phormidium sp., U10: Synechococcus sp., U22: Cephalothrix sp., U55: Oscillatoria sp., U58: Wetiellopsis sp., U13: Croococcidiopsis sp., U30: Limnothrix sp., U5: Lyngbya sp., U6: Anabaena sp., U15: Calothrix sp., U67: Limnothrix sp.
3.3. Total lipid in cyanobacteria
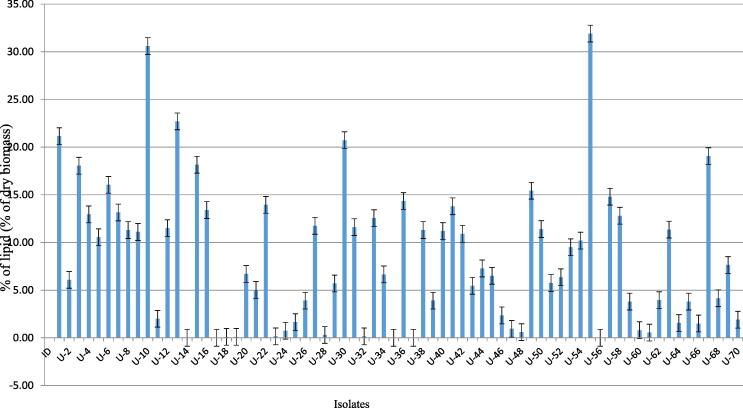
The lipid content was recorded maximum in the isolate U55 as Oscillatoria sp. (31.9 ± 2.01% of dry biomass) followed by U10 as Synechococcus sp. (30.6 ± 2.87%), U13 as Croococcidiopsis sp. (22.7 ± 1.36%), U1 as Leptolyngbya sp. (21.15 ± 1.99%), U30 as Limnothrix sp. (20.73 ± 3.26%), U67 as Limnothrix sp. (19.05 ± 0.78%), U15 as Calothrix sp. (18.15 ± 4.11%), U3 as Limnothrix sp. (18.05 ± 2.77%), U5 as Lyngbya sp. (16.05 ± 3.88%), U49 as Nostoc sp. (15.43 ± 3.89%), U57 as Plectonema sp. (14.8 ± 2.22%) respectively (Fig. 4). It was observed that the unicellular strains were proved as potential candidateswith high lipid content. However, filamentous strains such as Oscillatoria sp., Leptolyngbya sp., Limnothrix sp., and Lyngbya sp. were found to be highly lipid rich in the present study.
Fig. 4.
Lipid content (% of dry biomass) in different cyanobacterialisoaltes. The identities of the isolates denoted by the codes (U1, U2…) are shown in Annex 1.
Accordingly, the lipid content of the cyanobacterial culture reported in this study was found similar or higher than the recent values reported in some other studies (Cheng et al., 2013, Ehimen et al., 2010, Órpez et al., 2009, Song et al., 2013, Wahlen et al., 2011). The average lipid content of cyanobacteria and microalgae species is commonly 20–50% by weight of their dry biomass, although up to 80% of lipid contents have been reported (Chisti, 2007, Rajvanshi and Sharma, 2012). The lipid content is known to vary not only by the species of cyanobacterial cells, but also the growing conditions and the growth phase of the cells. These types of information are not usually available in the literature (Soydemir et al., 2016). However, Soydemir et al., (2016) reported that, mixed micro algal cultures was subjected to very low concentrations of nutrients at the time of the harvesting, the cells were at the stationary growth phase and N and P concentrations in water phase were about 3 mg L−1 and 0.1 mg L−1 respectively. Under such nutrient limiting conditions, the cells are expected to accumulate lipids (Soydemir et al., 2016). However, the nutritional requirements of cyanobacteria could be addressed by culturing them in wastewater. In Finland, researchers have explored the use of cyanobacteria to treat wastewater and feedstock for biodiesel production simultaneously. Cyanobacteria was reported best to clean municipal wastewater (Meghan Sapp, 2019).
Total glycerides (TG) are the parts of the lipids that are converted to biodiesel. For analysis of TGs, the preferred method involves esterification to form FAME. Using this approach in our studies, the TG content of the cyanobacterial lipid samples were measured gravimetrically or using GC. Once TG amount is quantified, an estimation was made as to how much of lipid can potentially be converted into biodiesel.
In the present study, it was observed that the cyanobacteria isolated from the dry zone are more competitive for lipid content. This can be due to their greater survival skills in high temperaure, pH and other physicochemical properties in that zone. The deposited fat might provide energy during dry periods for cyanobacterial survival.
3.4. Fatty acid compositions in cyanobacteria
The characterization of the FAME composition using GC analysis showed the presence of high Methyl palmitate, Linolelaidic acid methyl ester, Heptadecanoic acid methyl ester, Methyl octanoate, Methyl decanoate, Methyl laurate, Methyl tridecanoate, Methyl myristate, Myristoloeic acid, Cis-9-oleic acid methyl ester, Methyl arachidate, Cis-8,11,14-ecosatrieconoic acid methyl ester etc (Table 2). A representative scheme for GC analysis of Synechococcus sp. (U10) has been illustrated in Fig. 3. According to the American Society for Testing Materials (ASTM D6751) biodiesel standard, good quality biodiesel should have high oxidation stability, high cetane number and low iodine value. Another study on Amazonian cyanobacteria reported that Limnothrix sp. had a better lipid profile and produced high amounts of C16:0, which is favorable for the production of biodiesel. This strain also had better cetane number (58.06) above the minimum established by the ASTM (American Society for Tests and Materials) (de Oliveira et al., 2018).
Table 2.
Composition of fatty acid methyl esters related climatic zones and lipid % in cyanobacteria selected for the present study.
| Strain ID | Name of isolate | Type of FAME | Climatic zone | Lipid (% on dry biomass) |
|---|---|---|---|---|
| U-1 | Leptolyngbya sp. | Methyl behenate | Wet zone | 21.15 ± 1.99% |
| U-2 | Phormidium sp. | Methyl palmitoleate, Linolelaidic acid methyl ester, Cis-8,11,14-eicosatrienoic acid methyl ester | Wet zone | 6.08 ± 1.21% |
| U-7 | Phormidium sp. | Cis-10-heptadecanoic acid methyl ester, Methyl myristate, Methyl pentadecanoate | Dry Zone | 18.05 ± 2.11% |
| U-10 | Synechococcus sp. | Cis-10-heptadecanoic acid methyl ester, Methyl octanoate, Methyl decanoate, Methyl laurate, Methyl tridecanoate, Methyl myristate | Intermediate zone | 30.6 ± 2.87% |
| U-13 | Croococcidiopsis sp. | Undentified | Dry Zone | 22.7 ± 1.36% |
| U-16 | Croococcidiopsis sp. | Undentified | Wet zone | 13.40 ± 2.94% |
| U-22 | Cephalothrixsp. | Cis-10-heptadecanoic acid methyl ester | Dry Zone | 13.95 ± 4.27% |
| U-41 | Cephalothrix komarekiana | Cis-10-heptadecanoic acid methyl ester | Dry Zone | 13.8 ± 3.56% |
| U-42 | Synechocystis sp. | Methyl palmitoleate | Dry Zone | 10.90 ± 2.65% |
| U-55 | Oscillatoriales sp. | Methyl pentadeconoate, Methyl heptadeconoate, Linolaidic acid methyl ester, Methyl erucate | Dry Zone | 31.9 ± 2.01% |
| U-58 | Westiellopsisprolifica | Cis-10-heptadecanoic acid methyl ester | Intermediate zone | 12.80 ± 1.97% |
| U-67 | Limnothrix sp. | Methyl myristate, Myristoloeic acid, Methyl palmitate, Cis-9-oleic acid methyl ester, Methyl arachidate, Cis-8,11,14-ecosatrieconoic acid methyl ester | Dry Zone | 20.73 ± 3.26% |
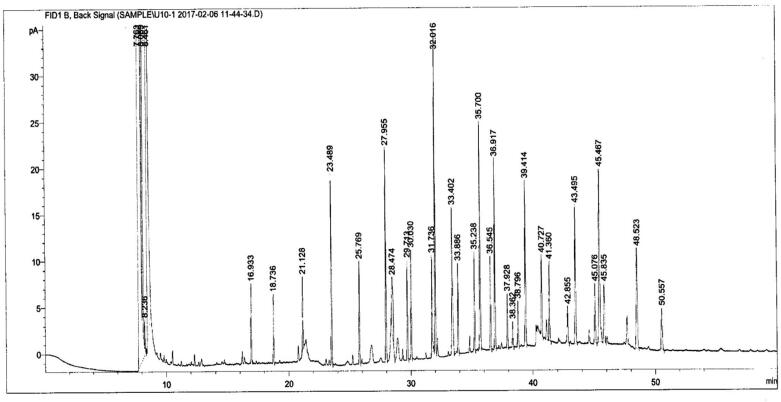
Fig. 3.
Gas chromatogram image of cyanobaccterial isolate Synechococcus sp. (U10).
It is reported that higher amount of palmitic and stearic acid result with high cetane number and lower iodine value (Kaur et al., 2012; Ramos et al., 2009). In the present study, some of the peaks were unidentified because of overlapping with several other peaks or shifting the retention time in the chromatogram in respect to the standard peaks (U13 and U16).
4. Conclusions
The present study was carried out to isolate cyanobacteria from fresh water bodies of Sri Lanka, to investigate them as a potential source for biodiesel and other value added products. A total 74 cyanobacterial monocultures were obtained. It was found that cyanobacteria are highly rich in lipid content and total lipid content was recorded highest in Oscillatoriales with sp.31.9 ± 2.01% of dry biomass. The Gas chromatograph of FAME confirmed the presence of different types of esters which indicated the potential of using cyanobacteria as a raw material for biodiesel industry. At the same time, the remaining biomass was found to be rich in high carbohydrate, high protein, antioxidant properties, antipathogenic properties, high sun protection factor value and high pigments content. Molecular identification key confirmed the presence of a novel species Cephalothrix komarekiana for the first time in Sri Lanka.
Further studies are required for optimization of mass culture conditions of cyanobacteria. To increase the lipid yeild, cyanobacterial mixed cultures can be considered. Attempts should be taken for identification and isolation of the active compounds responsible for antipathogenic and antioxidant properties. The procedures for biodiesel production in the current study should be followed by standard purification procedures to obtain high cetane number.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
Acknowledgement
The authors extend their appreciation to the Ministry of Higher Education, Sri Lankafor funding this Research work. The contribution of Professor Dr. Amin Chowdhury is also acknowledged for proof reading the final manuscript.
Authors’ contributions
Md. Fuad Hossain, R.R. Ratnayake and K.L. Wasantha Kumara designed the study, Md. Fuad Hossain collected the sample. Md. Fuad Hossain and D.N. Magana-Arachchi performed the molecular analysis and analyzed the data. Md. Fuad Hossain and Shamim Mahbub wrote the manuscript, prepared the first draft and finally Shamim Mahbub submitted with the contribution of the other authors.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
R.R. Ratnayake, Email: renukar@ifs.ac.lk.
Shamim Mahbub, Email: smahbub01@gmail.com.
References
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricaud A., Morel A., Babin M., Allali K., Claustre H. Variations of light absorption by suspended particles with chlorophyll a concentration in oceanic(case 1) waters- analysis and implications for bio-optical models. J. Geophys. Res. 1998;103(C13):31033–31044. [Google Scholar]
- Bryant D.A., Guglielmi G., de Marsac N.T., Castets A.-M., Cohen-Bazire G. The structure of cyanobacterial phycobilisomes: a model. Arch. Microbiol. 1979;123(2):113–127. [Google Scholar]
- Cheng, J., Yu, T., Li, T., Zhou, J., Cen, K., 2013. Using wet microalgae for direct biodiesel production via microwave irradiation. Bioresource technology, 131(531-35. [DOI] [PubMed]
- Chisti Y. Biodiesel from microalgae. Biotechnol. Adv. 2007;25(3):294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Cho S., Luong T.T., Lee D., Oh Y.-K., Lee T. Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour. Technol. 2011;102(18):8639–8645. doi: 10.1016/j.biortech.2011.03.037. [DOI] [PubMed] [Google Scholar]
- D’Alessandro E.B., Soares A.T., de Oliveira D’Alessandro N.C. Potential use of a thermal water cyanobacterium as raw material to produce biodiesel and pigments. Bioprocess Biosyst. Eng. 2019;42:2015–2022. doi: 10.1007/s00449-019-02196-5. [DOI] [PubMed] [Google Scholar]
- de Oliveira, D.T., Vasconcelos, C.T., Feitosa, A.M.T., Aboim, J.B., de Oliveira, A.D.N., Xavier, L.P., Santos, A.S., Gonçalves, E.C., da Rocha Filho, G.N. and do Nascimento, L.A.S., 2018. Lipid profile analysis of three new Amazonian cyanobacteria as potential sources of biodiesel. Fuel, 234, 785-788.Dos Santos, R.R., Moreira, D.M., Kunigami, C.N., Aranda, D.A.G., Teixeira, C.M.L.L., 2014. Comparison between several methods of total lipid extraction from< i> chlorella vulgaris</i> biomass. Ultrasonics Sonochemistry.
- Ehimen E., Sun Z., Carrington C. Variables affecting the in situ transesterification of microalgae lipids. Fuel. 2010;89(3):677–684. [Google Scholar]
- Ferdous K., Uddin M.R., Islam R., Khan M.R., Islam M. Potentiality of biodiesel production from non-edible oil: Bangladesh perspective. J. Chem. Eng. 2014;27(2):1–5. [Google Scholar]
- Geis S.W., Fleming K.L., Korthals E.T., Searle G., Reynolds L., Karner D.A. Modifications to the algal growth inhibition test for use as a regulatory assay. Environ. Toxicol. Chem. 2000;19(1):36–41. [Google Scholar]
- Gnansounou, E., Larroche, C., Pandey, A., 2008. Biofuels ii. Special Issue of J. Sci. Ind. Res., 67(837-1040.
- Halim R., Harun R., Webley P.A., Danquah M.K. Bioprocess engineering aspects of biodiesel and bioethanol production from microalgae. Adv. Biofuels Bioproducts. Springer. 2013:601–628. [Google Scholar]
- IEA . International Energy Agency; Paris(OECD/IEA: 2012. World energy outlook 2012. [Google Scholar]
- International Energy Agency, 2014. Fossil fuel energy consumption, sri lanka. http://www.iea.org/stats/index.asp, Online(Accessed 25 December 2016).
- Korres N.E., Singh A., Nizami A.S., Murphy J.D. Is grass biomethane a sustainable transport biofuel? Biofuels, Bioprod. Biorefin. 2010;4(3):310–325. [Google Scholar]
- Li Y., Horsman M., Wu N., Lan C.Q., Dubois-Calero N. Biofuels from microalgae. Biotechnol. Prog. 2008;24(4):815–820. doi: 10.1021/bp070371k. [DOI] [PubMed] [Google Scholar]
- Mutanda T., Ramesh D., Karthikeyan S., Kumari S., Anandraj A., Bux F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011;102(1):57–70. doi: 10.1016/j.biortech.2010.06.077. [DOI] [PubMed] [Google Scholar]
- Meghan Sapp., 2019. Finnish researcher looks at cyanobacteria to clean up wastewater and production biodiesel: Available at: https://www.biofuelsdigest.com/bdigest/2019/06/11/finnish-researcher-looks-at-cyanobacteria-to-clean-up-wastewater-and-production-biodiesel/ Retrieved on 21 Mar 2020.
- Mohammed A., et al. 2020. Triacylglycerol and phytyl ester synthesis in Synechocystis sp. PCC6803, Proceedings of the National Academy of Sciences (2020). DOI: 10.1073/pnas.1915930117. [DOI] [PMC free article] [PubMed]
- Olenina, I., Hajdu, S., Wasmund, N., Jurgensone, I., Gromisz, S., Kownacka, J., Toming, K., Olenin, S., 2009. Impacts of invasive phytoplankton species on the baltic sea ecosystem in 1980-2008. HELCOM Indicator Fact Sheets. [DOI] [PubMed]
- Órpez R., Martínez M.E., Hodaifa G., El Yousfi F., Jbari N., Sánchez S. Growth of the microalga botryococcus braunii in secondarily treated sewage. Desalination. 2009;246(1):625–630. [Google Scholar]
- Padovan A. Isolation and culture of five species of freshwater algae from the alligator rivers region, northern territory. Austr. Gov. Pub. Service. 1992 [Google Scholar]
- Pandey A. CRC Press; Boca Raton: 2009. Handbook of plant-based biofuels. [Google Scholar]
- Parmar A., Singh N.K., Pandey A., Gnansounou E., Madamwar D. Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour. Technol. 2011;102(22):10163–10172. doi: 10.1016/j.biortech.2011.08.030. [DOI] [PubMed] [Google Scholar]
- Pereira H., Barreira L., Mozes A., Florindo C., Polo C., Duarte C.V., Custódio L., Varela J. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels. 2011;4(1):1–12. doi: 10.1186/1754-6834-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posten C., Schaub G. Microalgae and terrestrial biomass as source for fuels—a process view. J. Biotechnol. 2009;142(1):64–69. doi: 10.1016/j.jbiotec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Rajvanshi S., Sharma M.P. Micro algae: a potential source of biodiesel. J. Sustain. Bioenergy Syst. 2012;2(03):49. [Google Scholar]
- Rippka R., Deruelles J., Waterbury J.B., Herdman M., Stanier R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979;111(1):1–61. [Google Scholar]
- Rodrigues L.H.R., Raya-Rodriguez M.T., Fontoura N.F. Algal density assessed by spectrophotometry: a calibration curve for the unicellular algae pseudokirchneriella subcapitata. J. Environ. Chem. Ecotoxicol. 2011;3(8):225–228. [Google Scholar]
- Santos-Ballardo, D.U., Rossi, S., Hernández, V., Gómez, R.V., del Carmen Rendón-Unceta, M., Caro-Corrales, J., Valdez-Ortiz, A., 2015. A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquaculture, 448(87-92.
- Song, M., Pei, H., Hu, W., Ma, G., 2013. Evaluation of the potential of 10 microalgal strains for biodiesel production. Bioresour. Technol., 141(245-51. [DOI] [PubMed]
- Soydemir G., Keris-Sen U.D., Sen U., Gurol M.D. Biodiesel production potential of mixed microalgal culture grown in domestic wastewater. Bioprocess Biosyst. Eng. 2016;39(1):45–51. doi: 10.1007/s00449-015-1487-3. [DOI] [PubMed] [Google Scholar]
- Stanier R., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order chroococcales) Bacteriol. Rev. 1971;35(2):171. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff, F.S., Karlberg, M., Graeve, M., Wulff, A., 2014. Cyanobacteria in scandinavian coastal waters—a potential source for biofuels and fatty acids? Algal Res., 5(42-51.
- Sumanta N., Haque C.I., Nishika J., Suprakash R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014;4(9):63–69. [Google Scholar]
- Tsygankov A., Fedorov A., Kosourov S., Rao K. Hydrogen production by cyanobacteria in an automated outdoor photobioreactor under aerobic conditions. Biotechnol. Bioeng. 2002;80(7):777–783. doi: 10.1002/bit.10431. [DOI] [PubMed] [Google Scholar]
- Valer R., Glock L. Quantificação de algas clorofíceas de interesse ecotoxicológico através do método espectrofotométrico. Acta Limnol. Bras. 1998;11(2):149–156. [Google Scholar]
- Wahlen B.D., Willis R.M., Seefeldt L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011;102(3):2724–2730. doi: 10.1016/j.biortech.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Wasmund N., Tuimala J., Suikkanen S., Vandepitte L., Kraberg A. Long-term trends in phytoplankton composition in the western and central baltic sea. J. Mar. Syst. 2011;87(2):145–159. [Google Scholar]
References
Further reading
- Alexandratos N., Bruinsma J. FAO; Rome: 2012. World agriculture towards 2030/2050: The 2012 revision. ESA Working paper. [Google Scholar]
- Heilig, G.K., 2012. World urbanization prospects the 2011 revision. United Nations, Department of Economic and Social Affairs (DESA), Population Division, Population Estimates and Projections Section, New York.
- Pandey V. Cyanobacterial natural products as antimicrobial agents. Int. J. Curr. Microbiol. App. Sci. 2015;4(1):310–317. [Google Scholar]